Ameliorative Effect of Medicarpin on Scopolamine-Induced Cognitive Impairment in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Administration
2.2. Chemicals and Enzymes
2.3. Animal Behavioral Experiments
2.3.1. Morris Water Maze (MWM)
2.3.2. Passive Avoidance Test (PAT)
2.3.3. Y-Maze
2.4. Enzyme Assays
2.5. Cell Culture
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Animal Behavioral Test
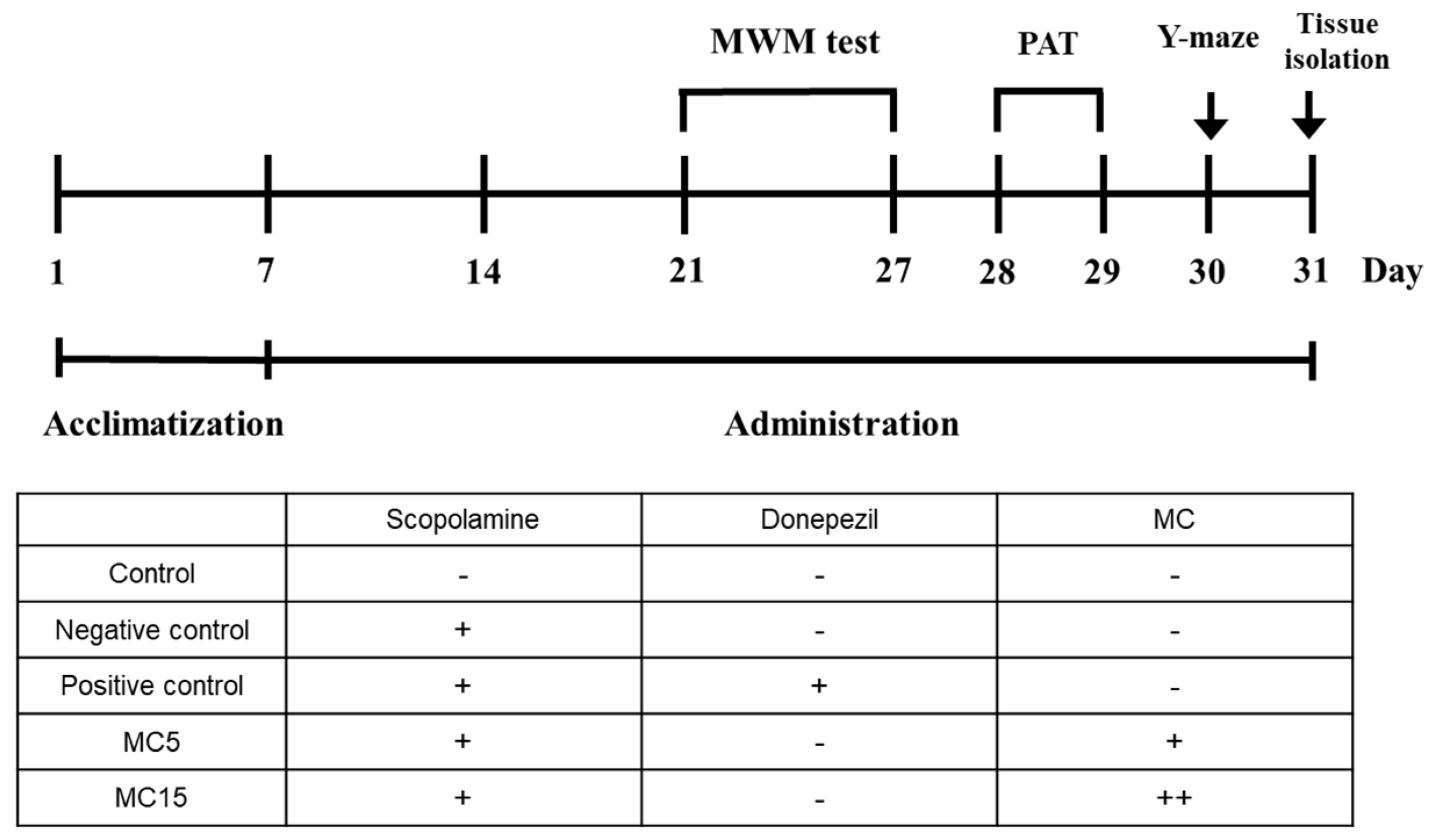
3.1.1. Animal Experiment Plan
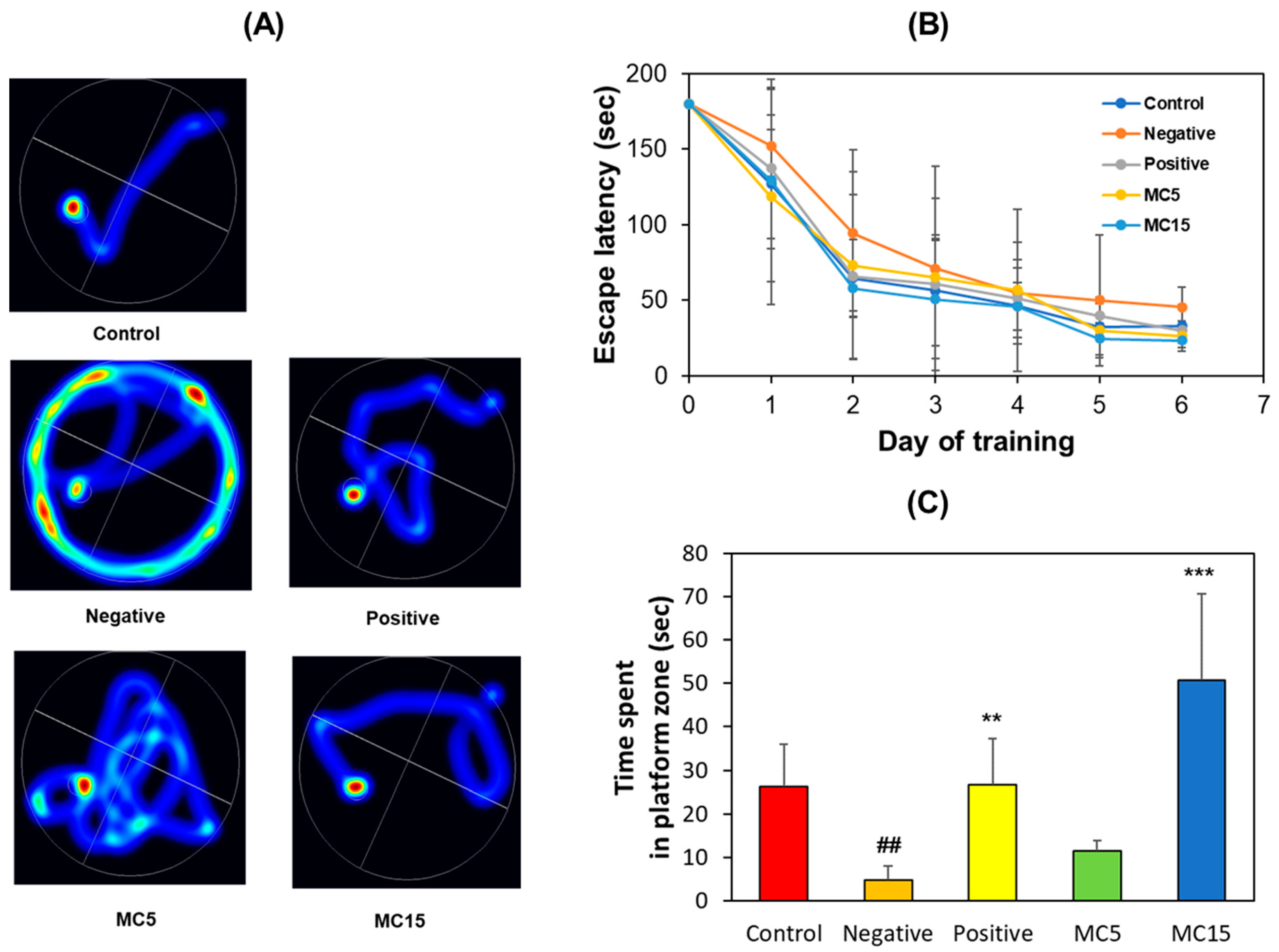
3.1.2. MWM
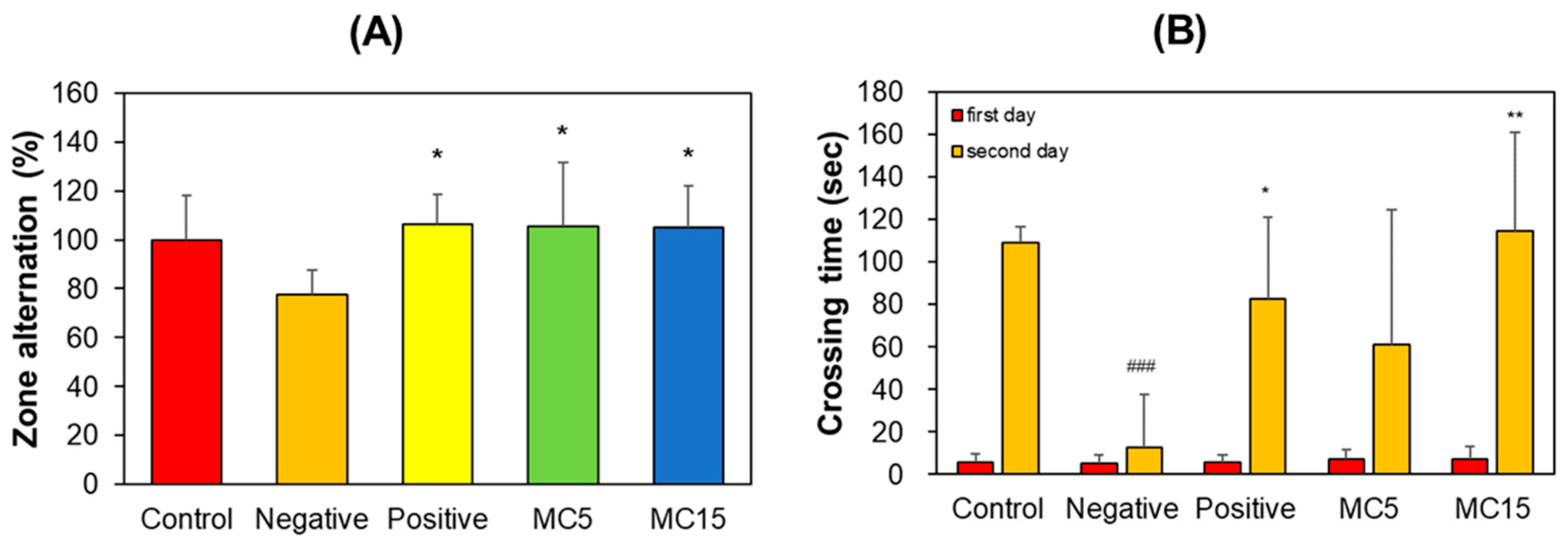
3.1.3. Y-Maze and PAT
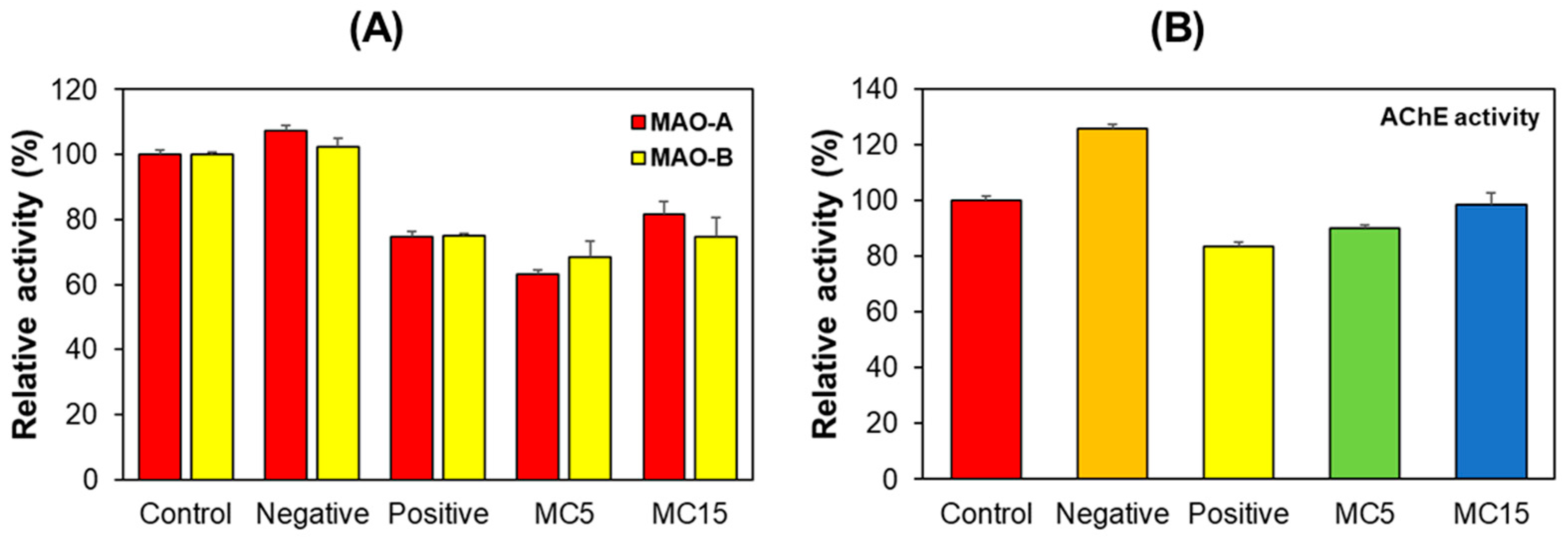
3.2. Enzyme Assays in Hippocampus Tissues
3.3. Enzyme Assays in SH-SY5Y Cells
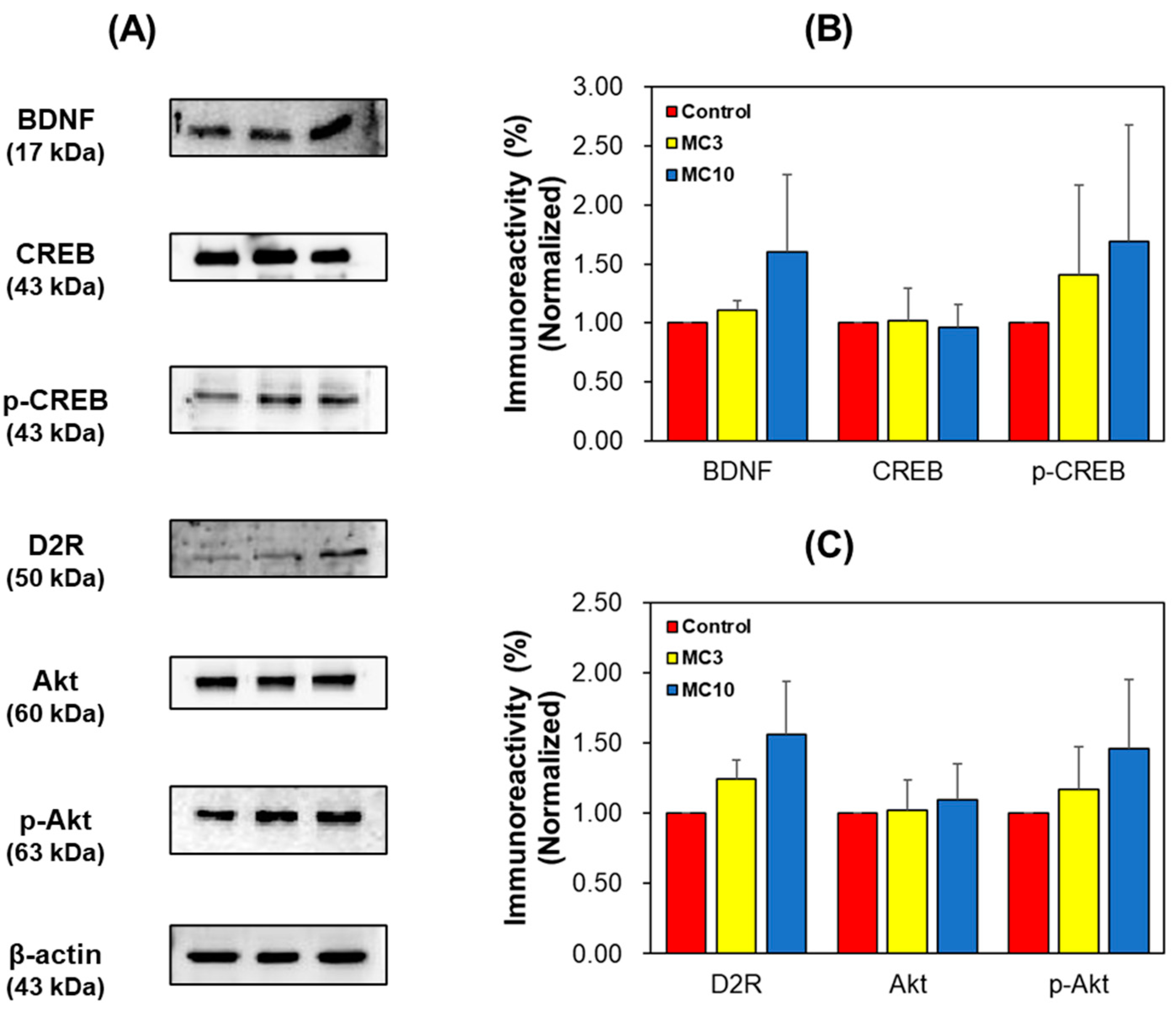
3.4. Western Blotting of Hippocampus Tissues and SH-SY5Y Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Se Thoe, E.; Fauzi, A.; Tang, Y.Q.; Chamyuang, S.; Chia, A.Y.Y. A review on advances of treatment modalities for Alzheimer’s disease. Life Sci. 2021, 276, 119129. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef] [PubMed]
- Moussa-Pacha, N.M.; Abdin, S.M.; Omar, H.A.; Alniss, H.; Al-Tel, T.H. BACE1 inhibitors: Current status and future directions in treating Alzheimer’s disease. Med. Res. Rev. 2020, 40, 339–384. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Dubey, R. Target enzyme in Alzheimer’s disease: Acetylcholinesterase inhibitors. Curr. Top Med. Chem. 2019, 19, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Ali, S.; Asad, M.H.H.B.; Maity, S.; Zada, W.; Rizvanov, A.A.; Iqbal, J.; Babak, B.; Hussain, I. Fluoro-benzimidazole derivatives to cure Alzheimer’s disease: In-silico studies, synthesis, structure-activity relationship and in vivo evaluation for β secretase enzyme inhibition. Bioorg. Chem. 2019, 88, 102936. [Google Scholar] [CrossRef] [PubMed]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B. A Review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharm. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. 2014, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Bronnick, K.; Williams-Gray, C.; Weintraub, D.; Marder, K.; Kulisevsky, J.; Burn, D.; Barone, P.; Pagonabarraga, J.; Allcock, L.; et al. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology 2010, 75, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Marino, B.L.B.; de Souza, L.R.; Sousa, K.P.A.; Ferreira, J.V.; Padilha, E.C.; da Silva, C.H.T.P.; Taft, C.A.; Hage-Melim, L.I.S. Parkinson’s disease: A review from pathophysiology to treatment. Mini Rev. Med. Chem. 2020, 20, 754–767. [Google Scholar] [CrossRef]
- Masato, A.; Plotegher, N.; Boassa, D.; Bubacco, L. Impaired dopamine metabolism in Parkinson’s disease pathogenesis. Mol. Neurodegener. 2019, 14, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.-M.; Gainetdinov, R.R.; Caron, M.G. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol. Sci. 2007, 28, 166–172. [Google Scholar] [CrossRef]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.-K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef] [PubMed]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015, 6, 331–341. [Google Scholar]
- Yan, L.; Xu, X.; He, Z.; Wang, S.; Zhao, L.; Qiu, J.; Wang, D.; Gong, Z.; Qiu, X.; Huang, H. Antidepressant-like effects and cognitive enhancement of coadministration of chaihu shugan san and fluoxetine: Dependent on the BDNF-ERK-CREB signaling pathway in the hippocampus and frontal cortex. Biomed. Res. Int. 2020, 2020, 2794263. [Google Scholar] [CrossRef] [Green Version]
- Lian, W.-W.; Zhou, W.; Zhang, B.-Y.; Jia, H.; Xu, L.-J.; Liu, A.-L.; Du, G.-H. DL0410 ameliorates cognitive disorder in SAMP8 mice by promoting mitochondrial dynamics and the NMDAR-CREB-BDNF pathway. Acta Pharmacol. Sin. 2021, 42, 1055–1068. [Google Scholar] [CrossRef]
- Guo, C.; Liu, Y.; Fang, M.-S.; Li, Y.; Li, W.; Mahaman, Y.A.R.; Zeng, K.; Xia, Y.; Ke, D.; Liu, R.; et al. ω-3PUFAs improve cognitive impairments through Ser133 phosphorylation of CREB upregulating BDNF/TrkB signal in Schizophrenia. Neurotherapeutics 2020, 17, 1271–1286. [Google Scholar] [CrossRef]
- Calabresi, P.; Picconi, B.; Parnetti, L.; Di Filippo, M. A convergent model for cognitive dysfunctions in Parkinson’s Disease: The critical dopamine-acetylcholine synaptic balance. Lancet Neurol. 2006, 5, 974–983. [Google Scholar] [CrossRef]
- Emre, M. Dementia associated with Parkinson’s disease. Lancet Neurol. 2003, 2, 229–237. [Google Scholar] [CrossRef]
- Nakano, I.; Hirano, A. Parkinson’s disease: Neuron loss in the nucleus basalis without concomitant Alzheimer’s disease. Ann. Neurol. 1984, 15, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.K.; Curtis, M.; Dick, D.J.; Candy, J.M.; Atack, J.R.; Bloxham, C.A.; Blessed, G.; Fairbairn, A.; Tomlinson, B.E.; Perry, R.H. Cholinergic correlates of cognitive impairment in Parkinson’s disease: Comparisons with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1985, 48, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesulam, M.; Guillozet, A.; Shaw, P.; Quinn, B. Widely spread butyrylcholinesterase can hydrolyze acetylcholine in the normal and Alzheimer brain. Neurobiol. Dis. 2002, 9, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016, 16, 247–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, F.C. Tacrine therapy for the dementia of Alzheimer’s disease. Am. Fam. Physician 1994, 50, 819–826. [Google Scholar]
- Al Mamun, A.; Uddin, M.S. KDS2010: A potent highly selective and reversible MAO-B inhibitor for Alzheimer’s disease. Comb. Chem. High Throughput Screen. 2020, 23, 836–841. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Albreht, A. Kinetics, mechanism, and inhibition of monoamine oxidase. J. Neural Transm. 2018, 125, 1659–1683. [Google Scholar] [CrossRef] [Green Version]
- Schedin-Weiss, S.; Inoue, M.; Hromadkova, L.; Teranishi, Y.; Yamamoto, N.G.; Wiehager, B.; Bogdanovic, N.; Winblad, B.; Sandebring-Matton, A.; Frykman, S.; et al. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with γ-secretase and regulates neuronal amyloid β-peptide levels. Alzheimers Res. Ther. 2017, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.M.; Gabr, M.T. Multitarget therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2019, 14, 437–440. [Google Scholar] [PubMed]
- Chowdhury, S.; Kumar, S. Inhibition of BACE1, MAO-B, Cholinesterase enzymes, and anti-amyloidogenic potential of selected natural phytoconstituents: Multi-target-directed ligand approach. J. Food Biochem. 2021, 45, e13571. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R.; Tipton, K.F. Assessment of Enzyme Inhibition: A review with examples from the development of monoamine oxidase and cholinesterase inhibitory drugs. Molecules 2017, 22, 1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, B.; Parambi, D.G.T.; Mathew, G.E.; Uddin, M.S.; Inasu, S.T.; Kim, H.; Marathakam, A.; Unnikrishnan, M.K.; Carradori, S. Emerging therapeutic potentials of dual-acting MAO and AChE inhibitors in Alzheimer’s and Parkinson’s diseases. Arch. Pharm. 2019, 352, e1900177. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.-J.; Lee, H.-J.; Yoo, E.-S.; Jung, D.-S.; Riu, K.-Z.; Lee, S.-J. Antioxidant effects and inhibitory effect on NO synthesis by extracts of Canavalia lineata. Kor. J. Pharmacogn. 2004, 35, 338–345. [Google Scholar]
- Hong, S.-J.; Kwon, O.-K.; Hwang, D.; Goo, S.H.; Kim, D.-Y.; Kim, M.H.; Kim, S.-Y.; Jang, H.-J.; Oh, S.-R. Anti-inflammatory activity of cajanin, an isoflavonoid derivative isolated from Canavalia lineata pods. Int. J. Mol. Sci. 2022, 23, 9492. [Google Scholar] [CrossRef]
- Oh, J.M.; Jang, H.-J.; Kang, M.-G.; Mun, S.-K.; Park, D.; Hong, S.-J.; Kim, M.H.; Kim, S.-Y.; Yee, S.-T.; Kim, H. Medicarpin and homopterocarpin isolated from Canavalia lineata as potent and competitive reversible inhibitors of human monoamine oxidase-B. Molecules 2022, 28, 258. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, Y.; Cao, Y.; Shan, M.; Song, D.; Ye, C.; Zhu, D. Studies on chemical composition of Pueraria lobata and its anti-tumor mechanism. Molecules 2022, 27, 7253. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Sanz, A.; Cifuentes, A.; Ibánez, E.; Paape, T.; Lucas, M.M.; Pueyo, J.J. Flavonoid accumulation varies in Medicago truncatula in response to mercury stress. Front. Plant Sci. 2022, 13, 933209. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.W.; Tan, H.B.; Li, B.L.; Qiu, S.X. Three new pterocarpans from the aerial parts of Abrus precatorius. Nat. Prod. Res. 2020, 34, 1836–1844. [Google Scholar] [CrossRef]
- Dixit, M.; Raghuvanshi, A.; Gupta, C.P.; Kureel, J.; Mansoori, M.N.; Shukla, P.; John, A.A.; Singh, K.; Purohit, D.; Awasthi, P.; et al. Medicarpin, a natural pterocarpan, heals cortical bone defect by activation of notch and wnt canonical signaling pathways. PLoS ONE 2015, 10, e0144541. [Google Scholar] [CrossRef] [Green Version]
- Ghribi, L.; Waffo-Téguo, P.; Cluzet, S.; Marchal, A.; Marques, J.; Mérillon, J.M.; Ben Jannet, H. Isolation and structure elucidation of bioactive compounds from the roots of the Tunisian Ononis angustissima L. Bioorganic Med. Chem. Lett. 2015, 25, 3825–3830. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kang, D.-M.; Cho, Y.-J.; Hyun, J.-W.; Ahn, M.-J. Medicarpin increases antioxidant genes by inducing NRF2 transcriptional level in HeLa cells. Antioxidants 2022, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.B.; Zhao, Y.Y.; Wang, B.; Zhang, Q.Y.; Zhang, L.; Tu, P. Quantification and stability studies on the flavonoids of Radix hedysari. J. Agric. Food Chem. 2006, 54, 6634–6639. [Google Scholar] [CrossRef] [PubMed]
- Chern, C.M.; Lu, C.K.; Liou, K.T.; Wang, Y.H.; Tsai, K.C.; Chang, C.L.; Chang, C.C.; Shen, Y.C. Medicarpin isolated from Radix hedysari ameliorates brain injury in a murine model of cerebral ischemia. J. Food Drug Anal. 2021, 29, 581–605. [Google Scholar] [CrossRef]
- Mansoori, M.N.; Raghuvanshi, A.; Shukla, P.; Awasthi, P.; Trivedi, R.; Goel, A.; Singh, D. Medicarpin Prevents arthritis in post-menopausal conditions by arresting the expansion of TH17 cells and pro-inflammatory cytokines. Int. Immunopharmacol. 2020, 82, 106299. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Maurya, R.; Mishra, D.P. Medicarpin, a legume phytoalexin sensitizes myeloid leukemia cells to TRAIL-induced apoptosis through the induction of DR5 and activation of the ROS-JNK-CHOP pathway. Cell Death Dis. 2014, 5, e1465. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Cai, C.; Liao, Y.; Wu, Q.; Ke, H.; Guo, P.; Wang, Q.; Ding, B.; Fang, J.; Fang, S. Systems pharmacology approach uncovers the therapeutic mechanism of medicarpin against scopolamine-induced memory loss. Phytomedicine 2021, 91, 153662. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Zhang, J.; Zhou, Q.; Liu, X.; Zhou, G. Medicarpin improves depressive-like behaviors in a chronic unpredictable mild stress-induced mouse model of depression by upregulating liver X receptor β expression in the amygdala. Neurotox. Res. 2022, 40, 1937–1947. [Google Scholar] [CrossRef]
- Oh, J.M.; Ji, M.; Lee, M.-J.; Jeong, G.S.; Paik, M.-J.; Kim, H.; Suh, J.-W. Antidepressant-like effects of ethanol extract of Ziziphus jujuba Mill seeds in Mice. Appl. Sci. 2020, 10, 7374. [Google Scholar] [CrossRef]
- Oh, J.M.; Lee, H.-S.; Baek, S.C.; Lee, J.P.; Jeong, G.S.; Paik, M.-J.; Kim, H. Antidepressant-like activities of hispidol and decursin in mice and analysis of neurotransmitter monoamines. Neurochem. Res. 2020, 45, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, C.; Zhang, X.; Chen, H.; Dong, J.; Lu, W.; Song, Z.; Zhou, W. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J. Neuroinflammation 2018, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Sunyer, B.; Höger, H.; Lubec, G. Evaluation of spatial memory of C57BL/6J and CD1 mice in the barnes maze, the multiple T-maze and in the Morris water maze. Behav. Brain Res. 2009, 198, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Zamansoltani, F.; Javadi, A.; Ganjvar, M. The effects of rutin on a passive avoidance test in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Izadpanah, F.; Arab, F.; Zarghami, A.; Bijani, A.; Kazemi, S.; Moghadamnia, A.A. The Effect of lamotrigine on learning in mice using the passive avoidance model. Epilepsy Behav. 2017, 69, 1–6. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Asai, M.; Hara, T. High-fat diet enhances working memory in the Y-maze test in male C57BL/6J mice with less anxiety in the elevated plus maze test. Nutrients 2020, 12, 2036. [Google Scholar] [CrossRef]
- Rao, S.S.; Lago, L.; Volitakis, I.; Shukla, J.J.; McColl, G.; Finkelstein, D.I.; Adlard, P.A. Deferiprone treatment in aged transgenic tau mice improves Y-maze performance and alters tau pathology. Neurotherapeutics 2021, 18, 1081–1094. [Google Scholar] [CrossRef]
- Oh, J.M.; Jang, H.-J.; Kim, W.J.; Kang, M.-G.; Baek, S.C.; Lee, J.P.; Park, D.; Oh, S.-R.; Kim, H. Calycosin and 8-O-methylretusin isolated from Maackia amurensis as potent and selective reversible inhibitors of human monoamine oxidase-B. Int. J. Biol. Macromol. 2020, 151, 441–448. [Google Scholar] [CrossRef]
- Lee, J.P.; Kang, M.-G.; Lee, J.Y.; Oh, J.M.; Baek, S.C.; Leem, H.H.; Park, D.; Cho, M.-L.; Kim, H. Potent inhibition of acetylcholinesterase by sargachromanol I from Sargassum siliquastrum and by selected natural compounds. Bioorg. Chem. 2019, 89, 103043. [Google Scholar] [CrossRef]
- Park, J.E.; Mun, S.-K.; Yee, S.-T.; Kim, H. Evaluation of inhibitory activities of Sophora flavescens and Angelica gigas Nakai root extracts against monoamine oxidases, cholinesterases, and beta-Secretase. Processes 2022, 10, 880. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Wu, Z.; Yu, X.; Yin, Y.; Qian, S.; Wang, Z.; Huang, J.; Wang, W.; Liu, T.; et al. Quercitrin rapidly alleviated depression-like behaviors in lipopolysaccharide-treated mice: The involvement of PI3K/AKT/NF-κB signaling suppression and CREB/BDNF signaling restoration in the hippocampus. ACS Chem. Neurosci. 2021, 12, 3387–3396. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.M.; Park, J.E.; Mun, S.-K.; Yee, S.-T.; Kim, H. Ameliorative Effect of Medicarpin on Scopolamine-Induced Cognitive Impairment in Mice. Processes 2023, 11, 385. https://doi.org/10.3390/pr11020385
Oh JM, Park JE, Mun S-K, Yee S-T, Kim H. Ameliorative Effect of Medicarpin on Scopolamine-Induced Cognitive Impairment in Mice. Processes. 2023; 11(2):385. https://doi.org/10.3390/pr11020385
Chicago/Turabian StyleOh, Jong Min, Jong Eun Park, Seul-Ki Mun, Sung-Tae Yee, and Hoon Kim. 2023. "Ameliorative Effect of Medicarpin on Scopolamine-Induced Cognitive Impairment in Mice" Processes 11, no. 2: 385. https://doi.org/10.3390/pr11020385
APA StyleOh, J. M., Park, J. E., Mun, S.-K., Yee, S.-T., & Kim, H. (2023). Ameliorative Effect of Medicarpin on Scopolamine-Induced Cognitive Impairment in Mice. Processes, 11(2), 385. https://doi.org/10.3390/pr11020385







