Thermodynamic Nature of SiO2 and FeO in Flux O Potential Control Subject to Submerged Arc Welding Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flux Preparation
2.2. Thermodynamic Calculation
- The FToxid database was selected to model the slag.
- The equilibrium temperature of 2000 °C was set.
- The measured slag compositions were set as the input compositions.
- FToxid, Fstel, and FactPS databases are selected. The solution phases of ASlag-liq all oxides, S (FToxid-SLAGA), and LIQUID (FStel-Liqu) were selected to model the molten slag and metal phases.
- The equilibrium temperature of 2000 °C was set.
- The flux formulas and nominal compositions (if the dilution value is not given, it may be assumed to be 0.5) were set as the input chemistries [30].
3. Results and Discussion
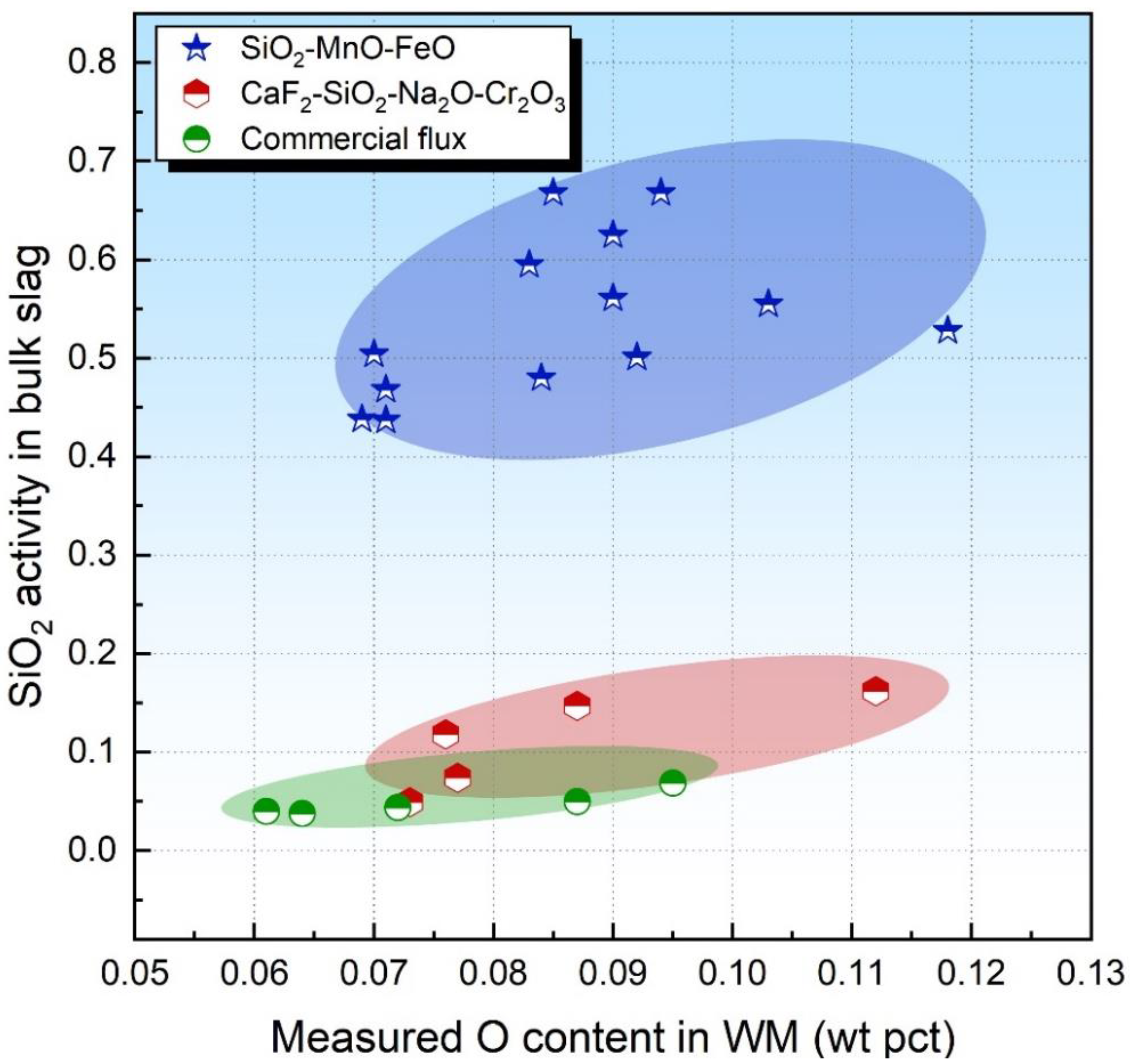
3.1. Flux O Potential vs. SiO2 and FeO Activities in Bulk Slag
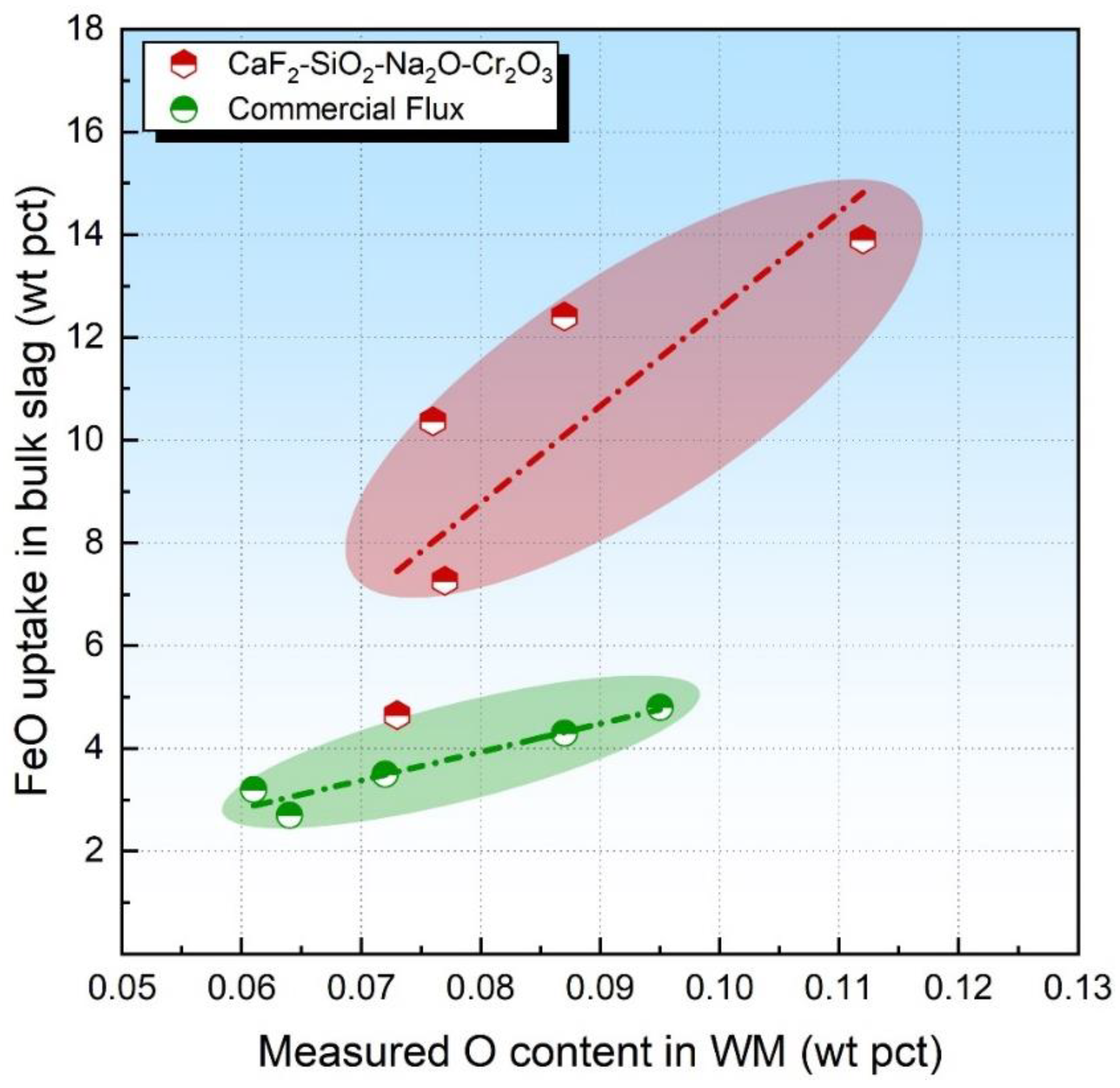
3.2. Flux O Potential vs. FeO Uptake in Bulk Slag
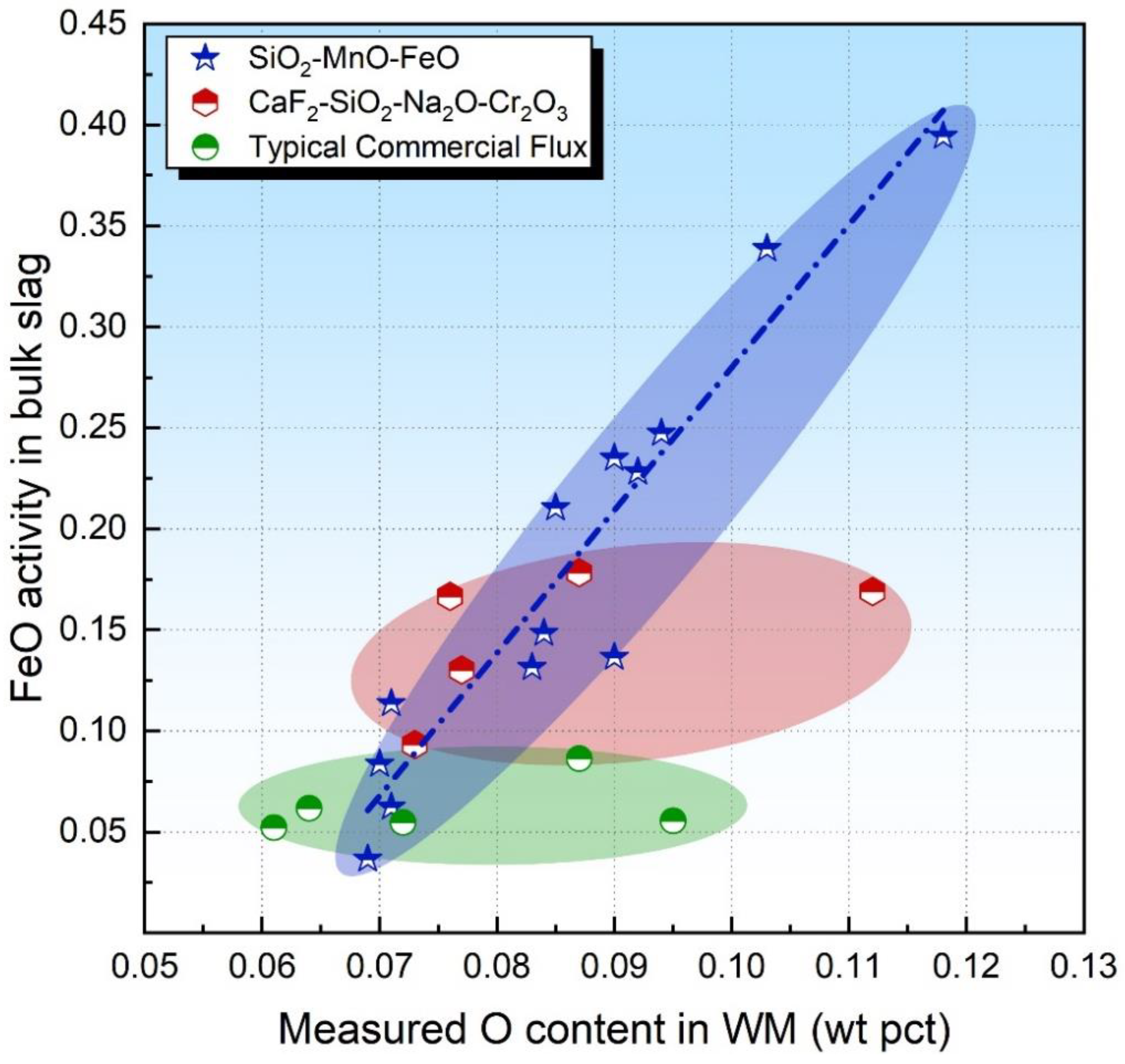
3.3. Flux O Potential FeO Activity at Slag–Metal Interface
- The molten slag and weld pool are shielded under the flux, making the FeO activity unmeasurable.
- The impact of gas formation on the transfer behaviors in SAW was not considered.
- The measurement in terms of the slag is needed to obtain the FeO uptake level.
4. Conclusions
- For SiO2-enriched acid fluxes, both activities of SiO2 and FeO in the bulk slag may be able to evaluate the flux O potential. For SiO2-enriched acid fluxes with FeO incorporated, the FeO activity in the bulk slag is more feasible than the SiO2 activity to evaluate the flux O potential since the transfer of FeO is less kinetically constrained than that of SiO2.
- For basic fluxes, the level of the FeO uptake in the slag is more suitable for the identification of the O potential in comparison to the FeO activity in the bulk slag since Reaction (4) generally proceeds forward under such circumstances.
- Although the equilibrium FeO activity is unmeasurable, the value can be simulated by the gas–slag–metal equilibrium model. Thermodynamic analysis shows that the simulated equilibrium FeO activity possesses a good generality in the evaluation of the flux potential for both acid and basic fluxes.
- In comparison to the oxide activity (or content) in the slag, no measurement of the slag composition is needed for the calculation of the equilibrium FeO activity, which may pave a vital way to save the experimental resources on the flux’s design and analysis.
Author Contributions
Funding
Conflicts of Interest
References
- Sengupta, V.; Havrylov, D.; Mendez, P. Physical Phenomena in the Weld Zone of Submerged Arc Welding—A Review. Weld. J. 2019, 98, 283–313. [Google Scholar] [CrossRef]
- Cong, W.; Zhang, J. Fine-tuning Weld Metal Compositions via Flux Optimization in Submerged Arc Welding: An Overview. Acta Metall. Sin. 2022, 57, 1126–1140. [Google Scholar] [CrossRef]
- Olson, D.; Liu, S.; Frost, R.; Edwards, G.; Fleming, D. Nature and Behavior of Fluxes Used for Welding. ASM International, ASM Handbook. 1993, 6, 55–63. [Google Scholar] [CrossRef]
- Natalie, C.A.; Olson, D.L.; Blander, M. Physical and Chemical Behavior of Welding Fluxes. Annu. Rev. Mater. Sci. 1986, 16, 389–413. [Google Scholar] [CrossRef]
- Dallam, C.; Liu, S.; Olson, D. Flux Composition Dependence of Microstructure and Toughness of Submerged Arc HSLA Weldments. Weld. J. 1985, 64, 140–151. [Google Scholar]
- Zhang, J.; Leng, J.; Wang, C. Tuning Weld Metal Mechanical Responses via Welding Flux Optimization of TiO2 Content: Application into EH36 Shipbuilding Steel. Mater. Trans. B 2019, 50, 2083–2087. [Google Scholar] [CrossRef]
- Lau, T.; Weatherly, G.; McLean, A. The Sources of Oxygen and Nitrogen Contamination in Submerged Arc Welding using CaO-Al2O3 Based Fluxes. Weld. J. 1985, 64, 343–347. [Google Scholar]
- Eagar, T. Sources of Weld Metal Oxygen Contamination during Submerged Arc Welding. Weld. J. 1978, 57, 76–80. [Google Scholar]
- Ricks, R.; Howell, P.; Barritte, G. The Nature of Acicular Ferrite in HSLA Steel Weld Metals. J. Mater. Sci. 1982, 17, 732–740. [Google Scholar] [CrossRef]
- Mills, A.; Thewlis, G.; Whiteman, J. Nature of Inclusions in Steel Weld Metals and Their Influence on Formation of Acicular Ferrite. Mater. Sci. Technol. 1987, 3, 1051–1061. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Slag Metal Reactions in Binary CaF2-Metal Oxide Welding Fluxes. Weld. J. 1982, 61, 229–232. [Google Scholar]
- Kou, S. Welding Metallurgy, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 22–122. [Google Scholar]
- Zhang, J.; Shao, G.; Guo, Y.; Xu, Q.; Liu, Z. Facilitating Flux Design Process Geared Towards Submerged Arc Welding via Thermodynamic Approach: Case Study into CaF2–SiO2–Na2O–Al2O3–TiO2 Agglomerated Flux. Calphad 2022, 79, 102483. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Coetsee, T. Assessment of Weld Metal Compositional Prediction Models Geared Towards Submerged Arc Welding: Case Studies Involving CaF2-SiO2-MnO and CaO-SiO2-MnO Fluxes. Mater. Trans. B 2021, 52, 2404–2415. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Basu, S.; Wang, C. Impact of Gas Formation on the Transfer of Ti and O From TiO2-bearing Basic-fluoride Fluxes to Submerged Arc Welded Metals: A Thermodynamic Approach. Calphad 2020, 71, 102195. [Google Scholar] [CrossRef]
- Tuliani, S.; Boniszewski, T.; Eaton, N. Notch Toughness of Commercial Submerged Arc Weld Metal. Weld. Met. Fabr. 1969, 37, 327–339. [Google Scholar]
- Indacochea, J.E.; Blander, M.; Christensen, N.; Olson, D.L. Chemical Reactions During Submerged Arc Welding with FeO-MnO-SiO2 Fluxes. Metall. Trans. B 1985, 16, 237–245. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Coetsee, T. Thermodynamic Evaluation of Element Transfer Behaviors for Fused CaO-SiO2-MnO Fluxes Subjected to High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2021, 52, 1937–1944. [Google Scholar] [CrossRef]
- Liby, A.; Dixon, R.; Olson, D. Welding: Theory and Practice; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1990; Volume 389. [Google Scholar]
- Belton, G.; Moore, T.; Tankins, E. Slag-metal Reactions in Submerged Arc Welding. Weld. J. 1963, 42, 289s–297s. [Google Scholar]
- Zhang, J.; Peng, L.; Zhou, L.; Chen, Y. On the Si Content Prediction for Submerged Arc Welded Metal via Calphad Technique: A Brief Discussion. J. Mater. Res. Technol. 2022, 21, 1856–1862. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag Metal Reactions during Submerged Arc Welding of Alloy Steels. Metall. Trans. A 1984, 15, 217–227. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions during Welding: Part I. Evaluation and Reassessment of Existing Theories. Metall. Trans. B 1991, 22, 65–71. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions During Welding: Part II. Theory. Metall. Trans. B 1991, 22, 73–81. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions during Welding: Part III. Verification of the Theory. Metall. Trans. B 1991, 22, 83–100. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J. Reprint of: FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Jung, I.-H. Overview of the Applications of Thermodynamic Databases to Steelmaking Processes. Calphad 2010, 34, 332–362. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q. Probing Element Transfer Behavior during the Submerged Arc Welding Process for CaF2-SiO2-Na2O-Cr2O3 Agglomerated Fluxes: A Thermodynamic Approach. Processes 2022, 10, 1900. [Google Scholar] [CrossRef]
- Coetsee, T.; Mostert, R.J.; Pistorius, P.G.H.; Pistorius, P.C. The Effect of Flux Chemistry on Element Transfer in Submerged Arc Welding: Application of Thermochemical Modelling. J. Mater. Res. Technol. 2021, 11, 2021–2036. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Slag-metal Equilibrium during Submerged Arc Welding. Metall. Trans. B 1981, 12, 539–547. [Google Scholar] [CrossRef]
- Indacochea, J.; Olson, D. Relationship of Weld-metal Microstructure and Penetration to Weld-metal Oxygen Content. J. Mater. Energy Syst. 1983, 5, 139–148. [Google Scholar] [CrossRef]
- Lau, T.; Weatherly, G.; McLean, A. Gas/metal/slag Reactions in Submerged Arc Welding Using CaO-Al2O3 Based Fluxes. Weld. J. 1986, 65, 31–38. [Google Scholar]
- Coetsee, T. Phase Chemistry of Submerged Arc Welding (SAW) Fluoride Based Slags. J. Mater. Res. Technol. 2020, 9, 9766–9776. [Google Scholar] [CrossRef]
- Mills, K.; Keene, B. Physicochemical properties of molten CaF2-based slags. Int. Met. Rev. 1981, 26, 21–69. [Google Scholar] [CrossRef]
- Mills, K. Slag atlas. VDEh, 2nd ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1995. [Google Scholar]
- Zhang, J.; Coetsee, T.; Wang, C. Element Transfer Behaviors of Fused CaF2-SiO2 Fluxes Subject to High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2020, 51, 16–21. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Dong, H.; Wang, C. Element Transfer Behaviors of Fused CaF2-SiO2-MnO Fluxes under High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2020, 51, 885–890. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Dong, H.; Wang, C. Element Transfer Behaviors of Fused CaF2-TiO2 Fluxes in EH36 Shipbuilding Steel During High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2020, 51, 1953–1957. [Google Scholar] [CrossRef]
- Chai, C.-S. Slag-metal Reactions during Flux Shielded Arc Welding. Massachusetts Institute of Technology. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, UK, 1980. [Google Scholar]
- Bale, C.W.; Chartrand, P.; Degterov, S.; Eriksson, G.; Hack, K.; Mahfoud, R.B.; Melançon, J.; Pelton, A.; Petersen, S. FactSage thermochemical software and databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Jones, J.; Bowman, B.; Lefrank, P. The Making, Shaping, and Treating of Steel—Steelmaking and Refining Volume; The AISE Steel Foundation: Pittsburgh, PA, USA, 1998. [Google Scholar]
- Shao, G.; Liu, Z.; Fan, J.; Guo, Y.; Xu, Q.; Zhang, J. Evaluation of Flux Basicity Concept Geared toward Estimation for Oxygen Content in Submerged Arc Welded Metal. Metals 2022, 12, 1530. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Yang, S.; Shao, G.; Liu, Z. Element Transfer Behavior for CaF2-Na2O-SiO2 Agglomerated Flux Subject in Submerged Arc Welding Process. Processes 2022, 10, 1847. [Google Scholar] [CrossRef]


| Flux | MnO | SiO2 | FeO | BI |
|---|---|---|---|---|
| F-1-1 | 60 | 40 | 0 | 0.75 |
| F-1-2 | 55 | 40 | 5 | 0.75 |
| F-1-3 | 50 | 40 | 10 | 0.75 |
| F-1-4 | 45 | 40 | 15 | 0.75 |
| F-1-5 | 40 | 40 | 20 | 0.75 |
| F-1-6 | 35 | 40 | 25 | 0.75 |
| F-1-7 | 30 | 40 | 30 | 0.75 |
| F-1-8 | 25 | 40 | 35 | 0.75 |
| F-1-9 | 20 | 40 | 40 | 0.75 |
| F-1-10 | 15 | 40 | 45 | 0.75 |
| F-1-11 | 10 | 40 | 50 | 0.75 |
| F-1-12 | 5 | 40 | 55 | 0.75 |
| F-1-13 | 0 | 40 | 60 | 0.75 |
| Flux | Cr2O3 | SiO2 | Na2O | CaF2 | BI |
|---|---|---|---|---|---|
| F-2-1 | 12.58 | 7.99 | 0.52 | 78.91 | 5.56 |
| F-2-2 | 22.89 | 7.56 | 0.48 | 69.07 | 3.66 |
| F-2-3 | 32.88 | 7.63 | 0.55 | 58.94 | 2.47 |
| F-2-4 | 41.99 | 7.88 | 0.61 | 49.52 | 1.74 |
| F-2-5 | 53.19 | 7.66 | 0.49 | 38.66 | 1.14 |
| Flux | F-3-1 | F-3-2 | F-3-3 | F-3-4 | F-3-5 |
|---|---|---|---|---|---|
| MnO | 0.9 | 5.8 | 1.1 | 6.8 | 12.3 |
| CaO | 5.8 | 3.7 | 6.7 | 0.1 | 2.3 |
| Al2O3 | 13.9 | 17.3 | 17.9 | 24.9 | 36 |
| SiO2 | 15.1 | 21.3 | 13.4 | 19.6 | 18.6 |
| CaF2 | 25.7 | 22.6 | 25.9 | 17.9 | 4.2 |
| MgO | 32.1 | 21.2 | 29.8 | 22.2 | 4.9 |
| FeO | 0.6 | 0.9 | 1 | 2.4 | 5.4 |
| TiO2 | 0.7 | 1.9 | 1.2 | 1 | 10.7 |
| ZrO2 | 0 | 0 | 0 | 0 | 0.2 |
| Na2O | 2 | 2.7 | 1.6 | 1.6 | 2.2 |
| K2O | 0.8 | 1.5 | 1.2 | 0.2 | 0.5 |
| BI | 3 | 1.8 | 2.9 | 1.4 | 0.5 |
| Flux | αNSiO2 | αNFeO | O Content in WM (wt pct) |
|---|---|---|---|
| F-1-1 | 0.438 | 0.037 | 0.069 |
| F-1-2 | 0.437 | 0.062 | 0.071 |
| F-1-3 | 0.504 | 0.083 | 0.07 |
| F-1-4 | 0.468 | 0.114 | 0.071 |
| F-1-5 | 0.480 | 0.148 | 0.084 |
| F-1-6 | 0.595 | 0.131 | 0.083 |
| F-1-7 | 0.625 | 0.137 | 0.09 |
| F-1-8 | 0.501 | 0.228 | 0.092 |
| F-1-9 | 0.561 | 0.235 | 0.09 |
| F-1-10 | 0.668 | 0.210 | 0.085 |
| F-1-11 | 0.668 | 0.247 | 0.094 |
| F-1-12 | 0.555 | 0.339 | 0.103 |
| F-1-13 | 0.528 | 0.395 | 0.118 |
| F-2-1 | 0.073 | 0.093 | 0.049 |
| F-2-2 | 0.077 | 0.130 | 0.074 |
| F-2-3 | 0.076 | 0.167 | 0.118 |
| F-2-4 | 0.087 | 0.178 | 0.147 |
| F-2-5 | 0.112 | 0.169 | 0.162 |
| F-3-1 | 0.040 | 0.034 | 0.04 |
| F-3-2 | 0.070 | 0.034 | 0.0377 |
| F-3-3 | 0.122 | 0.031 | 0.0437 |
| F-3-4 | 0.161 | 0.043 | 0.0499 |
| F-3-5 | 0.248 | 0.043 | 0.0687 |
| Flux | αFeO | O Content in WM (wt pct) |
|---|---|---|
| F-1-1 | 0.061 | 0.069 |
| F-1-2 | 0.075 | 0.071 |
| F-1-3 | 0.093 | 0.07 |
| F-1-4 | 0.116 | 0.071 |
| F-1-5 | 0.144 | 0.084 |
| F-1-6 | 0.175 | 0.083 |
| F-1-7 | 0.207 | 0.09 |
| F-1-8 | 0.241 | 0.092 |
| F-1-9 | 0.276 | 0.09 |
| F-1-10 | 0.310 | 0.085 |
| F-1-11 | 0.344 | 0.094 |
| F-1-12 | 0.376 | 0.103 |
| F-1-13 | 0.407 | 0.118 |
| F-2-1 | 0.183 | 0.049 |
| F-2-2 | 0.232 | 0.074 |
| F-2-3 | 0.243 | 0.118 |
| F-2-4 | 0.243 | 0.147 |
| F-2-5 | 0.239 | 0.162 |
| F-3-1 | 0.008 | 0.04 |
| F-3-2 | 0.024 | 0.038 |
| F-3-3 | 0.009 | 0.044 |
| F-3-4 | 0.030 | 0.05 |
| F-3-5 | 0.065 | 0.069 |
| Symbols | Explanations |
|---|---|
| BI | Basicity index |
| K | Equilibrium coefficient |
| f | Interaction coefficient |
| αNSiO2 | SiO2 activity in bulk slag |
| αNFeO | FeO activity in bulk slag |
| αFeO | Equilibrium FeO activity at interface |
| αO | Equilibrium O activity in weld pool |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, D.; Liu, P. Thermodynamic Nature of SiO2 and FeO in Flux O Potential Control Subject to Submerged Arc Welding Process. Processes 2023, 11, 400. https://doi.org/10.3390/pr11020400
Zhang J, Zhang D, Liu P. Thermodynamic Nature of SiO2 and FeO in Flux O Potential Control Subject to Submerged Arc Welding Process. Processes. 2023; 11(2):400. https://doi.org/10.3390/pr11020400
Chicago/Turabian StyleZhang, Jin, Dan Zhang, and Ping Liu. 2023. "Thermodynamic Nature of SiO2 and FeO in Flux O Potential Control Subject to Submerged Arc Welding Process" Processes 11, no. 2: 400. https://doi.org/10.3390/pr11020400





