Mechanical Properties and Water Resistance of Magnesium Oxychloride Cement–Solidified Residual Sludge
Abstract
1. Introduction
2. Experiments
2.1. Raw Materials
2.2. Solidification Experiment of Residual Sludge
2.3. Water Resistance Test of Solidified Blocks
2.4. Characterization
3. Results and Discussion
3.1. Characterization of Solidified Blocks
3.1.1. SEM Images of Cemented Blocks
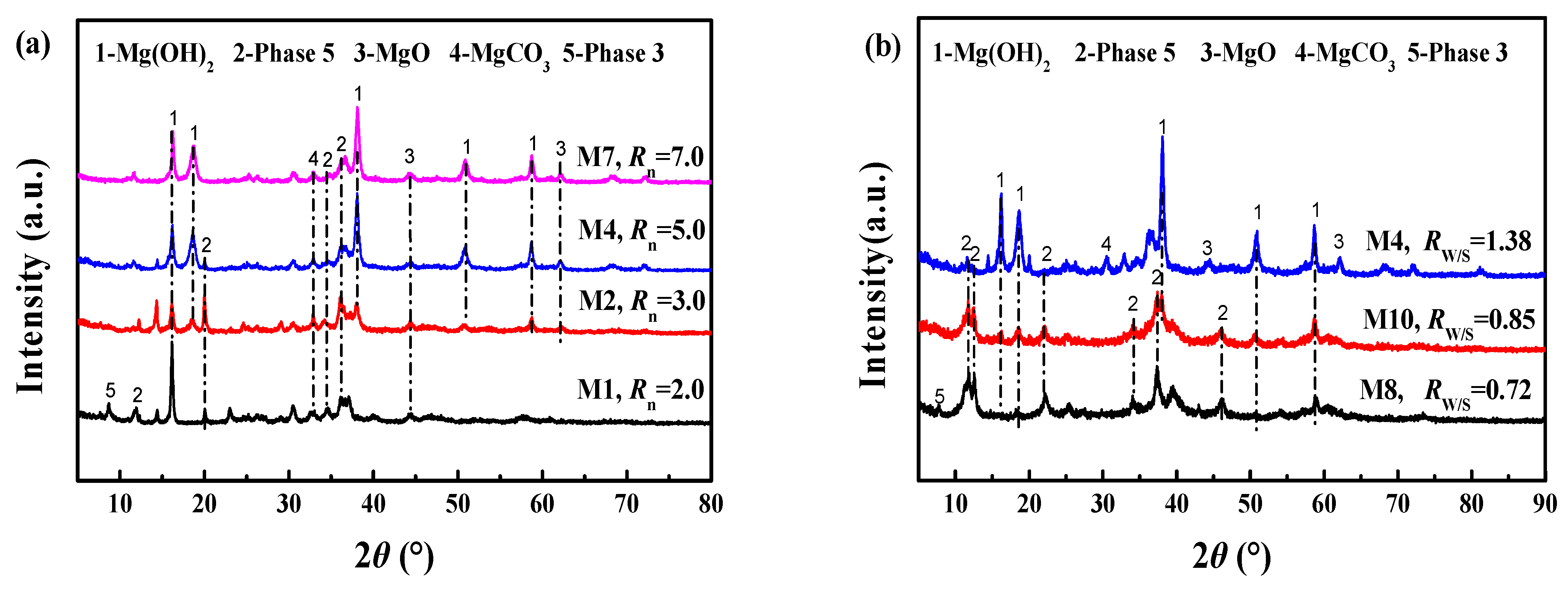
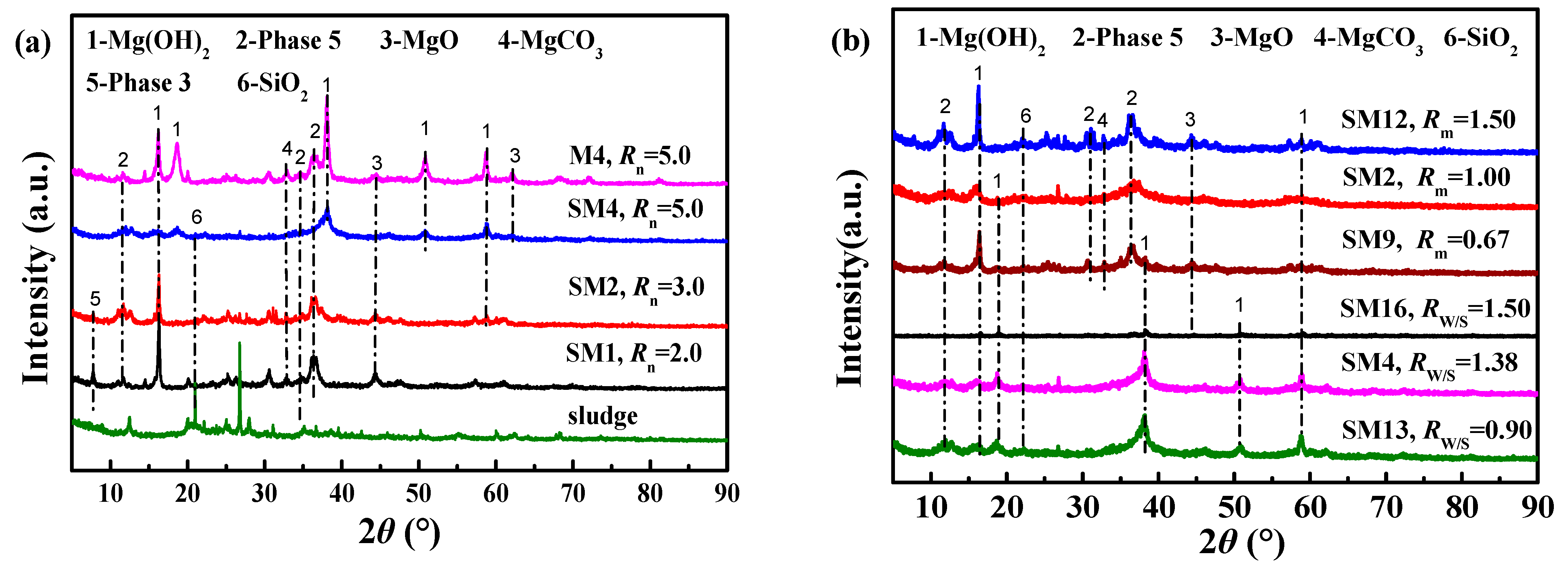
3.1.2. XRD Analysis of Cemented Blocks
3.2. Effects of Rn and Mass Ratio of RW/S on RC of MOC–Cemented Blocks
3.3. MOC–Solidified Residual Sludge
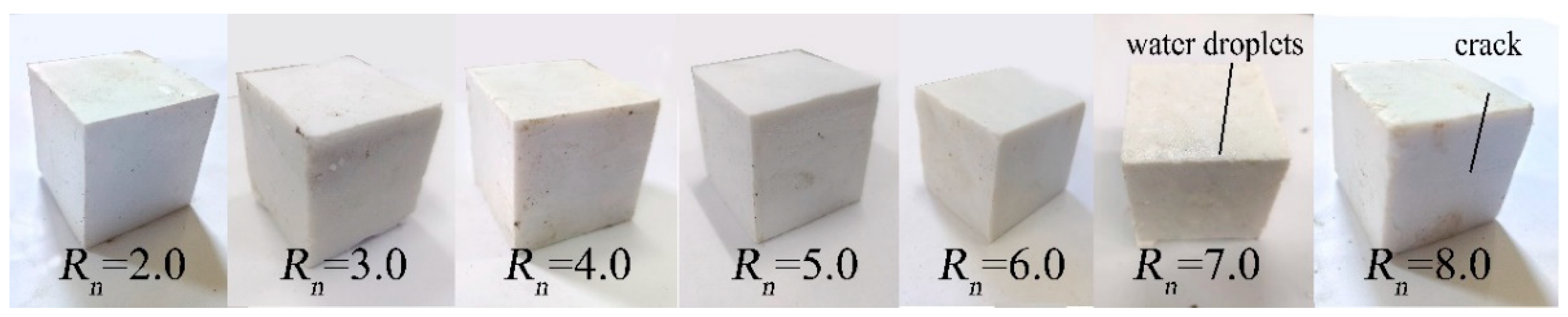
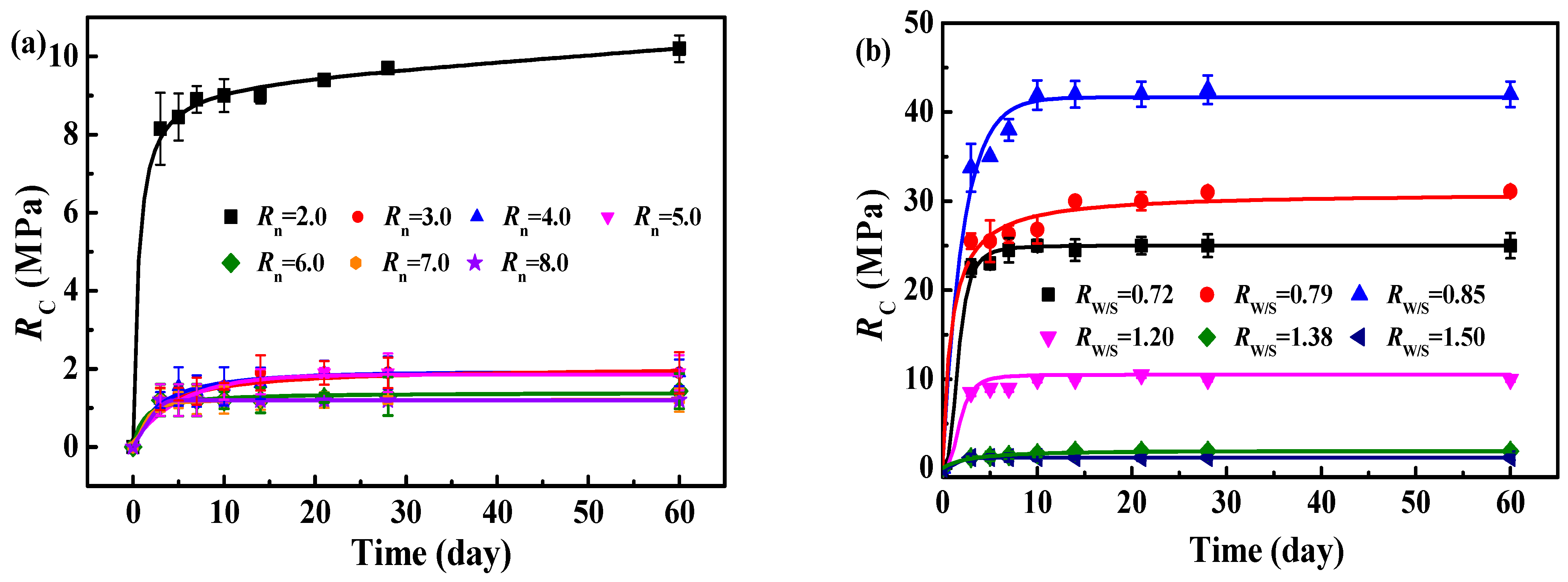
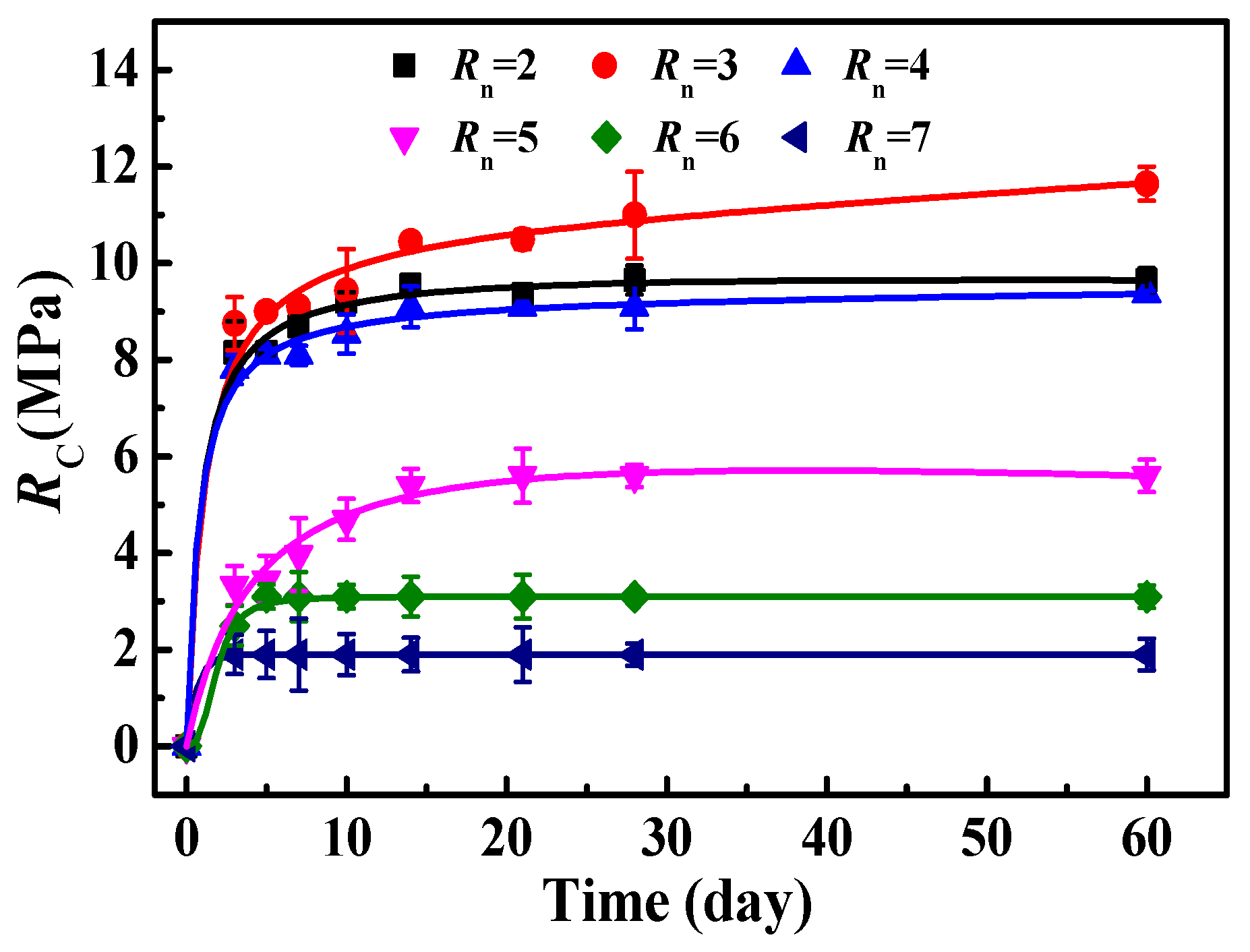
3.3.1. Influence of Rn on RC of MOC–Solidified Residual Sludge
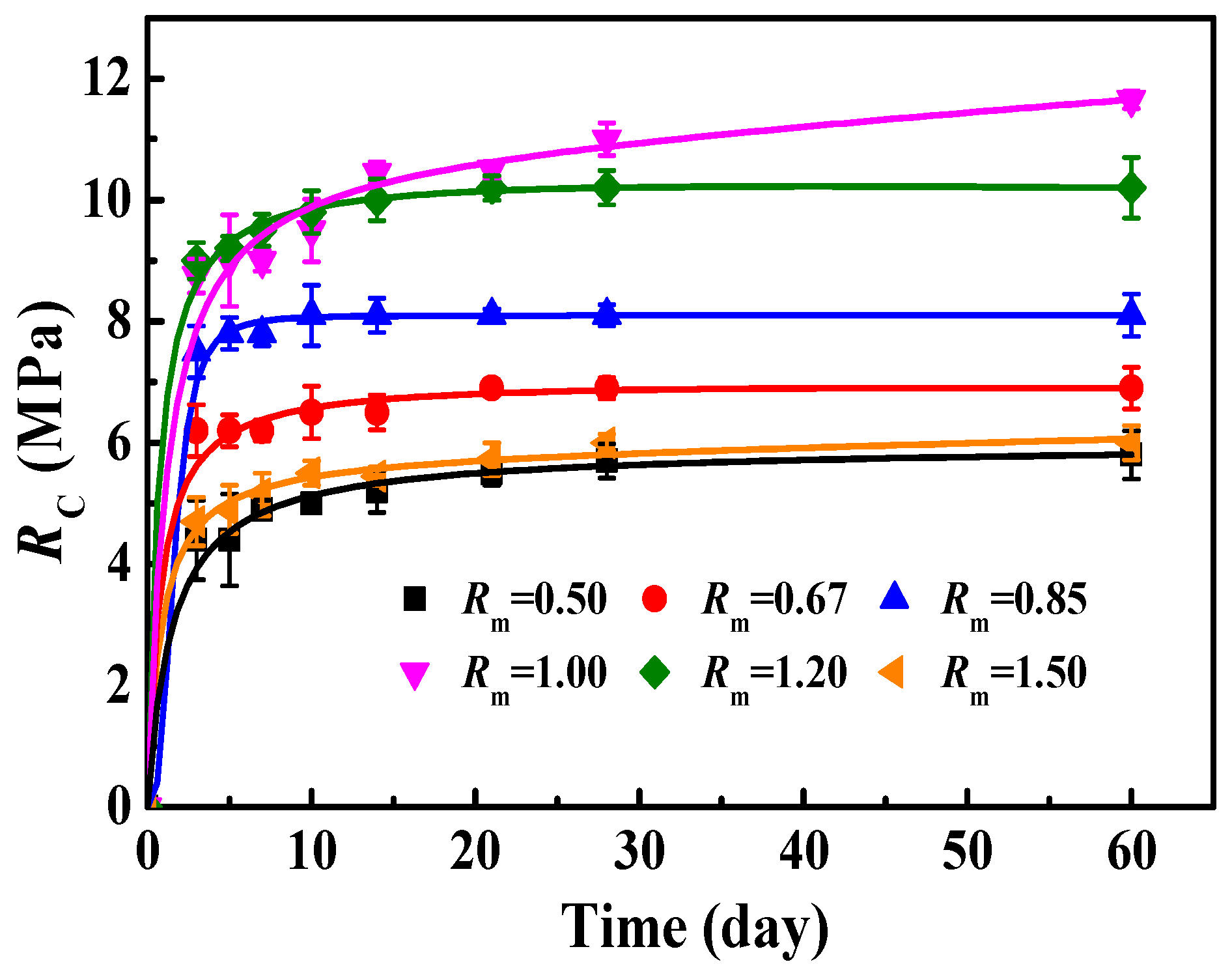
3.3.2. Influence of Rm on RC of MOC–Residual Sludge Solidified Blocks
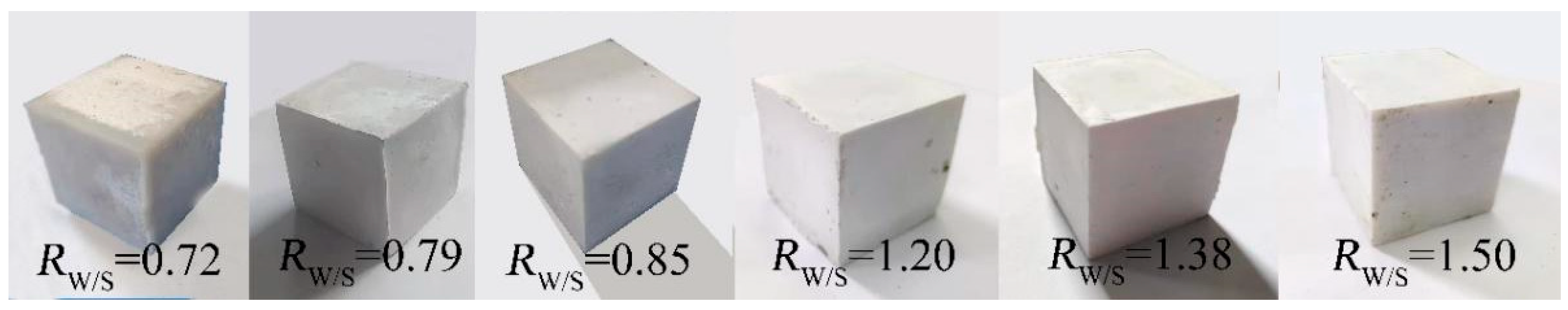
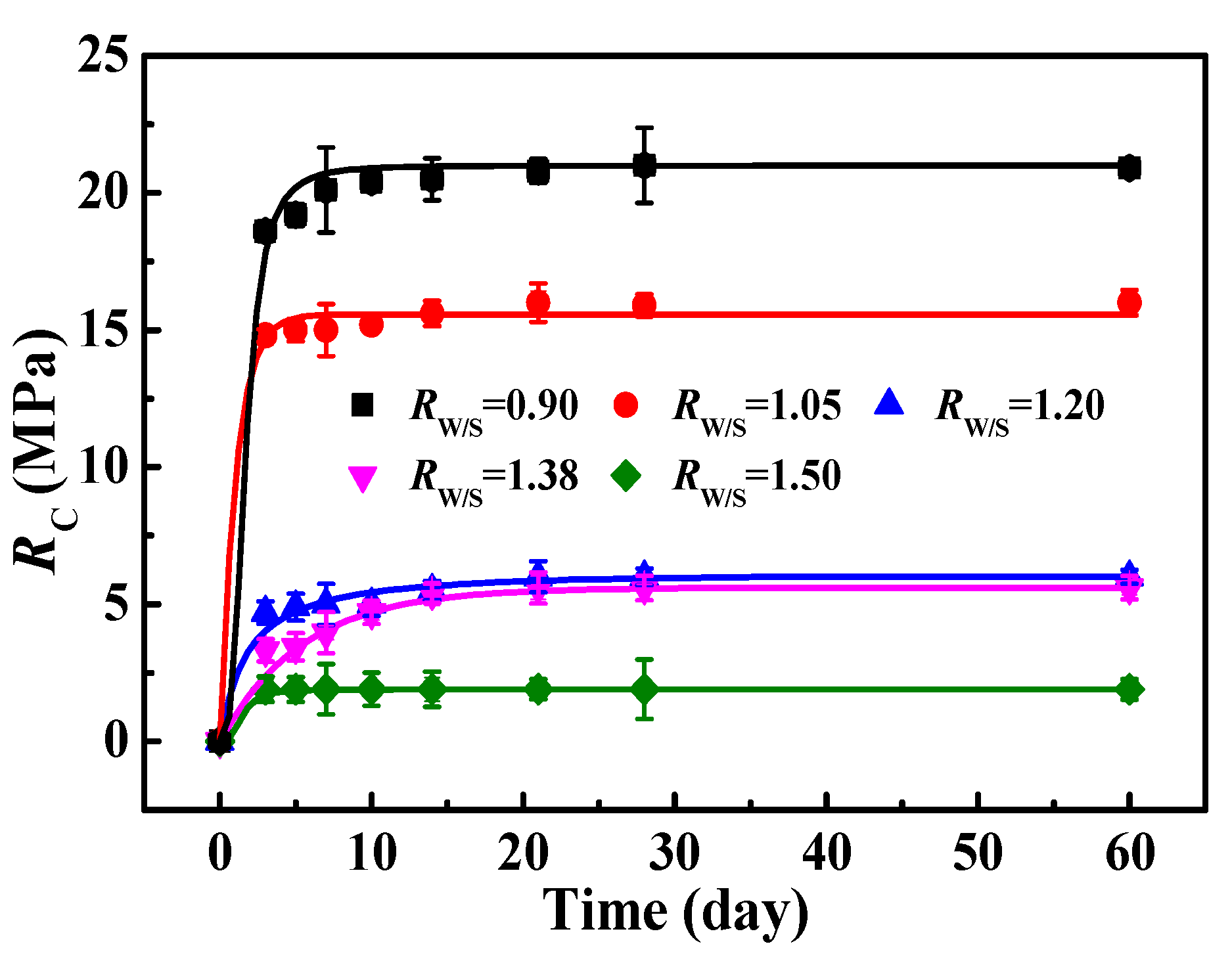
3.3.3. Influence of RW/S on RC of MOC–Residual Sludge Solidified Blocks
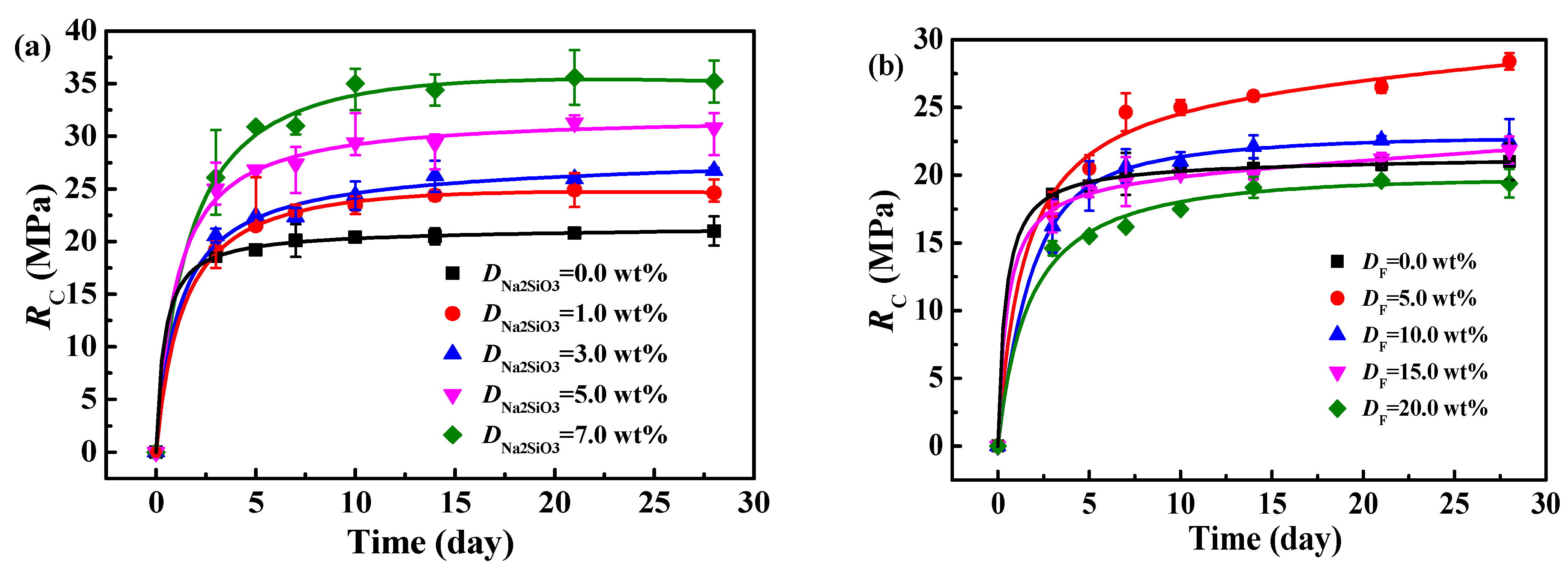
3.4. Influence of Na2SiO3 and Fly Ash on RC of MOC–Solidified Residual Sludge
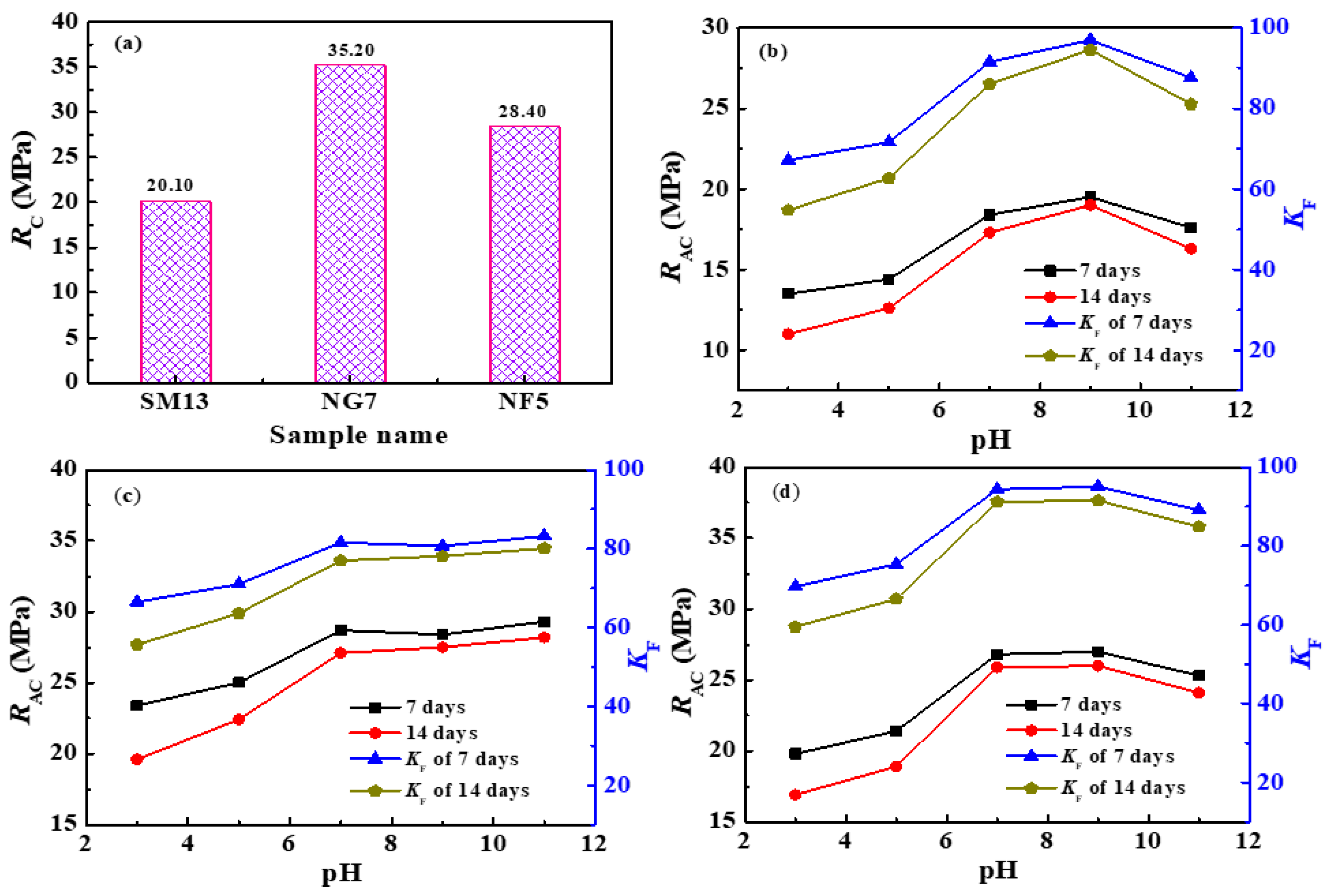
3.5. Water Resistance Test of Solidified Blocks in Different Aqueous Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gomes, S.D.C.; Zhou, J.; Li, W.; Qu, F. Recycling of raw water treatment sludge in cementitious composites: Effects on heat evolution, compressive strength and microstructure. Resour. Conserv. Recycl. 2020, 161, 104970. [Google Scholar] [CrossRef]
- Bonton, A.; Bouchard, C.; Barbeau, B.; Jedrzejak, S. Comparative life cycle assessment of water treatment plants. Desalination 2012, 284, 42–54. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.; Martínez-Barrera, G.; Gencel, O.; Bernal-Martínez, L.A.; Brostow, W. Processed wastewater sludge for improvement of mechanical properties of concretes. J. Hazard. Mater. 2011, 192, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Skousen, J.; Clinger, C. Sewage sludge land application program in West Virginia. J. Soil Water Conserv. 1996, 48, 145–151. [Google Scholar]
- Chang, Z.; Long, G.; Zhou, J.L.; Ma, C. Valorization of sewage sludge in the fabrication of construction and building materials: A review. Resour. Conserv. Recycl. 2020, 154, 104606. [Google Scholar] [CrossRef]
- Liang, J.; He, H.; Wei, J.; Han, T.; Wang, W.; Wang, L.; Han, J.; Zhang, L.; Zhang, Y.; Ma, H. Study of solidifying surplus sludge as building material using ordinary portland cement. Processes 2022, 10, 2234. [Google Scholar] [CrossRef]
- Liew, C.S.; Kiatkittipong, W.; Lim, J.W.; Lam, M.K.; Ho, Y.C.; Ho, C.D.; Ntwampe, S.K.O.; Mohamad, M.; Usman, A. Stabilization of heavy metals loaded sewage sludge: Reviewing conventional to state-of-the-art thermal treatments in achieving energy sustainability. Chemosphere 2021, 277, 130310. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, F.; Li, Q.; Xue, C.; Xia, X.; Yu, H.; Zhao, Q.; Jiang, J.; Bai, S. Development, current state and future trends of sludge management in China: Based on exploratory data and CO2-equivaient emissions analysis. Environ. Int. 2020, 144, 106093. [Google Scholar] [CrossRef]
- Liu, X.; Zhai, Y.; Li, S.; Wang, B.; Wang, T.; Liu, Y.; Qiu, Z.; Li, C. Hydrothermal carbonization of sewage sludge: Effect of feed-water pH on hydrochar’s physicochemical properties, organic component and thermal behavior. J. Hazard. Mater. 2020, 388, 122084. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Z.; Wu, G.; Wu, Q.; Zhang, F.; Niu, Z.; Hu, H.Y. Characteristics of water quality of municipal wastewater treatment plants in China: Implications for resources utilization and management. J. Clean. Prod. 2016, 131, 1–9. [Google Scholar] [CrossRef]
- Wang, T.; Shi, F.; Zhang, Q.; Qian, X.; Hashimoto, S. Exploring material stock efficiency of municipal water and sewage infrastructures in China. J. Clean. Prod. 2018, 181, 498–507. [Google Scholar] [CrossRef]
- Liew, C.S.; Yunus, N.M.; Chidi, B.S.; Lam, M.K.; Goh, P.S.; Mohamad, M.; Sin, J.C.; Lam, S.M.; Lim, J.W.; Lam, S.S. A review on recent disposal of hazardous sewage sludge via anaerobic digestion and novel composting. J. Hazard. Mater. 2022, 423, 126995. [Google Scholar] [CrossRef] [PubMed]
- Breulmann, M.; van Afferden, M.; Müller, R.A.; Schulz, E.; Fühner, C. Process conditions of pyrolysis and hydrothermal carbonization affect the potential of sewage sludge for soil carbon sequestration and amelioration. J. Anal. Appl. Pyrolysis 2017, 124, 256–265. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Efaq, A.N.; Bala, J.D.; Norli, I.; Abdel-Monem, M.O.; Ab. Kadir, M.O. Removal of pathogenic bacteria from sewage-treated effluent and biosolids for agricultural purposes. Appl. Water Sci. 2018, 8, 74. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, G.; Wang, H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Anders, A.; Weigand, H.; Cakir, H.; Kornhaas, U.; Platen, H. Phosphorus recycling from activated sludge of full-scale wastewater treatment plants by fast inversion of the biological phosphorus elimination mechanism. J. Environ. Chem. Eng. 2021, 9, 106403. [Google Scholar] [CrossRef]
- Shehu, M.S.; Abdul Manan, Z.; Alwi, S.R. Optimization of thermo-alkaline disintegration of sewage sludge for enhanced biogas yield. Bioresour. Technol. 2012, 114, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, J.; Liu, H.; Zhang, J. Sludge ozonation: Disintegration, supernatant changes and mechanisms. Bioresour. Technol. 2009, 100, 1505–1509. [Google Scholar] [CrossRef]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminations in sewage sludge-the current situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef]
- Peng, C.; Zhai, Y.; Zhu, Y.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Production of char from sewage sludge employing hydrothermal carbonization: Char properties, combustion behavior and thermal characteristics. Fuel 2016, 176, 110–118. [Google Scholar] [CrossRef]
- Suarez-Iglesias, O.; Urrea, J.L.; Oulego, P.; Collado, S.; Diaz, M. Valuable compounds from sewage sludge by thermal hydrolysis and wet oxidation-A review. Sci. Total. Environ. 2017, 584–585, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energ. Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Wei, L.; Li, J.; Xue, M.; Wang, S.; Li, Q.; Qin, K.; Jiang, J.; Ding, J.; Zhao, Q. Adsorption behaviors of Cu2+, Zn2+ and Cd2+ onto proteins, humic acid, and polysaccharides extracted from sludge EPS: Sorption properties and mechanisms. Bioresour. Technol. 2019, 291, 121868. [Google Scholar] [CrossRef]
- Minh Trang, N.T.; Ho, N.A.D.; Babel, S. Reuse of waste sludge from water treatment plants and fly ash for manufacturing of adobe bricks. Chemosphere 2021, 284, 131367. [Google Scholar] [CrossRef]
- Hii, K.; Baroutian, S.; Parthasarathy, R.; Gapes, D.J.; Eshtiaghi, N. A review of wet air oxidation and thermal hydrolysis technologies in sludge treatment. Bioresour. Technol. 2014, 155, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Manara, P.; Zabaniotou, A. Towards sewage sludge based biofuels via thermochemical conversion-A review. Renew. Sustain. Energ. Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Werle, S.; Wilk, R.K. A review of methods for the thermal utilization of sewage sludge: The polish perspective. Renew. Energ. 2010, 35, 1914–1919. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.T.; Feng, D.; Wu, C.L.; Wang, X.; Min, F.L. Effect of chemical conditioners on deep dewatering of urban dewatered sewage sludge in the temporary sludge lagoon. J. Environ. Eng. 2019, 145, 04019063. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Yang, W.N.; Ngo, H.H.; Guo, W.S.; Jin, P.K.; Dzakpasu, M.; Yang, S.J.; Wang, Q.; Wang, X.C.; Ao, D. Current status of urban wastewater treatment plants in China. Environ. Int. 2016, 92–93, 11–22. [Google Scholar] [CrossRef]
- Ministry of Construction of the People’s Republic of China. Determination Method for Municipal Sludge in Wastewater Treatment Plant; Ministry of Construction of the People’s Republic of China: Beijing, China, 2005. (In Chinese)
- Ye, Q.; Han, Y.; Zhang, S.; Gao, Q.; Zhang, W.; Chen, H.; Gong, S.; Shi, S.Q.; Xia, C.; Li, J. Bioinspired and biomineralized magnesium oxychloride cement with enhanced compressive strength and water resistance. J. Hazard. Mater. 2020, 383, 121099. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, C. The formation mechanism of the hydrate phases in magnesium oxychloride cement. Cem. Concr. Res. 1999, 29, 1365–1371. [Google Scholar]
- Hamood, A.; Khatib, J.M.; Williams, C. The effectiveness of using raw sewage sludge (RSS) as a water replacement in cement mortar mixes containing Unprocessed Fly Ash (u-FA). Constr. Build. Mater. 2017, 147, 27–34. [Google Scholar] [CrossRef]
- Monzó, J.; Payá, J.; Borrachero, M.V.; Peris-Mora, E. Mechanical behavior of mortars containing sewage sludge ash (SSA) and Portland cements with different tricalcium aluminate content. Cem. Concr. Res. 1999, 29, 87–94. [Google Scholar] [CrossRef]
- Cyr, M.; Coutand, M.; Clastres, P. Technological and environmental behavior of sewage sludge ash (SSA) in cement-based materials. Cem. Concr. Res. 2007, 37, 1278–1289. [Google Scholar] [CrossRef]
- Garcés, P.; Pérez Carrión, M.; García-Alcocel, E.; Payá, J.; Monzó, J.; Borrachero, M.V. Mechanical and physical properties of cement blended with sewage sludge ash. Waste Manag. 2008, 28, 2495–2502. [Google Scholar] [CrossRef]
- Valls, S.; Vàzquez, E. Leaching properties of stabilised/solidified cement-admixtures-sewage sludges systems. Waste Manag. 2002, 22, 37–45. [Google Scholar] [CrossRef]
- Cheilas, A.; Katsioti, M.; Georgiades, A.; Malliou, O.; Teas, C.; Haniotakis, E. Impact of hardening conditions on to stabilized/solidified products of cement–sewage sludge–jarosite/alunite. Cem. Concr. Compos. 2007, 29, 263–269. [Google Scholar] [CrossRef]
- Yague, A.; Valls, S.; Vázquez, E.; Albareda, F. Durability of concrete with addition of dry sludge from waste water treatment plants. Cem. Concr. Res. 2005, 35, 1064–1073. [Google Scholar] [CrossRef]
- Jordán, M.M.; Almendro-Candel, M.B.; Romero, M.; Rincó, J.M. Application of sewage sludge in the manufacturing of ceramic tile bodies. Appl. Clay Sci. 2005, 30, 219–224. [Google Scholar] [CrossRef]
- Favoni, C.; Minichelli, D.; Tubaro, F.; Brückner, S.; Bachiorrini, A.; Maschio, S. Ceramic processing of municipal sewage sludge (MSS) and steelworks slags (SS). Ceram. Int. 2005, 31, 697–702. [Google Scholar] [CrossRef]
- Montero, M.A.; Jordán, M.M.; Hernández-Crespo, M.S.; Sanfeliu, T. The use of sewage sludge and marble residues in the manufacture of ceramic tile bodies. Appl. Clay Sci. 2009, 46, 404–408. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, Q.; Li, R.; Yue, M.; Han, S.; Gao, B.; Li, Q.; Yu, H. Research on sludge-fly ash ceramic particles (SFCP) for synthetic and municipal wastewater treatment in biological aerated filter (BAF). Bioresour. Technol. 2009, 100, 4955–4962. [Google Scholar] [CrossRef] [PubMed]
- Cusidó, J.A.; Soriano, C. Valorization of pellets from municipal WWTP sludge in lightweight clay ceramics. Waste Manag. 2011, 31, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Moon, S.O.; Heo, J. Crystalline phase control of glass ceramics obtained from sewage sludge fly ash. Ceram. Int. 2003, 29, 223–227. [Google Scholar] [CrossRef]
- Merino, I.; Arévalo, L.F.; Romero, F. Preparation and characterization of ceramic products by thermal treatment of sewage sludge ashes mixed with different additives. Waste Manag. 2007, 27, 1829–1844. [Google Scholar] [CrossRef]
- Chen, L.; Lin, D.F. Applications of sewage sludge ash and nano-SiO2 to manufacture tile as construction material. Constr. Build. Mater. 2009, 23, 3312–3320. [Google Scholar] [CrossRef]
- Wang, K.S.; Chiou, I.J.; Chen, C.H.; Wang, D. Lightweight properties and pore structure of foamed material made from sewage sludge ash. Constr. Build. Mater. 2005, 19, 627–633. [Google Scholar] [CrossRef]
- Chiou, I.J.; Wang, K.S.; Chen, C.H.; Lin, Y.T. Lightweight aggregate made from sewage sludge and incinerated ash. Waste Manag. 2006, 26, 1453–1461. [Google Scholar] [CrossRef]
- Wang, X.; Jin, Y.; Wang, Z.; Nie, Y.; Huang, Q.; Wang, Q. Development of lightweight aggregate from dry sewage sludge and coal ash. Waste Manag. 2009, 29, 1330–1335. [Google Scholar] [CrossRef]
- Mun, K.J. Development and tests of lightweight aggregate using sewage sludge for nonstructural concrete. Constr. Build. Mater. 2007, 21, 1583–1588. [Google Scholar] [CrossRef]
- Cheeseman, C.R.; Virdi, G.S. Properties and microstructure of lightweight aggregate produced from sintered sewage sludge ash. Resour. Conserv. Recycl. 2005, 45, 18–30. [Google Scholar] [CrossRef]
- Theodoratos, P.; Moirou, A.; Xenidis, A.; Paspaliaris, I. The use of municipal sewage sludge for the stabilization of soil contaminated by mining activities. J. Hazard. Mater. 2000, 77, 177–191. [Google Scholar] [CrossRef]
- Lin, D.F.; Lin, K.L.; Hung, M.J.; Luo, H.L. Sludge ash/hydrated lime on the geotechnical properties of soft soil. J. Hazard. Mater. 2007, 145, 58–64. [Google Scholar] [CrossRef]
- Chen, L.; Lin, D.F. Stabilization treatment of soft subgrade soil by sewage sludge ash and cement. J. Hazard. Mater. 2009, 162, 321–327. [Google Scholar] [CrossRef]
- Pan, S.C.; Lin, C.C.; Tseng, D.H. Reusing sewage sludge ash as adsorbent for copper removal from wastewater. Resour. Conserv. Recycl. 2003, 39, 79–90. [Google Scholar] [CrossRef]
- Okol, R.E.; Balafoutas, G. Landfill sealing potentials of bottom ashes of sludge cakes. Soil Tillage Res. 1998, 46, 307–314. [Google Scholar] [CrossRef]
- Xu, Z.; Ye, D.; Dai, T.; Dai, Y. Research on preparation of coal waste-based geopolymer and its stabilization/solidification of heavy metals. Integr. Ferroelectr. 2021, 217, 214–224. [Google Scholar]
- Shen, Z.; Jin, F.; O’Connor, D.; Hou, D. Solidification/stabilization for soil remediation: An old technology with new vitality. Environ. Sci. Technol. 2019, 53, 11615–11617. [Google Scholar] [CrossRef]
- Tuncan, A.; Tuncan, M.; Koyuncu, H. Use of petroleum-contaminated drilling wastes as sub-base material for road construction. Waste Manag. Res. 2000, 18, 489–505. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Soe, K.; Pulham, M. Recent development in magnesium oxychloride cement. Struct. Concr. 2018, 19, 1290–1300. [Google Scholar] [CrossRef]
- Beaudoin, J.J.; Ramachandran, V.S. Strength development in magnesium oxychloride and other cements. Cem. Concr. Res. 1975, 5, 617–630. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Hu, C.; Li, Z. Influence of cenospheres on properties of magnesium oxychloride cement-based composites. Mater Struct. 2016, 49, 1319–1326. [Google Scholar] [CrossRef]
- Ma, J.L.; Zhao, Y.C.; Wang, J.; Wang, L. Effect of magnesium oxychloride cement on stabilization/solidification of sewage sludge. Constr. Build. Mater. 2010, 24, 79–83. [Google Scholar]
- Deng, D. The mechanism for soluble phosphates to improve the water resistance of magnesium oxychloride cement. Cem. Concr. Res. 2003, 33, 1311–1317. [Google Scholar] [CrossRef]
- Guan, B.; He, Z.; Wei, F.; Wang, F.; Yu, J. Effects of fly ash and hexadecyltrimethoxysilane on the compressive properties and water resistance of magnesium oxychloride cement. Polymers 2023, 15, 172. [Google Scholar] [CrossRef]
- Wang, D.; Di, S.; Gao, X.; Wang, R.; Chen, Z. Strength properties and associated mechanisms of magnesium oxychloride cement-solidified urban river sludge. Constr. Build. Mater. 2020, 250, 118933. [Google Scholar] [CrossRef]
- Li, G.; Yu, Y.; Li, J.; Wang, Y.; Liu, H. Experimental study on urban refuse/magnesium oxychloride cement compound floor tile. Cem. Concr. Res. 2003, 33, 1663–1668. [Google Scholar] [CrossRef]
- Matkovlć, B.; Popović, S.; Rogić, V.; Žunić, T.; Young, J.F. Reaction products in magnesium oxychloride cement pastes. System MgO-MgCl2-H2O. J. Am. Ceram. Soc. 1977, 60, 504–507. [Google Scholar] [CrossRef]
- Li, Z.; Chau, C.K. Influence of molar ratios on properties of magnesium oxychloride cement. Cem. Concr. Res. 2007, 37, 866–870. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-based cements: A journey of 150 years, and cements for the future? Chem. Rev. 2016, 116, 4186–4191. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, S.; Wang, H.; Chen, R. Effect of 5-phase seed crystal on the mechanical properties and microstructure of magnesium oxychloride cement. Constr. Build. Mater. 2017, 150, 409–417. [Google Scholar] [CrossRef]
- Urwongse, L.; Sorrell, C.A. The system MgO-MgCl2-H2O at 23 °C. J. Am. Ceram. Soc. 1980, 63, 501–504. [Google Scholar] [CrossRef]
- Cole, W.; Demediuk, T. X-Ray, thermal, and dehydration studies on magnesium oxychlorides. Aust. J. Chem. 1955, 8, 234–251. [Google Scholar] [CrossRef]
- Wang, D.; Gao, X.; Liu, X.; Zeng, G. Strength, durability and microstructure of granulated blast furnace slag-modified magnesium oxychloride cement solidified waste sludge. J. Clean. Prod. 2021, 292, 126072. [Google Scholar] [CrossRef]
- Wang, F.; Xu, X.; Zhou, B.; Zhong, S. Preparation of straw-magnesium oxychloride cement composites. J. Build. Mater. 2019, 22, 135–141. [Google Scholar]
- Chen, X.; Zhang, T.; Bi, W.; Cheeseman, C.R. Effect of tartaric acid and phosphoric acid on the water resistance of magnesium oxychloride (MOC) cement. Constr. Build. Mater. 2019, 213, 528–536. [Google Scholar] [CrossRef]
- Lv, Q.; Yu, J.; Ji, F.; Gu, L.; Chen, Y.; Shan, X. Mechanical property and microstructure of fly ash-based geopolymer activated by sodium silicate. KSCE J. Civ. Eng. 2021, 25, 1765–1777. [Google Scholar] [CrossRef]
- Hou, Y.F.; Wang, D.M.; Li, Q.; Lu, H.B. Effect of water glass performance on fly ash-based geopolymers. J. Chin. Ceram. Soc. 2008, 36, 61–64. (In Chinese) [Google Scholar]
- De Araujo, C.C.; Zhang, L.; Eckert, H. Sol-gel preparation of AlPO4-SiO2 glasses with high surface mesoporous structure. J. Mater. Chem. 2006, 16, 1323–1331. [Google Scholar] [CrossRef]
- Hao, Y.; Li, Y. Study on preparation and properties of modified magnesium oxychloride cement foam concrete. Constr. Build. Mater. 2021, 282, 122708. [Google Scholar] [CrossRef]
- Pivak, A.; Pavlikova, M.; Zaleska, M.; Lojka, M.; Jankovsky, O.; Pavlik, Z. Magnesium oxychloride cement composites with silica filler and coal fly ash admixture. Materials 2020, 13, 2537. [Google Scholar] [CrossRef] [PubMed]
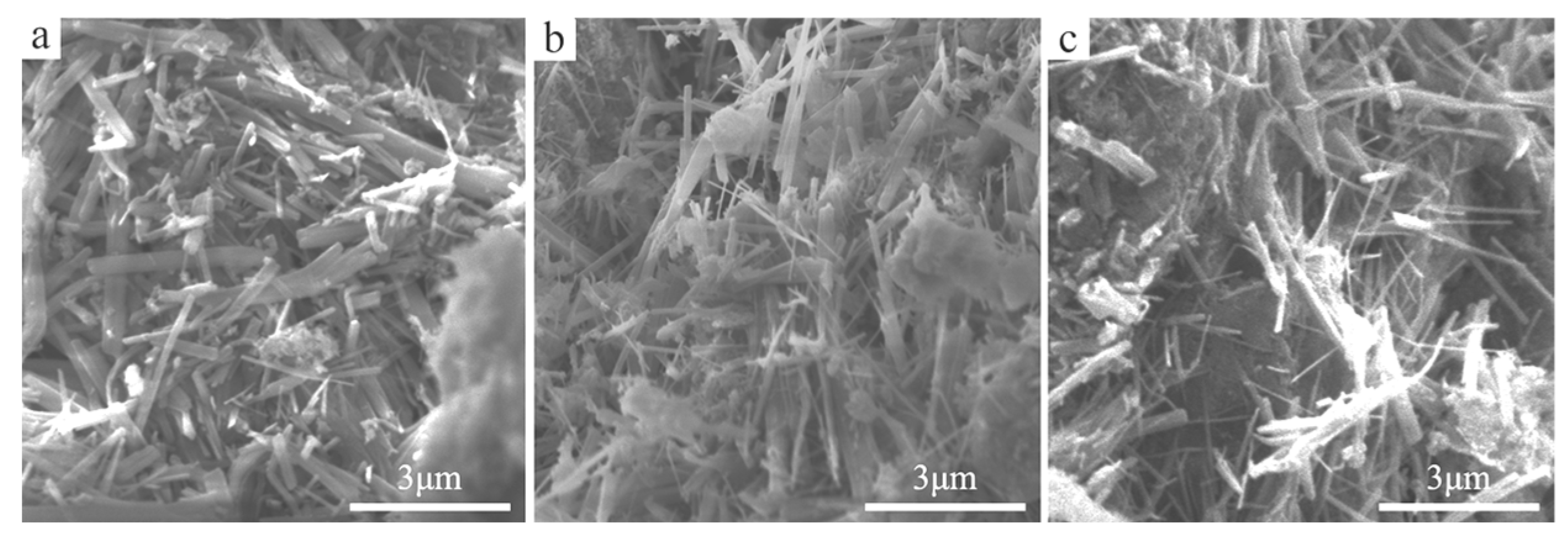


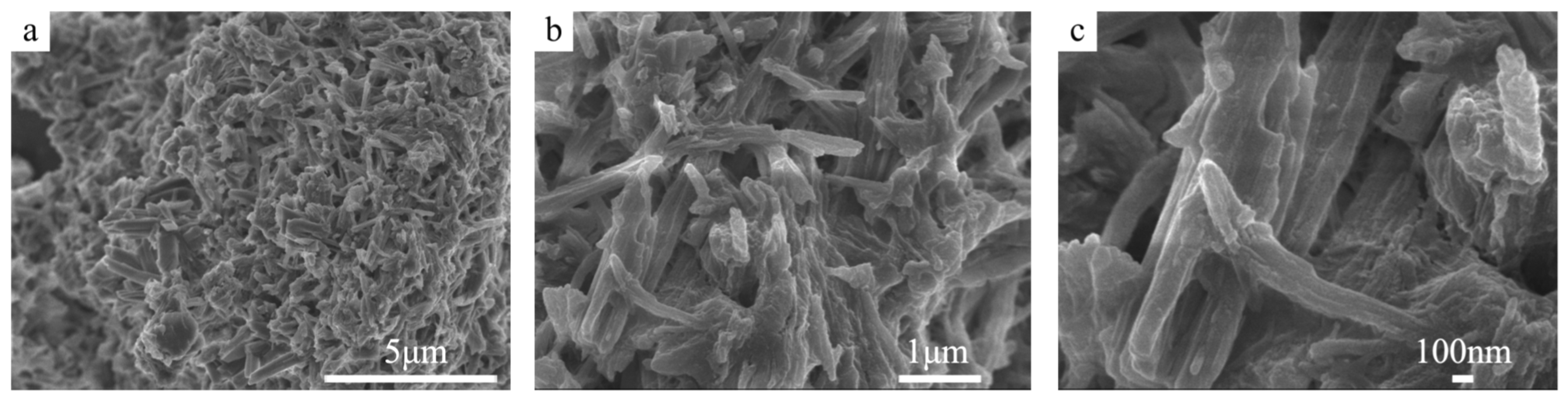
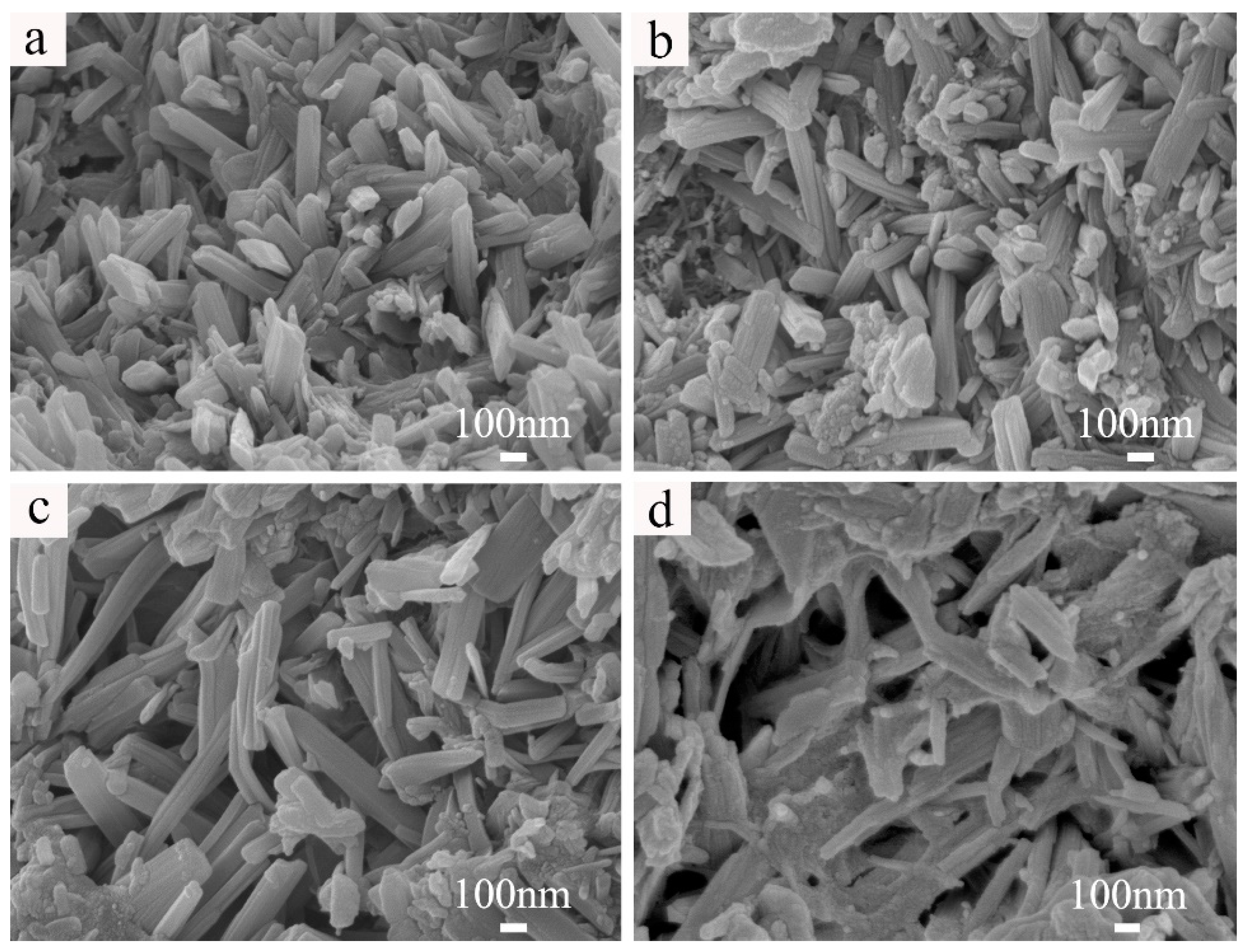
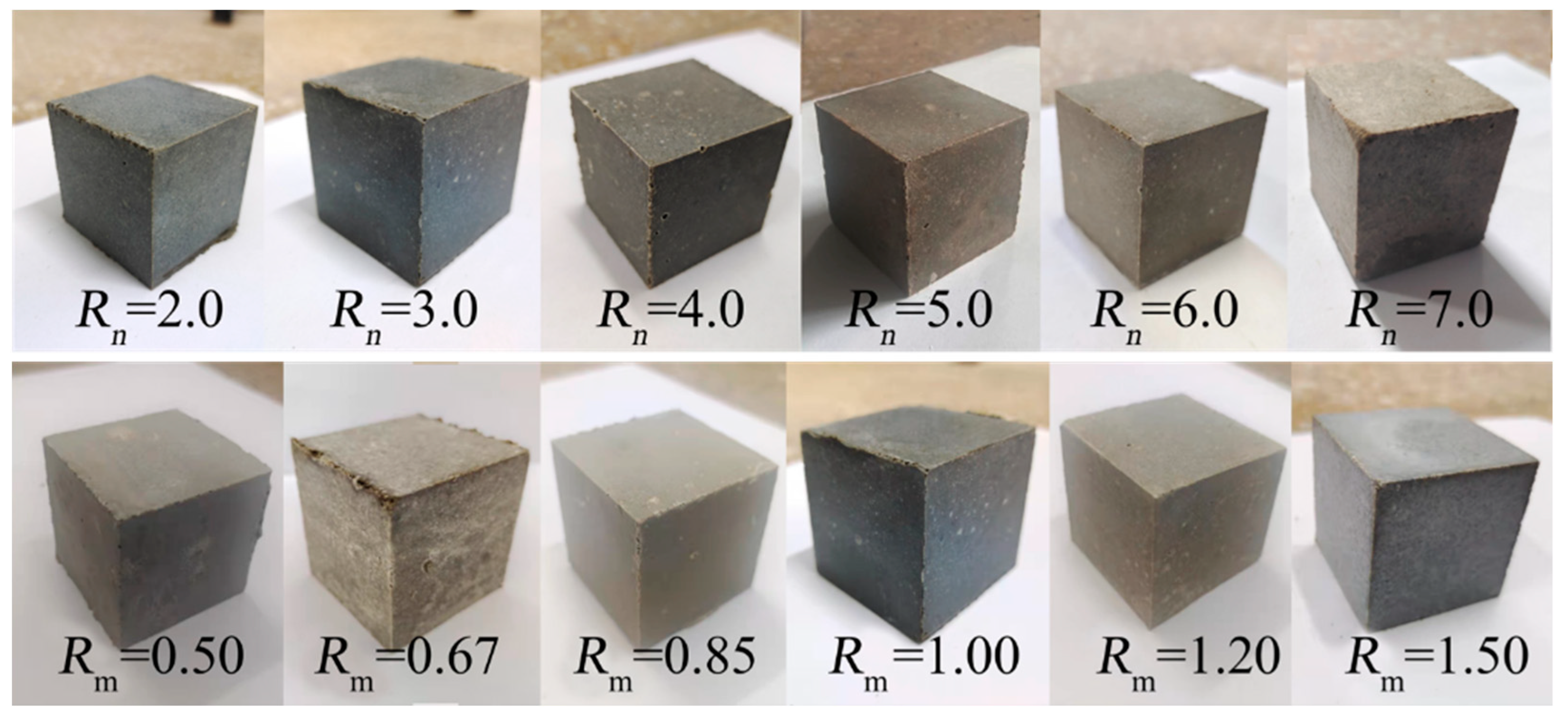
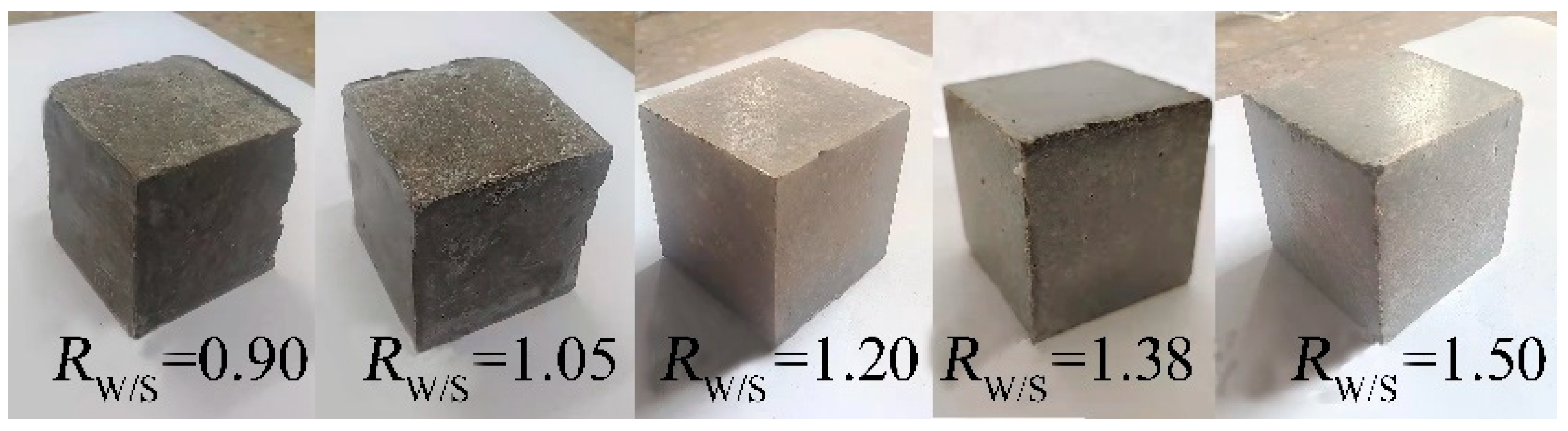

| Samples | DS (wt%) | DMgO (wt%) | DMgCl2 (wt%) | DMOC (wt%) | DW (wt%) | RW/S | Rn | Rm | DNa2SiO3 (wt%) | DF (wt%) |
|---|---|---|---|---|---|---|---|---|---|---|
| M1 | - | 19.31 | 22.78 | 42.09 | 57.91 | 1.38 | 2.0 | - | - | - |
| M2 | - | 23.55 | 18.54 | 42.09 | 57.91 | 1.38 | 3.0 | - | - | - |
| M3 | - | 26.44 | 15.65 | 42.09 | 57.91 | 1.38 | 4.0 | - | - | - |
| M4 | - | 28.57 | 13.52 | 42.09 | 57.91 | 1.38 | 5.0 | - | - | - |
| M5 | - | 30.20 | 11.89 | 42.09 | 57.91 | 1.38 | 6.0 | - | - | - |
| M6 | - | 31.46 | 10.63 | 42.09 | 57.91 | 1.38 | 7.0 | - | - | - |
| M7 | - | 32.48 | 9.61 | 42.09 | 57.91 | 1.38 | 8.0 | - | - | - |
| M8 | - | 39.47 | 18.67 | 58.14 | 41.86 | 0.72 | 5.0 | - | - | - |
| M9 | - | 37.93 | 17.94 | 55.87 | 44.13 | 0.79 | 5.0 | - | - | - |
| M10 | - | 36.70 | 17.36 | 54.06 | 45.94 | 0.85 | 5.0 | - | - | - |
| M11 | - | 30.86 | 14.60 | 45.46 | 54.54 | 1.20 | 5.0 | - | - | - |
| M12 | - | 27.15 | 12.85 | 40.00 | 60.00 | 1.50 | 5.0 | - | - | - |
| SM1 | 37.50 | 17.20 | 20.30 | 37.50 | 25.00 | 1.38 | 2.0 | 1.00 | - | - |
| SM2 | 37.50 | 20.98 | 16.52 | 37.50 | 25.00 | 1.38 | 3.0 | 1.00 | - | - |
| SM3 | 37.50 | 23.56 | 13.94 | 37.50 | 25.00 | 1.38 | 4.0 | 1.00 | - | - |
| SM4 | 37.50 | 25.46 | 12.04 | 37.50 | 25.00 | 1.38 | 5.0 | 1.00 | - | - |
| SM5 | 37.50 | 26.90 | 10.60 | 37.50 | 25.00 | 1.38 | 6.0 | 1.00 | - | - |
| SM6 | 37.50 | 28.03 | 9.47 | 37.50 | 25.00 | 1.38 | 7.0 | 1.00 | - | - |
| SM7 | 37.50 | 28.93 | 8.57 | 37.50 | 25.00 | 1.38 | 8.0 | 1.00 | - | - |
| SM8 | 19.83 | 22.19 | 17.47 | 39.66 | 40.51 | 1.38 | 3.0 | 0.50 | - | - |
| SM9 | 25.94 | 21.77 | 17.14 | 38.91 | 35.15 | 1.38 | 3.0 | 0.67 | - | - |
| SM10 | 32.40 | 21.33 | 16.79 | 38.12 | 29.48 | 1.38 | 3.0 | 0.85 | - | - |
| SM11 | 44.03 | 20.53 | 16.17 | 36.70 | 19.27 | 1.38 | 3.0 | 1.20 | - | - |
| SM12 | 53.33 | 19.89 | 15.67 | 35.56 | 11.11 | 1.38 | 3.0 | 1.50 | - | - |
| SM13 | 46.89 | 31.83 | 15.05 | 46.88 | 6.23 | 0.90 | 5.0 | 1.00 | - | - |
| SM14 | 43.46 | 29.50 | 13.95 | 43.45 | 13.09 | 1.05 | 5.0 | 1.00 | - | - |
| SM15 | 40.49 | 27.49 | 13.00 | 40.49 | 19.02 | 1.20 | 5.0 | 1.00 | - | - |
| SM16 | 35.63 | 24.20 | 11.44 | 35.64 | 28.73 | 1.50 | 5.0 | 1.00 | - | - |
| NG1 | 46.89 | 31.83 | 15.05 | 46.88 | 6.23 | 0.90 | 5.0 | 1.00 | 1.0 | - |
| NG3 | 46.89 | 31.83 | 15.05 | 46.88 | 6.23 | 0.90 | 5.0 | 1.00 | 3.0 | - |
| NG5 | 46.89 | 31.83 | 15.05 | 46.88 | 6.23 | 0.90 | 5.0 | 1.00 | 5.0 | - |
| NG7 | 46.89 | 31.83 | 15.05 | 46.88 | 6.23 | 0.90 | 5.0 | 1.00 | 7.0 | - |
| NF5 | 46.89 | 30.24 | 14.30 | 44.54 | 6.23 | 0.90 | 5.0 | 1.00 | - | 5.0 |
| NF10 | 46.89 | 28.65 | 13.55 | 42.20 | 6.23 | 0.90 | 5.0 | 1.00 | - | 10.0 |
| NF15 | 46.89 | 27.06 | 12.80 | 39.86 | 6.23 | 0.90 | 5.0 | 1.00 | - | 15.0 |
| NF20 | 46.89 | 25.47 | 12.04 | 37.51 | 6.23 | 0.90 | 5.0 | 1.00 | - | 20.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Liang, J.; Wang, L.; He, H.; Wang, W.; Han, T.; Xu, Z.; Han, J. Mechanical Properties and Water Resistance of Magnesium Oxychloride Cement–Solidified Residual Sludge. Processes 2023, 11, 413. https://doi.org/10.3390/pr11020413
Ma H, Liang J, Wang L, He H, Wang W, Han T, Xu Z, Han J. Mechanical Properties and Water Resistance of Magnesium Oxychloride Cement–Solidified Residual Sludge. Processes. 2023; 11(2):413. https://doi.org/10.3390/pr11020413
Chicago/Turabian StyleMa, Haiqiang, Jiling Liang, Lu Wang, Han He, Wenwu Wang, Tingting Han, Ziting Xu, and Jie Han. 2023. "Mechanical Properties and Water Resistance of Magnesium Oxychloride Cement–Solidified Residual Sludge" Processes 11, no. 2: 413. https://doi.org/10.3390/pr11020413
APA StyleMa, H., Liang, J., Wang, L., He, H., Wang, W., Han, T., Xu, Z., & Han, J. (2023). Mechanical Properties and Water Resistance of Magnesium Oxychloride Cement–Solidified Residual Sludge. Processes, 11(2), 413. https://doi.org/10.3390/pr11020413









