Removal of the Pigment Congo Red from Synthetic Wastewater with a Novel and Inexpensive Adsorbent Generated from Powdered Foeniculum Vulgare Seeds
Abstract
:1. Introduction
2. Methodology
2.1. Modification of FVSP
2.2. Adsorbent Characterizations
2.3. Experiments of Adsorption
2.3.1. Identifying the Ideal Adsorbent
2.3.2. Kinetic and Equilibrium Experiments
3. Results and Discussion
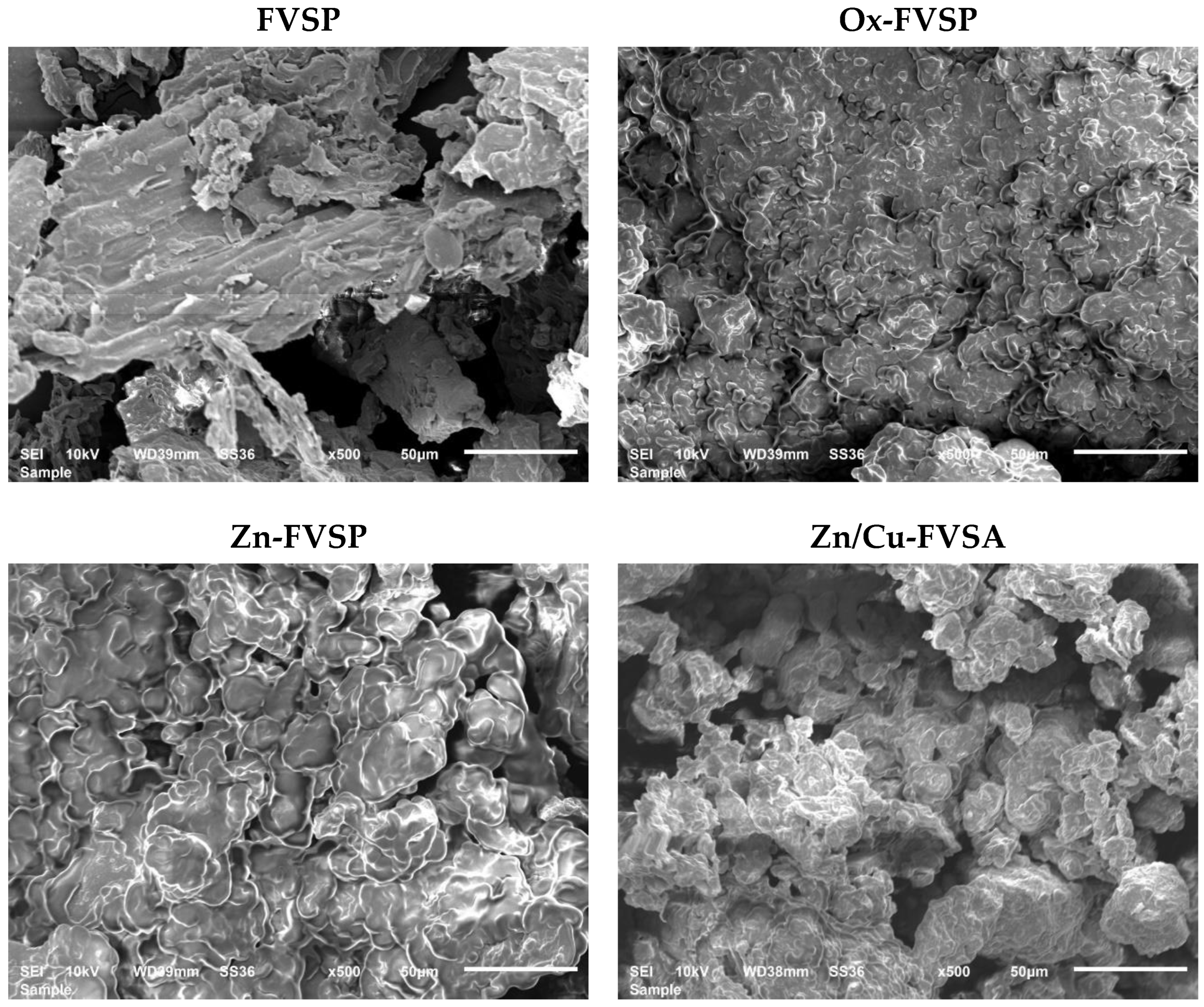
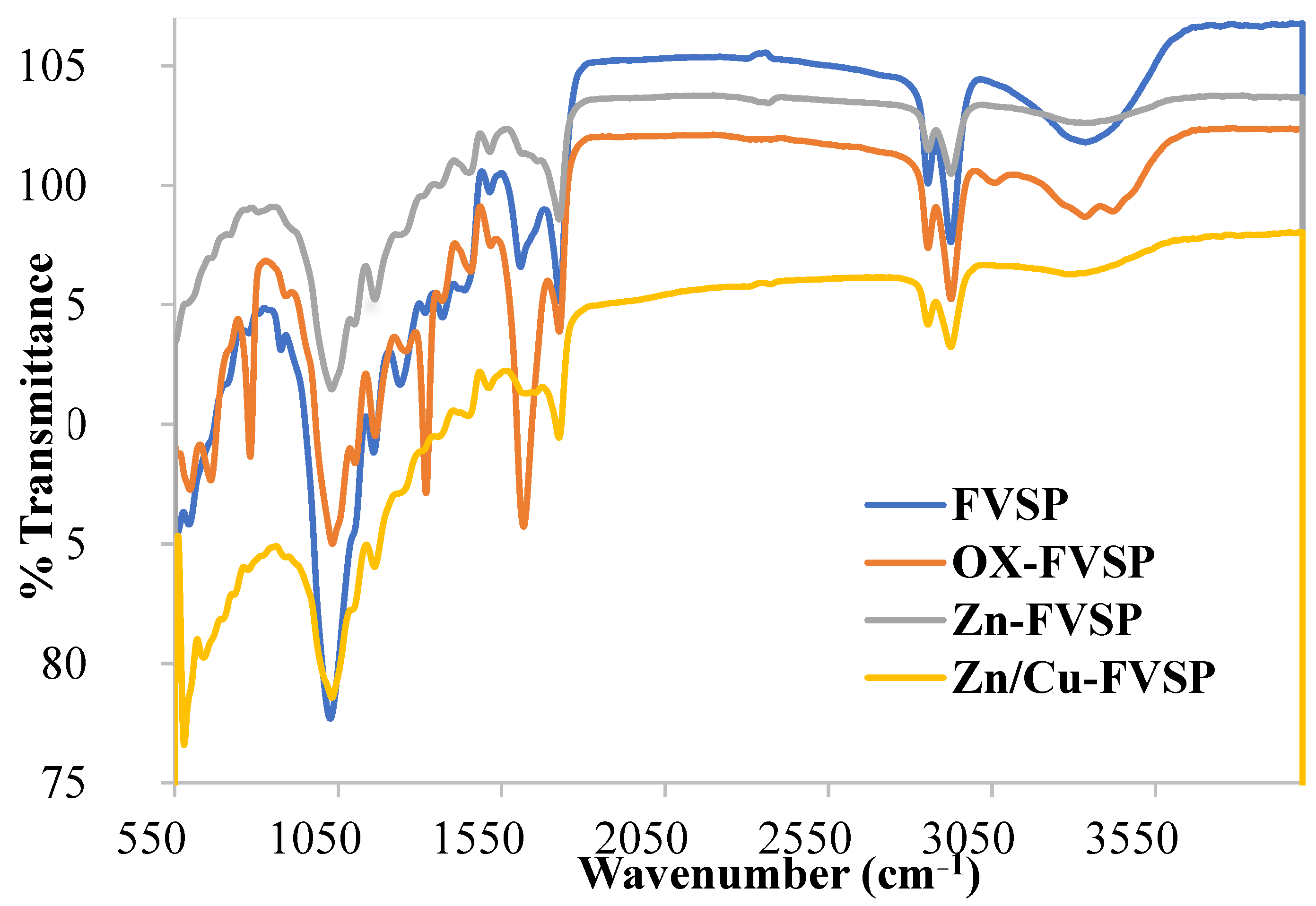
3.1. Characterization of the FVSP Adsorbents
3.2. Outcomes of Adsorption
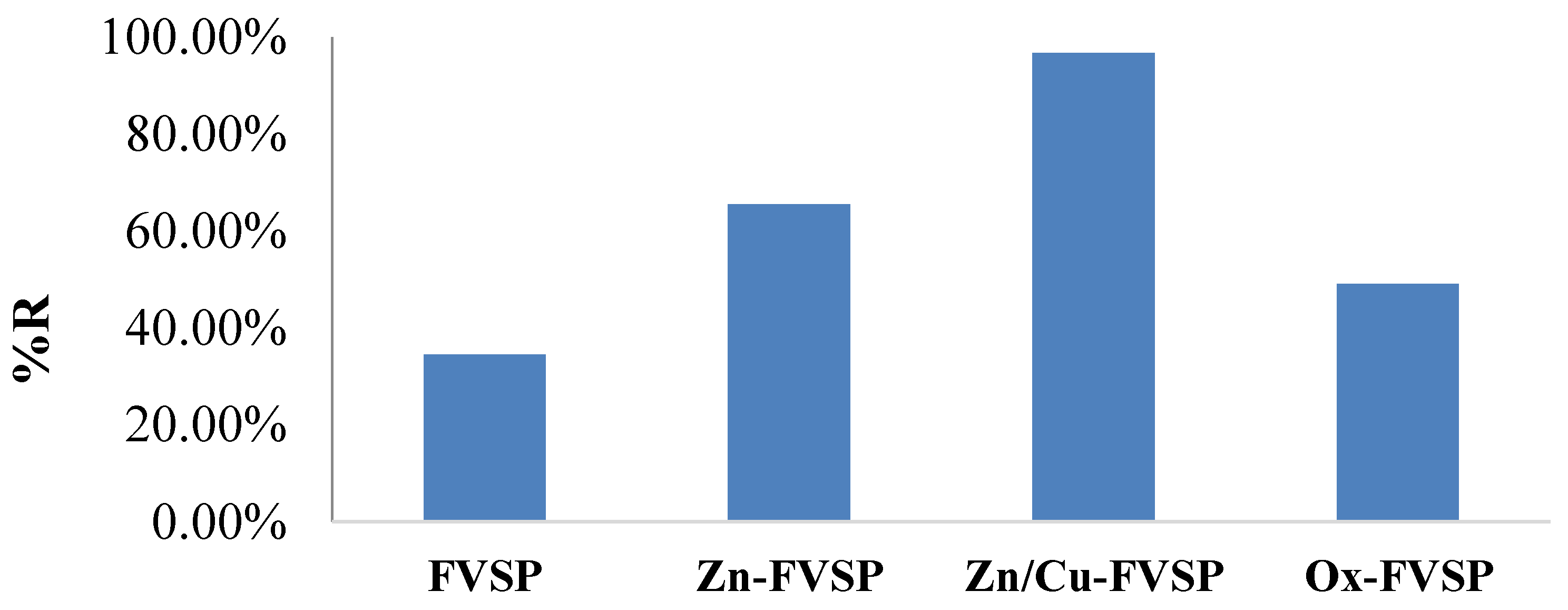
3.2.1. Effectiveness of Adsorbents
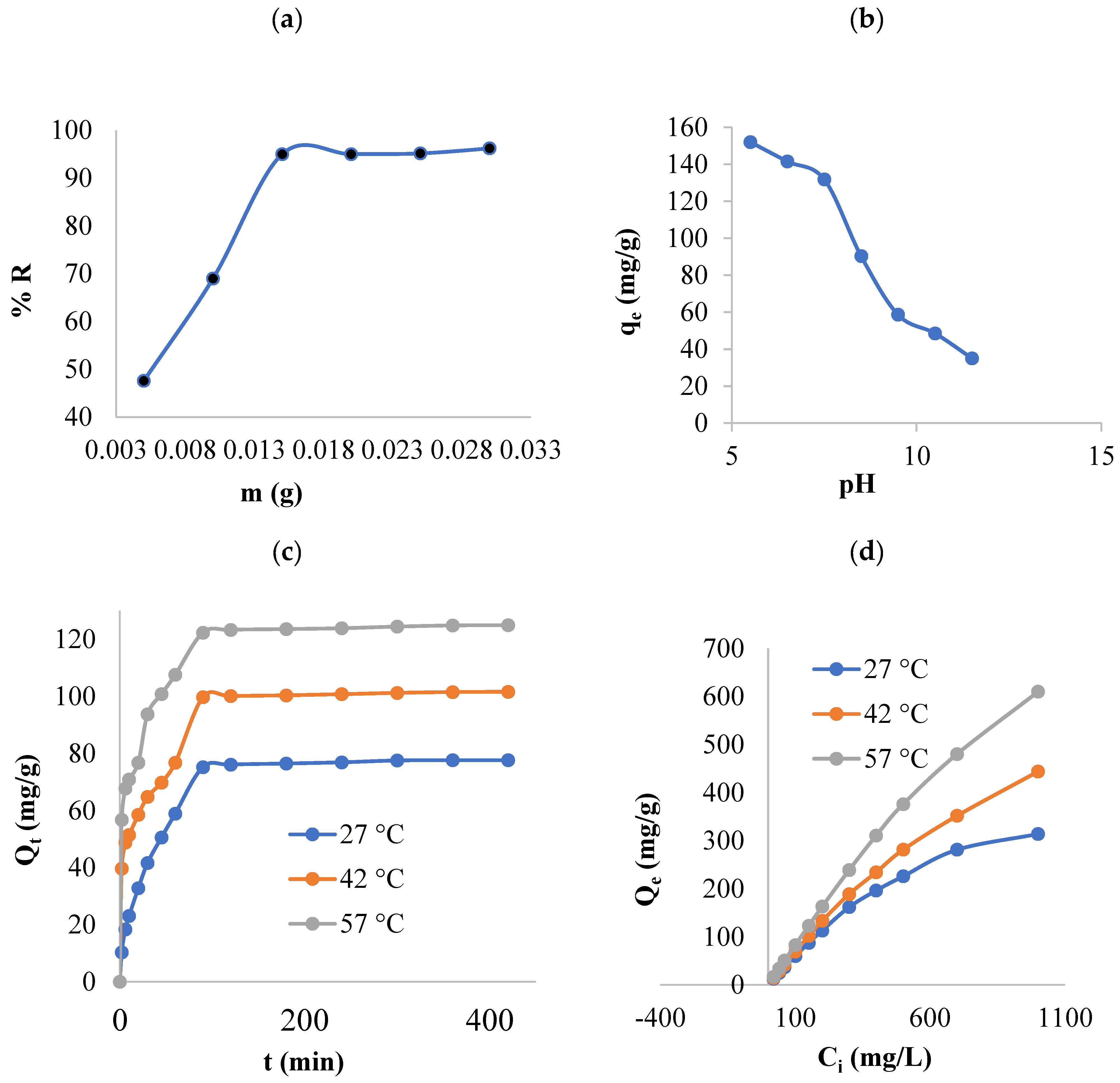
3.2.2. Impact of the Empirical Circumstances
3.2.3. Kinetics of Adsorption
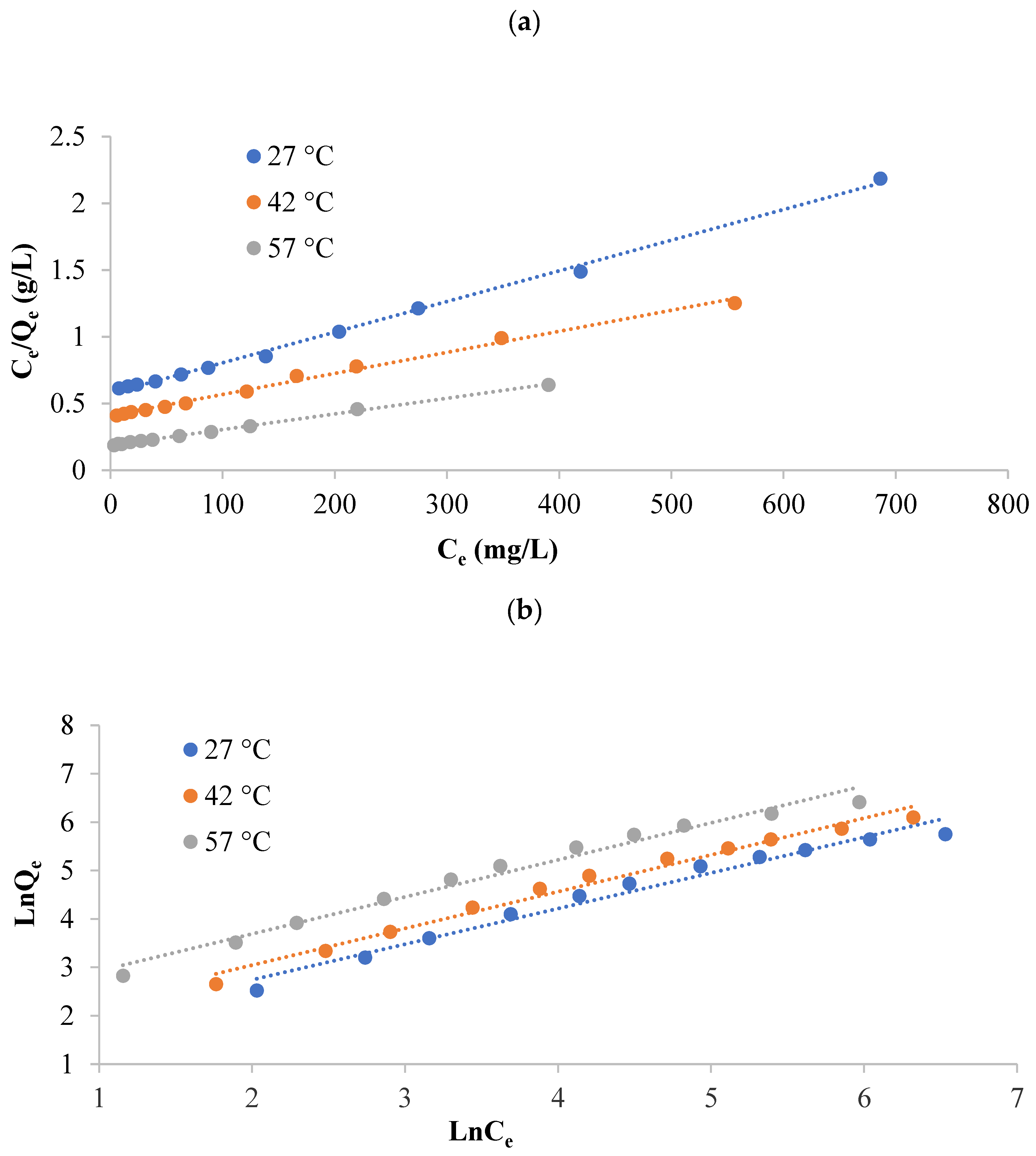
3.2.4. Adsorption Isotherms
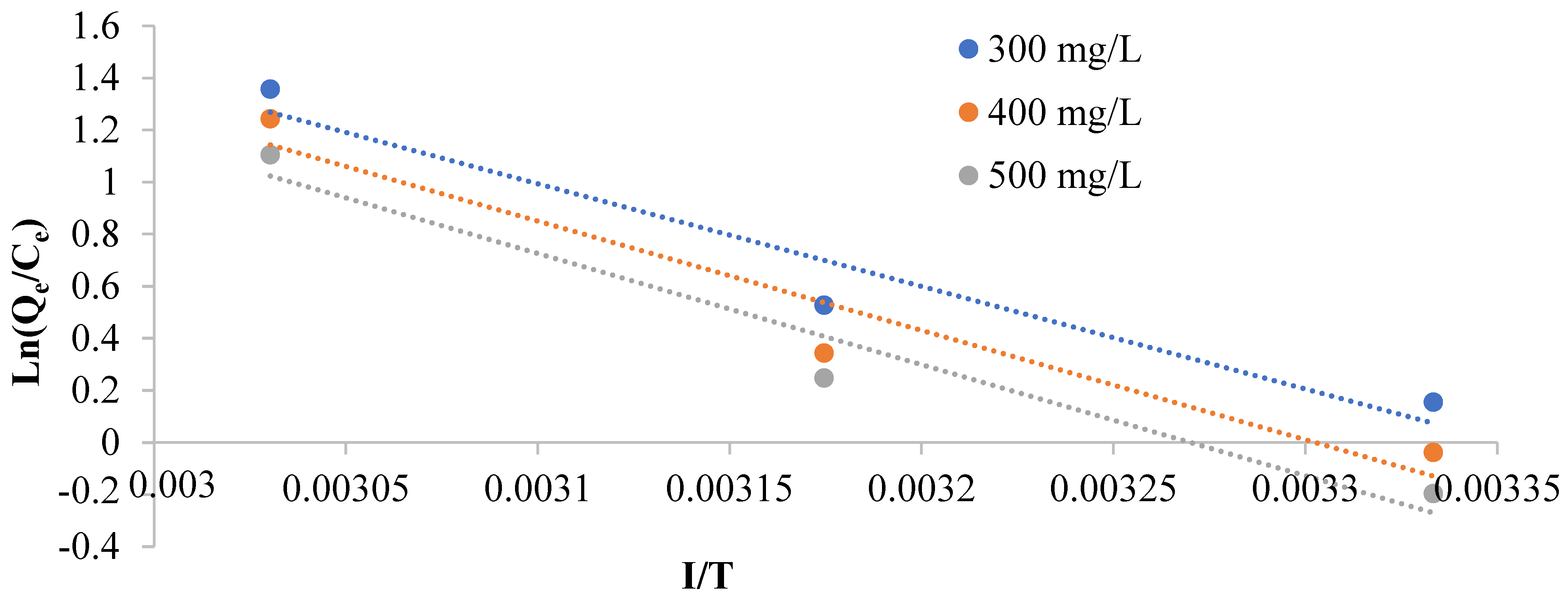
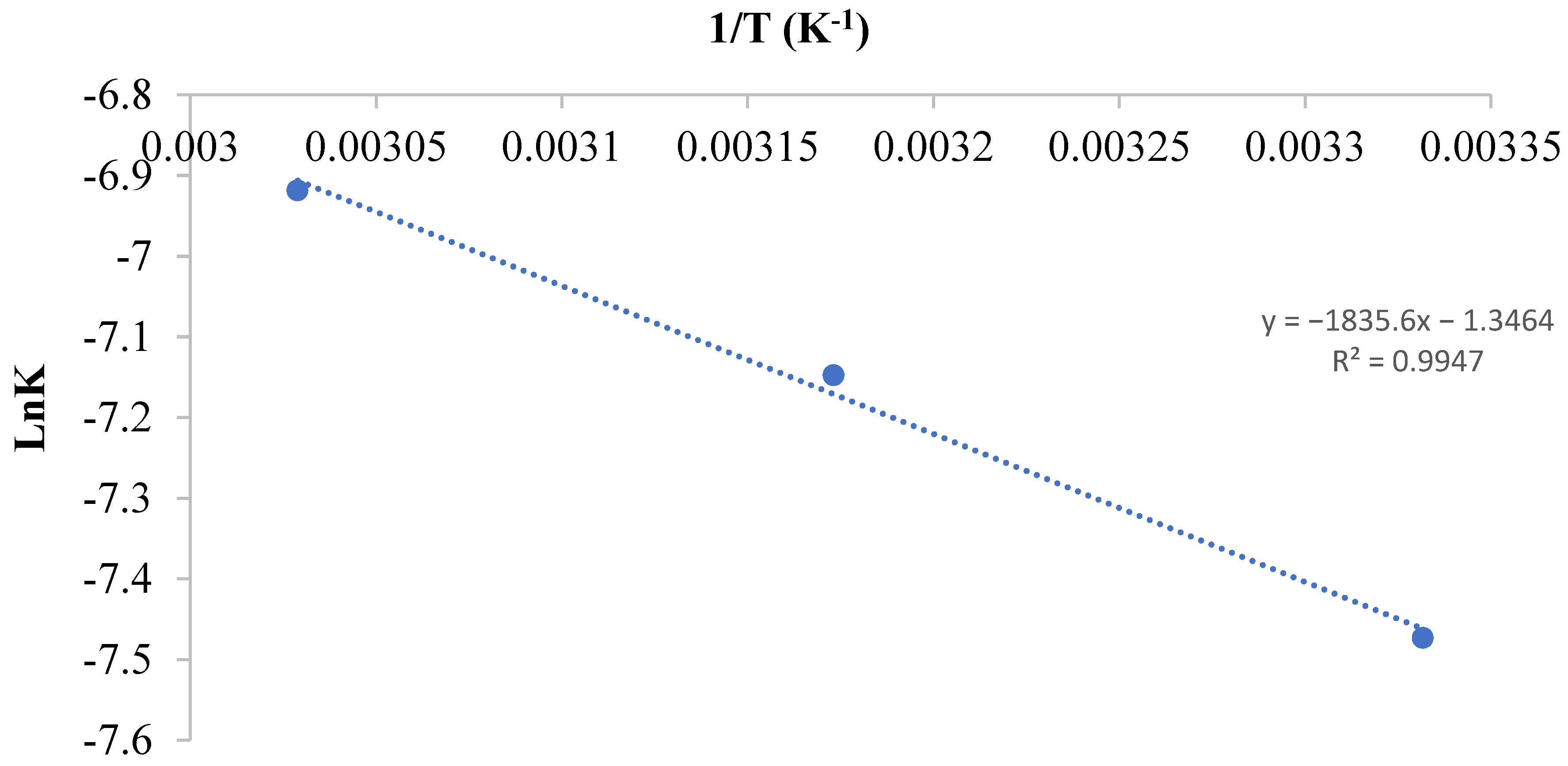
3.2.5. Thermodynamics of CR Adsorption
3.3. The Performance of Cheap Adsorbents toward CR
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Z.; Chen, H.; Ji, F.; Yuan, S. Removal of Congo Red from aqueous solution by cattail root. J. Hazard. Mater. 2010, 173, 292–297. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef] [PubMed]
- Ravi, K.; Deebika, B.; Balu, K. Decolorization of aqueous dye solutions by a novel adsorbent: Application of statistical designs and surface plots for the optimization and regression analysis. J. Hazard. Mater. 2005, B122, 75–83. [Google Scholar]
- McMullan, G.; Meehan, C.; Conneely, A.; Kirby, N.; Robinson, T.; Nigam, P.; Banat, I.; Marchant, R.; Smyth, W. Microbial decolorization and degradation of textiles dyes. Appl. Microbiol. Biotechnol. 2001, 56, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, S.P.; Thiruvenkatachari, R.; Shim, W.G.; Moon, H. Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dye. Pigment. 2006, 69, 196–203. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, L.; Qi, J. Biosorption of methylene blue from aqueous solution using a bioenergy forest waste: Xanthoceras sorbifolia seed coat. Clean-Soil Air Water 2009, 37, 642–648. [Google Scholar] [CrossRef]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of Pinus radiata. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Hameed, B.H.; Ahmad, A.L. Equilibrium and kinetic studies on basic dye adsorption by oil palm fiber activated carbon. Chem. Eng. J. 2007, 127, 111–119. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Ananthashankar, R.; Alhattab, M.; Ramakrishnan, V.V. Production, characterization and treatment of textile effluents: A critical review. J. Chem. Eng. Process Technol. 2014, 5, 1000182. [Google Scholar]
- Chen, K.C.; Wu, J.Y.; Huang, C.C.; Liang, Y.M.; Hwang, S.C.J. Decolorization of azo dye using PVA-immobilized microorganisms. J. Biotechnol. 2003, 101, 241–252. [Google Scholar] [CrossRef]
- Gong, R.; Ding, Y.; Li, M.; Yang, C.; Liu, H.; Sun, Y. Utilization of powdered peanut hull as biosorbent for removal of anionic dyes from aqueous solution. Dyes Pigment. 2005, 64, 187–192. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J. Adsorption study for removal of Congo red anionic dye using organo-atapulgite. Adsorption 2009, 15, 381–389. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Zhang, X.; Guo., X.; Jiang, X.; Li, T. Adsorption Behavior of Direct Red 80 and Congo Red onto Activated Carbon/Surfactant: Process Optimization, Kinetics and Equilibrium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 1126–1143. [Google Scholar] [CrossRef] [PubMed]
- Frid, P.; Anisimov., A.V.; Popovic., N. Congo red and protein aggregation in neurodegenerative diseases. Brain Res. Rev. 2007, 53, 135–160. [Google Scholar]
- Mittal, A.; Mittal, J.; Malviya, A.; Gupta, V.K. Adsorptive removal of hazardous anionic dye ‘‘Congo red” from wastewater using waste materials and recovery by desorption. J. Colloid Interface Sci. 2009, 340, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pinecone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1945. [Google Scholar] [CrossRef]
- Wasti, A.; Awan, M.A. Adsorption of Textile Dye onto Modified Immobilized Activated Alumina. J. Assoc. Arab. Univ. Basic Appl. Sci 2016, 20, 26–31. [Google Scholar] [CrossRef]
- Chakraborty, S.; Basak, B.; Dutta, S.; Bhunia, B.; Dey, A. Decolorization and biodegradation of congo red dye by a novel white rot fungus Alternata CMERI F6. Bioresour. Technol. 2013, 147, 662–666. [Google Scholar] [CrossRef]
- Sonwani, R.K.; Swain, G.; Giri, B.S.; Singh, R.S.; Rai, B.N. Biodegradation of Congo red dye in a moving bed biofilm reactor: Performance evaluation and kinetic modeling. Bioresour. Technol. 2020, 302, 122811. [Google Scholar] [CrossRef]
- Fowsiya, J.; Madhumitha, G.; Al-Dhabi, N.A.; Arasu, M.V. Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 162, 395–401. [Google Scholar] [CrossRef]
- Bhat, S.A.; Zafar, F.; Mondal, A.H.; Abdul, K.; Mirza, A.U.; Khan, S.; Mohammad, A.; Haq, Q.M.; Rizwanul, N.N. Photocatalytic degradation of carcinogenic Congo red dye in aqueous solution, antioxidant activity and bactericidal effect of NiO nanoparticles. J. Iran. Chem. Soc. 2020, 17, 215–227. [Google Scholar] [CrossRef]
- Ali, N.; Said, A.; Ali, F.; Raziq, F.; Ali, Z.; Bilal, M.; Reinert, L.; Begum, T.; Iqbal, H.M.N. Photocatalytic Degradation of Congo Red Dye from Aqueous Environment Using Cobalt Ferrite Nanostructures: Development, Characterization, and Photocatalytic Performance. Water Air Soil Pollut. 2020, 231, 50. [Google Scholar] [CrossRef]
- Ahmad, I.; Aslam, M.; Jabeen, U.; Zafar, M.N.; Malghani, M.N.K.; Alwadai, N.; Alshammari, F.H.; Almuslem, A.S.; Ullah, Z. ZnO and Ni-doped ZnO photocatalysts: Synthesis, characterization and improved visible light driven photocatalytic degradation of methylene blue. Inorg. Chim. Acta 2022, 543, 121167. [Google Scholar] [CrossRef]
- Velkova, Z.Y.; Kirova, G.K.; Stoytcheva, M.S.; Gochev, V.K. Biosorption of Congo red and methylene blue by pretreated waste Streptomyces fradiae biomass—Equilibrium, kinetic and thermodynamic studies. J. Serb. Chem. Soc. 2018, 83, 107–120. [Google Scholar] [CrossRef]
- Kristianto, H.; Tanuarto, M.Y.; Prasetyo, S.; Sugih, A.K. Magnetically assisted coagulation using iron oxide nanoparticles-Leucaena leucocephala seeds’ extract to treat synthetic Congo red wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 3561–3570. [Google Scholar] [CrossRef]
- Hou, T.; Guo, K.; Wang, Z.; Zhang, X.-F.; Feng, Y.; He, M.; Yao, J. Glutaraldehyde and polyvinyl alcohol crosslinked cellulose membranes for efficient methyl orange and Congo red removal. Cellulose 2019, 26, 5065–5074. [Google Scholar] [CrossRef]
- Mao-Xu, Z.; Li, L.; Hai-Hua, W.; Zheng, W. Removal of an anionic dye by adsorption/precipitation processes using alkaline white mud. J. Hazard. Mater. 2007, 149, 735–741. [Google Scholar]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Ikhazuangbe, P.M.O.; Adama, K.K.; Akintoye, G.I. Adsorption of Congo Red Dye onto Activated Carbon from Periwinkle Shell. Niger. J. Eng. Sci. Res. 2020, 3, 63–75. [Google Scholar]
- Lafi, R.; Montasser, I.; Hafiane, A. Adsorption of Congo red dye from aqueous solutions by prepared activated carbon with oxygen-containing functional groups and its regeneration. Adsorpt. Sci. Technol. 2019, 37, 160–181. [Google Scholar] [CrossRef]
- Khaniabadi, Y.O.; Mohammadi, M.J.; Shegerd, M.; Sadeghi, S.; Saeedi, S.; Basiri, H. Removal of Congo red dye from aqueous solutions by a low-cost adsorbent: Activated carbon prepared from Aloe vera leaves shell. Environ. Health Eng. Manag. 2017, 4, 29–35. [Google Scholar] [CrossRef]
- Darwish, A.; Rashad, M.; Al-Aoh, H.A. Methyl orange adsorption comparison on nanoparticles: Isotherm, kinetics, and thermodynamic studies. Dyes Pigment. 2019, 160, 563–571. [Google Scholar] [CrossRef]
- Zafar, B.; Shafqat, S.S.; Zafar, M.N.; Haider, S.; Sumrra, S.H.; Zubair, M.; Alwadai, N.; Alshammari, F.H.; Almuslem, A.S.; Akhtar, M.S. NaHCO3 assisted multifunctional Co3O4, CuO and Mn2O3 nanoparticles for tartrazine removal from synthetic wastewater and biological activities. Mater. Today Commun. 2022, 33, 104946. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Patra, T.; Sahoo, T.R. An efficient and comparative adsorption of Congo red and Trypan blue dyes on MgO nanoparticles: Kinetics, thermodynamics and isotherm studies. J. Magnes. Alloy. 2020, 16, 53. [Google Scholar] [CrossRef]
- Alamrani, N.A.; Al-Aoh, H.A. Elimination of Congo Red Dye from Industrial Wastewater Using Teucrium polium L. as a Low-Cost Local Adsorbent. Adsorpt. Sci. Technol. 2021, 2021, 5728696. [Google Scholar] [CrossRef]
- Foroughi-Dahr, M.; Abolghasemi, H.; Esmaili, M.; Shojamoradi, A.; Fatoorehchi, H. Adsorption Characteristics of Congo Red from Aqueous Solution onto Tea Waste. Chem. Eng. Commun 2014, 202, 181–193. [Google Scholar] [CrossRef]
- Zhou, Y.; Ge, L.; Fan, N.; Xia, M. Adsorption of Congo red from aqueous solution onto shrimp shell powder. Adsorp. Sci. Technol. 2018, 36, 1310–1330. [Google Scholar] [CrossRef]
- Yang, K.; Li, Y.; Zheng, H.; Luan, X.; Li, H.; Wang, Y.; Du, Q.; Sui, K.; Li, H.; Xia, Y. Adsorption of Congo red with hydrothermal treated shiitake mushroom. Mater. Res. Express 2020, 7, 015103. [Google Scholar] [CrossRef]
- Mushtaq, S.; Aslam, Z.; Ali, R.; Aslam, U.; Naseem, S.; Ashraf, M.; Bello, M.M. Surfactant modified waste ash for the removal of chloro and nitro group substituted benzene from wastewater. Water. Sci. Technol. 2022, 86, 1969–1980. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2013, 44, 437–445. [Google Scholar] [CrossRef]
- Purkayastha, S.; Narain, R.; Dahiya, P. Evaluation of antimicrobial and phytochemical screening of Fennel, Juniper and Kalonji essential oils against multi drug resistant clinical isolates. Asian Pac. J. Trop. Biomed. 2012, 2, S1625–S1629. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed. Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.H.; Qureshi, S.; Ageel, A.M. Toxicity studies in mice of ethanol extracts of Foeniculum vulgare fruit and Ruta chalepensis aerial parts. J. Ethnopharmacol. 1991, 34, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.K.; Kataria, J.; Sharma, S.A. Biomimetic synthesis of stable gold nanoparticles derived from aqueous extract of Foeniculum vulgare seeds and evaluation of their catalytic activity. Appl. Nanosci. 2017, 7, 439–447. [Google Scholar] [CrossRef]
- Yulizar, Y.; Apriandanu, D.O.B.; Al Jabbar, J.L. Facile one-pot preparation of V2O5-Fe2O3 nanocomposites using Foeniculum vulgare extracts and their catalytic property. Inorg. Chem. Commun. 2021, 123, 108320. [Google Scholar] [CrossRef]
- Bani-Atta, S.A. Potassium permanganate dye removal from synthetic wastewater using a novel, low-cost adsorbent, modified from the powder of Foeniculum vulgare seeds. Sci. Rep. 2022, 12, 4547. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H. Carboxyl functionalized Cinnamomum camphora for removal of heavy metals from synthetic wastewater-contribution to sustainability in agroforestry. J. Clean. Prod. 2018, 184, 921–928. [Google Scholar] [CrossRef]
- Huang, R.; Yang, J.; Cao, Y.; Dionysiou, D.D.; Wang, C. Peroxymonosulfate catalytic degradation of persistent organic pollutants by engineered catalyst of self-doped iron/carbon nanocomposite derived from waste toner powder. Sep. Purif. Technol. 2022, 291, 120963. [Google Scholar] [CrossRef]
- Kuchekar, S.R.; Patil, M.P.; Gaikwad, V.B.; Han, S.-H. Synthesis and characterization of silver nanoparticles using Azadirachta indica (Neem) leaf extract. Int. J. Eng. Sci. Invent. 2017, 6, 47–55. [Google Scholar]
- Nekouei, F.; Nekouei, S.; Tyagi, I.; Gupta, V.K. Kinetic, thermodynamic and isotherm studies for acid blue 129 removal from liquids using copper oxide nanoparticle-modified activated carbon as a novel adsorbent. J. Mol. Liq. 2015, 201, 124–133. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Saha, P.D. Adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohyd. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Du, Q.; Li, Q. Adsorption of Congo red from aqueous solutions by porous soybean curd xerogels. Pol. J. Chem. Technol. 2018, 20, 95–102. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.A. Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J. Hazard. Mater. 2009, 164, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Wanyonyi, W.C.; Onyari, J.M.; Shiundu, P.M. Adsorption of Congo red dye from aqueous solutions using roots of Eichhornia crassipes: Kinetic and equilibrium studies. Energy Procedia 2014, 50, 862–869. [Google Scholar] [CrossRef]
- Mustafa, S.K.; Al-Aoh, H.A.; Bani-Atta, S.A.; Alrawashdeh, L.R.; Aljohani, M.M.; Alsharif, M.A.; Darwish, A.; Al-Shehri, H.; Ahmad, M.A.; Al-Tweher, J.N.; et al. Enhance the adsorption behavior of methylene blue from wastewater by using ZnCl2 modified neem (Azadirachta indica) leaves powder. Desalination Water Treat. 2021, 209, 367–378. [Google Scholar] [CrossRef]
- Al-Aoh, H.A.; Aljohani, M.M.; Darwish, A.; Ahmad, M.A.; Bani-Atta, S.A.; Alsharif, M.A.; Mahrous, Y.; Mustafa, S.K.; Al-Shehri, H.; Alrawashdeh, L.R.; et al. A potentially low-cost adsorbent for methylene blue removal from synthetic wastewater. Desalination Water Treat. 2021, 213, 431–440. [Google Scholar] [CrossRef]
- Bani-Atta, S.A.; Al-Aoh, H.A.; Aljohani, M.M.H.; Keshk, A.A.; Al-Shehri, H.S.; Mustafa, S.K.; Alamrani, N.A.; Darwish, A.A.A.; Sobhi, M. Methylene Blue sorption by the chemically modified Ocimum basilicum leaves powder. Desalination Water Treat. 2021, 222, 237–245. [Google Scholar] [CrossRef]
- Ceglowski, M.; Schroeder, G. Removal of heavy metal ions with the use of chelating polymers obtained by grafting pyridine-pyrazole ligands onto polymethylhydrosiloxane. Chem. Eng. J. 2015, 259, 885–893. [Google Scholar] [CrossRef]
- Yaneva, Z.L.; Georgieva, N.V. Insights into Congo rerd adsorption on agro-industrial materials-Spectral, equilibrium, kinetic, thermodynamic, dynamic and desorption studies. Int. Rev. Chem. Eng. 2012, 4, 127–146. [Google Scholar]
- Cardoso, N.F.; Lima, E.C.; Royer, B.; Bach, G.L.; Dotto, M.V.; Pinto, L.A.A.; Calvete, T. Comparison of Spirulina platensis microalgae and commercial activated carbon as adsorbents for the removal of reactive Red 120 dye from aqueous effluents. J. Hazard. Mater. 2012, 241, 146–153. [Google Scholar] [CrossRef]
- Hou, H.; Zhou, R.; Wu, P.; Wu, L. Removal of Congo red dye from aqueous solution with hydroxyapatite/chitosan composite. Chem. Eng. J. 2012, 211, 336–342. [Google Scholar] [CrossRef]
- Rahchamani, J.; Mousavi, H.Z.; Behzad, M. Adsorption of methyl violet from aqueous solution by polyacrylamide as an adsorbent: Isotherm and kinetic studies. Desalination 2011, 267, 256–260. [Google Scholar] [CrossRef]
- Salahuddin, N.; Abdelwahab, M.A.; Akelah, A.; Elnagar, M. Adsorption of Congo red and crystal violet dyes onto cellulose extracted from Egyptian water hyacinth. Nat. Hazards 2021, 105, 1375–1394. [Google Scholar] [CrossRef]
- Kaur, S.; Rani, S.; Mahajan, R.K. Adsorption kinetics for the removal of hazardous dye Congo red by biowastes materials as adsorbents. J. Chem. 2013, 628582, 12. [Google Scholar] [CrossRef]


| Type of Empirical Condition | Concentration of CR (mg/L) | Contact Time (hour) | Adsorbent Dose (g) | pH | Temperature (°C) |
|---|---|---|---|---|---|
| Adsorbent dosage | 60 | 25 | 0.005–0.03 | 7 | 27 |
| Adsorption time and temp | 150 | 0–7 | 0.015 | 7 | 27–57 |
| Solution pH | 300 | 25 | 0.015 | 5.5–11.5 | 27 |
| CR conc | 20–1000 | 9 | 0.015 | 7 | 27–57 |
| FT-IR Peak | FVSP | Ox-FVSP | Zn-FVSP | Zn/Cu-FVSP | ||||
|---|---|---|---|---|---|---|---|---|
| Wavenumber (cm−1) | Assignment | Wavenumber (cm−1) | Assignment | Wavenumber (cm−1) | Assignment | Wavenumber (cm−1) | Assignment | |
| 1 | 3302.49 | Stretching of O-H, hydrogen bond | 3363.67 | Stretching of O-H, hydrogen bond | 3365.41 | Stretching of O-H, hydrogen bond | 3364.91 | Stretching of O-H, hydrogen bond |
| 2 | 2922.38 | Stretching of C-H (alkyl) | 2922.41 | Stretching of C-H (alkyl) | 2922.09 | Stretching of C-H (alkyl) | 2921.60 | Stretching of C-H (alkyl) |
| 3 | 2853.30 | Stretching of C-H | 2853.46 | Stretching of C-H | 2853.24 | Stretching of C-H | 2852.82 | Stretching of C-H |
| 4 | 1742.92 | Stretching of C=O (aliphatic aldehydes) | 1743.17 | Stretching of C=O (aliphatic aldehydes | 1743.11 | Stretching of C=O (aliphatic aldehydes | 1738.88 | Stretching of C=O (aliphatic aldehydes |
| 5 | 1603.69 | C=C stretching | 1620.36 | N-H (1°-amide) II band | 1629.16 | N-H (1°-amide) II band | 1625.67 | N-H (1°-amide) II band |
| 6 | ------- | -------- | 1457.37 | C-H scissoring | 1457.52 | C-H scissoring | -------- | -------- |
| 7 | 1375.62 | NO2 stretching | 1317.52 | Stretching of C-O | 1370.97 | NO2 stretching | -------- | -------- |
| 8 | 1029.09 | Bending of C-H in-plane | 1064.92 | C-O-C (ether) | 1064.94 | C-O-C (ether) | ------- | ----------- |
| 9 | -------- | -------- | 779.46 | Bending of C-H out-of-plane | -------- | -------- | -------- | -------- |
| Temperature (°C) | |||
|---|---|---|---|
| 27 | 42 | 57 | |
| Qe.exp (mg/g) | 77.616 | 101.6075 | 124.926 |
| Dynamic model of 1st order | |||
| Qe.cal (mg/g) | 40.766 | 35.213 | 35.221 |
| K1 (1/h) | 0.0173 | 0.0138 | 0.0136 |
| R2 | 0.761 | 0.784 | 0.801 |
| CFEF | 17.495 | 43.385 | 64.414 |
| X2 | 33.310 | 125.190 | 228.473 |
| Dynamic model of 2nd order | |||
| Qe.cal (mg/g) | 82.645 | 105.263 | 128.205 |
| K2 (g/mg. h) | 0.00057 | 0.00079 | 0.00099 |
| R2 | 0.998 | 0.998 | 0.999 |
| Rate | 0.047 | 0.083 | 0.127 |
| CFEF | 0.326 | 0.132 | 0.086 |
| X2 | 0.306 | 0.127 | 0.084 |
| Dynamic model of intra-particle diffusion (first region) | |||
| Kdif | 7.395 | 6.508 | 7.402 |
| B | 0.347 | 30.216 | 48.423 |
| R2 | 0.991 | 0.972 | 0.976 |
| Dynamic model of intra-particle diffusion (second region) | |||
| Kdif | 0.175 | 0.186 | 0.212 |
| B | 74.227 | 97.894 | 120.680 |
| R2 | 0.877 | 0.976 | 0.970 |
| Temperature (°C) | |||
|---|---|---|---|
| 27 | 42 | 57 | |
| Qe.exp (mg/g) | 313.92 | 443.70 | 609.57 |
| Langmuir | |||
| Qmax (mg/g) | 434.78 | 625.00 | 833.33 |
| Qe (mg/g) | 318.567 | 427.732 | 595.062 |
| KL (L/mg) | 0.003996 | 0.003898 | 0.006397 |
| Sf | 0.2002 | 0.2042 | 0.1352 |
| R2 | 0.998 | 0.994 | 0.999 |
| CFEF | 0.069 | 0.575 | 0.345 |
| X2 | 0.068 | 0.596 | 0.354 |
| Freundlich | |||
| Qe (mg/g) | 655.05 | 661.46 | 831.86 |
| KF (mg/g)(L/mg)1/n | 4.602 | 4.015 | 8.681 |
| n | 1.317 | 1.238 | 1.308 |
| 1/n | 0.7592 | 0.8075 | 0.7646 |
| R2 | 0.974 | 0.984 | 0.980 |
| CFEF | 370.714 | 106.877 | 81.058 |
| X2 | 177.655 | 71.691 | 59.398 |
| CR Concentration (mg/L) | ∆Ho (kJ/mol) | ∆So (kJ/mol) | ∆Go (KJ/mol) | R2 | ||
|---|---|---|---|---|---|---|
| 27 °C | 42 °C | 57 °C | ||||
| 300 | 32.767 | 0.1098 | −0.184 | −1.831 | −3.479 | 0.942 |
| 400 | 34.903 | 0.1153 | −0.323 | −1.406 | −3.135 | 0.936 |
| 500 | 35.495 | 0.1161 | −0.673 | −1.068 | −2.809 | 0.957 |
| Adsorbents | Qmax (mg/g) | References | |
|---|---|---|---|
| Zn/Cu-FVSP | 434.78 625.00 833.33 | 27 °C 42 °C 57 °C | This study |
| Cattail rot | 38.79 | [1] | |
| Pine bark | 3.90 | 50 °C | [2] |
| Shrimp shell powder | 232.00 264.60 288.20 | 30 °C 40 °C 50 °C | [37] |
| Modified shiitake mushroom | 217.90 211.90 209.40 | 20 °C 35 °C 50 °C | [38] |
| Soybean curd | 69.00 69.20 69.90 | 25 °C 40 °C 55 °C | [52] |
| Roots of Eichhornia crassipes | 1.60 | [54] | |
| Egyptian hyacinth cellulose | 230.00 | [63] | |
| Tea waste | 32.30 | [36] | |
| Charcoal of Eichhornia | 56.8 | [64] | |
| Charcoal of groundnut | 117.6 | [64] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL-Aoh, H.A. Removal of the Pigment Congo Red from Synthetic Wastewater with a Novel and Inexpensive Adsorbent Generated from Powdered Foeniculum Vulgare Seeds. Processes 2023, 11, 446. https://doi.org/10.3390/pr11020446
AL-Aoh HA. Removal of the Pigment Congo Red from Synthetic Wastewater with a Novel and Inexpensive Adsorbent Generated from Powdered Foeniculum Vulgare Seeds. Processes. 2023; 11(2):446. https://doi.org/10.3390/pr11020446
Chicago/Turabian StyleAL-Aoh, Hatem A. 2023. "Removal of the Pigment Congo Red from Synthetic Wastewater with a Novel and Inexpensive Adsorbent Generated from Powdered Foeniculum Vulgare Seeds" Processes 11, no. 2: 446. https://doi.org/10.3390/pr11020446
APA StyleAL-Aoh, H. A. (2023). Removal of the Pigment Congo Red from Synthetic Wastewater with a Novel and Inexpensive Adsorbent Generated from Powdered Foeniculum Vulgare Seeds. Processes, 11(2), 446. https://doi.org/10.3390/pr11020446






