Biotemplating of Al2O3-Doped, CaO-Based Material from Bamboo Fiber for Efficient Solar Energy Storage
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Materials
2.2. Sample Preparation Process
2.3. Cyclic Experiment
2.4. Characterization
3. Results and Discussion
3.1. Effect of Biotemplate Addition on Energy Storage Performance of CaO-Based Materials
3.2. Effect of Support Loading on Energy Storage Performance of CaO-Based Materials
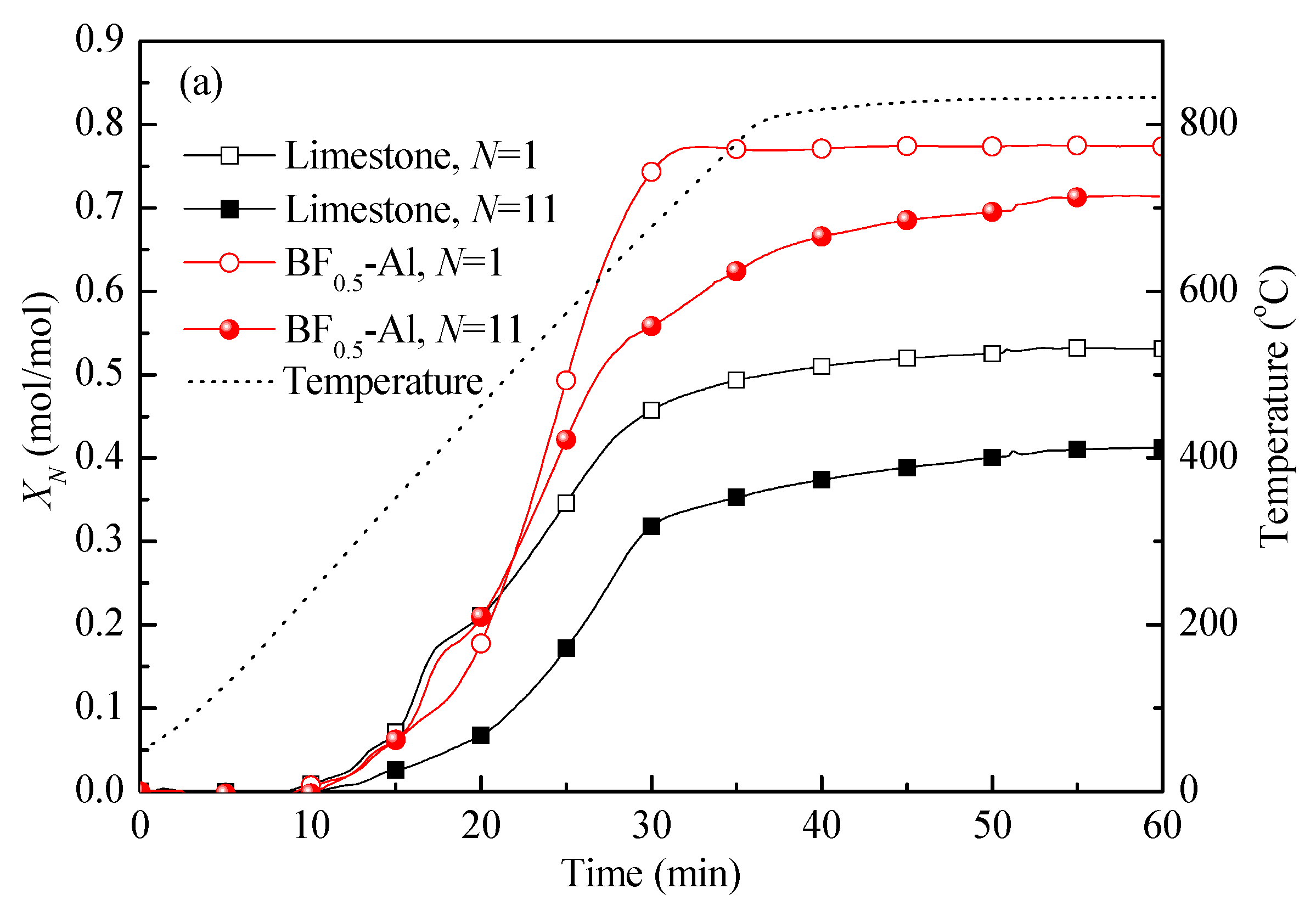
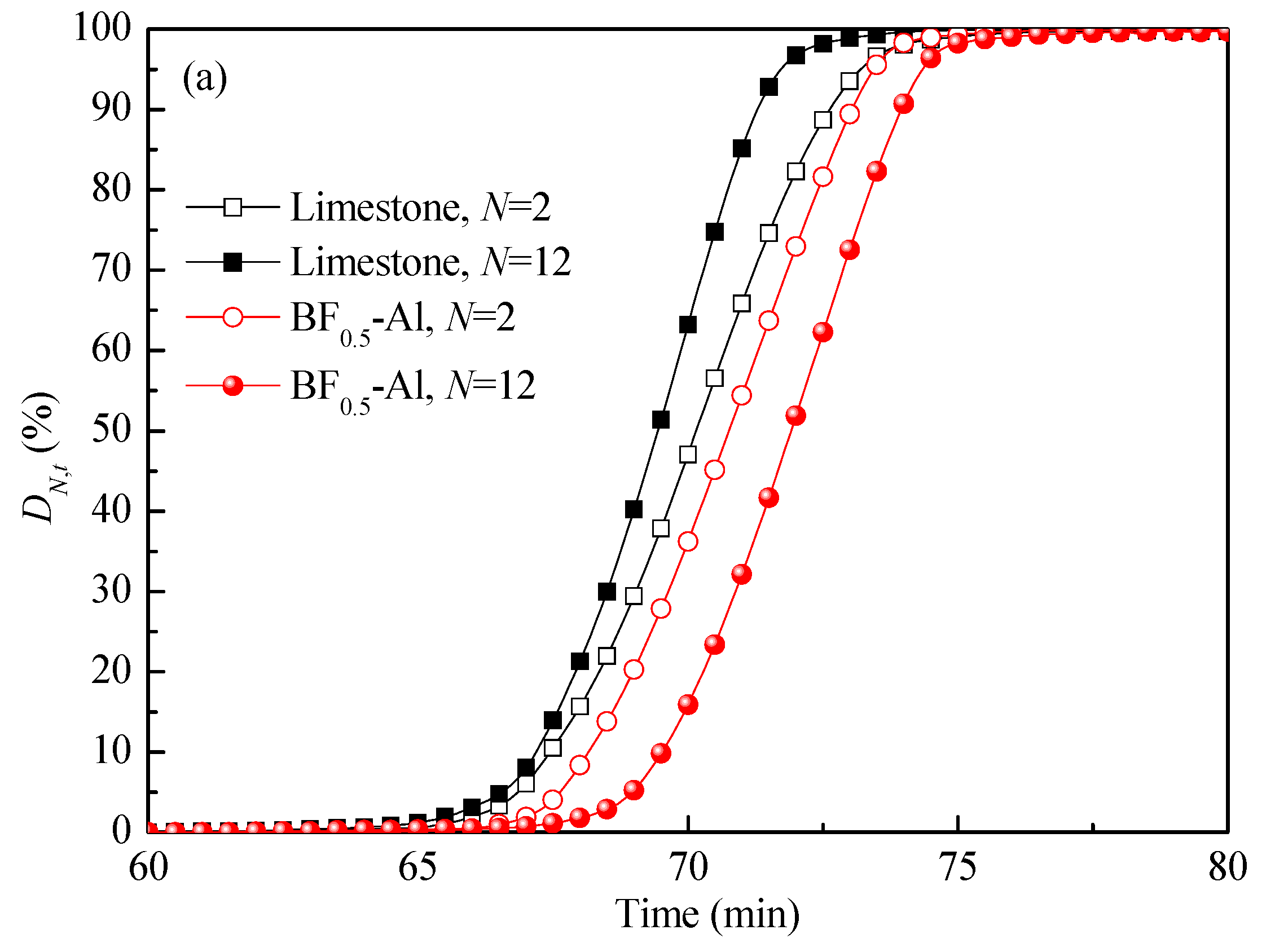
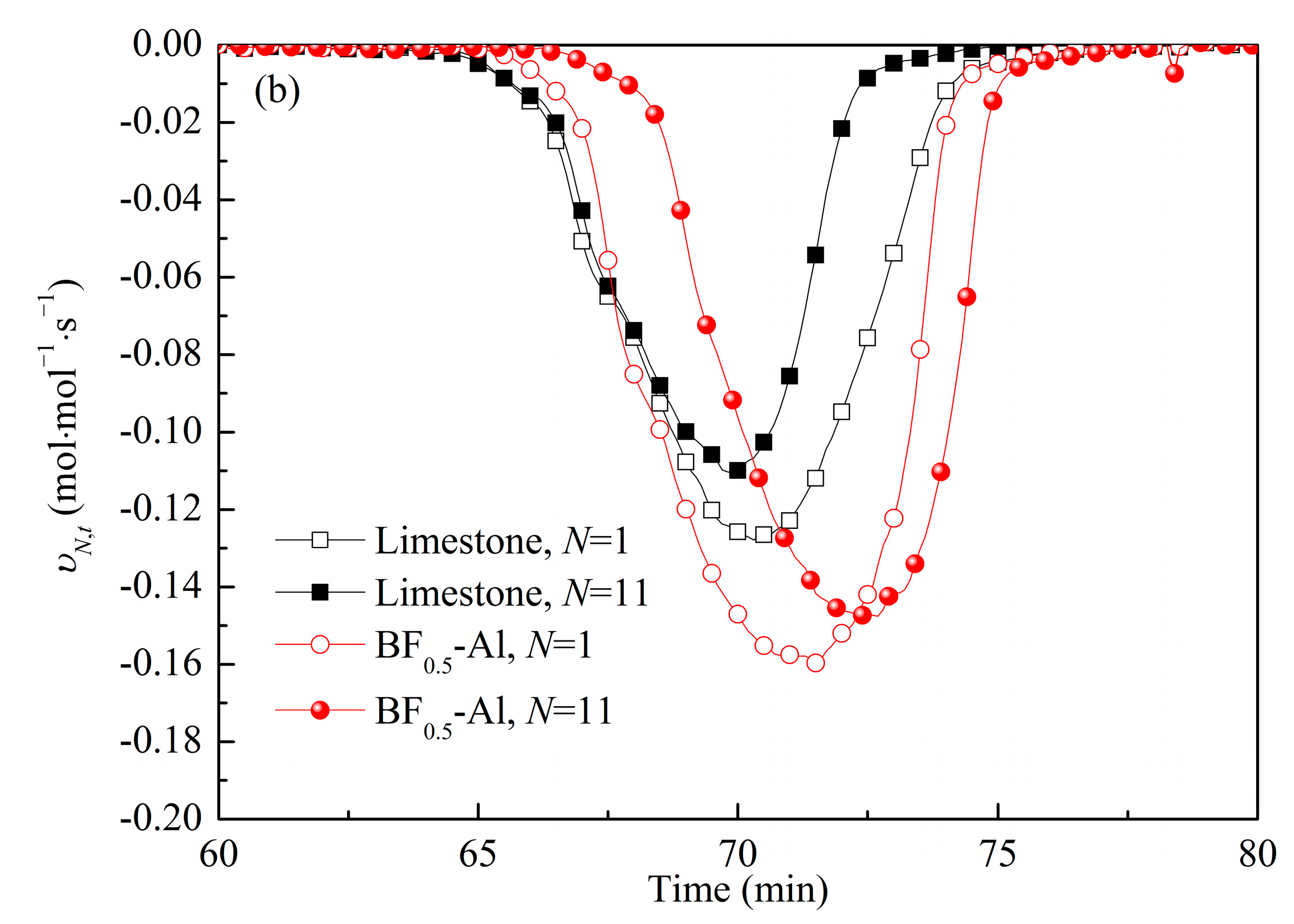
3.3. Comparison of Energy Storage Performances of Different Templated CaO-Based Materials with Doped Al2O3
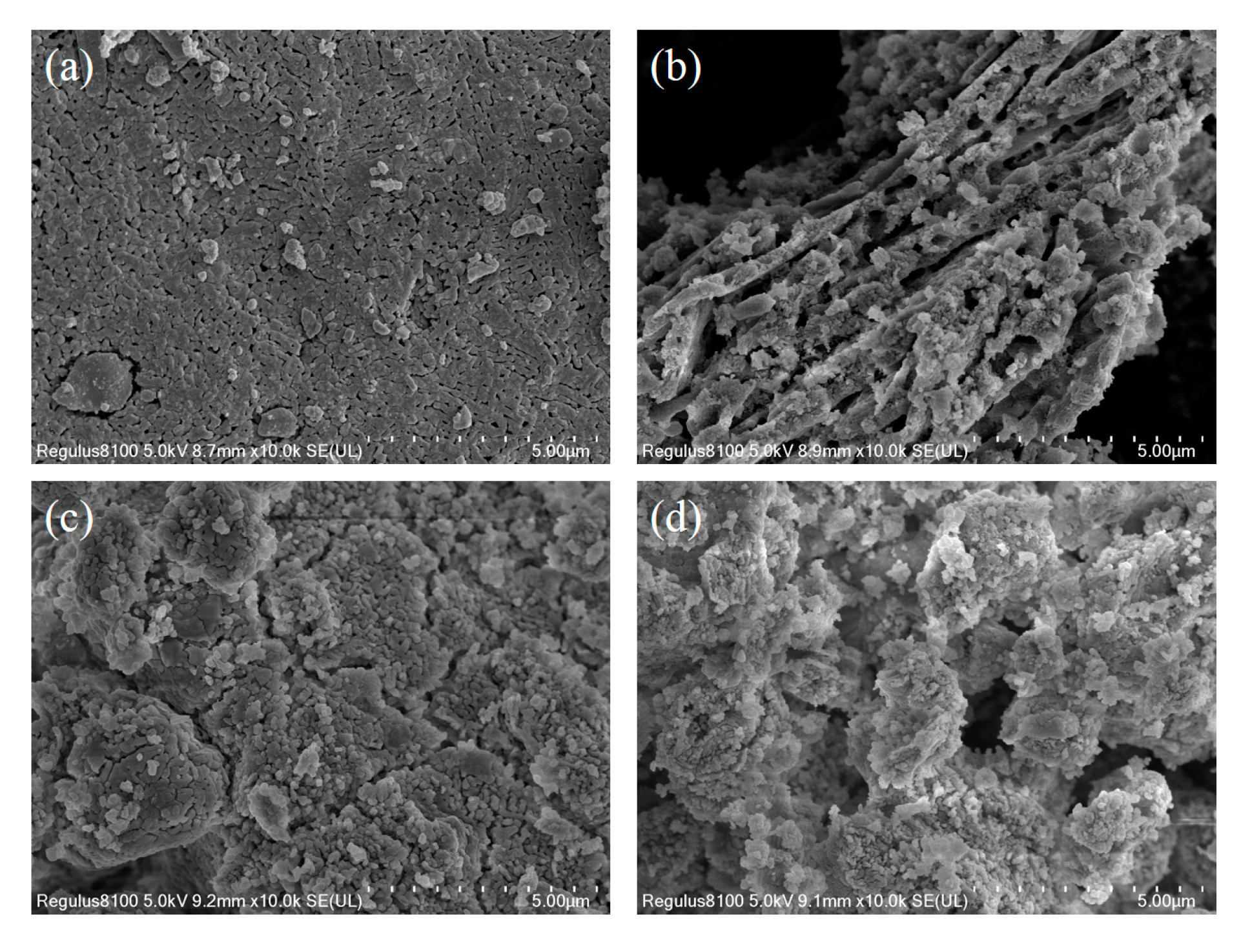
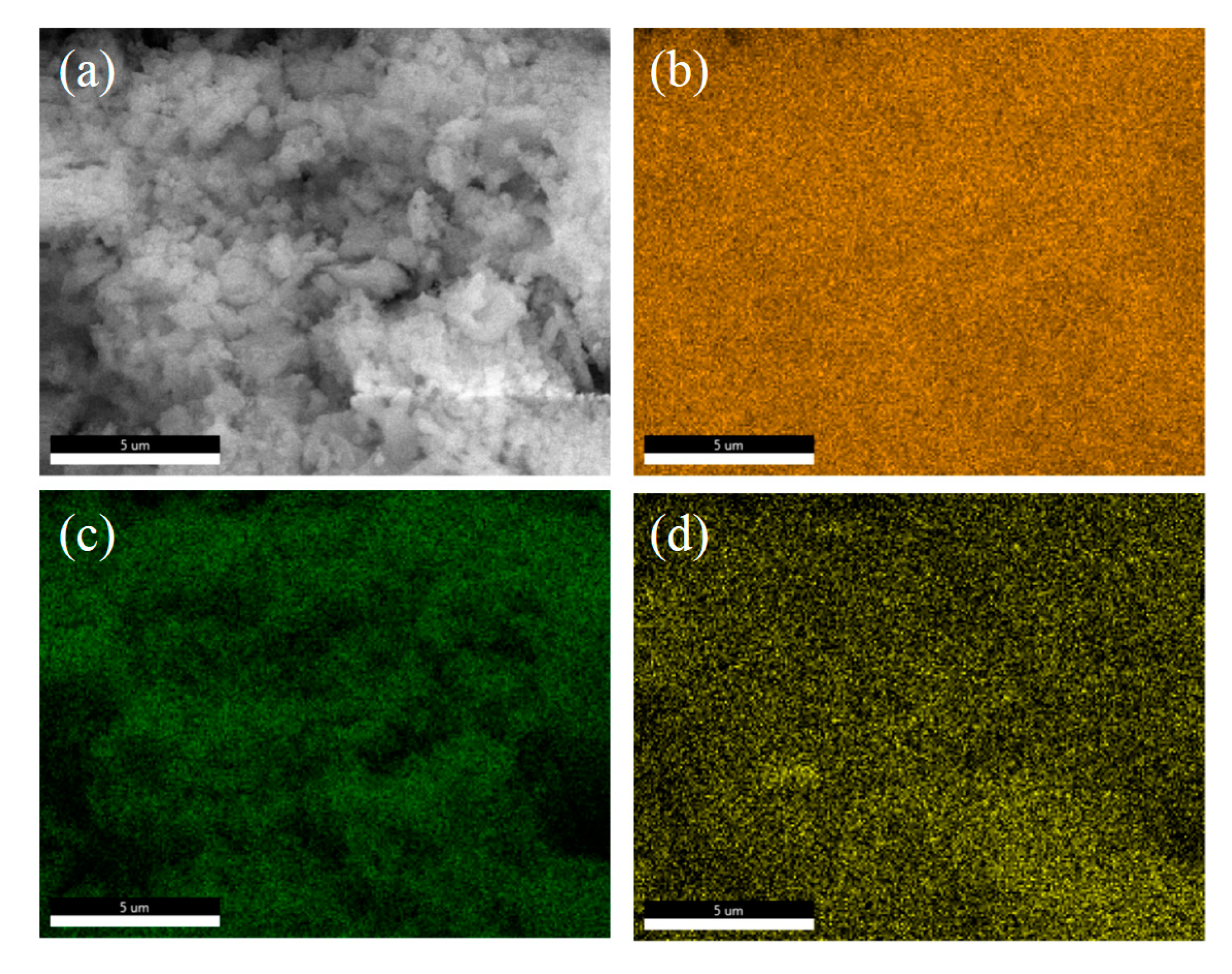
3.4. Reaction Kinetic Evaluation and Morphological Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holditch, S.A.; Chianelli, R.R. Factors that will influence oil and gas supply and demand in the 21st century. MRS Bull. 2008, 33, 317–323. [Google Scholar] [CrossRef]
- Magida, N.E.; Dugmore, G.; Ogunlaja, A.S. Coal-scenedesmus microalgae co-firing in a fixed bed combustion reactor: A study on CO2, SO2 and NOx emissions and ash. Processes 2022, 10, 2183. [Google Scholar] [CrossRef]
- Ramezani, R.; Di Felice, L.; Gallucci, F. A review on hollow fiber membrane contactors for carbon capture: Recent advances and future challenges. Processes 2022, 10, 2103. [Google Scholar] [CrossRef]
- Yan, X.; Li, Y.; Sun, C.; Zhang, C.; Yang, L.; Fan, X.; Chu, L. Enhanced H2 production from steam gasification of biomass by red mud-doped Ca-Al-Ce bi-functional material. Appl. Energy 2022, 312, 118737. [Google Scholar] [CrossRef]
- Li, S.; Tan, X.; Li, H.; Gao, Y.; Wang, Q.; Li, G.; Guo, M. Investigation on pore structure regulation of activated carbon derived from sargassum and its application in supercapacitor. Sci. Rep. 2022, 12, 10106. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Dou, B.; Ma, Y.; Liang, Y. Effects of TiO2 doping on the performance of thermochemical energy storage based on Mn2O3/Mn3O4 redox materials. RSC Adv. 2021, 11, 33744–33758. [Google Scholar] [CrossRef]
- Carmona-Martínez, A.A.; Fresneda-Cruz, A.; Rueda, A.; Birgi, O.; Khawaja, C.; Janssen, R.; Davidis, B.; Reumerman, P.; Vis, M.; Karampinis, E.; et al. Renewable power and heat for the decarbonisation of energy-intensive industries. Processes 2023, 11, 18. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Ma, Y.; Liang, Y. Heat transfer enhancement of indirect heat transfer reactors for Ca(OH)2/CaO thermochemical energy storage system. Processes 2021, 9, 1136. [Google Scholar] [CrossRef]
- Han, R.; Xing, S.; Wu, X.; Pang, C.; Lu, S.; Su, Y.; Liu, Q.; Song, C.; Gao, J. Compressing two-dimensional graphite-nanosheet-supported CaO for optimizing porous structures toward high-volumetric-performance heat storage. Energy Fuels 2021, 35, 10841–10849. [Google Scholar] [CrossRef]
- Valverde, J.M.; Barea-López, M.; Perejón, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Effect of thermal pretreatment and nanosilica addition on limestone performance at calcium-looping conditions for thermochemical energy storage of concentrated solar power. Energy Fuels 2017, 31, 4226–4236. [Google Scholar] [CrossRef]
- Irwin, L.; Stekli, J.; Pfefferkorn, C.; Pitchumani, R. 11-Thermochemical energy storage for concentrating solar thermal (CST) systems. Adv. Conc. Sol. Therm. Res. Technol. 2017, 2017, 247–267. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Yan, X.; Zhao, J.; Zhang, C. Development of thermochemical heat storage based on CaO/CaCO3 cycles: A review. Energies 2021, 14, 847. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Qi, C.; Ling, X.; Peng, H. State of the art on the high-temperature thermochemical energy storage systems. Energy Convers. Manage. 2018, 177, 792–815. [Google Scholar] [CrossRef]
- Sun, R.; Xiao, R.; Ye, J. Kinetic analysis about the CO2 capture capacity of lime mud from paper mill in calcium looping process. Energy Sci. Eng. 2020, 8, 4014–4024. [Google Scholar] [CrossRef]
- Chen, Z.; Song, H.S.; Portillo, M.; Lim, C.J.; Grace, J.R.; Anthony, E.J. Long-term calcination/carbonation cycling and thermal pretreatment for CO2 capture by limestone and dolomite. Energy Fuels 2009, 23, 1437–1444. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, Y.; Sun, Y.; Li, P.; Yu, J. Preparation of CaO-Al2O3 sorbent and CO2 capture performance at high temperature. Fuel 2013, 111, 636–642. [Google Scholar] [CrossRef]
- Wang, K.; Gu, F.; Clough, P.T.; Zhao, P.; Anthony, E.J. Porous MgO-stabilized CaO-based powders/pellets via a citric acid-based carbon template for thermochemical energy storage in concentrated solar power plants. Chem. Eng. J. 2020, 390, 124163. [Google Scholar] [CrossRef]
- Chen, X.; Jin, X.; Liu, Z.; Ling, X.; Wang, Y. Experimental investigation on the CaO/CaCO3 thermochemical energy storage with SiO2 doping. Energy 2018, 155, 128–138. [Google Scholar] [CrossRef]
- Yan, X.; Li, Y.; Sun, C.; Wang, Z. Hydrogen production from absorption-enhanced steam gasification of Enteromorpha prolifera and its char using Ce-doped CaO material. Fuel 2021, 287, 119554. [Google Scholar] [CrossRef]
- Bai, S.; Sun, J.; Zhou, Z.; Bu, C.; Chen, X.; Yang, Y.; Wang, R.; Guo, Y.; Zhao, C.; Liu, W. Structurally improved, TiO2-incorporated, CaO-based pellets for thermochemical energy storage in concentrated solar power plants. Sol. Energy Mater. Sol. Cells 2021, 226, 111076. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Leng, L.; Hu, Y. Thermochemical energy storage of concentrated solar power by novel Y2O3-doped CaO pellets. Energy Fuels 2021, 35, 12610–12618. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. Calcium-Looping performance of mechanically modified Al2O3-CaO composites for energy storage and CO2 capture. Chem. Eng. J. 2018, 334, 2343–2355. [Google Scholar] [CrossRef]
- Sarrion, B.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A.; Valverde, J.M. Pressure effect on the multicycle activity of natural carbonates and a Ca/Zr composite for energy storage of concentrated solar power. ACS Sustain. Chem. Eng. 2018, 6, 7849–7858. [Google Scholar] [CrossRef]
- Han, R.; Gao, J.; Wei, S.; Su, Y.; Su, C.; Li, J.; Liu, Q.; Qin, Y. High-performance CaO-based composites synthesized using a space-confined chemical vapor deposition strategy for thermochemical energy storage. Sol. Energy Mater. Sol. Cells 2020, 206, 110346. [Google Scholar] [CrossRef]
- Naeem, M.A.; Armutlulu, A.; Broda, M.; Lebedev, D.; Müller, C.R. The development of effective CaO-based CO2 sorbents via a sacrificial templating technique. Faraday Discuss. 2016, 192, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Deng, J.; Peng, D.; Wei, T.; Zhang, H.; Peng, R. Selective adsorption of Pb2+ in the presence of Mg2+ by layer-by-layer self-assembled MnO2/Mxene composite films. Processes 2022, 10, 641. [Google Scholar] [CrossRef]
- Chi, C.; Li, Y.; Zhang, W.; Wang, Z. Synthesis of a hollow microtubular Ca/Al sorbent with high CO2 uptake by hard templating. Appl. Energy 2019, 251, 113382. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, S.; Suzuki, Y. Experimental study on CO2 capture conditions of a fluidized bed limestone decomposition reactor. Fuel Process. Technol. 2010, 91, 958–963. [Google Scholar] [CrossRef]
- Sayyah, M.; Lu, Y.; Masel, R.I.; Suslick, K.S. Mechanical activation of CaO-based adsorbents for CO2 capture. ChemSusChem 2013, 6, 193–198. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Duan, L.; Anthony, E.; Liu, H. CO2 capture performance of calcium-based synthetic sorbent with hollow core-shell structure under calcium looping conditions. Appl. Energy 2018, 225, 402–412. [Google Scholar] [CrossRef] [Green Version]
- Arcenegui Troya, J.; Sánchez-Jiménez, P.; Perejon, A.; Moreno, V.; Valverde, J.; Pérez-Maqueda, L. Kinetics and cyclability of limestone (CaCO3) in presence of steam during calcination in the CaL scheme for thermochemical energy storage. Chem. Eng. J. 2021, 417, 129194. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Liu, L.; She, X.; Xu, R.; Sun, J.; Xu, M. Enhanced thermochemical energy storage stability of CaO-based composite pellets incorporated with a Zr-based stabilizer. Energy Fuels 2021, 35, 18778–18788. [Google Scholar] [CrossRef]
- Bian, Z.; Li, Y.; Ren, Y.; Fang, Y.; Zhao, J. Thermochemical heat storage performance of CaO particles under fluidization in coupled CaO/Ca(OH)2 cycles and CaO/CaCO3 cycles. J. Energy Storage 2022, 56, 106045. [Google Scholar] [CrossRef]
- Jia, L.; Hughes, R.; Lu, D.; Anthony, E.J.; Lau, I. Attrition of calcining limestones in circulating fluidized-bed systems. Ind. Eng. Chem. Res. 2007, 46, 5199–5209. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Yan, X.; Zhang, W.; Zhao, J.; Wang, Z. Preparation of a morph-genetic CaO-based sorbent using paper fibre as a biotemplate for enhanced CO2 capture. Chem. Eng. J. 2019, 361, 235–244. [Google Scholar] [CrossRef]
- Sher, F.; Chen, S.; Raza, A.; Rasheed, T.; Razmkhah, O.; Rashid, T.; Rafi-ul-Shan, P.M.; Erten, B. Novel strategies to reduce engine emissions and improve energy efficiency in hybrid vehicles. Clean. Eng. Technol. 2021, 2, 100074. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Bian, Z.; Yan, X.; Wang, Z.; Liu, W. Thermochemical energy storage performances of Ca-based natural and waste materials under high pressure during CaO/CaCO3 cycles. Energy Convers. Manag. 2019, 197, 111885. [Google Scholar] [CrossRef]
- Sher, F.; Hazafa, A.; Marintseva, K.; Rasheed, T.; Ali, U.; Rashid, T.; Babu, A.; Khzouz, M. Fully solar powered doncaster sheffield airport: Energy evaluation, glare analysis and CO2 mitigation. Sustain. Energy Technol. 2021, 45, 101122. [Google Scholar] [CrossRef]
- Grasa, G.S.; García, J.C.A. CO2 capture capacity of CaO in long series of carbonation/calcination cycles. Ind. Eng. Chem. Res. 2006, 45, 8846–8851. [Google Scholar] [CrossRef]
- Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. CO2 multicyclic capture of pretreated/doped CaO in the Ca-looping process. Theory and experiments. Phys. Chem. Chem. Phys. 2013, 15, 11775–11793. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Zhu, H.; Xiao, R. Enhancement of CO2 capture and microstructure evolution of the spent calcium-based sorbent by the self-reactivation process. Chin. J. Chem. Eng. 2021, 29, 160–166. [Google Scholar] [CrossRef]
- Sher, F.; Ilyas, M.; Ilyas, M.; Liaqat, U.; Lima, E.C.; Sillanpää, M.; Klemeš, J.J. Biorenewable nanocomposites as robust materials for energy storage applications. Am. Chem. Soc. 2022, 1410, 197–224. [Google Scholar] [CrossRef]
- Ullah, S.; Branquinho, R.; Mateus, T.; Martins, R.; Fortunato, E.; Rasheed, T.; Sher, F. Solution combustion synthesis of transparent conducting thin films for sustainable photovoltaic applications. Sustainability 2020, 12, 10423. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.M.; Perejon, A.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A. Low-cost Ca-based composites synthesized by biotemplate method for thermochemical energy storage of concentrated solar power. Appl. Energy 2018, 210, 108–116. [Google Scholar] [CrossRef]
- Fedunik-Hofman, L.; Bayon, A.; Gao, X.; Tricoli, A.; Donne, S.W. Dysprosium oxide-supported CaO for thermochemical energy storage. Front. Mater. 2021, 8, 670638. [Google Scholar] [CrossRef]
- Sher, F.; Iqbal, S.Z.; Liu, H.; Imran, M.; Snape, C.E. Thermal and kinetic analysis of diverse biomass fuels under different reaction environment: A way forward to renewable energy sources. Energy Convers. Manag. 2020, 203, 112266. [Google Scholar] [CrossRef]
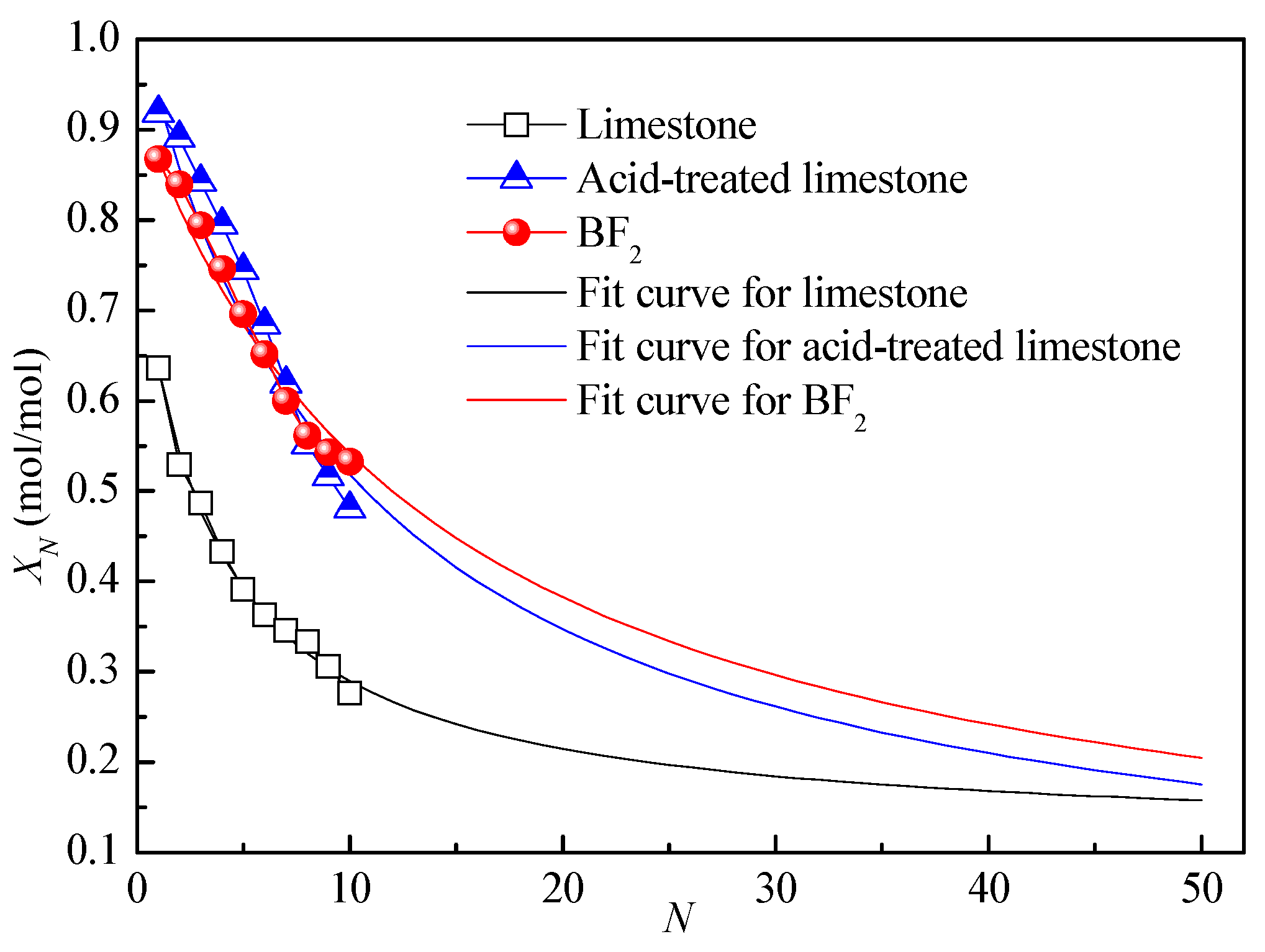
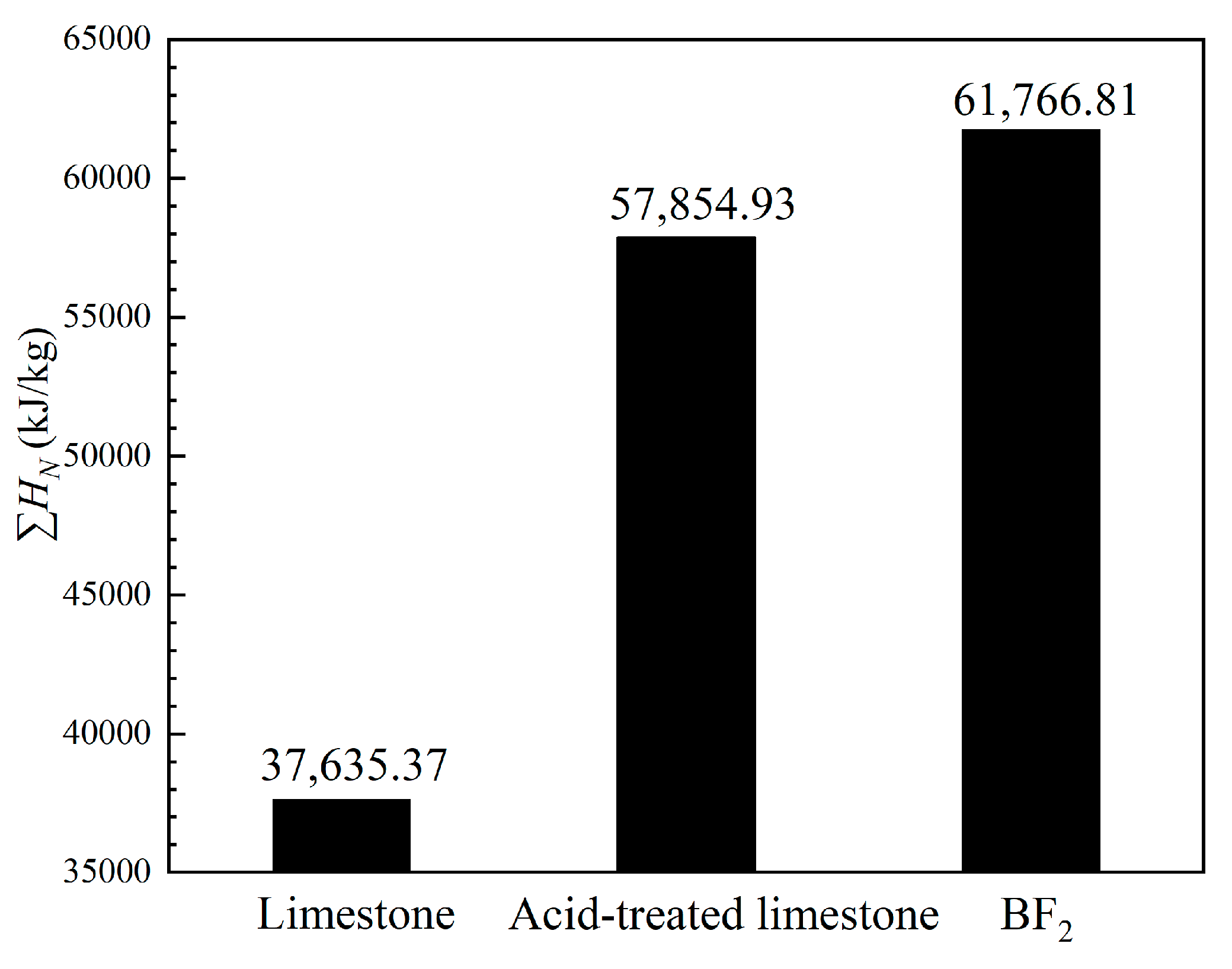
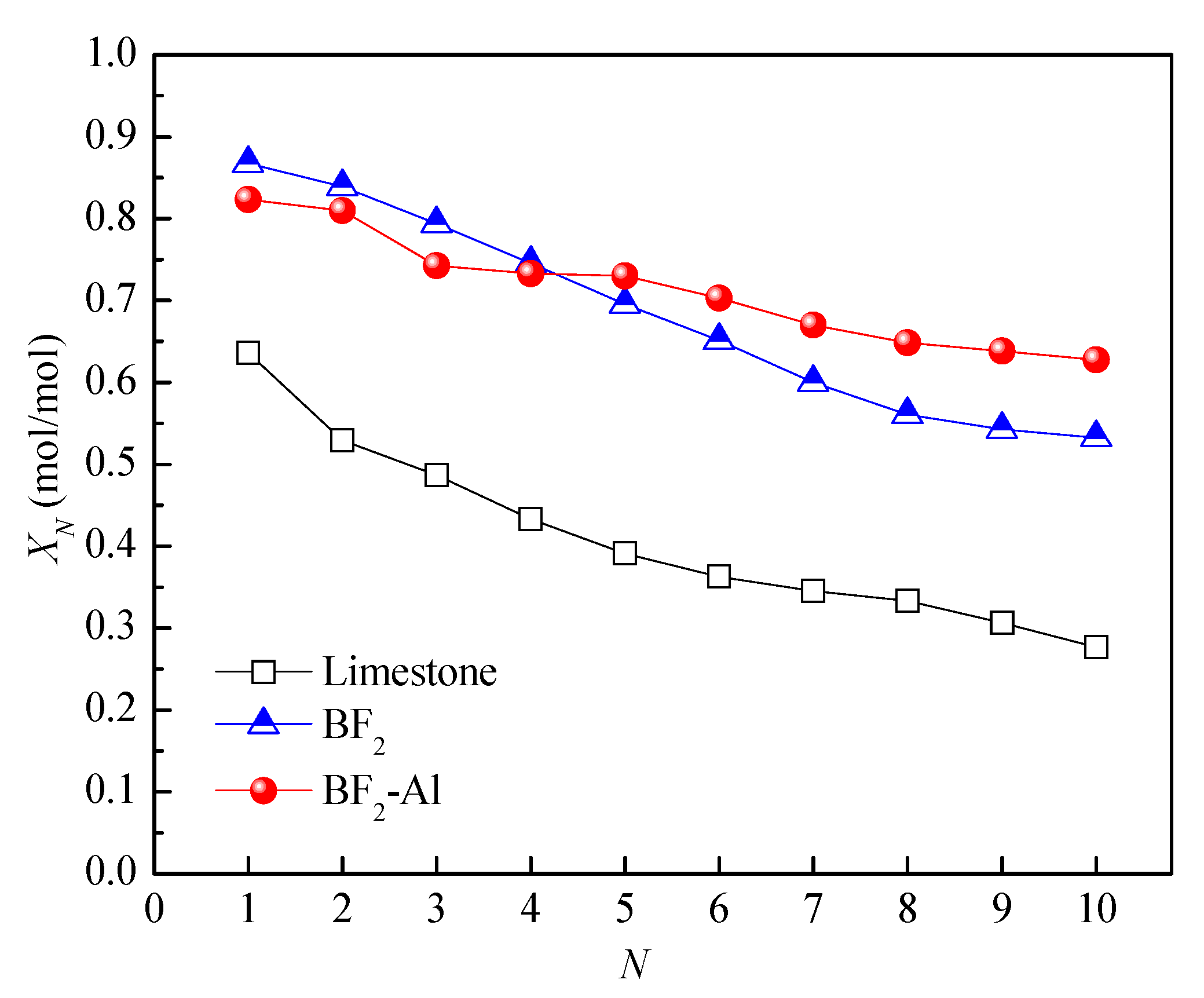

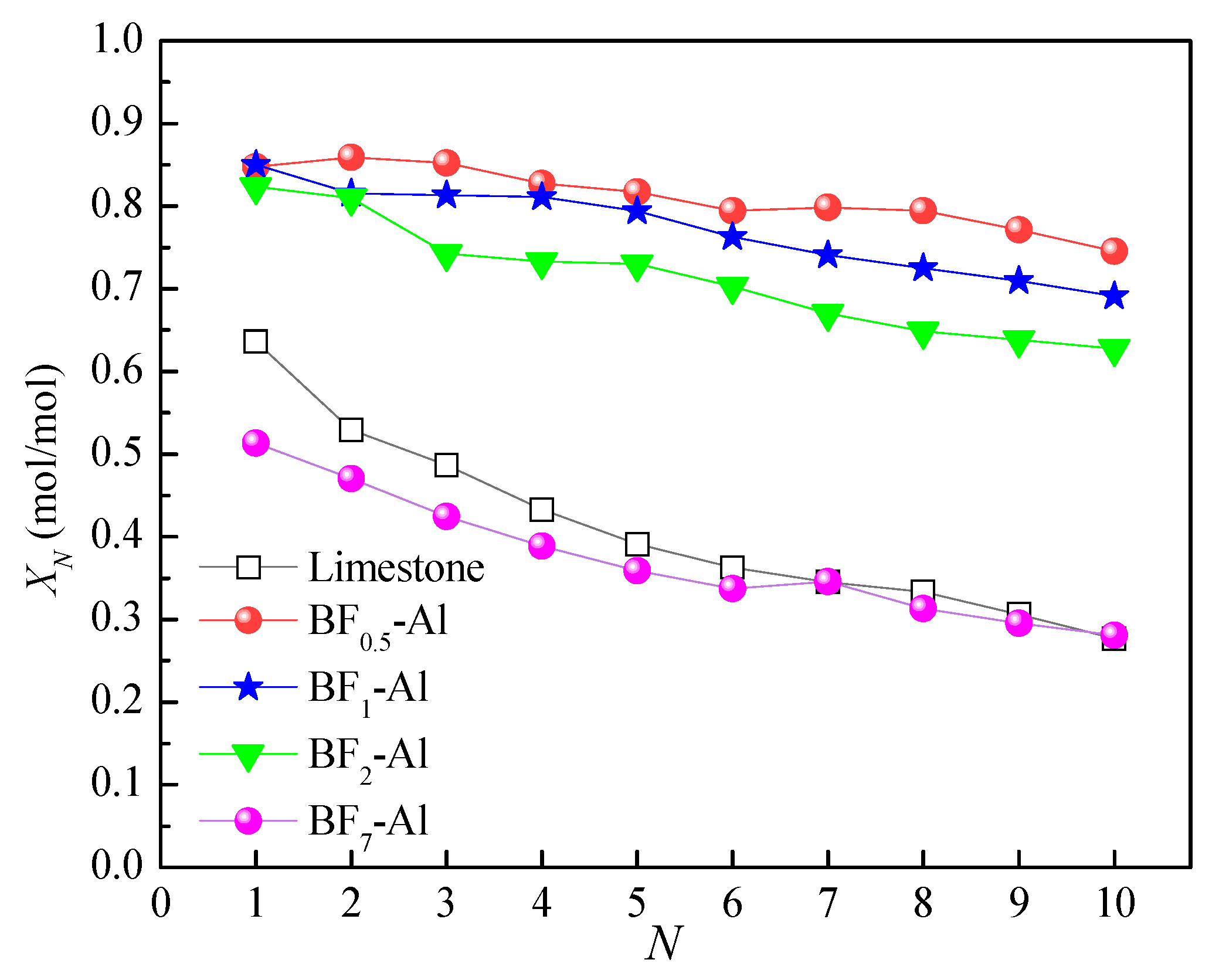

| Sample | CaO | MgO | SiO2 | Al2O3 | Fe2O3 | SO3 | TiO2 | K2O | Na2O | Cl | P2O5 | LOI | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcined limestone [33] | 94.77 | 1.84 | 2.58 | 0.24 | 0.1 | -- | 0.02 | 0.42 | -- | -- | -- | -- | 0.03 |
| Limestone from Alberta [34] | 47.8 | 2.3 | 9.05 | 1.61 | 1.47 | 0.59 | 0.13 | 0.65 | 0.07 | -- | -- | 38.52 | 0.01 |
| Limestone in this work | 50.69 | 2.2 | 2.63 | 0.55 | 0.24 | 0.78 | 0.02 | 0.11 | -- | 0.09 | 0.19 | 42.4 | 0.29 |
| Sample | X1 | Xr | k | Residual Error |
|---|---|---|---|---|
| Limestone | 0.64 | 0.11 | 0.27 | 0.01 |
| Acid-treated limestone | 0.93 | 0.003 | 0.09 | 0.06 |
| BF2 | 0.87 | 0.01 | 0.07 | 0.03 |
| Precursor and Methods | Reaction Conditions | Cycle No. | XN (mol/mol) | Reference |
|---|---|---|---|---|
| Calcium citrate hydrate and aluminum acetylacetonate, space-confined chemical vapor deposition method | Calcination: 750 °C, 100% N2, 10 min; carbonation: 850 °C, 100% CO2, 5 min | 20 | 0.75 | [6] |
| Calcium hydroxide and titanium dioxide, extrusion-spheronization method | Calcination: 750 °C, 100% N2, 10 min; carbonation: 850 °C, 100% CO2, 6 min | 20 | 0.575 | [20] |
| Limestone and powdered nanoalumina, mechanical mixing method | Calcination: 900 °C, 70% CO2/30% air, 5 min; carbonation: 850 °C, 100% CO2, 5 min | 20 | 0.55 | [22] |
| Limestone and ZrO2, mechanical milling method | Calcination: 1000 °C, 100% CO2; carbonation: 850 °C, 100% CO2 | 11 | 0.35 | [23] |
| Ca(NO3)2·4H2O and rice husk, biotemplate method | Calcination: 725 °C, 100% He, 5 min; carbonation: 850 °C, 100% CO2, 5 min | 20 | 0.34 | [44] |
| Ca(NO3)2, Dy(NO3)3 and Al(NO3)3, Pechini method | Calcination: 1000 °C, 100% CO2; carbonation: 850 °C, 100% CO2, 30 min | 40 | 0.51 | [45] |
| Limestone and Al(NO3)3·9H2O, template method | Calcination: 850 °C, 100% N2, 10 min; carbonation: 850 °C, 100% CO2, 5 min | 10 | 0.75 | This work |
| Element | Weight (%) | Atomic (%) |
|---|---|---|
| Ca | 45.34 | 25.16 |
| O | 52.68 | 73.22 |
| Al | 1.98 | 1.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Ma, X.; Huang, X.; Li, F.; Li, J.; Hu, X.; Wang, C. Biotemplating of Al2O3-Doped, CaO-Based Material from Bamboo Fiber for Efficient Solar Energy Storage. Processes 2023, 11, 460. https://doi.org/10.3390/pr11020460
Zhang H, Ma X, Huang X, Li F, Li J, Hu X, Wang C. Biotemplating of Al2O3-Doped, CaO-Based Material from Bamboo Fiber for Efficient Solar Energy Storage. Processes. 2023; 11(2):460. https://doi.org/10.3390/pr11020460
Chicago/Turabian StyleZhang, Haoran, Xiaotong Ma, Xingkang Huang, Fei Li, Jia Li, Xiude Hu, and Cuiping Wang. 2023. "Biotemplating of Al2O3-Doped, CaO-Based Material from Bamboo Fiber for Efficient Solar Energy Storage" Processes 11, no. 2: 460. https://doi.org/10.3390/pr11020460
APA StyleZhang, H., Ma, X., Huang, X., Li, F., Li, J., Hu, X., & Wang, C. (2023). Biotemplating of Al2O3-Doped, CaO-Based Material from Bamboo Fiber for Efficient Solar Energy Storage. Processes, 11(2), 460. https://doi.org/10.3390/pr11020460







