Evaluation of Dithiocarbamate-Modified Silica for Cisplatin Removal from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
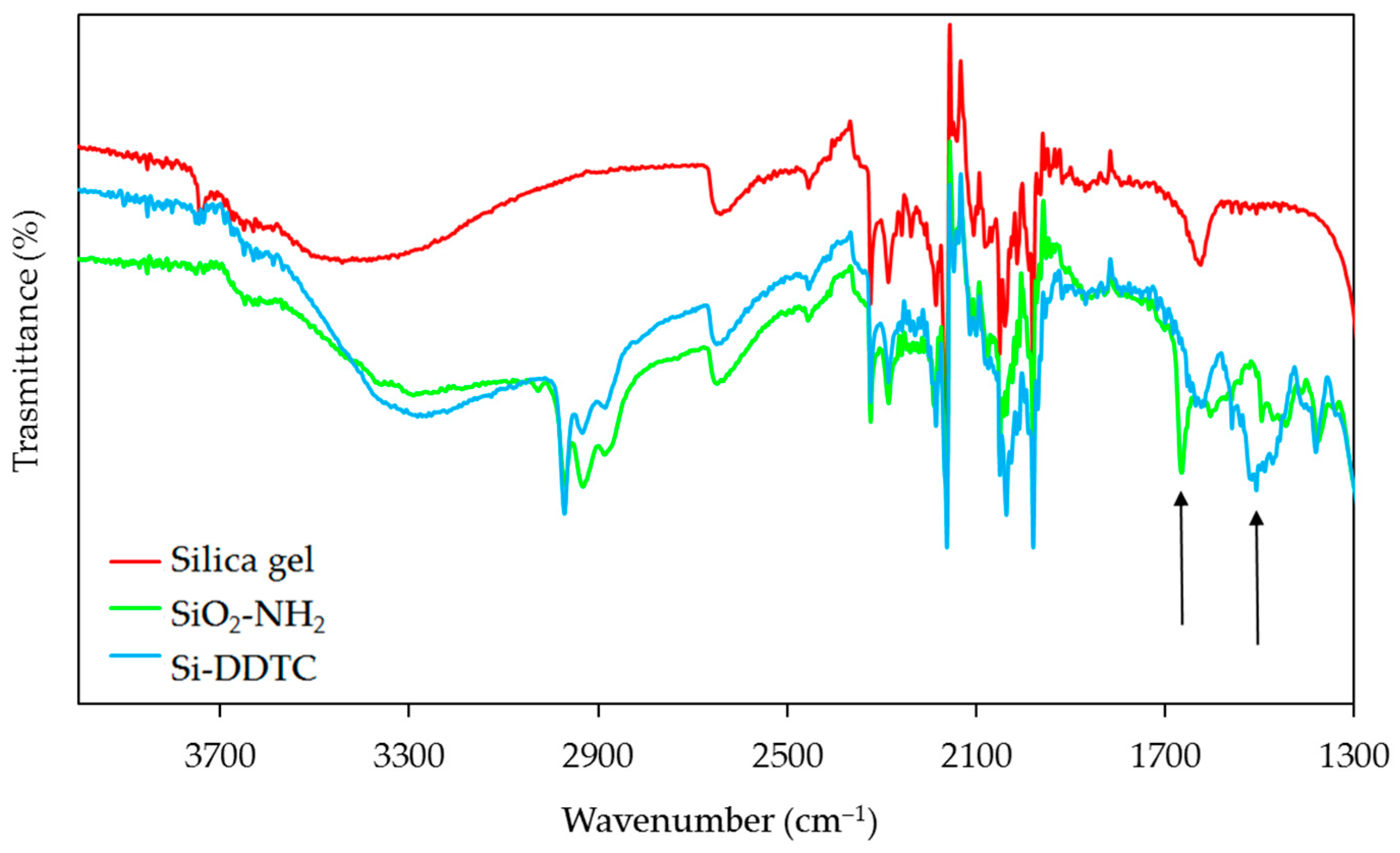
2.2. Material Preparation and Characterization
2.3. Determination of Platinum
2.4. Effect of pH
2.5. Adsorption Kinetics
2.6. Effect of Sorbent Dose
2.7. Adsorption Isotherms
2.8. Isothermal Titration Calorimetry
2.9. Desorption Experiments
3. Results and Discussion
3.1. Characterization of the Material
3.2. Cisplatin Adsorption
3.3. Adsorption Isotherms
3.4. Calorimetric Study
3.5. Study of Desorption of Platinum from Si-DTC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- WHO. IARC Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/en (accessed on 1 December 2022).
- Gouveia, T.I.A.; Alves, A.; Santos, M.S.F. New insights on cytostatic drug risk assessment in aquatic environments based on measured concentrations in surface waters. Environ. Int. 2019, 133, 105236. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Goddard, R.; Pörschke, K.R. 9,9-Difluorobispidine Analogues of Cisplatin, Carboplatin, and Oxaliplatin. Inorg. Chem. 2017, 56, 6712–6724. [Google Scholar] [CrossRef] [PubMed]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Devel. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anna, M.M.; Censi, V.; Carrozzini, B.; Caliandro, R.; Denora, N.; Franco, M.; Veclani, D.; Melchior, A.; Tolazzi, M.; Mastrorilli, P. Triphenylphosphane Pt(II) complexes containing biologically active natural polyphenols: Synthesis, crystal structure, molecular modeling and cytotoxic studies. J. Inorg. Biochem. 2016, 163, 346–361. [Google Scholar] [CrossRef]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Veclani, D.; Melchior, A.; Tolazzi, M.; Cerón-Carrasco, J.P. Using Theory to Reinterpret the Kinetics of Monofunctional Platinum Anticancer Drugs: Stacking Matters. J. Am. Chem. Soc. 2018, 140, 14024–14027. [Google Scholar] [CrossRef] [PubMed]
- Veclani, D.; Tolazzi, M.; Melchior, A. Molecular interpretation of pharmaceuticals’ adsorption on carbon nanomaterials: Theory meets experiments. Processes 2020, 8, 642. [Google Scholar] [CrossRef]
- Veclani, D.; Tolazzi, M.; Cerón-Carrasco, J.P.; Melchior, A. Intercalation Ability of Novel Monofunctional Platinum Anticancer Drugs: A Key Step in Their Biological Action. J. Chem. Inf. Model. 2021, 61, 4391–4399. [Google Scholar] [CrossRef]
- Melchior, A.; Marcos, E.S.; Pappalardo, R.R.; Martínez, J.M. Comparative study of the hydrolysis of a third- and a first-generation platinum anticancer complexes. Theor. Chem. Acc. 2011, 128, 627–638. [Google Scholar] [CrossRef]
- Melchior, A.; Tolazzi, M.; Martínez, J.M.; Pappalardo, R.R.; Sánchez Marcos, E. Hydration of two cisplatin aqua-derivatives studied by quantum mechanics and molecular dynamics simulations. J. Chem. Theory Comput. 2015, 11, 1735–1744. [Google Scholar] [CrossRef]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. 2005, 4, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.; Hann, S.; Koellensperger, G.; Stefanka, Z.; Stingeder, G.; Weissenbacher, N.; Mahnik, S.N.; Fuerhacker, M. Presence of cancerostatic platinum compounds in hospital wastewater and possible elimination by adsorption to activated sludge. Sci. Total Environ. 2005, 345, 141–152. [Google Scholar] [CrossRef]
- Lenz, K.; Mahnik, S.N.; Weissenbacher, N.; Mader, R.M.; Krenn, P.; Hann, S.; Koellensperger, G.; Uhl, M.; Knasmüller, S.; Ferk, F.; et al. Monitoring, removal and risk assessment of cytostatic drugs in hospital wastewater. Water Sci. Technol. 2007, 56, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Hori, A.; Shimura, M.; Iida, Y.; Yamada, K.; Nohara, K.; Ichinose, T.; Yamashita, A.; Shirataki, J.; Hagiwara, S. Occupational exposure of platinum-based anti-cancer drugs: Five-year monitoring of hair and environmental samples in a single hospital. J. Occup. Med. Toxicol. 2020, 15, 29. [Google Scholar] [CrossRef]
- Nygren, O.; Lundgren, C. Determination of platinum in workroom air and in blood and urine from nursing staff attending patients receiving cisplatin chemotherapy. Int. Arch. Occup. Environ. Health 1997, 70, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, C.; Radon, K.; Pethran, A.; Schierl, R.; Hauff, K.; Grimm, C.-H.; Boos, K.-S.; Nowak, D. Uptake of antineoplastic agents in pharmacy personnel. Part II: Study of work-related risk factors. Int. Arch. Occup. Environ. Health 2003, 76, 11–16. [Google Scholar] [CrossRef]
- Lancharro, P.M.; De Castro-Acuña Iglesias, N.; González-Barcala, F.J.; González, J.D.M. Evidence of exposure to cytostatic drugs in healthcare staff: A review of recent literature. Farm. Hosp. 2016, 40, 604–621. [Google Scholar] [CrossRef]
- Raghavan, R.; Burchett, M.; Loffredo, D.; Mulligan, J.A. Low-Level (PPB)Determination of Cisplatin in Cleaning Validation (Rinse Water) Samples. II. A High-Performance Liquid Chromatogrphic Method. Drug Dev. Ind. Pharm. 2000, 26, 429–440. [Google Scholar] [CrossRef]
- Heath, E.; Isidori, M.; Kosjek, T.; Filipič, M. Fate and Effects of Anticancer Drugs in the Environment; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; ISBN 9783030210489. [Google Scholar]
- Franquet-Griell, H.; Gómez-Canela, C.; Ventura, F.; Lacorte, S. Predicting concentrations of cytostatic drugs in sewage effluents and surface waters of Catalonia (NE Spain). Environ. Res. 2015, 138, 161–172. [Google Scholar] [CrossRef]
- Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D. Incidence of anticancer drugs in an aquatic urban system: From hospital effluents through urban wastewater to natural environment. Environ. Pollut. 2014, 193, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel, Y.; González Alonso, S.; Rodríguez-Gil, J.L.; Gil, A.; Catalá, M. Detection of pharmaceutically active compounds in the rivers and tap water of the Madrid Region (Spain) and potential ecotoxicological risk. Chemosphere 2011, 84, 1336–1348. [Google Scholar] [CrossRef] [PubMed]
- Queirós, V.; Azeiteiro, U.M.; Soares, A.M.V.M.; Freitas, R. The antineoplastic drugs cyclophosphamide and cisplatin in the aquatic environment—Review. J. Hazard. Mater. 2021, 412, 125028. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, V.W.C.; Giannis, A.; Wang, J.Y. Removal of cytostatic drugs from aquatic environment: A review. Sci. Total Environ. 2013, 445–446, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Roque-Diaz, Y.; Sanadar, M.; Han, D.; López-Mesas, M.; Valiente, M.; Tolazzi, M.; Melchior, A.; Veclani, D. The dark side of platinum based cytostatic drugs: From detection to removal. Processes 2021, 9, 1873. [Google Scholar] [CrossRef]
- Wormington, A.M.; De María, M.; Kurita, H.G.; Bisesi, J.H.; Denslow, N.D.; Martyniuk, C.J. Antineoplastic Agents: Environmental Prevalence and Adverse Outcomes in Aquatic Organisms. Environ. Toxicol. Chem. 2020, 39, 967–985. [Google Scholar] [CrossRef]
- Nassour, C.; Nabhani-Gebara, S.; Barton, S.J.; Barker, J. Aquatic ecotoxicology of anticancer drugs: A systematic review. Sci. Total Environ. 2021, 800, 149598. [Google Scholar] [CrossRef]
- Kajander, V.; Sewell, G.; Turner, A. Bioaccumulation and toxicity of oxaliplatin in fresh water: A study with Lemna minor. Environ. Adv. 2021, 3, 100030. [Google Scholar] [CrossRef]
- Iordache, A. A Fugacity Based Model for the Assessment of Pollutant Dynamic Evolution of VOCS and BTEX in the Olt River Basin (Romania). Rev. Chim. 2019, 70, 3456–3463. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [Green Version]
- Veclani, D.; Melchior, A. Adsorption of ciprofloxacin on carbon nanotubes: Insights from molecular dynamics simulations. J. Mol. Liq. 2020, 298, 111977. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, J.; Liang, X.; Liu, Z. Adsorption of platinum(IV) and palladium(II) from aqueous solution by magnetic cross-linking chitosan nanoparticles modified with ethylenediamine. J. Hazard. Mater. 2010, 182, 518–524. [Google Scholar] [CrossRef]
- Sicupira, D.; Campos, K.; Vincent, T.; Leao, V.; Guibal, E. Palladium and platinum sorption using chitosan-based hydrogels. Adsorption 2010, 16, 127–139. [Google Scholar] [CrossRef]
- Sharififard, H.; Soleimani, M.; Ashtiani, F.Z. Evaluation of activated carbon and bio-polymer modified activated carbon performance for palladium and platinum removal. J. Taiwan Inst. Chem. Eng. 2012, 43, 696–703. [Google Scholar] [CrossRef]
- Gu, W.; Zhu, X. Nanoparticles of type Fe3O4-SiO2-graphene oxide and coated with an amino acid-derived ionic liquid for extraction of Al(III), Cr(III), Cu(II), Pb(II) prior to their determination by ICP-OES. Microchim. Acta 2017, 184, 4279–4286. [Google Scholar] [CrossRef]
- Park, H.N.; Choi, H.A.; Won, S.W. Fibrous polyethylenimine/polyvinyl chloride crosslinked adsorbent for the recovery of Pt(IV) from acidic solution: Adsorption, desorption and reuse performances. J. Clean. Prod. 2018, 176, 360–369. [Google Scholar] [CrossRef]
- Melchior, A.; Lanas, S.G.; Valiente, M.; Tolazzi, M. Thermodynamics of sorption of platinum on superparamagnetic nanoparticles functionalized with mercapto groups. J. Therm. Anal. Calorim. 2018, 134, 1261–1266. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, B.; He, M.; Wang, H.; Hu, B. A porous organic polymer with magnetic nanoparticles on a chip array for preconcentration of platinum(IV), gold(III) and bismuth(III) prior to their on-line quantitation by ICP-MS. Microchim. Acta 2019, 186, 107. [Google Scholar] [CrossRef]
- Yousif, A.M.; Labib, S.A.; Ibrahim, I.A.; Atia, A.A. Recovery of Pt (IV) from aqueous solutions using magnetic functionalized cellulose with quaternary amine. Sep. Sci. Technol. 2018, 54, 1257–1268. [Google Scholar] [CrossRef]
- Hajizadeh, S.; Ye, L. Hierarchical macroporous material with dual responsive copolymer brushes and phenylboronic acid ligands for bioseparation of proteins and living cells. Sep. Purif. Technol. 2019, 224, 95–105. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, X.; Wang, D.; Song, C.; Wang, Y. Development of silica-gel-supported polyethylenimine sorbents for CO2 capture from flue gas. AIChE J. 2012, 58, 2495–2502. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Niculescu, V.-C. Fly Ash, from Recycling to Potential Raw Material for Mesoporous Silica Synthesis. Nanomaterials 2020, 10, 474. [Google Scholar] [CrossRef]
- Lanas, S.G.; Valiente, M.; Aneggi, E.; Trovarelli, A.; Tolazzi, M.; Melchior, A. Efficient fluoride adsorption by mesoporous hierarchical alumina microspheres. RSC Adv. 2016, 6, 42288–42296. [Google Scholar] [CrossRef]
- Venkatesan, K.A.; Srinivasan, T.G.; Vasudeva Rao, P.R. Cobalt-extraction studies on dithiocarbamate grafted on silica gel surface. Colloids Surfaces A Physicochem. Eng. Asp. 2001, 180, 277–284. [Google Scholar] [CrossRef]
- Qu, R.; Wang, M.; Sun, C.; Zhang, Y.; Ji, C.; Chen, H.; Meng, Y.; Yin, P. Chemical modification of silica-gel with hydroxyl- or amino-terminated polyamine for adsorption of Au(III). Appl. Surf. Sci. 2008, 255, 3361–3370. [Google Scholar] [CrossRef]
- Qu, R.; Niu, Y.; Liu, J.; Sun, C.; Zhang, Y.; Chen, H.; Ji, C. Adsorption and desorption behaviors of Pd(II) on silica-gel functionalized with ester- and amino-terminated dendrimer-like polyamidoamine polymers. React. Funct. Polym. 2008, 68, 1272–1280. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, R.; Sun, C.; Chen, H.; Wang, C.; Ji, C.; Yin, P.; Sun, Y.; Zhang, H.; Niu, Y. Comparison of synthesis of chelating resin silica-gel-supported diethylenetriamine and its removal properties for transition metal ions. J. Hazard. Mater. 2009, 163, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.A.; Salama, R.S.; El-Hakam, S.A.; Khder, A.S.; Ahmed, A.I. Synthesis of 12-tungestophosphoric acid supported on Zr/MCM-41 composite with excellent heterogeneous catalyst and promising adsorbent of methylene blue. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 631, 127753. [Google Scholar] [CrossRef]
- Salama, R.S.; El-Bahy, S.M.; Mannaa, M.A. Sulfamic acid supported on mesoporous MCM-41 as a novel, efficient and reusable heterogenous solid acid catalyst for synthesis of xanthene, dihydropyrimidinone and coumarin derivatives. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 628, 127261. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, A.; Sharma, G.; Naushad, M.; Dhiman, P.; Kumari, A.; Stadler, F.J. Recent advances in nano-Fenton catalytic degradation of emerging pharmaceutical contaminants. J. Mol. Liq. 2019, 290, 111177. [Google Scholar] [CrossRef]
- Lanas, S.G.; Valiente, M.; Tolazzi, M.; Melchior, A. Thermodynamics of Hg2+ and Ag+ adsorption by 3-mercaptopropionic acid-functionalized superparamagnetic iron oxide nanoparticles. J. Therm. Anal. Calorim. 2019, 136, 1153–1162. [Google Scholar] [CrossRef]
- Bai, L.; Hu, H.; Fu, W.; Wan, J.; Cheng, X.; Zhuge, L.; Xiong, L.; Chen, Q. Synthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ions. J. Hazard. Mater. 2011, 195, 261–275. [Google Scholar] [CrossRef]
- Yusoff, S.N.M.; Kamari, A.; Putra, W.P.; Ishak, C.F.; Mohamed, A.; Hashim, N.; Isa, I.M. Removal of Cu(II), Pb(II) and Zn(II) Ions from Aqueous Solutions Using Selected Agricultural Wastes: Adsorption and Characterisation Studies. J. Environ. Prot. 2014, 5, 289–300. [Google Scholar] [CrossRef]
- Losev, V.N.; Elsuf’Ev, E.V.; Trofimchuk, A.K.; Legenchuk, A.V. Low-temperature sorption-luminescence determination of platinum using silica chemically modified with dithiocarbamate groups. J. Anal. Chem. 2012, 67, 772–777. [Google Scholar] [CrossRef]
- Losev, V.N.; Parfenova, V.V.; Elsuf’ev, E.V.; Borodina, E.V.; Metelitsa, S.I.; Trofimchuk, A.K. Separation and preconcentration followed by ICP-OES and ICP-MS determination of precious metals using silica gel chemically modified with dithiocarbamate groups. Sep. Sci. Technol. 2020, 55, 2659–2669. [Google Scholar] [CrossRef]
- Goubert-Renaudin, S.; Gaslain, F.; Marichal, C.; Lebeau, B.; Schneider, R.; Walcarius, A. Synthesis of dithiocarbamate-functionalized mesoporous silica-based materials: Interest of one-step grafting. New J. Chem. 2009, 33, 528–537. [Google Scholar] [CrossRef]
- Farías, T.; Hajizadeh, S.; Ye, L. Cryogels with high cisplatin adsorption capacity: Towards removal of cytotoxic drugs from wastewater. Sep. Purif. Technol. 2020, 235, 116203. [Google Scholar] [CrossRef]
- Hann, S.; Koellensperger, G.; Stefánka, Z.; Stingeder, G.; Fürhacker, M.; Buchberger, W.; Mader, R.M. Application of HPLC-ICP-MS to speciation of cisplatin and its degradation products in water containing different chloride concentrations and in human urine. J. Anal. At. Spectrom. 2003, 18, 1391–1395. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I. Determination of sorbent point zero charge: Usefulness in sorption studies. Environ. Chem. Lett. 2009, 7, 79–84. [Google Scholar] [CrossRef]
- Ghafuri, Y.; Yunesian, M.; Nabizadeh, R.; Mesdaghinia, A.; Dehghani, M.H.; Alimohammadi, M. Platinum cytotoxic drugs in the municipal wastewater and drinking water, a validation method and health risk assessment. Hum. Ecol. Risk Assess. 2018, 24, 784–796. [Google Scholar] [CrossRef]
- Nassour, C.; Zacharauskas, Z.; Nabhani-Gebara, S.; Barton, S.J.; Barker, J. Development and Validation of an ICP-MS Method for the Detection of Platinum in the Lebanese Aquatic Environment. Water 2022, 14, 2631. [Google Scholar] [CrossRef]
- Andrews, P.A.; Wung, W.E.; Howell, S.B. A high-performance liquid chromatographic assay with improved selectivity for cisplatin and active platinum (II) complexes in plasma ultrafiltrate. Anal. Biochem. 1984, 143, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Bannister, S.J.; Sternson, L.A.; Repta, A.J. Urine analysis of platinum species derived from cis-dichlorodiammineplatinum(II) by high-performance liquid chromatography following derivatization with sodium diethyldithiocarbamate. J. Chromatogr. A 1979, 173, 333–342. [Google Scholar] [CrossRef]
- Suzuki, M. Adsorption Engineering; Kodansha Ltd.: Tokyo, Japan; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1990; Volume 25. [Google Scholar]
- Comuzzi, C.; Polese, P.; Melchior, A.; Portanova, R.; Tolazzi, M. Solverstat: A new utility for multipurpose analysis. An application to the investigation of dioxygenated Co(II) complex formation in dimethylsulfoxide solution. Talanta 2003, 59, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Polese, P.; Tolazzi, M.; Melchior, A. cEST: A flexible tool for calorimetric data analysis. J. Therm. Anal. Calorim. 2018, 134, 1317–1326. [Google Scholar] [CrossRef]
- Endrizzi, F.; Di Bernardo, P.; Zanonato, P.L.; Tisato, F.; Porchia, M.; Isse, A.A.; Melchior, A.; Tolazzi, M. Cu(i) and Ag(i) complex formation with the hydrophilic phosphine 1,3,5-triaza-7-phosphadamantane in different ionic media. How to estimate the effect of a complexing medium. Dalton Trans. 2017, 46, 1455–1466. [Google Scholar] [CrossRef]
- Simon, B.; Bouyer, C.; De Sio, S.; Berthon, C.; Boubals, N.; Miserque, F.; Brackx, E.; Raymond, N.; Chagnes, A.; Berthon, L. Characterization of palladium species after γ-irradiation of a TBP–alkane–Pd(NO3)2 system. RSC Adv. 2018, 8, 21513–21527. [Google Scholar] [CrossRef]
- Shaibu, S.E.; Adekola, F.A.; Adegoke, H.I.; Ayanda, O.S. A comparative study of the adsorption of methylene blue onto synthesized nanoscale zero-valent iron-bamboo and manganese-bamboo composites. Materials 2014, 7, 4493–4507. [Google Scholar] [CrossRef]
- Miller, S.E.; House, D.A. The hydrolysis products of cis-diamminedichloroplatinum(II). I. The kinetics of formation and anation of the cis-diammine(aqua)chloroplatinum(II) cation in acidic aqueous solution. Inorg. Chim. Acta 1989, 161, 131–137. [Google Scholar] [CrossRef]
- Lagergren, S. About Theory of So-Called Adsorption of Soluble Substances. K. Sven. Vetenskapsakad. Handl. 1898, 24, 1–39. [Google Scholar]
- Gosset, T.; Trancart, J.-L.; Thévenot, D.R. Batch metal removal by peat. Kinetics and thermodynamics. Water Res. 1986, 20, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Jiang, R.; Fu, Y.Q.; Jiang, J.H.; Xiao, L.; Zeng, G.M. Preparation, characterization and dye adsorption properties of γ-Fe2O3/SiO2/chitosan composite. Appl. Surf. Sci. 2011, 258, 1337–1344. [Google Scholar] [CrossRef]
- Miller, S.E.; House, D.A. The hydrolysis products of cis-dichlorodiammineplatinum(II) 2. The kinetics of formation and anation of the cis-diamminedi(aqua)platinum(II) cation. Inorg. Chim. Acta 1989, 166, 189–197. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. II. Liquids. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef]
- Ma, H.; Liao, X.; Liu, X.; Shi, B. Recovery of platinum(IV) and palladium(II) by bayberry tannin immobilized collagen fiber membrane from water solution. J. Memb. Sci. 2006, 278, 373–380. [Google Scholar] [CrossRef]










| Pseudo-First Order | Pseudo-Second Order | ||
|---|---|---|---|
| K1 | R2 | K2 | R2 |
| 0.0336 ± 0.0034 | 0.894 | 0.098 ± 0.018 | 0.871 |
| Value | R2 | ||
|---|---|---|---|
| Langmuir | KL (L mg−1) | 0.0055 ± 0.0025 | 0.944 |
| b (mg g−1) | 15.6 ± 4.7 | ||
| Freundlich | KF (mg g−1/n) | 0.19 ± 0.08 | 0.935 |
| n | 1.38 ± 0.17 |
| Condition | Concentration (M) | Recovery % |
|---|---|---|
| HCl | 0.1 | 60 ± 2 |
| 0.5 | 42 ± 5 | |
| HNO3 | 0.1 | 48 ± 10 |
| NaCl-acetate buffer | 0.1 | 47 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombana Fraguela, R.; Ricardo Garcia, J.A.; Villanueva Tagle, M.E.; Pomares Alfonso, M.S.; Cracchiolo, M.; Kovačević, A.; Tolazzi, M.; Melchior, A.; Sanadar, M. Evaluation of Dithiocarbamate-Modified Silica for Cisplatin Removal from Water. Processes 2023, 11, 472. https://doi.org/10.3390/pr11020472
Lombana Fraguela R, Ricardo Garcia JA, Villanueva Tagle ME, Pomares Alfonso MS, Cracchiolo M, Kovačević A, Tolazzi M, Melchior A, Sanadar M. Evaluation of Dithiocarbamate-Modified Silica for Cisplatin Removal from Water. Processes. 2023; 11(2):472. https://doi.org/10.3390/pr11020472
Chicago/Turabian StyleLombana Fraguela, Rachel, José Alejandro Ricardo Garcia, Margarita Edelia Villanueva Tagle, Mario Simeón Pomares Alfonso, Maria Cracchiolo, Anđela Kovačević, Marilena Tolazzi, Andrea Melchior, and Martina Sanadar. 2023. "Evaluation of Dithiocarbamate-Modified Silica for Cisplatin Removal from Water" Processes 11, no. 2: 472. https://doi.org/10.3390/pr11020472
APA StyleLombana Fraguela, R., Ricardo Garcia, J. A., Villanueva Tagle, M. E., Pomares Alfonso, M. S., Cracchiolo, M., Kovačević, A., Tolazzi, M., Melchior, A., & Sanadar, M. (2023). Evaluation of Dithiocarbamate-Modified Silica for Cisplatin Removal from Water. Processes, 11(2), 472. https://doi.org/10.3390/pr11020472











