Impact of Non-Saccharomyces Yeast Fermentation in Madeira Wine Chemical Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spontaneous Fermentations for Yeast Isolation
2.1.1. Winery Samples
2.1.2. Vineyard Samples
2.2. Yeast Counting and Isolation
2.2.1. Yeast Identification
2.2.2. Yeast Inoculation and Wine Production
2.3. Characterization of the Selected Non-Saccharomyces Species
2.3.1. Ethanol, Reducing Sugars, and Organic Acids
2.3.2. Polyphenolic Composition
2.3.3. Antioxidant Potential
2.4. Data Processing
3. Results and Discussion
3.1. Identification of Non-Saccharomyces Derived from Wineries and Vineyards
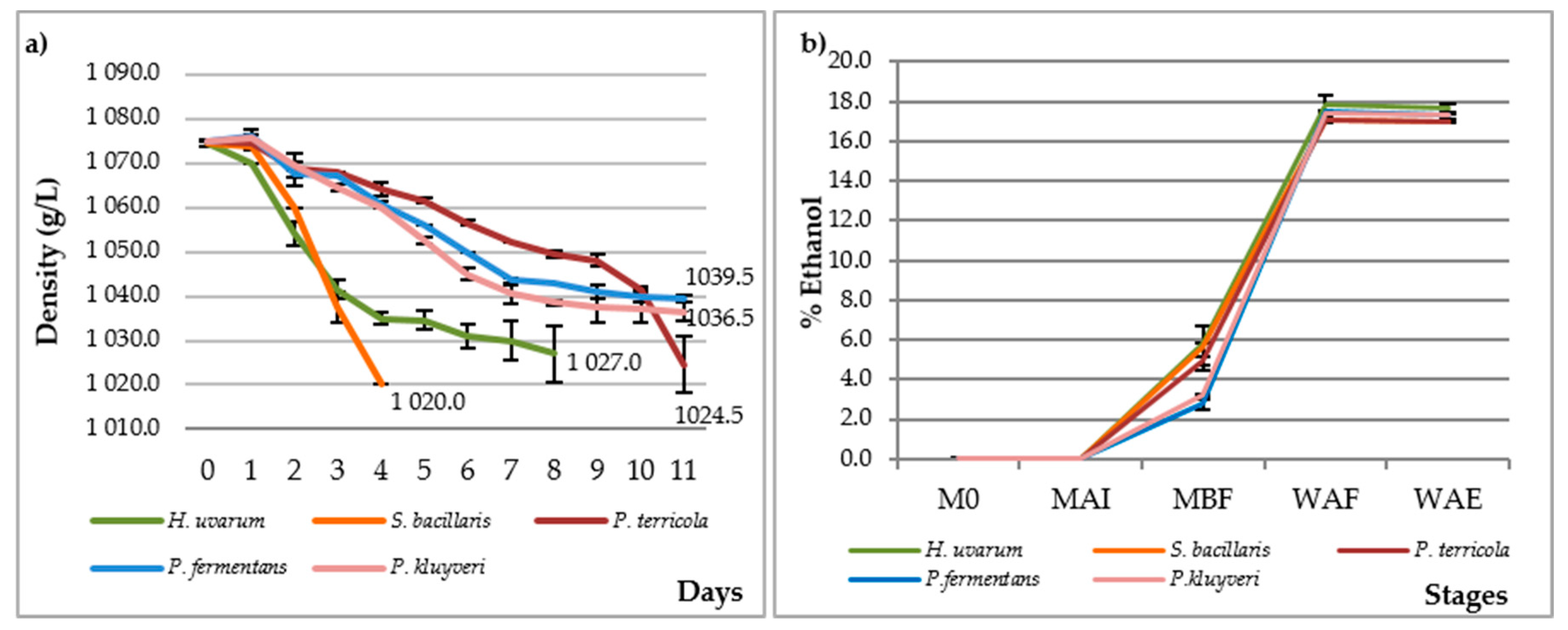
3.2. Analytical Characterization
3.3. Antioxidant Potential and Total Polyphenols
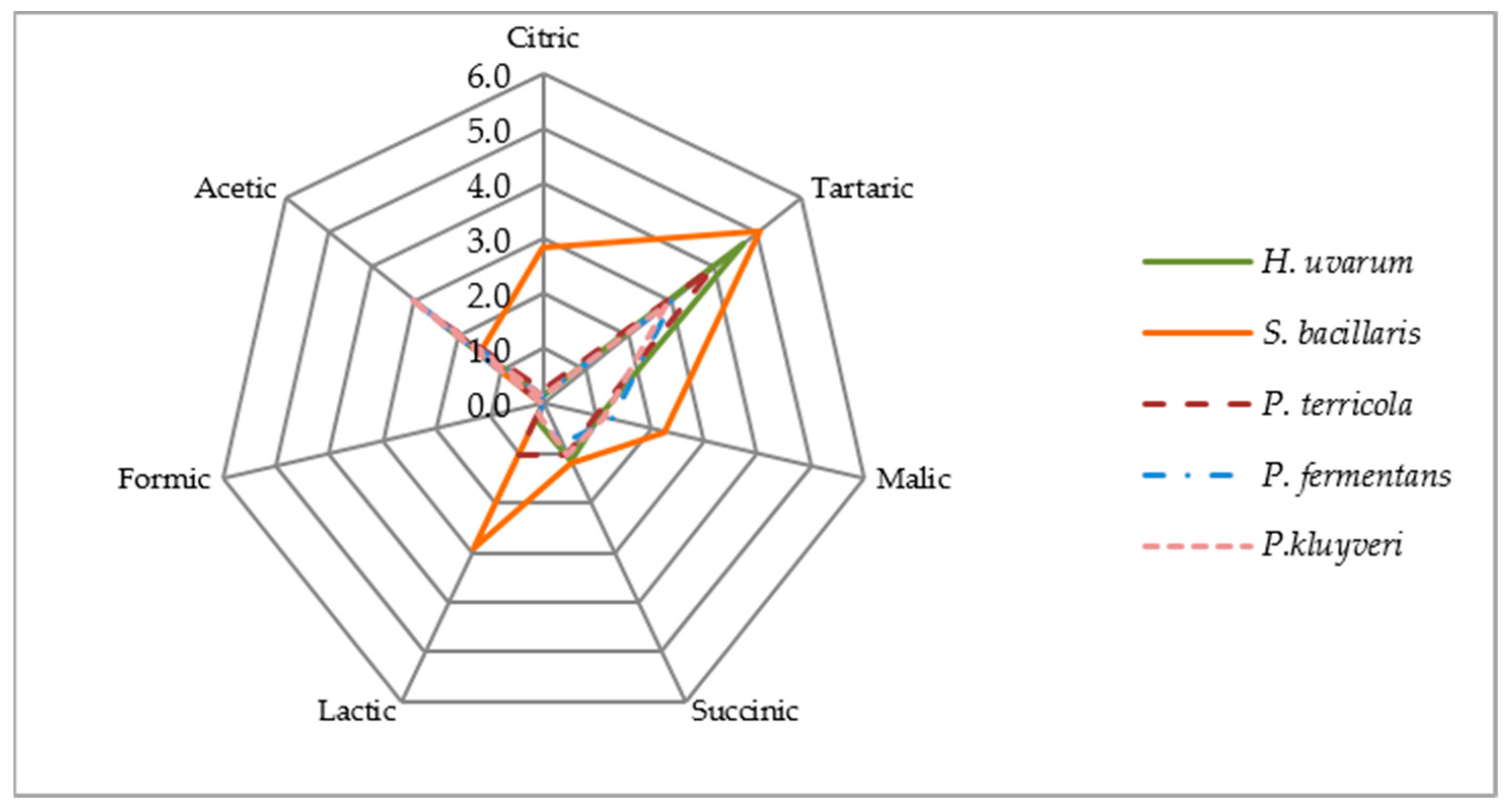
3.4. Polyphenolic Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2009, 10, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Wine yeasts for the future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Borneman, A.R. Yeasts found in vineyards and wineries. Yeast 2017, 34, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The microbial ecology of wine grape berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Beltran, G.; Torija, M.J.; Novo, M.; Poblet, M.; Guillamon, J.M.; Rozés, N.; Mas, A. Analysis of yeast populations during alcoholic fermentation: A six year follow-up study. Syst. Appl. Microbiol. 2002, 25, 287–293. [Google Scholar] [CrossRef]
- Borren, E.; Tian, B. The Important Contribution of Non-Saccharomyces Yeasts to the Aroma Complexity of Wine: A Review. Foods 2020, 10, 13. [Google Scholar] [CrossRef]
- Ocón, E.; Gutiérrez, A.R.; Garija, P.; López, R.; Santamaría, P. Presence of non-Saccharomyces yeasts in cellar equipment and grape juice during harvest time. Food Microbiol. 2010, 27, 1023–1027. [Google Scholar] [CrossRef]
- Sabate, J.; Cano, J.; Esteve-Zarzoso, B.; Guillamón, J.M. Isolation and identification of yeasts associated with vineyard and winery by RFLP analysis of ribosomal genes and mitochondrial DNA. Microbiol. Res. 2002, 157, 267–274. [Google Scholar] [CrossRef]
- Grangeteau, C.; Gerhards, D.; Wallbrunn, C.; Alexandre, H.; Rousseaux, S. Persistence of Two Non-Saccharomyces Yeasts (Hanseniaspora and Starmerella) in the Cellar. Front. Microbiol. 2016, 7, 268. [Google Scholar] [CrossRef]
- Santamaría, P.; Garijo, P.; López, R.; Tenorio, C.; Gutiérrez, A.R. Analysis of yeast population during spontaneous alcoholic fermentation: Effect of the age of the cellar and the practice of inoculation. Int. J. Food Microbiol. 2005, 103, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Englezos, V.; Rantsiou, K.; Torchio, F.; Rolle, L.; Gerbi, V. Exploitation of the non-Saccharomyces yeast Starmerella bacillaris (synonym Candida zemplinina) in wine fermentation: Physiological and molecular characterizations. Int. J. Food Microbiol. 2015, 199, 33–40. [Google Scholar] [CrossRef]
- Mateus, D.; Sousa, S.; Coimbra, C.; Rogerson, F.S.; Simões, J. Identification and Characterization of Non-Saccharomyces Species Isolated from Port Wine Spontaneous Fermentations. Foods 2020, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Russo, P.; Beneduce, L.; Massa, S.; Spano, G.; Capozzi, V. Non-Saccharomyces biodiversity in wine and the ‘microbial terroir’: A survey on Nero di Troia wine from the Apulian region, Italy. Ann. Microbiol. 2016, 66, 143–150. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; Portu, J.; Garijo, P.; López, R.; Santamaria, P.; López-Alfaro, I.; Gutiérrez, A.R.; González-Arenzana, L. Effect of the Sequential Inoculation of Non-Saccharomyces/Saccharomyces on the Anthocyans and Stilbenes Composition of Tempranillo Wines. Front. Microbiol. 2019, 10, 773. [Google Scholar] [CrossRef]
- Li, S.; Bi, P.; Sun, N.; Gao, Z.; Chen, X.; Guo, J. Characterization of different non-Saccharomyces yeasts via mono-fermentation to produce polyphenol-enriched and fragrant kiwi wine. Food Microbiol. 2022, 103, 103867. [Google Scholar] [CrossRef]
- Vilela, A. Use of Nonconventional Yeasts for Modulating Wine Acidity. Fermentation 2019, 5, 27. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Gonçalves, C.; Castillo, M.; Câmara, J.S. An Approach of the Madeira Wine Chemistry. Beverages 2020, 6, 12. [Google Scholar] [CrossRef]
- Miranda, A.; Pereira, V.; Pontes, M.; Albuquerque, F.; Marques, J.C. Acetic acid and ethyl acetate in Madeira wines: Evolution with ageing and assessment of the odour rejection threshold. Ciência Téc. Vitiv. 2017, 32, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Benito, Á.; Calderón, F.; Benito, S. The Influence of Non-Saccharomyces Species on Wine Fermentation Quality Parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef]
- Rodrigues, N.; Gonçalves, G.; Pereira-da-Silva, S.; Malfeito-Ferreira, M.; Loureiro, V. Development and use of a new medium to detect yeasts of the genera Dekkera/Brettanomyces. J. Appl. Microbiol. 2001, 90, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Schuller, D.; Côrte-Real, M.; Leão, C. A Differential Medium for the Enumeration of the Spoilage Yeast Zygosaccharomyces bailii in Wine. J. Food Prot. 2000, 63, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Benito, S. The Combined Use of Schizosaccharomyces pombe and Lachancea thermotolerans-Effect on the Anthocyanin Wine Composition. Molecules 2017, 22, 739. [Google Scholar] [CrossRef]
- Pereira, V.; Albuquerque, F.; Cacho, J.; Marques, J.C. Polyphenols, Antioxidant Potential and Color of Fortified Wines during Accelerated Ageing: The Madeira Wine Case Study. Molecules 2013, 18, 2997–3017. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-A.; Park, H.-D. Role of non-Saccharomyces yeasts in Korean wines produced from Campbell Early grapes: Potential use of Hanseniaspora uvarum as a starter culture. Food Microbiol. 2013, 34, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Cheng, C.; Li, Z.; Chen, J.-Y.; Yan, B.; Han, B.-H.; Reeves, M. Yeast species associated with wine grapes in China. Int. J. Food Microbiol. 2010, 138, 85–90. [Google Scholar] [CrossRef]
- Nemcová, K.; Breierová, E.; Vadkertiová, R.; Molnárová, J. The diversity of yeasts associated with grapes and musts of the Strekov winegrowing region, Slovakia. Folia Microbiol. 2015, 60, 103–109. [Google Scholar] [CrossRef]
- Binati, R.L.; Junior, W.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Šuranská, H.; Vránová, D.; Omelková, J.; Vadkertiová, R. Monitoring of yeast population isolated during spontaneous fermentation of Moravian wine. Chem. Pap. 2012, 66, 861–868. [Google Scholar] [CrossRef]
- Martini, A. Origin and domestication of the wine yeast Saccharomyces cerevisiae. J. Wine Res. 1993, 4, 165–176. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.A.; Broock, M.; Querol, A.; Caballero, A.C. Saccharomyces cerevisiae wine yeast populations in a cold region in Argentinean Patagonia. A study at different fermentation scales. J. Appl. Microbiol. 2002, 93, 608–615. [Google Scholar] [CrossRef]
- Mortimer, R.; Polsinelli, M. On the origins of wine yeast. Microbiol. Res. 1999, 150, 199–204. [Google Scholar] [CrossRef]
- Englezos, V.; Giacosa, S.; Rantsiou, K.; Cocolin, L. Starmerella bacillaris in winemaking: Opportunities and risks. Curr. Opin. Food Sci. 2017, 17, 30–35. [Google Scholar] [CrossRef]
- Vicente, J.; Calderón, F.; Santos, A.; Marqina, D.; Bento, S. High Potential of Pichia kluyveri and Other Pichia Species in Wine Technology. Int. J. Mol. Sci. 2021, 22, 1196. [Google Scholar] [CrossRef]
- Aponte, M.; Blaiotta, G. Potential Role of Yeast Strains Isolated from Grapes in the Production of Taurasi DOCG. Front. Microbiol. 2016, 7, 809. [Google Scholar] [CrossRef]
- Junior, W.; Nadai, C.; Crepalde, L.; Oliveira, V.; Mantos, A.; Giacomino, A.; Corich, V. Potential use of Starmerella bacillaris as fermentation starter for the production of low-alcohol beverages obtained from unripe grapes. Int. J. Food Microbiol. 2019, 303, 1–8. [Google Scholar] [CrossRef]
- Pereira, V.; Santos, M.; Cacho, J.; Marques, J.C. Assessment of the development of browning, antioxidant activity and volatile organic compounds in thermally processed sugar model wines. LWT 2017, 75, 719–726. [Google Scholar] [CrossRef]
- Pereira, V.; Câmara, J.S.; Marques, J.C. HPLC-DAD methodology for the quantification of organic acids, furans and polyphenols by direct injection of wine samples. J. Sep. Sci. 2010, 33, 1204–1215. [Google Scholar] [CrossRef] [Green Version]
- Caridi, A.; Cufari, A.; Lovino, R.; Palumbo, R.; Tedesco, I. Influence of Yeast on Polyphenol Composition of Wine. Food Technol. Biotechnol. 2004, 42, 37–40. [Google Scholar]
- Silva, C.L.; Gonçalves, J.; Câmara, J.S. A sensitive microextraction by packed sorbent-based methodology combined with ultra-high pressure liquid chromatography as a powerful technique for analysis of biologically active flavonols in wines. Anal. Chim. Acta 2012, 739, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R. Practical interventions that influence the sensory attributes of red wines related to the phenolic composition of grapes: A review. Int. J. Food Sci. 2018, 53, 3–18. [Google Scholar] [CrossRef]
- Gonçalves, J.; Silva, C.; Castilho, P.; Câmara, J.S. An attractive, sensitive and high-throughput strategy based on microextraction by packed sorbent followed by UHPLC-PDA analysis for quantification of hydroxybenzoic and hydroxycinnamic acids in wines. Microchem. J. 2013, 106, 129–138. [Google Scholar] [CrossRef]
- Mark, L.; Nikfardjam MS, P.; Avar, P.; Ohmacht, R. A Validated HPLC Method for the Quantitative Analysis of Trans-Resveratrol and Trans-Piceid in Hungarian Wines. J. Chromatogr. Sci. 2005, 43, 445–449. [Google Scholar] [CrossRef]
- Gaensly, F.; Agustini, B.; Silva, G.; Picheth, G.; Bordin, B. Autochthonous yeasts with β-glucosidase activity increase resveratrol concentration during the alcoholic fermentation of Vitis labrusca grape must. J. Funct. Foods 2015, 19, 288–295. [Google Scholar] [CrossRef]
- Romboli, Y.; Mangani, S.; Buscioni, G.; Vincenzini, M. Effect of Saccharomyces cerevisiae and Candida zemplinina on quercetin, vitisin A and hydroxytyrosol contents in Sangiovese wines. World J. Microbiol. Biotechnol. 2015, 31, 1137–1145. [Google Scholar] [CrossRef]

| Wineries | Vineyard Locations | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Yeast Strain | W 1 | W 2 | W 3 | L1 | L2 | L3 | L4 | L5 | L6 |
| M0 stage | |||||||||
| Pichia terricola | 18 | ||||||||
| Starmerella bacillaris | 32 | 43 | 9 | 7 | 5 | 6 | |||
| Pichia kluyveri | 12 | 2 | 13 | ||||||
| Candida apicola | 14 | 19 | |||||||
| Cystobasidium minutum | 3 | ||||||||
| Hanseniaspora uvarum | 21 | 32 | 83 | 91 | 98 | 86 | 95 | 94 | 100 |
| Pichia fermentans | 6 | 2 | 1 | ||||||
| Cystobasidium slooffiae | 8 | ||||||||
| Total Ascomycetes | 79 | 100 | 92 | 100 | 100 | 100 | 100 | 100 | 100 |
| MBF Stage | |||||||||
| Pichia fermentans | 3 | 1 | |||||||
| Saccharomyces cerevisiae | 94 | 68 | 85 | 72 | 5 | 10 | 3 | 11 | |
| Wicheramomyces anomalus | 3 | ||||||||
| Hanseniaspora uvarum | 31 | 15 | 28 | 99 | 95 | 90 | 38 | 89 | |
| Torulaspora delbrueckii | 1 | ||||||||
| Hanseniospora opuntiae | 59 | ||||||||
| H. uvarum | S. bacillaris | P. terricola | P. fermentans | P. kluyveri | |
|---|---|---|---|---|---|
| TP (mg GAE/L) | |||||
| M0 | 398.52 ± 0.00 a | 398.52 ± 0.00 a | 398.52 ± 0.00 a | 398.52 ± 0.00 a | 398.52 ± 0.00 a |
| MAI | 255.94 ± 3.74 a | 248.44 ± 6.82 ab | 269.27 ± 8.31 c | 252.30 ± 0.00 ab | 217.00 ± 3.65 d |
| MBF | 174.35 ± 0.00 a | 215.48 ± 2.65 b | 186.09 ± 0.00 c | 177.98 ± 0.00 d | 186.17 ± 0.00 c |
| WAF | 201.92 ± 0.00 a | 185.94 ± 0.00 b | 212.15 ± 0.00 c | 201.09 ± 0.00 d | 205.94 ± 0.00 e |
| WAE | 294.95 ± 2.04 a | 263.74 ± 3.93 b | 356.85 ± 1.70 c | 361.85 ± 3.65 cd | 367.68 ± 8.10 d |
| DPPH (mg Trolox/L) | |||||
| M0 | 209.91 ± 0.00 a | 209.91 ± 0.00 a | 209.91 ± 0.00 a | 209.91 ± 0.00 a | 209.91 ± 0.00 a |
| MAI | 159.18 ± 0.00 a | 145.21 ± 0.00 b | 161.48 ± 0.00 c | 159.87 ± 0.00 d | 167.56 ± 0.00 e |
| MBF | 141.37 ± 0.00 a | 147.28 ± 0.00 b | 136.03 ± 0.00 c | 136.77 ± 0.00 d | 131.60 ± 0.00 e |
| WAF | 126.15 ± 0.00 a | 111.58 ± 0.00 b | 121.46 ± 0.00 c | 124.98 ± 0.00 d | 134.15 ± 0.00 e |
| WAE | 133.60 ± 0.00 a | 137.61 ± 0.00 b | 120.95 ± 0.00 c | 129.96 ± 0.00 d | 125.27 ± 0.00 e |
| M0 Stage | WAE Stage | |||||
|---|---|---|---|---|---|---|
| H. uvarum | S. bacillaris | P. terricola | P. fermentans | P. kluyveri | ||
| Non-flavonoids | ||||||
| Hydroxybenzoics | ||||||
| Gallic | 0.46 ± 0.04 a | 0.622 ± 0.004 b | 1.2 ± 0.1 c | 1.502 ± 0.001 d | 1.21 ± 0.02 c | 0.83 ± 0.04 e |
| Protocatechuic acid | 1.09 ± 0.05 a | 1.95 ± 0.03 b | 2.10 ± 0.03 b | 1.9 ± 0.2 b | 1.90 ± 0.08 b | 2.025 ± 0.004 b |
| Syringaldehyde | n.q. | 1.5 ± 0.1 a | 1.09 ± 0.04 b | 0.40 ± 0.01 c | 0.680 ± 0.002 d | 1.288 ± 0.002 e |
| Syringic | n.q. | 4.5 ± 0.24 a | 4.19 ± 0.01 b | 4.7 ± 0.12 c | 5.19 ± 0.01 d | 5.89 ± 0.07 e |
| Vanillic acid | n.q. | 1.92 ± 0.04 a | 2.4 ± 0.2 b | 3.2 ± 0.2 c | 2.7 ± 0.1 d | 3.29 ± 0.06 c |
| p-Hydroxybenzoic acid | n.q. | 1.18 ± 0.01 a | 0.436 ± 0.001 b | 0.97 ± 0.09 c | 2.5 ± 0.3 d | 2.2 ± 0.1 e |
| Ellagic | n.d. | 0.99 ± 0.09 a | 1.07 ± 0.08 b | 0.88 ± 0.03 c | 0.88 ± 0.02 c | n.q. |
| Total | 1.55 | 12.63 | 12.42 | 13.49 | 15.11 | 15.53 |
| Hydroxycinnamates | ||||||
| Caffeic acid | n.q. | 1.08 ± 0.08 a | 1.28 ± 0.08 b | 1.54 ± 0.01 c | 0.89 ± 0.06 d | 1.05 ± 0.07 a |
| trans-Caftaric acid | 4.89 ± 0.04 a | 3.551 ± 0.003 b | 3.7 ± 0.1 c | 3.69 ± 0.09 c | 3.26 ± 0.07 d | 3.14 ± 0.06 e |
| Ferrulic acid | 0.78 ± 0.06 a | 0.98 ± 0.08 b | 1.26 ± 0.01 c | 0.86 ± 0.04 a | 0.85 ± 0.04 a | 0.805 ± 0.001 a |
| Sinapic acid | n.d. | 0.23 ± 0.01 a | 0.36 ± 0.01 b | 0.24 ± 0.02 a | 0.26 ± 0.02 c | n.q. |
| p-Coumaric acid | n.q. | n.q. | 0.41 ± 0.01 a | 0.639 ± 0.002 b | 0.40 ± 0.04 a | 0.410 ± 0.003 a |
| cis-Coutaric | 0.13 ± 0.01 a | 0.142 ± 0.001 b | 0.139 ± 0.003 b | 0.15 ± 0.01 c | 0.114 ± 0.001 d | 0.135 ± 0.001 b |
| trans-Coutaric | 1.66 ± 0.01 a | 2.05 ± 0.08 b | 2.22 ± 0.04 c | 2.01 ± 0.06 b | 2.01 ± 0.02 b | 2.12 ± 0.00 d |
| trans-Fertaric | 0.43 ± 0.02 a | 0.44 ± 0.04 a | 0.468 ± 0.001 b | 0.228 ± 0.001 c | 0.377 ± 0.001 d | 0.390 ± 0.003 d |
| Total | 7.89 | 8.47 | 9.82 | 9.36 | 8.16 | 8.05 |
| Stilbene | ||||||
| trans-Resveratrol | n.q. | 0.341 ± 0.005 a | 0.265 ± 0.002 b | 0.29 ± 0.01 c | 0.344 ± 0.003 a | 0.36 ± 0.02 d |
| Flavonoids | ||||||
| Flavan-3-ols | ||||||
| (+)-Catechin | 1.16 ± 0.07 a | 14.3 ± 0.8 b | 10.8 ± 0.4 c | 19.7 ± 0.6 d | 17 ± 2 e | 17 ± 2 e |
| (−)-Epicatechin | 6.8 ± 0.2 a | 1.5 ± 0.1 b | 1.36 ± 0.08 b | 1.28 ± 0.01 b | 1.6 ± 0.1 b | 1.76 ± 0.06 b |
| (−)-Epigallocatechin | 0.15 ± 0.01 a | 2.1 ± 0.1 a | 1.36 ± 0.06 a | 2.37 ± 0.05 a | 1.15 ± 0.05 a | 1.6 ± 0.1 a |
| (−)-Epigallocatechin gallate | 1.68 ± 0.04 a | 1.85 ± 0.05 a | 2.0 ± 0.1 a | 1.40 ± 0.01 a | 1.70 ± 0.02 a | 1.45 ± 0.02 a |
| Total | 9.83 | 19.82 | 15.54 | 24.75 | 21.88 | 22.07 |
| Flavonols | ||||||
| Kaempferol | n.q. | n.q. | n.q. | n.q. | n.q. | n.q. |
| Myricetin | n.q. | 0.30 ± 0.03 a | 0.32 ± 0.02 a | 0.24 ± 0.01 b | 0.29 ± 0.01 c | n.q. |
| Quercetin | n.d. | 1.01 ± 0.03 a | 0.57 ± 0.01 b | 0.24 ± 0.01 c | 1.21 ± 0.02 d | 1.45 ± 0.02 e |
| Rutin | n.d. | 0.66 ± 0.05 a | 0.65 ± 0.01 a | 0.50 ± 0.01 b | 0.54 ± 0.01 c | 0.506 ± 0.003 b |
| Total | -- | 4.98 | 4.59 | 3.61 | 5.03 | 4.99 |
| Total polyphenols | 19.27 | 46.24 | 42.64 | 51.50 | 50.52 | 51.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, A.; Pereira, V.; Jardim, H.; Malfeito-Ferreira, M.; Marques, J.C. Impact of Non-Saccharomyces Yeast Fermentation in Madeira Wine Chemical Composition. Processes 2023, 11, 482. https://doi.org/10.3390/pr11020482
Miranda A, Pereira V, Jardim H, Malfeito-Ferreira M, Marques JC. Impact of Non-Saccharomyces Yeast Fermentation in Madeira Wine Chemical Composition. Processes. 2023; 11(2):482. https://doi.org/10.3390/pr11020482
Chicago/Turabian StyleMiranda, Andreia, Vanda Pereira, Humberto Jardim, Manuel Malfeito-Ferreira, and José Carlos Marques. 2023. "Impact of Non-Saccharomyces Yeast Fermentation in Madeira Wine Chemical Composition" Processes 11, no. 2: 482. https://doi.org/10.3390/pr11020482





