The Prospects of Algae-Derived Vitamins and Their Precursors for Sustainable Cosmeceuticals
Abstract
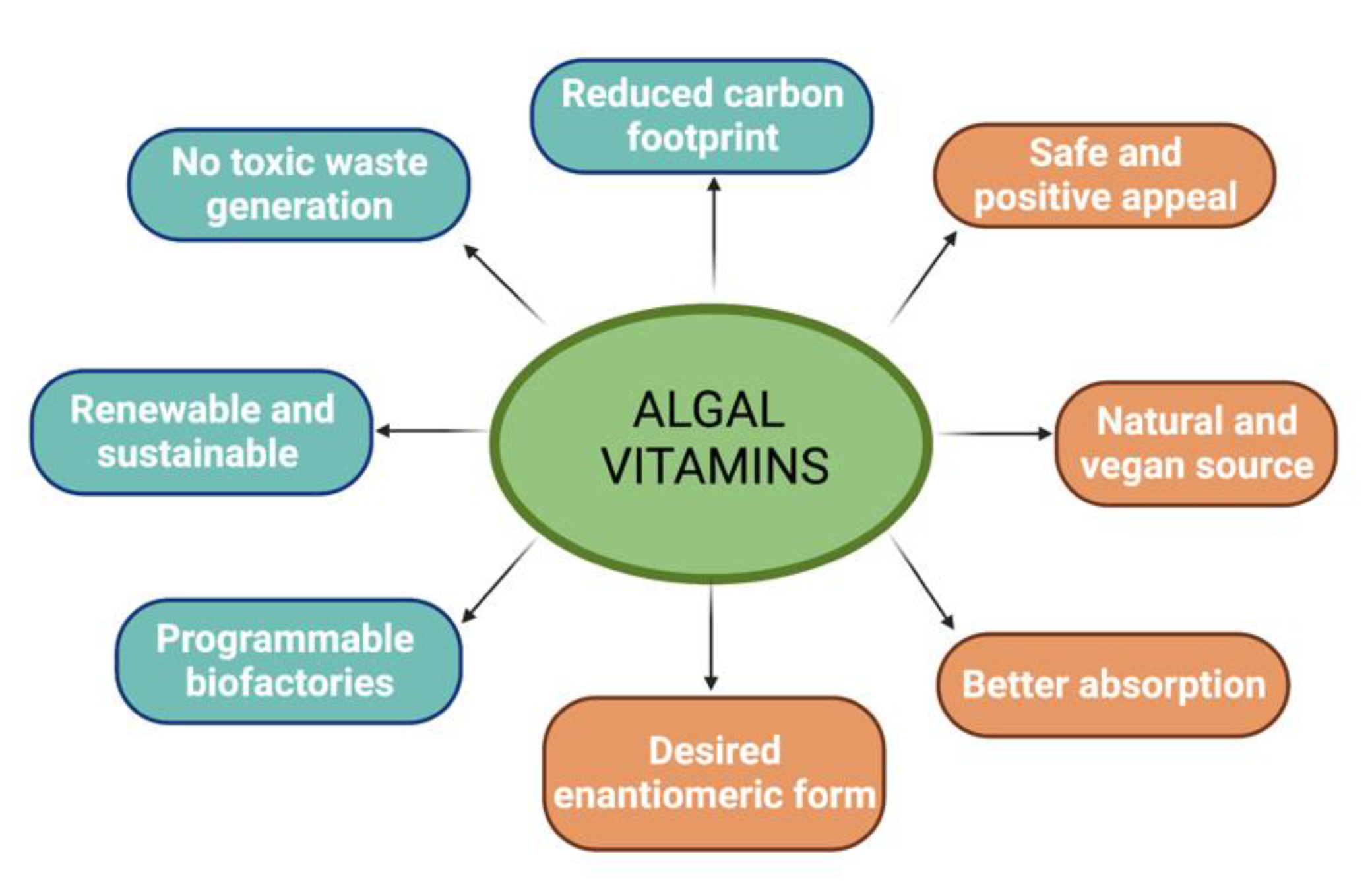
:1. Introduction
| Vitamin (Generic Name) | Vitamin Chemical Name(s)/Class | Market Size (USD Billion) | CAGR | Natural Sources | Reference |
|---|---|---|---|---|---|
| A/provitamin A | Retinol, retinal, α-carotene, β-carotene, γ-carotene, xanthophylls, β-cryptoxanthin | 0.52/0.47 | 4.81/6.9 | Leafy vegetables, spinach, carrots | [12,13] |
| B1 | Thiamine, thiamine pyrophosphate | 0.17 | 13.9 | Potatoes, vegetables | [14] |
| B2 | Riboflavin, flavin mononucleotide, flavin adenine dinucleotide | 1.3 | 6.1 | Vegetables, fruits | [15] |
| B3 | Niacin (nicotinic acid), niacinamide | 0.37 | 2.5 | Yeast, nuts | [16] |
| B5 | Pantothenic acid, panthenol, pantetheine | 0.46 | 6.2 | Pulses, grains | [17] |
| B7 | Biotin | 1.9 | 5.11 | Nuts | [18] |
| C | Ascorbic acid, dehydroascorbic acid, calcium ascorbate, sodium ascorbate | 1.3 | 6.1 | Citrus fruits, cabbage, paprika | [19] |
| D | Calcitriol, ergocalciferol (D2), cholecalciferol (D3) | 1.3 | 7.1 | Yeast, wheat germ oil, cabbage, citrus fruits | [20] |
| E | Tocopherols (α, β, γ, ∆), tocotrienols (α, β, γ, ∆) | 0.67 | 7.54 | Nuts, seeds, grains | [21] |
| K | Phylloquinone (K1), menaquinones (K2) and menadiones (K3) | 0.79 | 6.89 | Green leafy vegetables | [22] |
2. Vitamins Used in the Cosmeceutical Industry
3. Microalgae as Biofactories for Vitamin Production
3.1. Synthesis of Provitamin A (β-Carotene) by Algae
3.2. Synthesis of Other Key Vitamins by Algae
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levasseur, W.; Perré, P.; Pozzobon, V. A Review of High Value-Added Molecules Production by Microalgae in Light of the Classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef] [PubMed]
- Calijuri, M.L.; Silva, T.A.; Magalhães, I.B.; Pereira, A.S.A.d.P.; Marangon, B.B.; Assis, L.R.d.; Lorentz, J.F. Bioproducts from Microalgae Biomass: Technology, Sustainability, Challenges and Opportunities. Chemosphere 2022, 305, 135508. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Sharma, H.; Rai, A.K.; Nigam, P.S. Application of Biological Systems and Processes Employing Microbes and Algae to Reduce, Recycle, Reuse (3Rs) for the Sustainability of Circular Bioeconomy. AIMSMICRO 2022, 8, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Agrawal, S.; Nawaz, T.; Pan, S.; Selvaratnam, T. A Review of Algae-Based Produced Water Treatment for Biomass and Biofuel Production. Water 2020, 12, 2351. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Jin, Z.; Zhang, D. Microbial Cell Factories for Green Production of Vitamins. Front. Bioeng. Biotechnol. 2021, 9, 661562. [Google Scholar] [CrossRef]
- Acevedo-Rocha, C.G.; Gronenberg, L.S.; Mack, M.; Commichau, F.M.; Genee, H.J. Microbial Cell Factories for the Sustainable Manufacturing of B Vitamins. Curr. Opin. Biotechnol. 2019, 56, 18–29. [Google Scholar] [CrossRef]
- Revuelta, J.L.; Buey, R.M.; Ledesma-Amaro, R.; Vandamme, E.J. Microbial Biotechnology for the Synthesis of (pro)Vitamins, Biopigments and Antioxidants: Challenges and Opportunities. Microb. Biotechnol. 2016, 9, 564–567. [Google Scholar] [CrossRef]
- Allied Market Research, Cosmetics Market (by Category: Skin & Sun Care Products, Hair Care Products, Deodorants & Fragrances, Makeup & Color Cosmetics; by Gender: Men, Women, Unisex; by Distribution Channel: Hypermarkets/Supermarkets, Specialty Stores, Pharmacies, Online Sales Channels, Other)-Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2021–2030. 2021. Available online: https://www.alliedmarketresearch.com/cosmetics-market (accessed on 15 January 2023).
- Milito, A.; Castellano, I.; Damiani, E. From Sea to Skin: Is There a Future for Natural Photoprotectants? Mar. Drugs 2021, 19, 379. [Google Scholar] [CrossRef]
- Martínez-Ruiz, M.; Martínez-González, C.A.; Kim, D.-H.; Santiesteban-Romero, B.; Reyes-Pardo, H.; Villaseñor-Zepeda, K.R.; Meléndez-Sánchez, E.R.; Ramírez-Gamboa, D.; Díaz-Zamorano, A.L.; Sosa-Hernández, J.E.; et al. Microalgae Bioactive Compounds to Topical Applications Products—A Review. Molecules 2022, 27, 3512. [Google Scholar] [CrossRef]
- Yarkent, Ç.; Gürlek, C.; Oncel, S.S. Potential of Microalgal Compounds in Trending Natural Cosmetics: A Review. Sustain. Chem. Pharm. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Maximize Market Research, Vitamin a Market–Global Industry Analysis and Trends (2022–2029) Report. 2022. Available online: https://www.maximizemarketresearch.com/market-report/global-vitamin-a-market/121081/ (accessed on 15 January 2023).
- Research and Markets, Global Beta-Carotene Market (2021–2026) by Source, Application, and Geography Competitive Analysis and the Impact of COVID-19 with Ansoff Analysis. 2021. Available online: https://www.globenewswire.com/en/news-release/2022/01/11/2364624/28124/en/The-Worldwide-Beta-Carotene-Industry-is-Expected-to-Reach-609-Million-by-2026.html#:~:text=The%20Global%20Beta%2DCarotene%20Market,at%20a%20CAGR%20of%206.9%25.&text=Beta%2Dcarotene%20is%20an%20unrefined,found%20in%20plants%20and%20fruits (accessed on 15 January 2023).
- Market Analysis Report, Thiamine Market Size, Share & Trends Analysis Report by Application (Food & Beverages, Animal Feed, Pharmaceuticals, Dietary Supplements), by Region, and Segment Forecasts, 2022–2030. 2022. Available online: https://www.grandviewresearch.com/industry-analysis/thiamine-market-report (accessed on 15 January 2023).
- Market Watch, Riboflavin Market-Growth, Trends, and Forecasts (2023–2028). 2022. Available online: https://www.marketwatch.com/press-release/vitamin-b2-riboflavin-market-2023-to-2028-industry-trends-size-growth-insight-share-competitive-analysis-with-statistics-2022-12-21 (accessed on 15 January 2023).
- Research and Markets, Global Vitamin B3 Market Report 2020–2025: Opportunities in Increased Demand for Nutrition Supplements for Monogastric Animals. 2020. Available online: https://www.globenewswire.com/en/news-release/2020/12/22/2149324/28124/en/Global-Vitamin-B3-Market-Report-2020-2025-Opportunities-in-Increased-Demand-for-Nutrition-Supplements-for-Monogastric-Animals.html (accessed on 15 January 2023).
- Research and Markets, at 6.2% CAGR, Global Pantothenic Acid Market to reach us$ 750.7 million by 2028, Says Coherent Market Insights (CMI). 2021. Available online: https://www.globenewswire.com/en/news-release/2021/12/14/2351835/0/en/At-6-2-CAGR-Global-Pantothenic-Acid-Market-to-Reach-US-750-7-Million-by-2028-Says-Coherent-Market-Insights-CMI.html (accessed on 15 January 2023).
- Research and Markets, Biotin Supplements Market-Global Industry Assessment & Forecast. 2022. Available online: https://www.globenewswire.com/en/news-release/2022/07/13/2478866/0/en/Biotin-Supplements-Market-to-Reach-USD-3-4-Billion-by-2028-Consumer-Preference-Towards-Dietary-Supplements-and-Growing-Number-of-Health-Conscious-Customers-across-the-Globe-Drives-.html (accessed on 15 January 2023).
- Vantage Market Research, Global Vitamin C market Size & Share to Surpass $1.8 bn by 2028 | China Produces 80% of Commercial Vitamin C. 2022. Available online: https://www.globenewswire.com/en/news-release/2022/11/08/2550571/0/en/Global-Vitamin-C-Market-Size-Share-to-Surpass-1-8-Bn-by-2028-China-Produces-80-of-Commercial-Vitamin-C-Vantage-Market-Research.html (accessed on 15 January 2023).
- Markets and Markets, Vitamin D Market by Analog (Vitamin D2, Vitamin D3), form (Dry, Liquid), Application (Feed & Pet food, Pharma, Functional Food, and Personal Care), End Users (Adults, Pregnant Women, and Children), IU Strength and Region-Global Forecast to 2027. 2022. Available online: https://www.marketsandmarkets.com/Market-Reports/vitamin-d-market-22034298.html (accessed on 15 January 2023).
- Market Research Report, Natural Vitamin E Market Size, Share & Industry Analysis, by Type (Tocopherols, and Tocotrienolas), Application (Dietary Supplements, Food and Beverages, Cosmetics, and Others). And Regional Forecasts 2019–2026. 2018. Available online: https://www.fortunebusinessinsights.com/industry-reports/natural-vitamin-e-market-101591 (accessed on 15 January 2023).
- Global Market Insights, Global Vitamin K Market–Industry Trends and Forecast to 2029. 2019. Available online: https://www.databridgemarketresearch.com/reports/global-vitamin-k-market (accessed on 15 January 2023).
- Pimentel, F.; Alves, R.; Rodrigues, F.; P. P. Oliveira, M. Macroalgae-Derived Ingredients for Cosmetic Industry—An Update. Cosmetics 2017, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falqué, E.; Domínguez, H. Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics. Mar. Drugs 2021, 19, 552. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.-S.H. Natural Anti-Aging Skincare: Role and Potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Temova Rakuša, Ž.; Roškar, R. Quality Control of Vitamins A and E and Coenzyme Q10 in Commercial Anti-Ageing Cosmetic Products. Cosmetics 2021, 8, 61. [Google Scholar] [CrossRef]
- Kosari, P.; Alikhan, A.; Sockolov, M.; Feldman, S.R. Vitamin E and Allergic Contact Dermatitis. Dermatitis 2010, 21, 148–153. [Google Scholar] [CrossRef]
- Keen, M.; Hassan, I. Vitamin E in Dermatology. Indian Derm. Online J. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Rapisarda, L.; Ministeri, C.; Puglisi, G. Effects of Lipids and Emulsifiers on the Physicochemical and Sensory Properties of Cosmetic Emulsions Containing Vitamin E. Cosmetics 2015, 2, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, L.; Campos, P. Photostability and Efficacy Studies of Topical Formulations Containing UV-Filters Combination and Vitamins A, C and E. Int. J. Pharm. 2007, 343, 181–189. [Google Scholar] [CrossRef]
- Zasada, M.; Budzisz, E. Retinoids: Active Molecules Influencing Skin Structure Formation in Cosmetic and Dermatological Treatments. Pdia 2019, 36, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, S.S.; Saliou, C. Role of Vitamins in Skin Care. Nutrition 2001, 17, 839–844. [Google Scholar] [CrossRef]
- Rousselle, C. Opinion of the Scientific Committee on Consumer Safety (SCCS)–Final Version of the Opinion on Vitamin A (Retinol, Retinyl Acetate and Retinyl Palmitate) in Cosmetic Products. Regul. Toxicol. Pharmacol. 2017, 84, 102–104. [Google Scholar] [CrossRef]
- Burke, K.E. Interaction of Vitamins C and E as Better Cosmeceuticals: Vitamins C and E. Dermatol. Ther. 2007, 20, 314–321. [Google Scholar] [CrossRef]
- Ramos-e-Silva, M.; Celem, L.R.; Ramos-e-Silva, S.; Fucci-da-Costa, A.P. Anti-Aging Cosmetics: Facts and Controversies. Clin. Dermatol. 2013, 31, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Manela-Azulay, M.; Bagatin, E. Cosmeceuticals Vitamins. Clin. Dermatol. 2009, 27, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J. Vitamin D and the Skin: An Ancient Friend, Revisited. Exp. Dermatol. 2007, 16, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A Global Perspective for Health. Derm.-Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draelos, Z.D. Cosmetics, Diet, and the Future: Cosmetics, Diet, and the Future. Dermatol. Ther. 2012, 25, 267–272. [Google Scholar] [CrossRef]
- El-Hamd, M.A.; El Taieb, M.A.; Ibrahim, H.M.; Aly, S.S. Vitamin D Levels in Acne Vulgaris Patients Treated with Oral Isotretinoin. J. Cosmet. Dermatol. 2019, 18, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Marty, J.; Lafforgue, C.; Grossiord, J.; Soto, P. Rheological Properties of Three Different Vitamin D Ointments and Their Clinical Perception by Patients with Mild to Moderate Psoriasis. J. Eur. Acad. Dermatol. Venerol. 2005, 19, 7–10. [Google Scholar] [CrossRef]
- Vállez-Gomis, V.; Peris-Pastor, G.; Benedé, J.L.; Chisvert, A.; Salvador, A. Green Determination of Eight Water-Soluble B Vitamins in Cosmetic Products by Liquid Chromatography with Ultraviolet Detection. J. Pharm. Biomed. Anal. 2021, 205, 114308. [Google Scholar] [CrossRef]
- Heath, R.S.; Ruscoe, R.E.; Turner, N.J. The Beauty of Biocatalysis: Sustainable Synthesis of Ingredients in Cosmetics. Nat. Prod. Rep. 2022, 39, 335–388. [Google Scholar] [CrossRef] [PubMed]
- Sunita, A.; Siddharth, G.; Shalini, S.; Praneeta, S.; Aparajita, S.; Garishma, G.; Anmol, J. Vitamin K2: An Emerging Essential Nutraceutical and Its Market Potential. J. Appl. Biol. Biotech. 2022, 10, 173–184. [Google Scholar] [CrossRef]
- Shah, N.S.; Lazarus, M.C.; Bugdodel, R.; Hsia, S.L.; He, J.; Duncan, R.; Baumann, L. The Effects of Topical Vitamin K on Bruising after Laser Treatment. J. Am. Acad. Dermatol. 2002, 47, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.B.; Speretta, F.F.F.; Bentley, M.V.L.B. Enhancement of Skin Penetration of Vitamin K Using Monoolein-Based Liquid Crystalline Systems. Eur. J. Pharm. Sci. 2007, 32, 209–215. [Google Scholar] [CrossRef]
- Ren, L.; Peng, C.; Hu, X.; Han, Y.; Huang, H. Microbial Production of Vitamin K2: Current Status and Future Prospects. Biotechnol. Adv. 2020, 39, 107453. [Google Scholar] [CrossRef]
- Fang, H.; Kang, J.; Zhang, D. Microbial Production of Vitamin B12: A Review and Future Perspectives. Microb. Cell Fact. 2017, 16, 15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Lyu, S. Microbial Interactions in a Vitamin C Industrial Fermentation System: Novel Insights and Perspectives. Appl. Environ. Microbiol. 2022, 88, e01212-22. [Google Scholar] [CrossRef]
- Abu-Ghosh, S.; Dubinsky, Z.; Verdelho, V.; Iluz, D. Unconventional High-Value Products from Microalgae: A Review. Bioresour. Technol. 2021, 329, 124895. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-Value Products from Microalgae—Their Development and Commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Singh, R.V.; Sambyal, K. An Overview of β-Carotene Production: Current Status and Future Prospects. Food Biosci. 2022, 47, 101717. [Google Scholar] [CrossRef]
- Borowitzka, L.J.; Borowitzka, M.A. β-carotene (provitamin A) production with algae. In Biotechnology of Vitamins, Pigments, and Growth Factors, 1st ed.; Vandamme, E.J., Ed.; Springer Dordrecht Elsevier Science Publishers Ltd.: Dordrecht, The Netherlands, 1989; pp. 15–26. [Google Scholar]
- Harvey, P.J.; Ben-Amotz, A. Towards a Sustainable Dunaliella Salina Microalgal Biorefinery for 9-Cis β-Carotene Production. Algal Res. 2020, 50, 102002. [Google Scholar] [CrossRef]
- Mojaat, M.; Pruvost, J.; Foucault, A.; Legrand, J. Effect of Organic Carbon Sources and Fe2+ Ions on Growth and β-Carotene Accumulation by Dunaliella Salina. Biochem. Eng. J. 2008, 39, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Han, S.-I.; Kim, S.; Lee, C.; Choi, Y.-E. Blue-Red LED Wavelength Shifting Strategy for Enhancing Beta-Carotene Production from Halotolerant Microalga, Dunaliella Salina. J. Microbiol. 2019, 57, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Garbayo, I.; Cuaresma, M.; Vílchez, C.; Vega, J.M. Effect of Abiotic Stress on the Production of Lutein and β-Carotene by Chlamydomonas Acidophila. Process Biochem. 2008, 43, 1158–1161. [Google Scholar] [CrossRef]
- Morowvat, M.H.; Ghasemi, Y. Culture Medium Optimization for Enhanced β-Carotene and Biomass Production by Dunaliella salina in Mixotrophic Culture. Biocatal. Agric. Biotechnol. 2016, 7, 217–223. [Google Scholar] [CrossRef]
- Xi, Y.; Wang, J.; Chu, Y.; Chi, Z.; Xue, S. Effects of Different Light Regimes on Dunaliella salina Growth and β-Carotene Accumulation. Algal Res. 2020, 52, 102111. [Google Scholar] [CrossRef]
- Mousavi, P.; Morowvat, M.; Montazeri-Najafabady, N.; Abolhassanzadeh, Z.; Mohagheghzadeh, A.; Hamidi, M.; Niazi, A.; Ghasemi, Y. Investigating the Effects of Phytohormones on Growth and Beta-Carotene Production in a Naturally Isolates Stain of Dunaliella salina. J. Appl. Pharm. Sci. 2016, 6, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, R.; Kumar, V.A.; Kumar, D.; Ramesh, N.; Babu, S.; Gothandam, K.M. Effect of Dissolved Inorganic Carbon on β-Carotene and Fatty Acid Production in Dunaliella sp. Appl. Biochem. Biotechnol. 2015, 175, 2895–2906. [Google Scholar] [CrossRef]
- Fazeli, M.R.; Tofighi, H.; Samadi, N.; Jamalifar, H. Effects of Salinity on β-Carotene Production by Dunaliella tertiolecta DCCBC26 Isolated from the Urmia Salt Lake, North of Iran. Bioresour. Technol. 2006, 97, 2453–2456. [Google Scholar] [CrossRef]
- Keramati, A.; Pajoum Shariati, F.; Tavakoli, O.; Akbari, Z.; Rezaei, M. The Effect of Audible Sound Frequency on the Growth and Beta-Carotene Production of Dunaliella salina. S. Afr. J. Bot. 2021, 141, 373–382. [Google Scholar] [CrossRef]
- Luo, S.-W.; Alimujiang, A.; Cui, J.; Chen, T.-T.; Balamurugan, S.; Zheng, J.-W.; Wang, X.; Yang, W.-D.; Li, H.-Y. Molybdenum Disulfide Nanoparticles Concurrently Stimulated Biomass and β-Carotene Accumulation in Dunaliella salina. Bioresour. Technol. 2021, 320, 124391. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Yin, L.; Chi, Z.Y.; Luo, G. Characterization and RNA-Seq Transcriptomic Analysis of a Scenedesmus obliqnus Mutant with Enhanced Photosynthesis Efficiency and Lipid Productivity. Sci. Rep. 2021, 11, 11795. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Roy, A.; Kumar, L.; Bharadvaja, N. Media Optimization Using Box Behnken Design for Enhanced Production of Biomass, Beta-Carotene and Lipid from Dunaliella salina. Vegetos 2020, 33, 31–39. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Jiang, J.-G. Continuous Cultivation of Dunaliella salina in Photobioreactor for the Production of β-Carotene. Eur. Food Res. Technol. 2008, 227, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.; Xue, S.; Cao, X.; Chi, Z.; Wang, J. Quantitative Analysis on Photon Numbers Received per Cell for Triggering β-Carotene Accumulation in Dunaliella salina. Bioresour. Bioprocess. 2021, 8, 104. [Google Scholar] [CrossRef]
- Ben-Amotz, A. New Mode of Dunaliella Biotechnology: Two-Phase Growth for β-Carotene Production. J. Appl. Phycol. 1995, 7, 65–68. [Google Scholar] [CrossRef]
- Xu, Y.; Ibrahim, I.; Wosu, C.; Ben-Amotz, A.; Harvey, P. Potential of New Isolates of Dunaliella salina for Natural β-Carotene Production. Biology 2018, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, A.; Moslemi, M.; Pajoum Shariati, F.; Delavari Amrei, H. Beta-carotene Production within Dunaliella salina Cells under Salt Stress Condition in an Indoor Hybrid Helical-tubular Photobioreactor. Can. J. Chem. Eng. 2020, 98, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Dasan, Y.K.; Lam, M.K.; Yusup, S.; Lim, J.W.; Lee, K.T.; Show, P.L.; Tan, I.S.; Foo, H.C.Y. Cultivation of Chlorella vulgaris in Sequential Flow Photobioreactor System: Influence of Recycled Culture Medium on Growth, Lipid and Protein Content. Earth Env. Sci. 2021, 721, 012013. [Google Scholar] [CrossRef]
- Wongsnansilp, T.; Yokthongwattana, K.; Roytrakul, S.; Juntawong, N. β-Carotene Production of UV-C Induced Dunaliella salina Under Salt Stress. J. Pure Appl. Microbiol. 2019, 13, 193–200. [Google Scholar] [CrossRef]
- Zarandi-Miandoab, L.; Hejazi, M.-A.; Bagherieh-Najjar, M.-B.; Chaparzadeh, N. Statistical Optimization of The Four Key Factors on β-Carotene Production by Dunaliella salina Under Laboratory Conditions Using Response Surface Methodology. IJPR 2019, 18, 1566. [Google Scholar] [CrossRef] [PubMed]
- Tafreshi, A.H.; Shariati, M. Pilot Culture of Three Strains of Dunaliella Salina for β-Carotene Production in Open Ponds in the Central Region of Iran. World J. Microbiol. Biotechnol. 2006, 22, 1003–1006. [Google Scholar] [CrossRef]
- Hu, L.; Feng, S.; Liang, G.; Du, J.; Li, A.; Niu, C. CRISPR/Cas9-Induced β-Carotene Hydroxylase Mutation in Dunaliella salina CCAP19/18. AMB Expr. 2021, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Lu, H.; Zhao, Y.; Xu, H.; Zhang, Y.; Li, B. Two-Step Strategy for Obtaining Dunaliella sp. Biomass and β-Carotene from Anaerobically Digested Poultry Litter Wastewater. Int. Biodeterior. Biodegrad. 2019, 143, 104714. [Google Scholar] [CrossRef]
- Mogedas, B.; Casal, C.; Forján, E.; Vílchez, C. β-Carotene Production Enhancement by UV-A Radiation in Dunaliella bardawil Cultivated in Laboratory Reactors. J. Biosci. Bioeng. 2009, 108, 47–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Batghare, A.H.; Choudhury, B.J.; Goyal, A.; Moholkar, V.S. Microalgae Based Biorefinery: Assessment of Wild Fresh Water Microalgal Isolate for Simultaneous Biodiesel and β-Carotene Production. Bioresour. Technol. Rep. 2020, 11, 100440. [Google Scholar] [CrossRef]
- Singh, N.; Roy, K.; Goyal, A.; Moholkar, V.S. Investigations in Ultrasonic Enhancement of β-Carotene Production by Isolated Microalgal Strain Tetradesmus obliquus SGM19. Ultrason. Sonochemistry 2019, 58, 104697. [Google Scholar] [CrossRef]
- Schüler, L.M.; Bombo, G.; Duarte, P.; Santos, T.F.; Maia, I.B.; Pinheiro, F.; Marques, J.; Jacinto, R.; Schulze, P.S.C.; Pereira, H.; et al. Carotenoid Biosynthetic Gene Expression, Pigment and n-3 Fatty Acid Contents in Carotenoid-Rich Tetraselmis striata CTP4 Strains under Heat Stress Combined with High Light. Bioresour. Technol. 2021, 337, 125385. [Google Scholar] [CrossRef]
- Tran, Q.-G.; Cho, K.; Kim, U.; Yun, J.-H.; Cho, D.; Heo, J.; Park, S.-B.; Kim, J.W.; Lee, Y.J.; Ramanan, R.; et al. Enhancement of β-Carotene Production by Regulating the Autophagy-Carotenoid Biosynthesis Seesaw in Chlamydomonas reinhardtii. Bioresour. Technol. 2019, 292, 121937. [Google Scholar] [CrossRef]
- Couso, I.; Vila, M.; Rodriguez, H.; Vargas, M.A.; León, R. Overexpression of an Exogenous Phytoene Synthase Gene in the Unicellular Alga Chlamydomonas reinhardtii Leads to an Increase in the Content of Carotenoids. Biotechnol. Prog. 2011, 27, 54–60. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Y.; Xiao, L.; Li, Y.; Zou, S.; Han, D. Co-Production of Lutein, Zeaxanthin, and β-Carotene by Utilization of a Mutant of the Green Alga Chromochloris zofingiensis. Algal Res. 2022, 68, 102882. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Kao, A.-L.; Tsai, Z.-C.; Shen, Y.-M.; Kao, P.-H.; Ng, I.-S.; Chang, J.-S. Expression of Synthetic Phytoene Synthase Gene to Enhance β-Carotene Production in Scenedesmus Sp. CPC2. Biotechnol. J. 2017, 12, 1700204. [Google Scholar] [CrossRef] [PubMed]
- Cen, S.-Y.; Li, D.-W.; Huang, X.-L.; Huang, D.; Balamurugan, S.; Liu, W.-J.; Zheng, J.-W.; Yang, W.-D.; Li, H.-Y. Crucial Carotenogenic Genes Elevate Hyperaccumulation of Both Fucoxanthin and β-Carotene in Phaeodactylum tricornutum. Algal Res. 2022, 64, 102691. [Google Scholar] [CrossRef]
- Ben-Amotz, A. Production of P-Carotene and Vitamins by the Halotolerant Alga Dunaliella. In Marine Biotechnology, Pharmaceutical and Bioactive Natural Products, 1st ed.; Attaway, D.J., Zaborsky, O.R., Eds.; Plenum Press: New York, NY, USA, 1993; pp. 411–417. [Google Scholar]
- Fabregas, J.; Herrero, C. Vitamin Content of Four Marine Microalgae. Potential Use as Source of Vitamins in Nutrition. J. Ind. Microbiol. 1990, 5, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.R.; Mular, M.; Miller, I.; Farmer, C.; Trenerry, C. The Vitamin Content of Microalgae Used in Aquaculture. J. Appl. Phycol. 1999, 11, 247–255. [Google Scholar] [CrossRef]
- Bolinder, A.E.; Larsen, B. Studies on the Microbiological Determination of Niacin in Some Marine Algae. Acta Chem. Scanidanvica 1961, 15, 823–838. [Google Scholar] [CrossRef] [Green Version]
- Carlucci, A.F.; Bowes, P.M. Production of Vitamin B12, Thiamine, and Biotin by Phytoplankton. J. Phycol. 1970, 6, 351–357. [Google Scholar] [CrossRef]
- Edelmann, M.; Aalto, S.; Chamlagain, B.; Kariluoto, S.; Piironen, V. Riboflavin, Niacin, Folate and Vitamin B12 in Commercial Microalgae Powders. J. Food Compos. Anal. 2019, 82, 103226. [Google Scholar] [CrossRef]
- Brown, M.R.; Miller, K.A. The Ascorbic Acid Content of Eleven Species of Microalgae Used in Mariculture. J. Appl. Phycol. 1992, 4, 205–215. [Google Scholar] [CrossRef]
- Abalde, J.; Fabregas, J. Fi-Carotene, Vitamin C and Vitamin E Content of the Marine Microalga Dunaliella tertiolecta Cultured with Different Nitrogen Sources. Bioresour. Technol. 1991, 38, 121–125. [Google Scholar] [CrossRef]
- Running, J.A.; Huss, R.J.; Olson, P.T. Heterotrophic Production of Ascorbic Acid by Microalgae. J. Appl. Phycol. 1994, 6, 99–104. [Google Scholar] [CrossRef]
- Abe, K.; Nishimura, N.; Hirano, M. Simultaneous Production of β-Carotene, Vitamin E and Vitamin C by the Aerial Microalga Trentepohlia aurea. J. Appl. Phycol. 1999, 11, 331–336. [Google Scholar] [CrossRef]
- Mudimu, O.; Koopmann, I.K.; Rybalka, N.; Friedl, T.; Schulz, R.; Bilger, W. Screening of Microalgae and Cyanobacteria Strains for α-Tocopherol Content at Different Growth Phases and the Influence of Nitrate Reduction on α-Tocopherol Production. J. Appl. Phycol. 2017, 29, 2867–2875. [Google Scholar] [CrossRef]
- Durmaz, Y. Vitamin E (α-Tocopherol) Production by the Marine Microalgae Nannochloropsis oculata (Eustigmatophyceae) in Nitrogen Limitation. Aquaculture 2007, 272, 717–722. [Google Scholar] [CrossRef]
- Carballo-Cárdenas, E.C.; Tuan, P.M.; Janssen, M.; Wijffels, R.H. Vitamin E (α-Tocopherol) Production by the Marine Microalgae Dunaliella tertiolecta and Tetraselmis suecica in Batch Cultivation. Biomol. Eng. 2003, 20, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Bandarra, N.M.; Pereira, P.A.; Batista, I.; Vilela, M.H. Fatty Acids, Sterols and α-Tocopherol in Isochrysis galbana. J. Food Lipids 2003, 10, 25–34. [Google Scholar] [CrossRef]
- Mokrosnop, V.M.; Polishchuk, A.V.; Zolotareva, E.K. Accumulation of α-Tocopherol and β-Carotene in Euglena gracilis Cells Under Autotrophic and Mixotrophic Culture Conditions. Appl. Biochem. Microbiol. 2016, 52, 216–221. [Google Scholar] [CrossRef]
- Donato, M.; Vilela, M.H.; Bandarra, N.M. Fatty Acids, Sterols, α-Tocopherol and Total Carotenoids Composition of Diacronema vlkianum. J. Food Lipids 2003, 10, 267–276. [Google Scholar] [CrossRef]
- Tarento, T.D.C.; McClure, D.D.; Vasiljevski, E.; Schindeler, A.; Dehghani, F.; Kavanagh, J.M. Microalgae as a Source of Vitamin K1. Algal Res. 2018, 36, 77–87. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Y.; Zhang, H.; Wang, Y.; Qin, S. Genome-Wide Analysis of Biotin Biosynthesis in Eukaryotic Photosynthetic Algae. Plant Mol. Biol. Rep. 2012, 30, 421–432. [Google Scholar] [CrossRef]
- Ljubic, A.; Jacobsen, C.; Holdt, S.L.; Jakobsen, J. Microalgae Nannochloropsis Oceanica as a Future New Natural Source of Vitamin D3. Food Chem. 2020, 320, 126627. [Google Scholar] [CrossRef] [PubMed]
- Ljubic, A.; Thulesen, E.T.; Jacobsen, C.; Jakobsen, J. UVB Exposure Stimulates Production of Vitamin D3 in Selected Microalgae. Algal Res. 2021, 59, 102472. [Google Scholar] [CrossRef]

| Algae | Conditions | Yield | References |
|---|---|---|---|
| Dunaliella salina | Media enriched with acetate (67.5 mM), FeSO4 (450 µM) | 70 pg/cell | [55] |
| Sound frequency (200 Hz) and limited nitrate (0.25 g/L) | 12.5 mg/g | [63] | |
| Molybdenum disulfide nanoparticles (50 µg/L) and high light (600 µmol photons/m2s) | 0.15 mg/mg | [64] | |
| Optimized glucose (15 g/L), nitrate (1.8 g/L), and phosphate (0.013 g/L) | 8.12 mg/g | [58] | |
| 8-h illumination with 400 µmol photons/m2s | 7.24 % | [65] | |
| Optimized glucose (13.23 g/L), KNO3 (2.145 g/L), and NaCl (35.6 g/L) | 6.07 mg/g | [66] | |
| Floating photobioreactor (PBR) using seawater desalination concentrate | 14.4 g/m3 of culture | [67] | |
| Stimulated diurnal irradiance in summer (14/10, light/dark and 2000 µmol photons/m2s maximum irradiance) | 67.54 mg/L | [59] | |
| Flashing light (50 Hz) | 16 mg/L | [68] | |
| Kinetin (1 µM) | 23.03 mg/L | [60] | |
| Indole-3-acetic acid (1 µM) | 23.03 mg/L | ||
| Two-stage cultivation in raceway ponds | 15 mg/L | [69] | |
| Helix tube PBR, continuous cultivation | 30 mg/L | [67] | |
| Modified Johnson’s media with 10 mM NaHCO3 and high light intensity (1000 µmol photons/m2s) | 5.9 pg/cell | [70] | |
| Hybrid PBR | 4.85 mg/g | [71] | |
| Modified Johnson’s media with nitrate (5 mM) and 20% NaCl | 0.261 ng/cell | [72] | |
| UV-C mutants cultivated in 2.5 M NaCl under light intensity of 200 µmol photons/m2s | 3.06 mg/g | [73] | |
| Light intensity (200 µmol photons/m2s) with 0.9 mM nitrate and 3.8 M NaCl | 0.19 pg/cell | [74] | |
| Open raceway ponds | 7.1 mg/L | [75] | |
| 100 mM NaHCO3 | 0.18 mg/g | [61] | |
| ALE, blue light adaptation in blue–red shifting wavelengths | 33.94 µM | [56] | |
| CRISPR-Cas9 (silencing of β-carotene hydroxylase) | 1.4 mg/L | [76] | |
| Dunaliella tertiolecta DCCB26 | Artificial sea water with 0.5 M NaCl | 2.45 mg/g | [62] |
| Dunaliella FACHB-558 | Two-stage cultivation, anaerobically digested poultry water (first stage) + BG-11 (second stage) | 7.26 mg/L | [77] |
| Dunaliella bardawil | 8.7 W/m2 of UV-A radiation with 250 W/m2 PAR and nitrogen deprivation | 51.5 pg/cell | [78] |
| Tetraselmis obliquus SGM09 | BG-11 minimal media | 0.55 mg/g | [79] |
| Tetraselmis obliquus SGM19 | 0.67 mg/g | ||
| BG-11 with nitrate (1.5 g/L) and ultrasound (33 kHz and 1.4 bar at 10% duty cycle) | 0.77 mg/g | [80] | |
| Tetraselmis striata CTP4 | Ethyl methyl sulphonate mutant | 4.20 mg/g | [81] |
| Chlamydomonas reinhardtii | Silencing of autophagy gene (ATG8) and nitrogen starvation | 23.75 mg/g | [82] |
| Overexpression of phytoene synthase (PSY) gene | 1.2 mg/g | [83] | |
| Chlamydomonas acidophila | Light intensity (240 µmol photons/m2s) | 8.3 mg/L | [57] |
| Chromochloris zofingiensis | Ethyl methylsulphonate mutant with addition of gibberellin acid-3 (10 mg/L) in two-stage cultivation (C/N = 180/1 and 200 mM NaCl) | 0.52 g/L | [84] |
| Scenedesmus sp. CPC2 | Overexpression of PSY gene | 31.8 mg/g | [85] |
| Phaeodactylum tricornutum | Co-overexpression of 1-deoxy-D-xylulose 5-phosphate and lycopene cyclase | 4.34 mg/g | [86] |
| Vitamin | Algae | Conditions | Yield | References |
|---|---|---|---|---|
| Vitamin B1 | T. suecica | No stress | 32.3 µg/g | [88] |
| Isochrysis galbana | 14 µg/g | |||
| D. teriolecta | 29 µg/g | |||
| Chlorella stigmatophora | 14.6 µg/g | |||
| Tetraselmis sp. | 109 mg/g | [89] | ||
| Nannochloropsis sp. | Continuous light and harvesting in log phase | 70 mg/g | ||
| Vitamin B2 | T. suecica | No stress | 19.1 µg/g | [88] |
| Isochrysis galbana | 30 µg/g | |||
| D. teriolecta | 31.2 µg/g | |||
| Chlorella stigmatophora | 19.6 µg/g | |||
| Nannochloropsis sp. | Continuous light and harvesting in log phase | 62 mg/g | [89] | |
| Nannochloropsis gaditana | Commercialized powder | 22.1 µg/g | [92] | |
| Chlorella sp. | 20.7–33.6 µg/g | |||
| Vitamin B3 | Nannochloropsis gaditana | 0.24 mg/g | ||
| Chlorella sp. | 0.11 mg/g | |||
| Vitamin B5 | T. suecica | No stress | 37.7 µg/g | [88] |
| Isochrysis galbana | 9.1 µg/g | |||
| D. teriolecta | 13.2 µg/g | |||
| Chlorella stigmatophora | 21.4 µg/g | |||
| Vitamin B7 | T. suecica | No stress | 0.8 µg/g | |
| Isochrysis galbana | 1.0 µg/g | |||
| D. teriolecta | 0.9 µg/g | |||
| Chlorella stigmatophora | 1.1 µg/g | |||
| Vitamin C | Chaetoceros calcitrans | Harvesting at logarithmic phase | 44 fg/cell | [93] |
| Chaetoceros gracilis | 510 fg/cell | |||
| Chroomonas salina | 295 fg/cell | |||
| Nannochloropsis oculata | 61 fg/cell | |||
| T. suecica | 530 fg/cell | |||
| Isochrysis sp. | 76 fg/cell | |||
| Thalassiosira pseudonana | 46 fg/cell | |||
| Nannochloris atomus | No stress | 90.5 fg/cell | ||
| Pavlova lutheri | 56 fg/cell | |||
| Skletonema costatum | 700 fg/cell | |||
| D. teriolecta | 315 fg/cell | |||
| Nitrogen source (urea, 2 mg atom N/L) | 2.31 mg/g | [94] | ||
| Chlorella pyrenoidosa | Heterotrophic cultivation with glucose (5 g/L) in fermenters | 1 mg/g | [95] | |
| Mutant strain | 1–2 mg/g | |||
| T. suecica | No stress | 191 µg/g | [88] | |
| Isochrysis galbana | 119 µg/g | |||
| D. teriolecta | 163 µg/g | |||
| Chlorella stigmatophora | 100.2 µg/g | |||
| Tetraselmis sp. | 3 mg/g | [89] | ||
| Stichoccus sp. | 2.5 mg/g | |||
| Trentepholia aurea | Nitrogen source (NH4Cl, 120 mg/L) and light intensity (43 to 143 µmol photons/m2s, two stage) | 0.3 mg/g | [96] | |
| Vitamin E | N. oculata SAG 38.85 | Nitrogen limitation and harvesting at stationary phase | 1445.66 µg/g | [97] |
| Haematococcus pluvialis | 1179.91 µg/g | |||
| Microchloropsis salina | 1094 µg/g | |||
| Coccomyxa sp. | 1062 µg/g | |||
| Chlorococcum novae-angliae | No stress | 785 µg/g | ||
| Chlamydomonas nivalis | 719.73 µg/g | |||
| N. oculata | Nitrogen deficiency (441 µM/L) | 2325 µg/g | [98] | |
| T. suecica | No stress | 421.8 µg/g | [88] | |
| Isochrysis galbana | 58.2 µg/g | |||
| Chlorella stigmatophora | 669.0 µg/g | |||
| D. teriolecta | 116.3 µg/g | |||
| Nitrate source (urea, 2 mg nitrogen/L) | 1.81 mg/g | [94] | ||
| Harvesting in logarithmic phase | 0.37 mg/g | [99] | ||
| T. suecica | 0.39 mg/g | |||
| I galbana | Harvesting in late stationary phase | 55.4 µg/g | [100] | |
| Trentepholia aurea | Nitrogen source (NH4Cl, 120 mg/L) and light intensity (43 to 143 µmol photons/m2s, two stage) | 2.4 mg/g | [96] | |
| Euglena gracilis | Autotrophic | 1.422 mg/g | [101] | |
| Diacronema vlkianum | Harvesting in late stationary phase | 551.3 µg/g | [102] | |
| Vitamin K1 | Anabaena cylindrica | Optimization: light intensity (320 µmol photons/m2s), NaNO3 (1700 mg/L), K2HPO4 (34.6 mg/L) | 22 µg/L day | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arora, N.; Philippidis, G.P. The Prospects of Algae-Derived Vitamins and Their Precursors for Sustainable Cosmeceuticals. Processes 2023, 11, 587. https://doi.org/10.3390/pr11020587
Arora N, Philippidis GP. The Prospects of Algae-Derived Vitamins and Their Precursors for Sustainable Cosmeceuticals. Processes. 2023; 11(2):587. https://doi.org/10.3390/pr11020587
Chicago/Turabian StyleArora, Neha, and George P. Philippidis. 2023. "The Prospects of Algae-Derived Vitamins and Their Precursors for Sustainable Cosmeceuticals" Processes 11, no. 2: 587. https://doi.org/10.3390/pr11020587
APA StyleArora, N., & Philippidis, G. P. (2023). The Prospects of Algae-Derived Vitamins and Their Precursors for Sustainable Cosmeceuticals. Processes, 11(2), 587. https://doi.org/10.3390/pr11020587








