Continuous-Flow Hydrogenation of Nitroaromatics in Microreactor with Mesoporous Pd@SBA-15
Abstract
1. Introduction
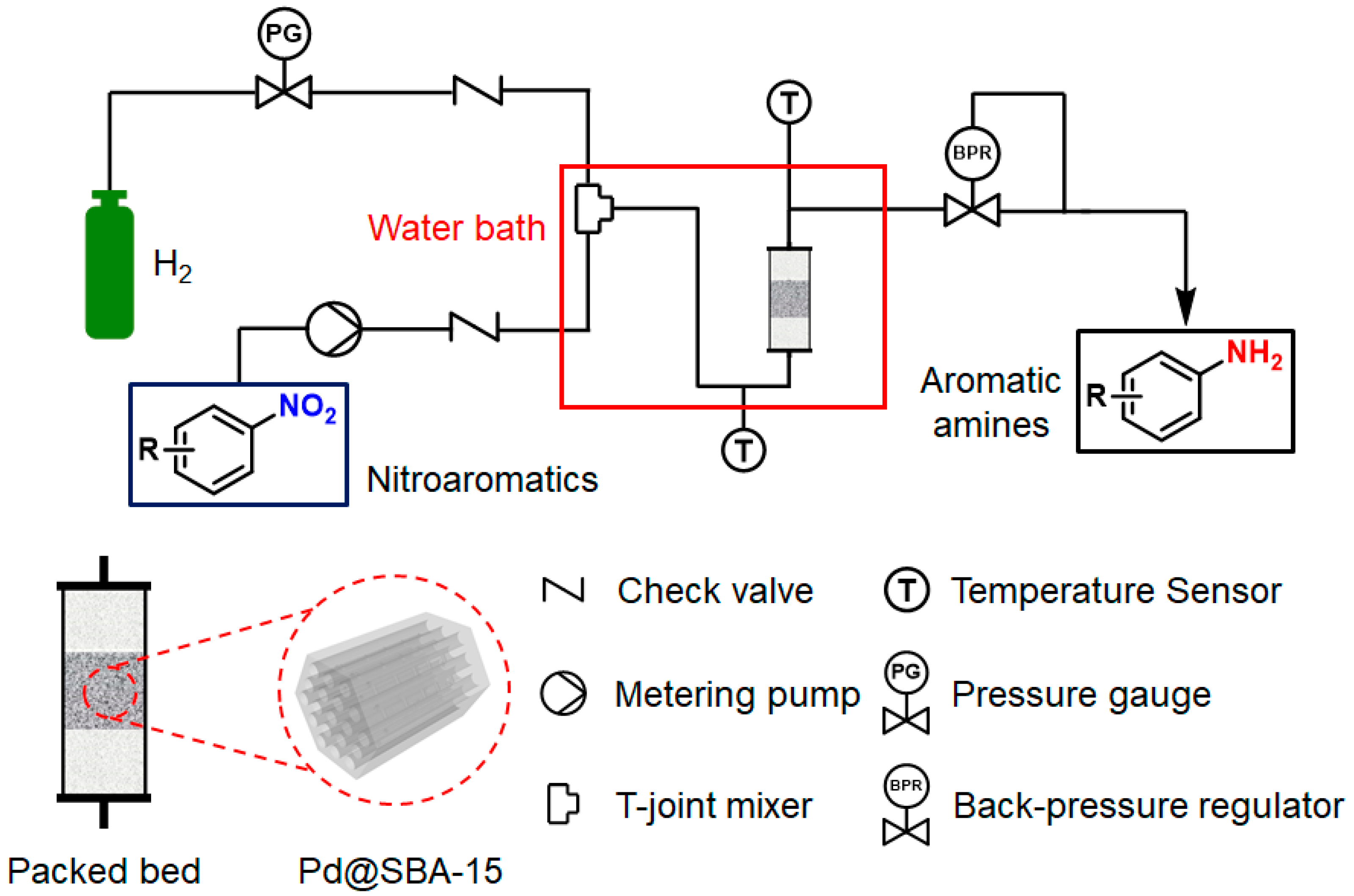
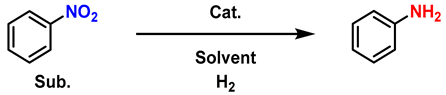
2. Materials and Methods
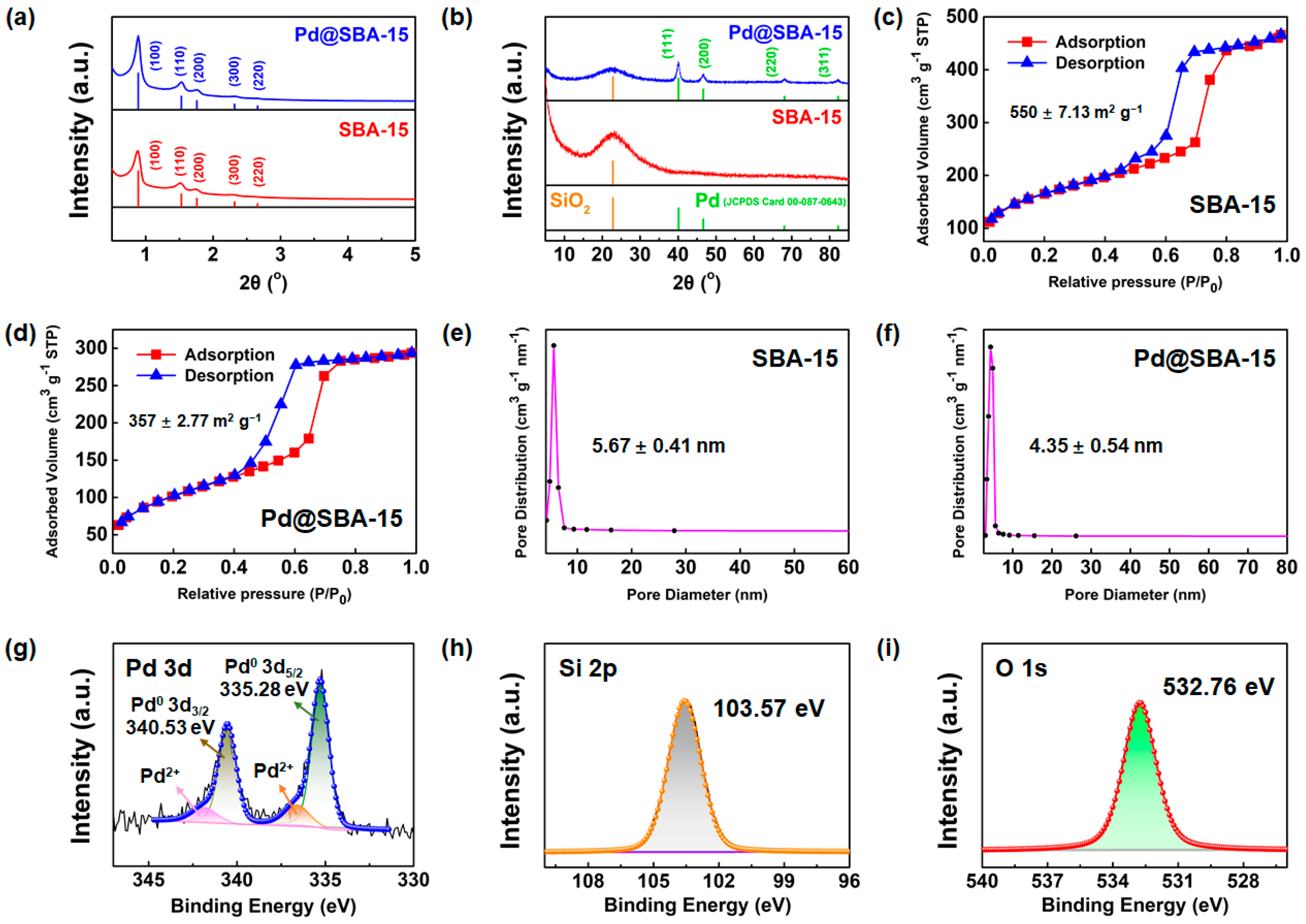
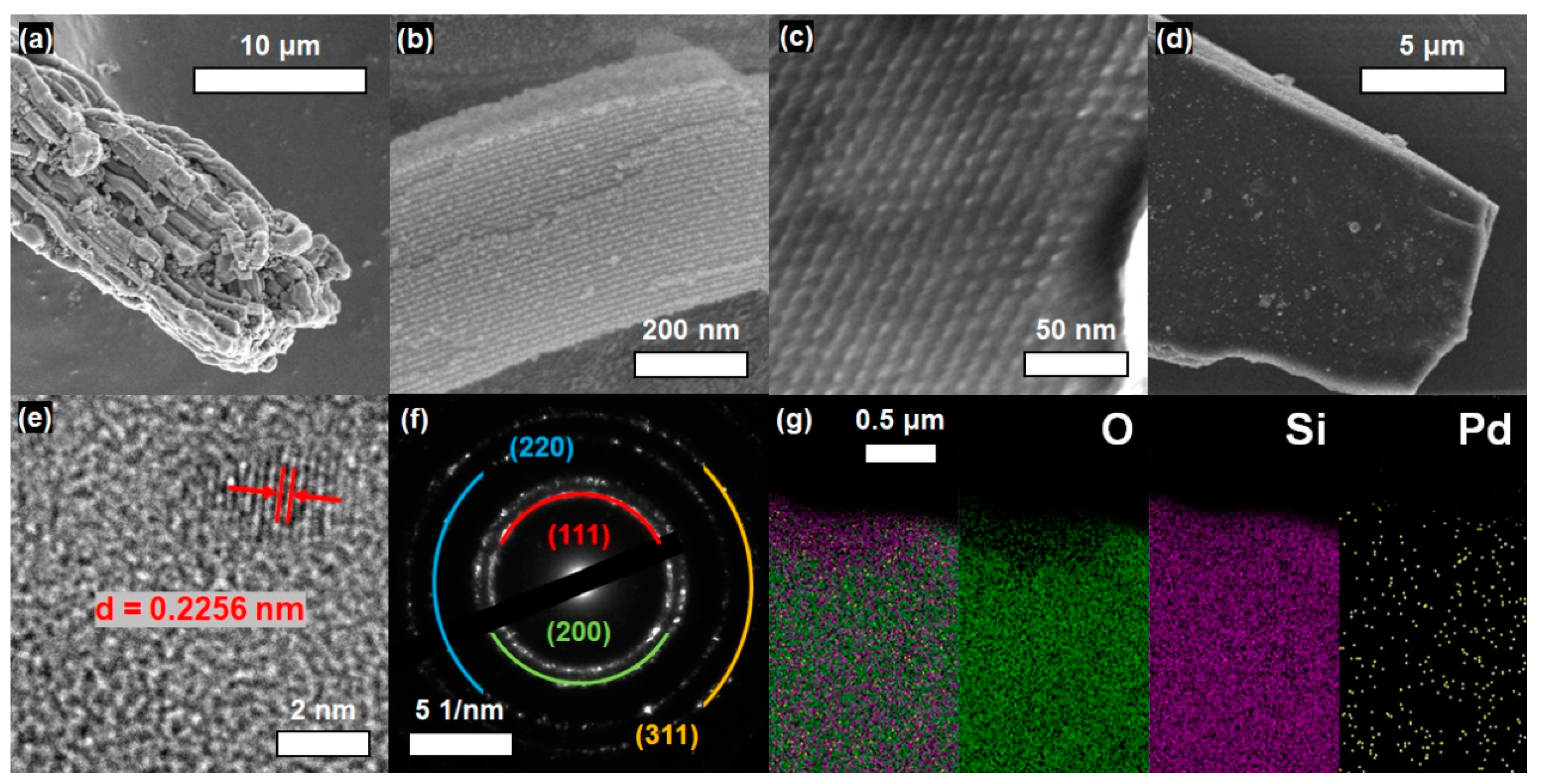
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H.H.; Wang, L.G.; Xu, S.; Hui, X.; Cao, Y.; He, P.; Li, Y.; Li, H.Q. Highly dispersed Ru anchored on nanosheet N-doped carbon for efficient and chemoselective hydrogenation of nitroaromatics to aromatic amines under mild conditions. Chem. Eng. J. 2022, 431, 133863. [Google Scholar] [CrossRef]
- Wu, S.T.; Huang, X.; Zhang, H.L.; Wei, Z.D.; Wang, M. Efficient Electrochemical Hydrogenation of Nitroaromatics into Arylamines on a CuCo2O4 Spinel Cathode in an Alkaline Electrolyte. ACS Catal. 2022, 12, 58–65. [Google Scholar] [CrossRef]
- Zhang, L.K.; Shang, N.Z.; Gao, S.T.; Wang, J.M.; Meng, T.; Du, C.C.; Shen, T.D.; Huang, J.Y.; Wu, Q.H.; Wang, H.J.; et al. Atomically Dispersed Co Catalyst for Efficient Hydrodeoxygenation of Lignin-Derived Species and Hydrogenation of Nitroaromatics. ACS Catal. 2020, 10, 8672–8682. [Google Scholar] [CrossRef]
- Sun, L.Q.; Zhu, L.; Qian, K.D.; Qin, B.J.; Huang, L.; Chen, C.H.; Lee, K.H.; Xie, L. Design, Synthesis, and Preclinical Evaluations of Novel 4-Substituted 1,5-Diarylanilines as Potent HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI) Drug Candidates. J. Med. Chem. 2012, 55, 7219–7229. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Liu, Q.; Luo, B.L.; Chen, M.H.; Pi, R.B.; Huang, P.; Wen, S.J. Synthesis of Carbazoles via One-Pot Copper-Catalyzed Amine Insertion into Cyclic Diphenyleneiodoniums as a Strategy to Generate a Drug-Like Chemical Library. Adv. Synth. Catal. 2013, 355, 2172–2178. [Google Scholar] [CrossRef]
- Eli, L.D.; Kavuri, S.M.; Liu, Z.Y.; Su, J.; Xiao, Y.; Qiao, B.T.; Ding, Y.J. NiOx-promoted Cu-based catalysts supported on AlSBA-15 for chemoselective hydrogenation of nitroarenes. J. Catal. 2022, 416, 332–343. [Google Scholar] [CrossRef]
- Sui, D.J.; Mao, F.; Fan, H.P.; Qi, Z.L.; Huang, J. General Reductive Amination of Aldehydes and Ketones with Amines and Nitroaromatics under H2 by Recyclable Iridium Catalysts. Chin. J. Chem. 2017, 35, 1371–1377. [Google Scholar] [CrossRef]
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-Flow Technology—A Tool for the Safe Manufacturing of Active Pharmaceutical Ingredients. Angew. Chem. Int. Ed. 2015, 54, 6688–6728. [Google Scholar] [CrossRef]
- Kreissl, H.; Jin, J.; Lin, S.H.; Schuette, D.; Stortte, S.; Levin, N.; Chaudret, B.; Vorholt, A.J.; Bordet, A.; Leitner, W. Commercial Cu2Cr2O5 Decorated with Iron Carbide Nanoparticles as a Multifunctional Catalyst for Magnetically Induced Continuous-Flow Hydrogenation of Aromatic Ketones. Angew. Chem. Int. Ed. 2021, 60, 26639–26646. [Google Scholar] [CrossRef]
- Wen, Z.H.; Yang, M.; Zhao, S.N.; Zhou, F.; Chen, G.W. Kinetics study of heterogeneous continuous-flow nitration of trifluoromethoxybenzene. React. Chem. Eng. 2018, 3, 379–387. [Google Scholar] [CrossRef]
- Wang, F.J.; Chen, A.; Ling, S.D.; Xu, J.H. Continuous-flow diazotization of red base KD hydrochloride suspensions in a microreaction system. React. Chem. Eng. 2021, 6, 1462–1474. [Google Scholar] [CrossRef]
- Lebl, R.; Zhu, Y.T.; Ng, D.; Hornung, C.H.; Cantillo, D.; Kappe, C.O. Scalable continuous flow hydrogenations using Pd/Al2O3-coated rectangular cross-section 3D-printed static mixers. Catal. Today 2022, 383, 55–63. [Google Scholar] [CrossRef]
- Braun, M.; Esposito, D. Hydrogenation Properties of Nanostructured Tungsten Carbide Catalysts in a Continuous-Flow Reactor. ChemCatChem 2017, 9, 393–397. [Google Scholar] [CrossRef]
- Davis, B.A.; Bennett, J.A.; Genzer, J.; Efimenko, K.; Abolhasani, M. Intensified Hydrogenation in Flow Using a Poly(beta-cyclodextrin) Network-Supported Catalyst. ACS Sustain. Chem. Eng. 2022, 10, 15987–15998. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.J.; Wang, X.L.; Sun, Q.D.; He, X.H.; Ji, H.B. Enhanced Hydrogenation Properties of Pd Single Atom Catalysts with Atomically Dispersed Ba Sites as Electronic Promoters. ACS Catal. 2022, 12, 15091–15096. [Google Scholar] [CrossRef]
- Huang, L.; Tang, F.Y.; Hao, F.; Zhao, H.; Liu, W.Y.; Lv, Y.; Liu, P.L.; Xiong, W.; Luo, H.A. Tuning the Electron Density of Metal Nickel via Interfacial Electron Transfer in Ni/MCM-41 for Efficient and Selective Catalytic Hydrogenation of Halogenated Nitroarenes. ACS Sustain. Chem. Eng. 2022, 10, 2947–2959. [Google Scholar] [CrossRef]
- Couto, C.S.; Madeira, L.M.; Nunes, C.P.; Araujo, P. Commercial catalysts screening for liquid phase nitrobenzene hydrogenation. Appl. Catal. A 2016, 522, 152–164. [Google Scholar] [CrossRef]
- Brandi, F.; Baumel, M.; Molinari, V.; Shekova, I.; Lauermann, I.; Heil, T.; Antonietti, M.; Al-Naji, M. Nickel on nitrogen-doped carbon pellets for continuous-flow hydrogenation of biomass-derived compounds in water. Green Chem. 2020, 22, 2755–2766. [Google Scholar] [CrossRef]
- Cova, C.M.; Zuliani, A.; Manno, R.; Sebastian, V.; Luque, R. Scrap waste automotive converters as efficient catalysts for the continuous-flow hydrogenations of biomass derived chemicals. Green Chem. 2020, 22, 1414–1423. [Google Scholar] [CrossRef]
- Geier, D.; Schrnitz, P.; Walkowiak, J.; Leitner, W.; Francio, G. Continuous Flow Asymmetric Hydrogenation with Supported Ionic Liquid Phase Catalysts Using Modified CO2 as the Mobile Phase: From Model Substrate to an Active Pharmaceutical Ingredient. ACS Catal. 2018, 8, 3297–3303. [Google Scholar] [CrossRef]
- Wei, J.; Sun, Z.K.; Luo, W.; Li, Y.H.; Elzatahry, A.A.; Al-Enizi, A.M.; Deng, Y.H.; Zhao, D.Y. New Insight into the Synthesis of Large-Pore Ordered Mesoporous Materials. J. Am. Chem. Soc. 2017, 139, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Consolati, G.; Ferragut, R.; Galarneau, A.; Renzo, F.; Quasso, F. Mesoporous materials for antihydrogen production. Chem. Soc. Rev. 2013, 42, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Ha, C.S. Hollow Mesoporous Functional Hybrid Materials: Fascinating Platforms for Advanced Applications. Adv. Funct. Mater. 2018, 28, 1703814. [Google Scholar] [CrossRef]
- Chen, C.; Li, B.X.; Zhou, L.J.; Xia, Z.F.; Feng, N.J.; Ding, J.; Wang, L.; Wan, H.; Guan, G.F. Synthesis of Hierarchically Structured Hybrid Materials by Controlled Self-Assembly of Metal Organic Framework with Mesoporous Silica for CO2 Adsorption. ACS Appl. Mater. Interfaces 2017, 9, 23060–23071. [Google Scholar] [CrossRef]
- Szczesniak, B.; Choma, J.; Jaroniec, M. Effect of graphene oxide on the adsorption properties of ordered mesoporous carbons toward H2, C6H6, CH4 and CO2. Microporous Mesoporous Mater. 2018, 261, 105–110. [Google Scholar] [CrossRef]
- Yao, J.F.; Dong, F.; Xu, X.; Wen, M.; Ji, Z.Y.; Feng, H.; Wang, X.L.; Tang, Z.C. Rational Design and Construction of Monolithic Ordered Mesoporous Co3O4@SiO2 Catalyst by a Novel 3D Printed Technology for Catalytic Oxidation of Toluene. ACS Appl. Mater. Interfaces 2022, 14, 22170–22185. [Google Scholar] [CrossRef]
- Bhat, A.; Hill, A.J.; Fisher, G.B.; Schwank, J.W. Improving the thermal stability and n-butanol oxidation activity of Ag-TiO2 catalysts by controlling the catalyst architecture and reaction conditions. Appl. Catal. B 2021, 297, 120476. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.Y.; Gu, F.N.; Wang, X.Y.; Lu, X.P.; Li, H.F.; Xu, G.W.; Su, F.B. CO methanation on ordered mesoporous Ni-Cr-Al catalysts: Effects of the catalyst structure and Cr promoter on the catalytic properties. J. Catal. 2016, 337, 221–232. [Google Scholar] [CrossRef]
- Xun, S.H.; Hou, C.Z.; Li, H.P.; He, M.Q.; Ma, R.L.; Zhang, M.; Zhu, W.S.; Li, H.M. Synthesis of WO3/mesoporous ZrO2 catalyst as a high-efficiency catalyst for catalytic oxidation of dibenzothiophene in diesel. J. Mater. Sci. 2018, 53, 15927–15938. [Google Scholar] [CrossRef]
- Yang, P.D.; Zhao, D.Y.; Chmelka, B.F.; Stucky, G.D. Triblock-copolymer-directed syntheses of large-pore mesoporous silica fibers. Chem. Mater. 1998, 10, 2033–2036. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Huo, Q.S.; Feng, J.L.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Roucher, A.; Bentaleb, A.; Laurichesse, E.; Dourges, M.A.; Emo, M.; Schmitt, V.; Blin, J.L.; Backov, R. First Macro-Mesocellular Silica SBA-15-Si(HIPE) Monoliths: Conditions for Obtaining Self-Standing Materials. Chem. Mater. 2018, 30, 864–873. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, L.; Pan, D.H.; Guo, Y.N.; Zhou, L.; Yang, J.; Zou, J.; Yu, C.Z. A systematic study of long-range ordered 3D-SBA-15 materials by electron tomography. New J. Chem. 2011, 35, 2456–2461. [Google Scholar] [CrossRef]
- Rettenmaier, C.; Aran-Ais, R.M.; Timoshenko, J.; Rizo, R.; Jeon, H.S.; Kuhl, S.; Chee, S.W.; Bergmann, A.; Roldan Cuenya, B. Enhanced Formic Acid Oxidation over SnO2-decorated Pd Nanocubes. ACS Catal. 2020, 10, 14540–14551. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Bai, Y.; Choi, S.M.; Tong, L.; Aleisa, R.M.; Li, Z.; Liu, X.; Yu, R.; Myung, N.V.; Yin, Y. Mesoporous TiO2 nanospheres loaded with highly dispersed Pd nanoparticles for pH-universal hydrogen evolution reaction. Mater. Today Nano 2019, 6, 100038. [Google Scholar] [CrossRef]
- Kirsch, P.D.; Ekerdt, J.G. Interfacial chemistry of the Sr/SiOxNy/Si(100) nanostructure. J. Vac. Sci. Technol. A Vac. Surf. Film. 2001, 19, 2222–2231. [Google Scholar] [CrossRef]
- Celebioglu, A.; Topuz, F.; Uyar, T. Facile and green synthesis of palladium nanoparticles loaded into cyclodextrin nanofibers and their catalytic application in nitroarene hydrogenation. New J. Chem. 2019, 43, 3146–3152. [Google Scholar] [CrossRef]
- Navaladian, S.; Viswanathan, B.; Varadarajan, T.K.; Viswanath, R.P. A Rapid Synthesis of Oriented Palladium Nanoparticles by UV Irradiation. Nanoscale Res. Lett. 2009, 4, 181–186. [Google Scholar] [CrossRef]
- Liang, W.K.; Wang, Y.W.; Zhao, L.; Guo, W.; Li, D.; Qin, W.; Wu, H.H.; Sun, Y.H.; Jiang, L. 3D Anisotropic Au@Pt-Pd Hemispherical Nanostructures as Efficient Electrocatalysts for Methanol, Ethanol, and Formic Acid Oxidation Reaction. Adv. Mater. 2021, 33, 2100713. [Google Scholar] [CrossRef]
- Kim, M.C.; Han, G.H.; Xiao, X.Y.; Song, J.; Hong, J.; Jung, E.; Kim, H.K.; Ahn, J.P.; Han, S.S.; Lee, K.Y.; et al. Anisotropic growth of Pt on Pd nanocube promotes direct synthesis of hydrogen peroxide. Appl. Surf. Sci. 2021, 562, 150031. [Google Scholar] [CrossRef]
- Rajadhyaksha, R.A.; Karwa, S.L. Solvent effects in catalytic hydrogenation. Chem. Eng. Sci. 1986, 41, 1765–1770. [Google Scholar] [CrossRef]
- Ding, S.M.; Cheng, D.; Xiao, W.M.; Ma, X.H.; Zeng, R.; Liu, S.Q.; Liang, S.Q.; Chen, C.; Song, W.G. Nickel Nanoparticles Encapsulated in Carbon Nanotubes as an Efficient and Robust Catalyst for Hydrogenation of Nitroarenes. Catal. Lett. 2023, 153, 595–604. [Google Scholar] [CrossRef]
- Krishnan, S.; Patel, P.N.; Balasubramanian, K.K.; Chadha, A. Yeast supported gold nanoparticles: An efficient catalyst for the synthesis of commercially important aryl amines. New J. Chem. 2021, 45, 1915–1923. [Google Scholar] [CrossRef]
- Kara, B.Y.; Kilbas, B.; Goksu, H. Selectivity and activity in catalytic hydrogenation of azido groups over Pd nanoparticles on aluminum oxy-hydroxide. New J. Chem. 2016, 40, 9550–9555. [Google Scholar] [CrossRef]
- Zhang, R.K.; Chen, T.; Wang, G.S.; Guan, Y.Q.; Yan, G.Y.; Chen, Z.P.; Hu, J.S. Magnetic Recyclable Cu/ZnFe2O4 for Catalytic Reduction of Nitroarenes and C-N Bond Formation Reactions. Catal. Lett. 2022, 152, 3506–3516. [Google Scholar] [CrossRef]
- Qu, Z.H.; Chen, X.; Zhong, S.; Deng, G.J.; Huang, H.W. NaI/PPh3-Mediated Photochemical Reduction and Amination of Nitroarenes. Org. Lett. 2021, 23, 5349–5353. [Google Scholar] [CrossRef]
- Almeida, A.F.; Ataide, F.A.P.; Loureiro, R.M.S.; Moreira, R.; Rodrigues, T. Augmenting Adaptive Machine Learning with Kinetic Modeling for Reaction Optimization. J. Org. Chem. 2021, 86, 14192–14198. [Google Scholar] [CrossRef]
- Guo, B.B.; de Vries, J.G.; Otten, E. Hydration of nitriles using a metal-ligand cooperative ruthenium pincer catalyst. Chem. Sci. 2019, 10, 10647–10652. [Google Scholar] [CrossRef]
- Quinn, J.F.; Bryant, C.E.; Golden, K.C.; Gregg, B.T. Rapid reduction of heteroaromatic nitro groups using catalytic transfer hydrogenation with microwave heating. Tetrahedron Lett. 2010, 51, 786–789. [Google Scholar] [CrossRef]
- Chang, J.B.; Hwang, J.H.; Park, J.S.; Kim, J.P. The effect of dye structure on the dyeing and optical properties of dichroic dyes for PVA polarizing film. Dye. Pigm. 2011, 88, 366–371. [Google Scholar] [CrossRef]
- Uberman, P.M.; Garcia, C.S.; Rodriguez, J.R.; Martin, S.E. PVP-Pd nanoparticles as efficient catalyst for nitroarene reduction under mild conditions in aqueous media. Green Chem. 2017, 19, 739–748. [Google Scholar] [CrossRef]
- Duan, X.N.; Wang, X.P.; Chen, X.K.; Zhang, J.S. Continuous and Selective Hydrogenation of Heterocyclic Nitroaromatics in a Micropacked Bed Reactor. Org. Process Res. Dev. 2021, 25, 2100–2109. [Google Scholar] [CrossRef]
- Yue, S.N.; Wang, X.G.; Li, S.T.; Sheng, Y.; Zou, X.J.; Lu, X.G.; Zhang, C.L. Highly selective hydrogenation of halogenated nitroarenes over Ru/CN nanocomposites by in situ pyrolysis. New J. Chem. 2020, 44, 11861–11869. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Li, Z.H.; Xia, T.Y.; Du, Y.L.; Mao, X.M.; Li, Y.Q. Molecular mechanism of azoxy bond formation for azoxymycins biosynthesis. Nat. Commun. 2019, 10, 4420. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Wen, Z.H.; Zhao, F.; Kuhn, S.; Noël, T. Scale-up of micro- and milli-reactors: An overview of strategies, design principles and applications. Chem. Eng. Sci. X 2021, 10, 100097. [Google Scholar] [CrossRef]
- Mohammad, N.; Chukwudoro, C.; Bepari, S.; Basha, O.; Aravamudhan, S.; Kuila, D. Scale-up of high-pressure F-T synthesis in 3D printed stainless steel microchannel microreactors: Experiments and modeling. Catal. Today 2022, 397, 182–196. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Yang, L.; Yu, Y.X.; Zhang, L.J.; Tao, S.Y. Scale-up Design of a Fluorescent Fluid Photochemical Microreactor by 3D Printing. ACS Omega 2020, 5, 7666–7674. [Google Scholar] [CrossRef]


 | |||||
|---|---|---|---|---|---|
| Entry a | Flow Rate (mL/min) | H2 Pres. (MPa) | Temp. (°C) | Solvent | Yield (%) b |
| 1 | 0.50 | 1.0 | 40 | MeOH | 85 |
| 2 | 0.50 | 1.0 | 50 | MeOH | 96 |
| 3 | 0.50 | 1.0 | 60 | MeOH | 99 |
| 4 | 0.50 | 1.0 | 70 | MeOH | 99 |
| 5 | 0.25 | 1.0 | 60 | MeOH | 99 |
| 6 | 0.75 | 1.0 | 60 | MeOH | 95 |
| 7 | 1.00 | 1.0 | 60 | MeOH | 88 |
| 8 | 0.50 | 2.0 | 40 | MeOH | 85 |
| 9 | 0.50 | 3.0 | 40 | MeOH | 85 |
| 10 | 0.50 | 1.0 | 60 | EtOH | 23 |
| 11 | 0.50 | 1.0 | 60 | EA | 31 |
| 12 | 0.50 | 1.0 | 60 | MeCN | 52 |
| 13 | 0.50 | 1.0 | 60 | THF | trace |
| 14 | 0.50 | 1.0 | 60 | DMF | trace |
| 15 c | 0.50 | 1.0 | 60 | MeOH | 83 |
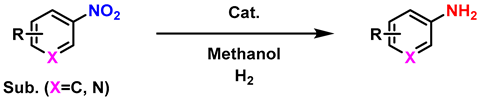 | ||||
|---|---|---|---|---|
| Entry a | Substrate | Product | Yield (%) b | |
| 1 | 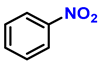 |  | (2a) | 99 |
| 2 |  | 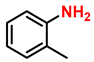 | (2b) | 99 |
| 3 | 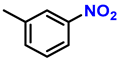 |  | (2c) | 99 |
| 4 | 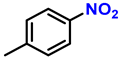 |  | (2d) | 99 |
| 5 | 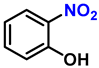 |  | (2e) | 99 |
| 6 | 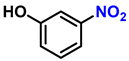 | 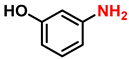 | (2f) | 99 |
| 7 |  |  | (2g) | 99 |
| 8 | 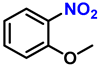 |  | (2h) | 99 |
| 9 |  | 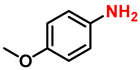 | (2i) | 99 |
| 10 | 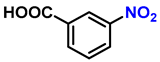 |  | (2j) | 99 |
| 11 | 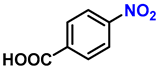 |  | (2k) | 99 |
| 12 | 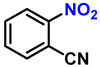 | 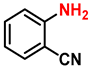 | (2l) | 98 |
| 13 |  | 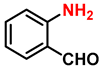 | (2m) | 98 |
| 14 | 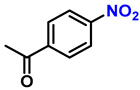 | 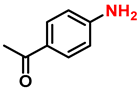 | (2n) | 99 |
| 15 | 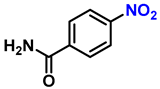 |  | (2o) | 98 |
| 16 | 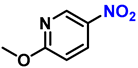 |  | (2p) | 99 |
| 17 | 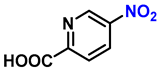 |  | (2q) | 99 |
| 18 c | 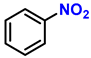 | 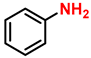 | (2a′) | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, K.; Shen, R.; Qi, T.; Chen, J.; Su, W.; Su, A. Continuous-Flow Hydrogenation of Nitroaromatics in Microreactor with Mesoporous Pd@SBA-15. Processes 2023, 11, 1074. https://doi.org/10.3390/pr11041074
Chai K, Shen R, Qi T, Chen J, Su W, Su A. Continuous-Flow Hydrogenation of Nitroaromatics in Microreactor with Mesoporous Pd@SBA-15. Processes. 2023; 11(4):1074. https://doi.org/10.3390/pr11041074
Chicago/Turabian StyleChai, Kejie, Runqiu Shen, Tingting Qi, Jianli Chen, Weike Su, and An Su. 2023. "Continuous-Flow Hydrogenation of Nitroaromatics in Microreactor with Mesoporous Pd@SBA-15" Processes 11, no. 4: 1074. https://doi.org/10.3390/pr11041074
APA StyleChai, K., Shen, R., Qi, T., Chen, J., Su, W., & Su, A. (2023). Continuous-Flow Hydrogenation of Nitroaromatics in Microreactor with Mesoporous Pd@SBA-15. Processes, 11(4), 1074. https://doi.org/10.3390/pr11041074







