Liquid–Liquid Phase Separation of Two Non-Dissolving Liquids—A Mini Review
Abstract
:1. Introduction
2. Thermodynamic Basis
2.1. Phase Equilibrium and Activity Coefficient
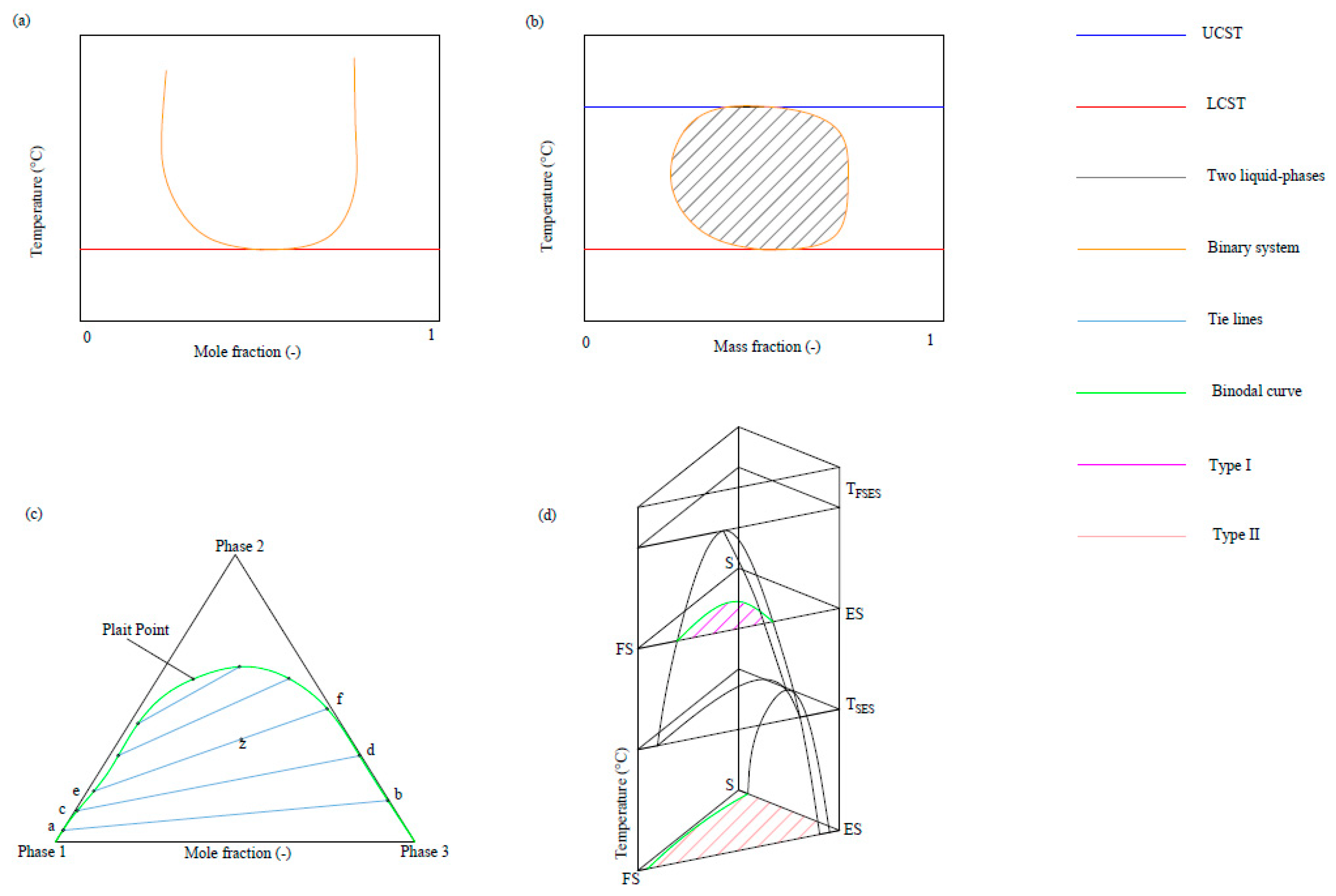
2.2. Phase Diagrams
- type I—one binary pair with restricted miscibility;
- type II—two binary pairs with restricted miscibility.
2.3. Liquid Substance (Thermodynamic) Data
- the spinning drop method (one liquid drop is held in suspension within a tube contacting second liquid and rotating) [113].
2.4. Liquid–Liquid Mixture Fundamentals
Dispersed Phase
2.5. Shake Test
3. Liquid–Liquid Phase Separation Equipment
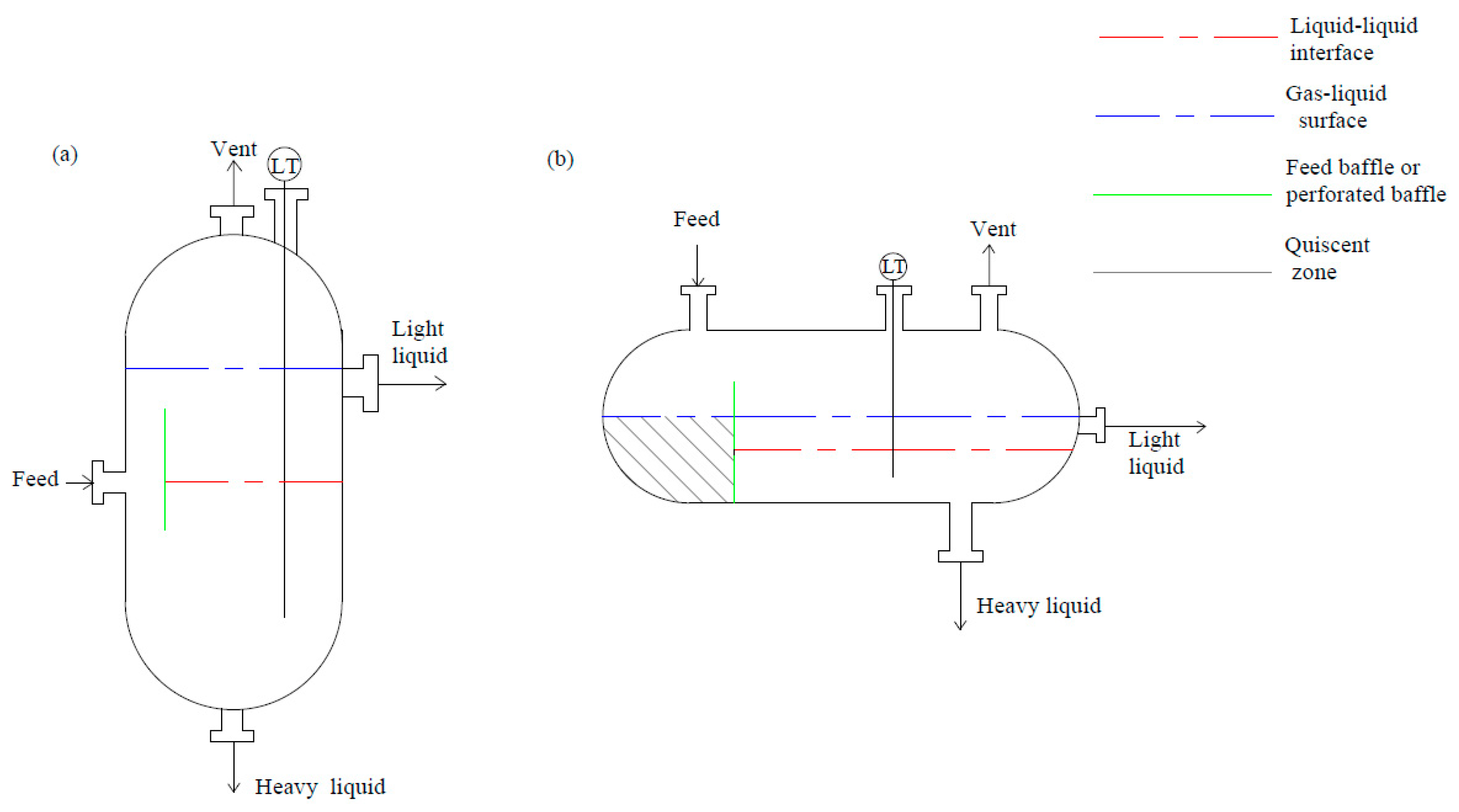
3.1. Gravity Decanters (Settlers)
3.1.1. Stokes’ Law Design Method
3.1.2. Vented Decanters
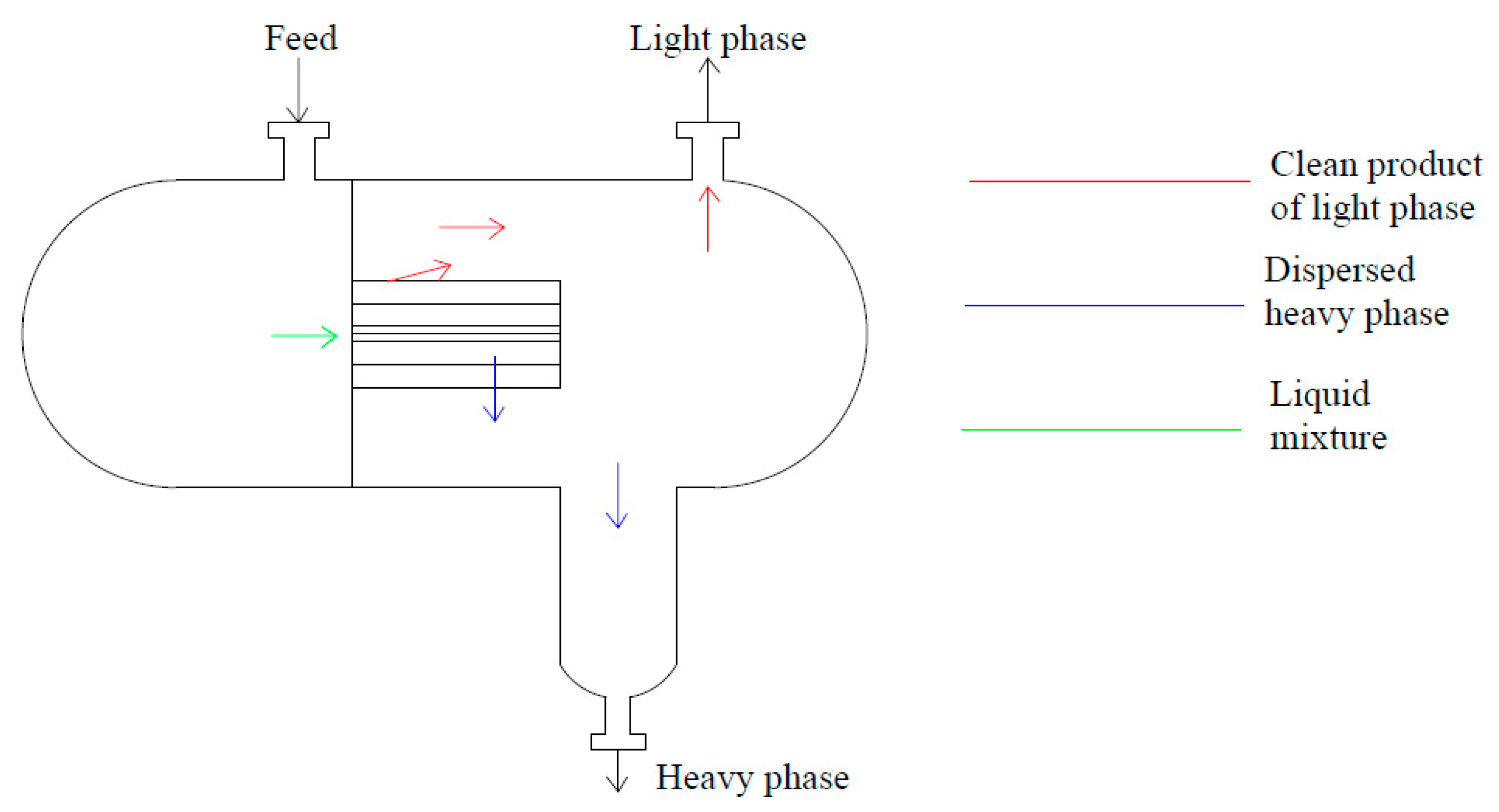
3.1.3. Coalescing Internals
3.2. Coalescers
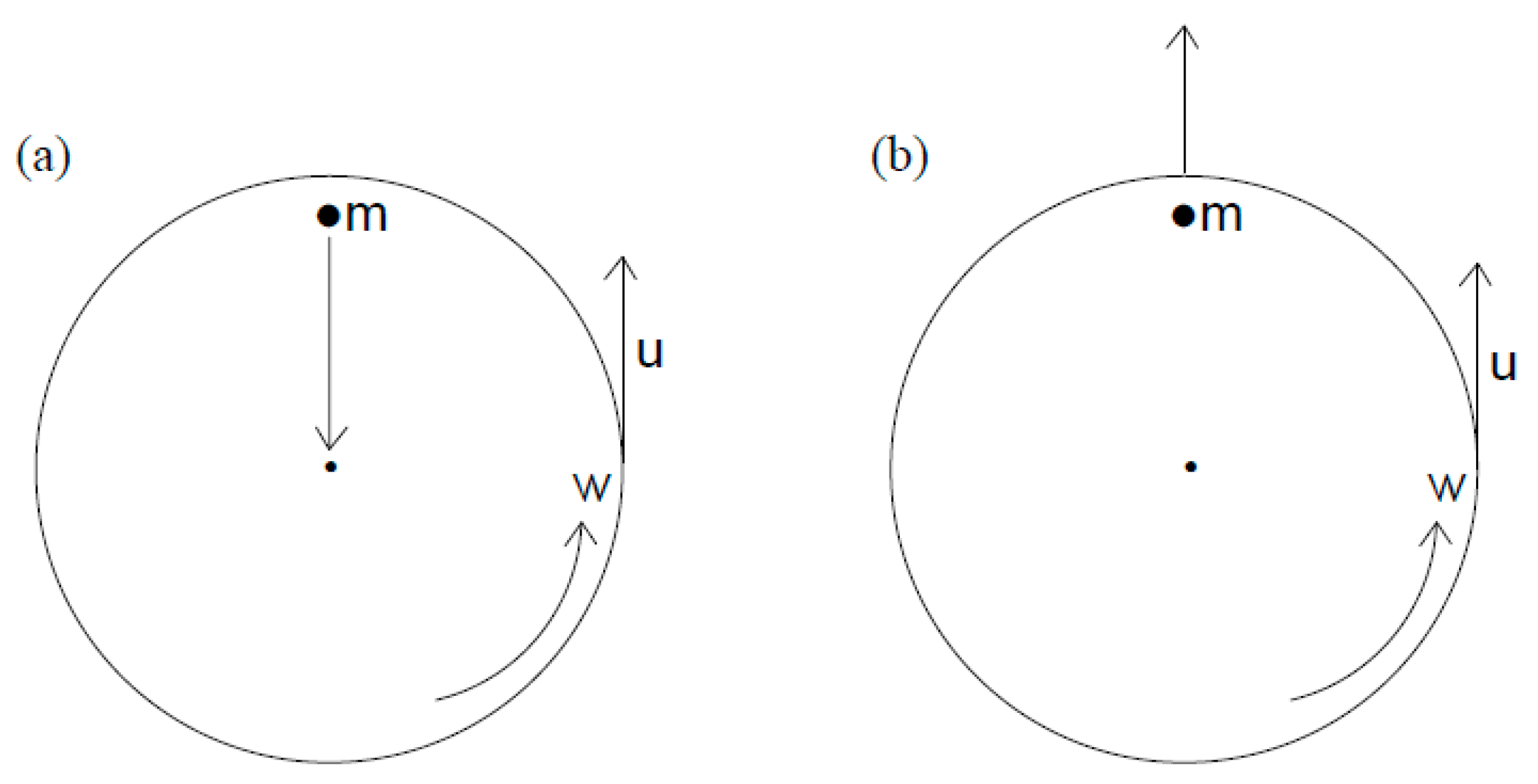
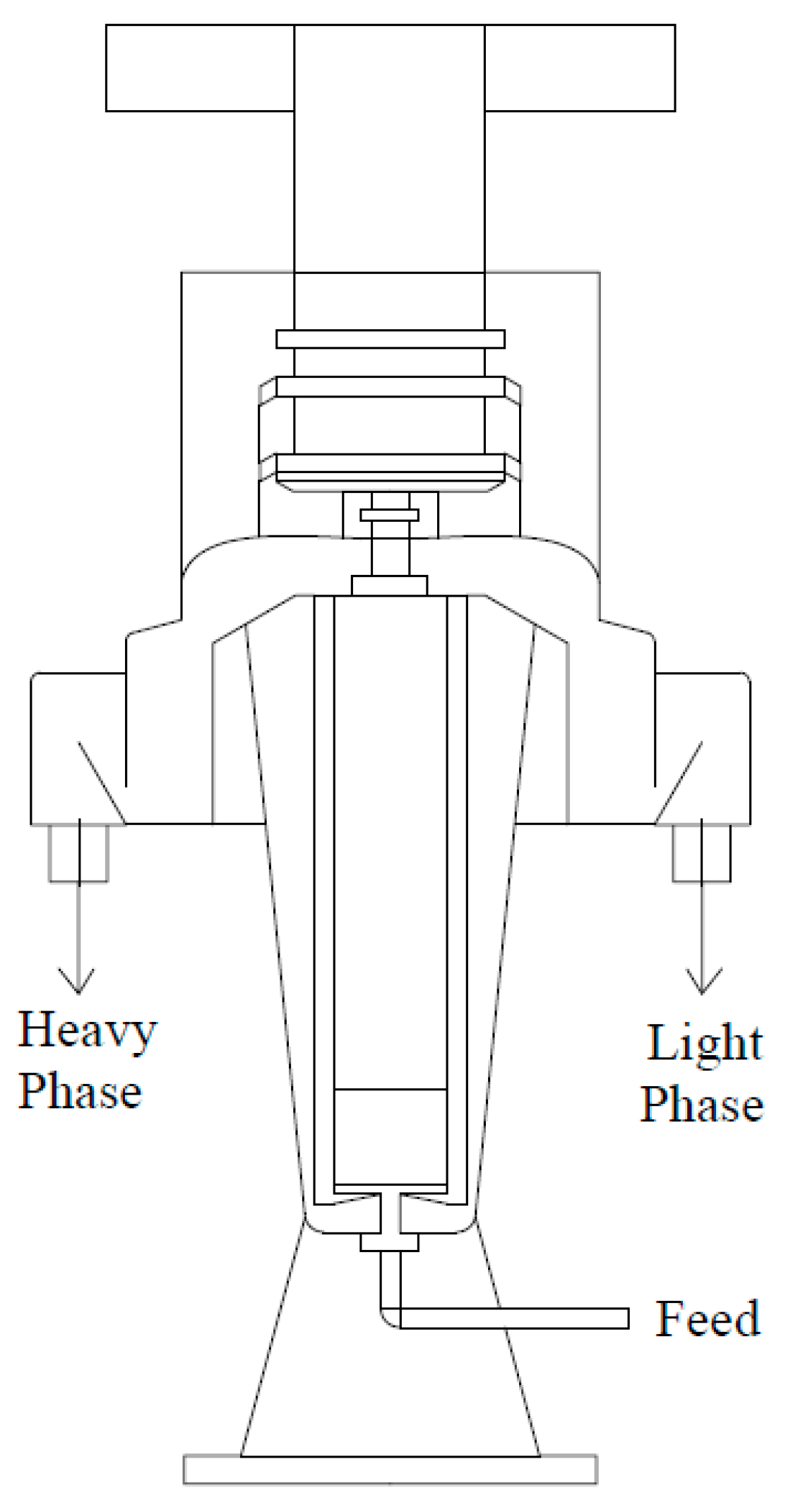
3.3. Centrifugal Separators
- the difference in density between the phases is small;
- immediate contact is needed to prevent degradation;
- feed and solvent emulsify easily;
- specific required throughput due to capacity restrictions.
- maintaining high-speed rotating machinery—even though the high acceleration in operation makes a good performance, in some cases, this can promote back-mixing or emulsification;
- presence of solids—some of these devices are designed to handle feeds containing solids, such as whole fermentation broth. However, if solids are present in the feed, there is a potential for plugging, e.g., in separators with close internal clearances.
3.3.1. Centrifugal Force
3.3.2. Centrifuges
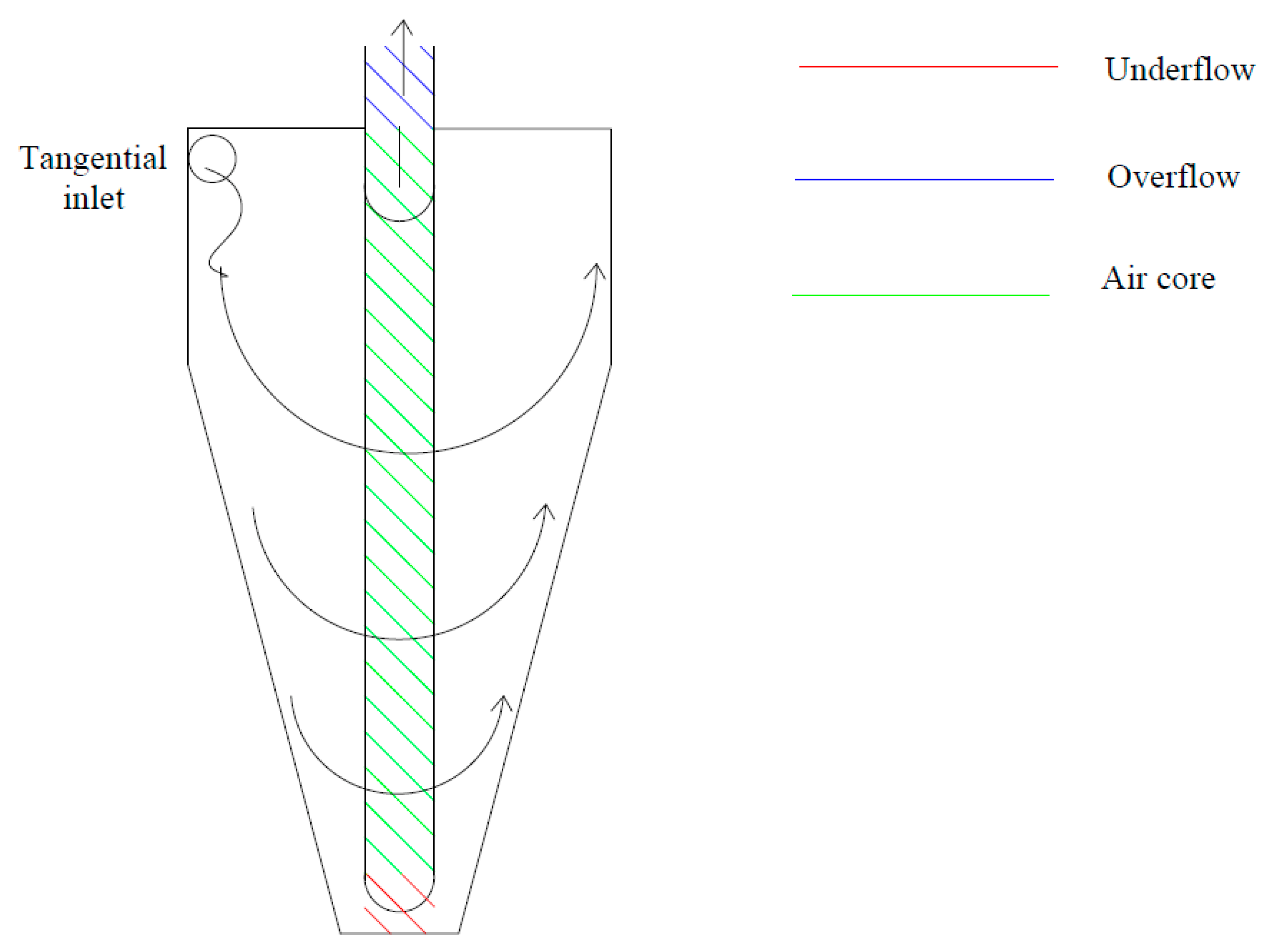
3.3.3. Hydrocyclones
3.4. Other Devices
3.4.1. Ultrafiltration Membranes
3.4.2. Electrostatic Coalescers
4. Recycling of Phase-Forming Components
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Diuzheva, A.; Balogh, J.; Jekő, J.; Cziáky, Z. Application of liquid-liquid microextraction for the effective separation and simultaneous determination of 11 pharmaceuticals in wastewater samples using high-performance liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2018, 41, 2870–2877. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, K.; Li, H.; Lian, S.; Han, C.; Zhu, Z.; Lu, Y.; Qi, J.; Wang, Y. Mechanism analysis and liquid-liquid equilibrium of methyl tert-butyl ether separation from petroleum wastewater azeotrope by green mixed solvent. J. Environ. Chem. Eng. 2023, 11, 109389. [Google Scholar] [CrossRef]
- Jiao, T.; Zhuang, X.; He, H.; Li, C.; Chen, H.; Zhang, S. Separation of Phenolic Compounds from Coal Tar via Liquid–Liquid Extraction Using Amide Compounds. Ind. Eng. Chem. Res. 2015, 54, 2573–2579. [Google Scholar] [CrossRef]
- Jiao, T.; Li, C.; Zhuang, X.; Cao, S.; Chen, H.; Zhang, S. The new liquid–liquid extraction method for separation of phenolic compounds from coal tar. Chem. Eng. J. 2015, 266, 148–155. [Google Scholar] [CrossRef]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent extraction: The coordination chemistry behind extractive metallurgy. Chem. Soc. Rev. 2014, 43, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, Y.-S.; Niewersch, C.; Lenz, M.; Kül, Z.Z.; Corvini, P.F.-X.; Schäffer, A.; Wintgens, T. Recycling of indium from CIGS photovoltaic cells: Potential of combining acid-resistant nanofiltration with liquid-liquid extraction. Environ. Sci. Technol. 2014, 48, 13412–13418. [Google Scholar] [CrossRef] [PubMed]
- Wongsawa, T.; Traiwongsa, N.; Pancharoen, U.; Nootong, K. A review of the recovery of precious metals using ionic liquid extractants in hydrometallurgical processes. Hydrometallurgy 2020, 198, 105488. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Olea, F.; Sepúlveda, R.; Castillo, J.; Cabezas, R.; Merlet, G.; Romero, J. Possibilities and challenges for ionic liquids in hydrometallurgy. Sep. Purif. Technol. 2020, 251, 117289. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, F.; Su, Z.; Liu, S.; Anderson, C.; Jiang, T. Hydrometallurgical Recovery of Rare Earth Elements from NdFeB Permanent Magnet Scrap: A Review. Metals 2020, 10, 841. [Google Scholar] [CrossRef]
- Wommer, L.; Meiers, P.; Kockler, I.; Ulber, R.; Kampeis, P. Development of a 3D-printed single-use separation chamber for use in mRNA-based vaccine production with magnetic microparticles. Eng. Life Sci. 2021, 21, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.; Healey, R.; Adly, F.G. Current trends in separation of plasmid DNA vaccines: A review. Anal. Chim. Acta 2013, 760, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Naseri, M.T.; Babri, M. Quantification of candidate prostate cancer metabolite biomarkers in urine using dispersive derivatization liquid-liquid microextraction followed by gas and liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2013, 81–82, 65–75. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ebrahim, H.; Mohyeldin, M.; Qusa, M.; Batarseh, Y.; Fayyad, A.; Tajmim, A.; Nazzal, S.; Kaddoumi, A.; El Sayed, K. Novel liquid-liquid extraction and self-emulsion methods for simplified isolation of extra-virgin olive oil phenolics with emphasis on (-)-oleocanthal and its oral anti-breast cancer activity. PLoS ONE 2019, 14, e0214798. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, T.I.A.; Silva, A.M.T.; Ribeiro, A.R.; Alves, A.; Santos, M.S.F. Liquid-liquid extraction as a simple tool to quickly quantify fourteen cytostatics in urban wastewaters and access their impact in aquatic biota. Sci. Total Environ. 2020, 740, 139995. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Cao, Y.; Liu, N.; Feng, L.; Jiang, L. Special wettable materials for oil/water separation. J. Mater. Chem. A 2014, 2, 2445–2460. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/water separation with selective superantiwetting/superwetting surface materials. Angew. Chem. Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Buarque, F.S.; Barreto, V.S.; Soares, C.M.F.; Souza, R.L.; Pereira, M.M.; Lima, Á.S. Selective extraction of female hormones using aqueous two-phase system composed of double protic ionic liquid + acetonitrile. Fluid Phase Equilibria 2020, 508, 112443. [Google Scholar] [CrossRef]
- Albertsson, P.A. Partition of cell particles and macromolecules in polymer two-phase systems. Adv. Protein Chem. 1970, 24, 309–341. [Google Scholar] [CrossRef]
- Freire, M.G. Ionic-Liquid-Based Aqueous Biphasic Systems: Fundamentals and Applications; Freire, M.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 3662528738. [Google Scholar]
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Canongia Lopes, J.N.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Belwal, T.; Cravotto, G.; Luo, Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrason. Sonochemistry 2020, 60, 104726. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, W.; Zhao, S.; Yang, X.; Xu, W.; Guo, M.; Xu, E.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted extraction of lipids as food components: Mechanism, solvent, feedstock, quality evaluation and coupled technologies—A review. Trends Food Sci. Technol. 2022, 122, 83–96. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Castillo-Peinado, L.S. 3—Microwave-Assisted Extraction of Food Components. In Innovative Food Processing Technologies: Extraction, Separation, Component Modification and Process Intensification; Knoerzer, K., Juliano, P., Smithers, G.W., Eds.; Woodhead Publishing: Duxford, UK, 2016; pp. 57–110. ISBN 978-0-08-100294-0. [Google Scholar]
- Antony, F.M.; Pal, D.; Wasewar, K. Separation of bio-products by liquid–liquid extraction. Phys. Sci. Rev. 2021, 6, 20180065. [Google Scholar] [CrossRef]
- Aerstin, F.; Street, G. Decanters. In Applied Chemical Process Design; Springer: Boston, MA, USA, 1978; pp. 35–38. [Google Scholar] [CrossRef]
- Sinnott, R.K. Coulson & Richardson’s Chemical Engineering: Fourth Edition Coulson & Richardson’s Chemical Engineering Series; Pergamon Press: Oxford, UK; New York, NY, USA, 1993; ISBN 9781483294704. [Google Scholar]
- Bai, C.; Park, H.; Wang, L. Modelling solid-liquid separation and particle size classification in decanter centrifuges. Sep. Purif. Technol. 2021, 263, 118408. [Google Scholar] [CrossRef]
- Hazlett, R.N. Fibrous Bed Coalescence of Water. Steps in the Coalescence Process. Ind. Eng. Chem. Fundam. 1969, 8, 625–632. [Google Scholar] [CrossRef]
- Madia, J.R.; Fruh, S.M.; Miller, C.A.; Beerbower, A. Granular packed bed coalescer: Influence of packing wettability on coalescence. Environ. Sci. Technol. 1976, 10, 1044–1046. [Google Scholar] [CrossRef]
- Sokolović, D.; Laminger, T.; Mauschitz, G.; Höflinger, W. Novel coalescer design with bed of waste polymer fibers for liquid aerosol separation. Sep. Purif. Technol. 2021, 263, 118187. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Pan, Z. Experimental Study on the Performance of a Novel Compact Electrostatic Coalescer with Helical Electrodes. Energies 2021, 14, 1733. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P. Handbook of Chemical Processing Equipment: Chapter 6. Mechanical Separation Equipment. Elsevier Butterworth-Heinemann: Woburn, MA, USA, 2000; ISBN 0-7506-7126-2. [Google Scholar]
- Herivel, J.W. Newton’s Discovery of the Law of Centrifugal Force. Isis 1960, 51, 546–553. [Google Scholar] [CrossRef]
- Leung, W.W.-F. Centrifugal Separations in Biotechnology, 2nd ed.; Elsevier Butterworth-Heinemann: San Diego, CA, USA, 2020; ISBN 9780081026359. [Google Scholar]
- Bradley, D. The Hydrocyclone, 1st ed.; Pergamon Press: Oxford, UK; New York, NY, USA, 1965; ISBN 9781483155708. [Google Scholar]
- Svarovsky, L.; Thew, M.T. Hydrocyclones: Analysis and Applications. In Proceedings of the 4th International Conference: Papers, Southampton, UK, 23–25 September 1992; ISBN 0792318765. [Google Scholar]
- Sheng, H.P. Separation of Liquids in a Conventional Hydrocyclone. Sep. Purif. Methods 1977, 6, 89–127. [Google Scholar] [CrossRef]
- Ji, L.; Paul, P.; Shanbhag, B.K.; Dixon, I.; Kuang, S.; He, L. Emerging application of hydrocyclone in biotechnology and food processing. Sep. Purif. Technol. 2023, 309, 122992. [Google Scholar] [CrossRef]
- He, L.; Ji, L.; Sun, X.; Chen, S.; Kuang, S. Investigation of mini-hydrocyclone performance in removing small-size microplastics. Particuology 2022, 71, 1–10. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, Q.; Cui, B.; Wei, D.; Song, Z.; Feng, Y. Geometrical configuration of hydrocyclone for improving the separation performance. Adv. Powder Technol. 2022, 33, 103419. [Google Scholar] [CrossRef]
- Ross, W.R.; Barnard, J.P.; Le Roux, J.; de Villiers, H.A. Application of ultrafiltration membranes for solids liquid separation in anaerobic digestion systems: The ADUF process. Water SA 1990, 16, 85–91. [Google Scholar]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef]
- Munirasu, S.; Haija, M.A.; Banat, F. Use of membrane technology for oil field and refinery produced water treatment—A review. Process Saf. Environ. Prot. 2016, 100, 183–202. [Google Scholar] [CrossRef]
- Moslehyani, A.; Mobaraki, M.; Ismail, A.F.; Othman, M.H.D.; Mayahi, A.; Shamsaei, E.; Abdullah, M.S.; Razis, M. PVDF membrane for oil-in-water separation via cross-flow ultrafiltration process. J. Teknol. 2015, 78, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Mercelat, A.Y.J. Fundamental Study of Hydrophobic Microporous Membrane Contactors for the Recovery of Insoluble Oil from Oil-Water Mixtures. Ph.D. Dissertation, The University of Texas at Austin, Austin, TX, USA, 2016. [Google Scholar]
- Eow, J.S.; Ghadiri, M.; Sharif, A.O.; Williams, T.J. Electrostatic enhancement of coalescence of water droplets in oil a review of current understaning. Chem. Eng. J. 2001, 84, 173–192. [Google Scholar] [CrossRef]
- Scott, T.C.; Wham, R.M. An electrically driven multistage countercurrent solvent extraction device: The emulsion-phase contactor. Ind. Eng. Chem. Res. 1989, 28, 94–97. [Google Scholar] [CrossRef]
- Xiang, H.; Kadet, V.V.; Evtyuhin, A.V.; Vasneva, G.I. Scientific Base and the Results of Electrotreatment Technology for EOR. In Proceedings of the International Field Exploration and Development Conference, Chengdu, China, 21–22 September 2017; Springer: Singapore, 2019; pp. 908–920. [Google Scholar]
- Singh, V.K.; Qureshi, D.; Nayak, S.K.; Pal, K. 10-Bigels. In Polymeric Gels: Characterization, Properties and Biomedical Applications; Pal, K., Banerjee, I., Eds.; Woodhead Publishing: Oxford, UK, 2018; pp. 265–282. ISBN 978-0-08-102179-8. [Google Scholar]
- Kooij, S.; Sijs, R.; Denn, M.M.; Villermaux, E.; Bonn, D. What Determines the Drop Size in Sprays? Phys. Rev. X 2018, 8, 31019. [Google Scholar] [CrossRef] [Green Version]
- Rossi, C.C.R.S.; Cardozo-Filho, L.; Guirardello, R. Gibbs free energy minimization for the calculation of chemical and phase equilibrium using linear programming. Fluid Phase Equilibria 2009, 278, 117–128. [Google Scholar] [CrossRef]
- Luo, Q.; Guo, Y.; Liu, B.; Feng, Y.; Zhang, J.; Li, Q.; Chou, K. Thermodynamics and kinetics of phase transformation in rare earth–magnesium alloys: A critical review. J. Mater. Sci. Technol. 2020, 44, 171–190. [Google Scholar] [CrossRef]
- André, A.A.M.; Spruijt, E. Liquid-Liquid Phase Separation in Crowded Environments. Int. J. Mol. Sci. 2020, 21, 5908. [Google Scholar] [CrossRef]
- Heidemann, R.A.; Mandhane, J.M. Ternary liquid—Liquid equilibria: The van laar equation. Chem. Eng. Sci. 1975, 30, 425–434. [Google Scholar] [CrossRef]
- Zhang, X.; You, J.B.; Arends, G.F.; Qian, J.; Chen, Y.; Lohse, D.; Shaw, J.M. Propelling microdroplets generated and sustained by liquid-liquid phase separation in confined spaces. Soft Matter 2021, 17, 5362–5374. [Google Scholar] [CrossRef] [PubMed]
- Zeegers-Huyskens, T.; Huyskens, P. Intermolecular Forces; Springer: Berlin, Heidelberg, 1991. [Google Scholar]
- Chatzigiannakis, E.; Jaensson, N.; Vermant, J. Thin liquid films: Where hydrodynamics, capillarity, surface stresses and intermolecular forces meet. Curr. Opin. Colloid Interface Sci. 2021, 53, 101441. [Google Scholar] [CrossRef]
- Cinar, H.; Fetahaj, Z.; Cinar, S.; Vernon, R.M.; Chan, H.S.; Winter, R.H.A. Temperature, Hydrostatic Pressure, and Osmolyte Effects on Liquid-Liquid Phase Separation in Protein Condensates: Physical Chemistry and Biological Implications. Chem. A Eur. J. 2019, 25, 13049–13069. [Google Scholar] [CrossRef]
- Dignon, G.L.; Zheng, W.; Kim, Y.C.; Mittal, J. Temperature-Controlled Liquid-Liquid Phase Separation of Disordered Proteins. ACS Cent. Sci. 2019, 5, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Dignon, G.L.; Best, R.B.; Mittal, J. Biomolecular Phase Separation: From Molecular Driving Forces to Macroscopic Properties. Annu. Rev. Phys. Chem. 2020, 71, 53–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J.; Jiang, C.; Chen, H.; Shen, Y.; Zhang, S.; Wang, L.; Chen, J. Novel Biphasic Solvent with Tunable Phase Separation for CO2 Capture: Role of Water Content in Mechanism, Kinetics, and Energy Penalty. Environ. Sci. Technol. 2019, 53, 4470–4479. [Google Scholar] [CrossRef] [PubMed]
- Babinchak, W.M.; Surewicz, W.K. Liquid-Liquid Phase Separation and Its Mechanistic Role in Pathological Protein Aggregation. J. Mol. Biol. 2020, 432, 1910–1925. [Google Scholar] [CrossRef]
- Searle, M.S.; Westwell, M.S.; Williams, D.H. Application of a generalised enthalpy–entropy relationship to binding co-operativity and weak associations in solution. J. Chem. Soc. Perkin Trans. 2 1995, 141–151. [Google Scholar] [CrossRef]
- Pezzotti, S.; König, B.; Ramos, S.; Schwaab, G.; Havenith, M. Liquid-Liquid Phase Separation? Ask the Water! J. Phys. Chem. Lett. 2023, 14, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Amigó, J.M.; Balogh, S.G.; Hernández, S. A Brief Review of Generalized Entropies. Entropy 2018, 20, 813. [Google Scholar] [CrossRef] [Green Version]
- Derimow, N.; Abbaschian, R. Liquid Phase Separation in High-Entropy Alloys-A Review. Entropy 2018, 20, 890. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Miquel, M.; Massel, M.; DeSilva, A.; Palomar, J.; Rodriguez, F.; Brennecke, J.F. Excess enthalpy of monoethanolamine + ionic liquid mixtures: How good are COSMO-RS predictions? J. Phys. Chem. B 2014, 118, 11512–11522. [Google Scholar] [CrossRef]
- Zobel, N.; Anca-Couce, A. Influence of intraparticle secondary heterogeneous reactions on the reaction enthalpy of wood pyrolysis. J. Anal. Appl. Pyrolysis 2015, 116, 281–286. [Google Scholar] [CrossRef]
- Campbell, J.A. Le Chatelier’s Principle, Temperature Effects, and Entropy. J. Chem. Educ. 1985, 62, 231–232. [Google Scholar] [CrossRef]
- Thomas, J.M. The existence of endothermic adsorption. J. Chem. Educ. 1961, 38, 138. [Google Scholar] [CrossRef]
- Chiban, M.; Carja, G.; Lehutu, G.; Sinan, F. Equilibrium and thermodynamic studies for the removal of As(V) ions from aqueous solution using dried plants as adsorbents. Arab. J. Chem. 2016, 9, S988–S999. [Google Scholar] [CrossRef] [Green Version]
- Green, D.W.; Southard, M.Z. (Eds.) Perry’s Chemical Engineers’ Handbook, 9th ed.; 85th anniversary edition; McGraw Hill Education: New York, NY, USA, 2019; ISBN 9780071834094. [Google Scholar]
- Mousavi, N.S.; Romero-Martínez, A.; Ramírez-Verduzco, L.F. Predicting the surface tension of mixtures of fatty acid ethyl esters and biodiesel fuels using UNIFAC activity coefficients. Fluid Phase Equilibria 2020, 507, 112430. [Google Scholar] [CrossRef]
- Wang, D.; Ji, C.; Wang, S.; Yang, J.; Wang, Z. Numerical study of the premixed ammonia-hydrogen combustion under engine-relevant conditions. Int. J. Hydrog. Energy 2021, 46, 2667–2683. [Google Scholar] [CrossRef]
- Gillespie, D.; Giri, J.; Fill, M. Reinterpreting the anomalous mole fraction effect: The ryanodine receptor case study. Biophys. J. 2009, 97, 2212–2221. [Google Scholar] [CrossRef] [Green Version]
- Voutsas, E.C.; Tassios, D.P. Prediction of Infinite-Dilution Activity Coefficients in binary Mixtures with UNIFAC. A Critical Evaluation. Ind. Eng. Chem. Res. 1996, 35, 1438–1445. [Google Scholar] [CrossRef]
- Kollau, L.J.B.M.; Vis, M.; van den Bruinhorst, A.; de With, G.; Tuinier, R. Activity modelling of the solid–liquid equilibrium of deep eutectic solvents. Pure Appl. Chem. 2019, 91, 1341–1349. [Google Scholar] [CrossRef]
- Wilson, G.M. Vapor-Liquid Equilibrium. XI. A New Expression for the Excess Free Energy of Mixing. J. Am. Chem. Soc. 1964, 86, 127–130. [Google Scholar] [CrossRef]
- Renon, H.; Prausnitz, J.M. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]
- Abrams, D.S.; Prausnitz, J.M. Statistical thermodynamics of liquid mixtures: A new expression for the excess Gibbs energy of partly or completely miscible systems. AIChE J. 1975, 21, 116–128. [Google Scholar] [CrossRef]
- Fredenslund, A.; Jones, R.L.; Prausnitz, J.M. Group-contribution estimation of activity coefficients in nonideal liquid mixtures. AIChE J. 1975, 21, 1086–1099. [Google Scholar] [CrossRef]
- Larsen, B.L.; Rasmussen, P.; Fredenslund, A. A modified UNIFAC group-contribution model for prediction of phase equilibria and heats of mixing. Ind. Eng. Chem. Res. 1987, 26, 2274–2286. [Google Scholar] [CrossRef]
- Weidlich, U.; Gmehling, J. A modified UNIFAC model. 1. Prediction of VLE, hE, and.gamma.infin. Ind. Eng. Chem. Res. 1987, 26, 1372–1381. [Google Scholar] [CrossRef]
- Kang, J.W.; Diky, V.; Frenkel, M. New modified UNIFAC parameters using critically evaluated phase equilibrium data. Fluid Phase Equilibria 2015, 388, 128–141. [Google Scholar] [CrossRef]
- Gambini Pereira, C. Thermodynamics of Phase Equilibria in Food Engineering; Academic Press: London, UK, 2019; ISBN 9780128115572. [Google Scholar]
- Zhang, Q.; Dong, S.; Zhang, M.; Huang, F. Supramolecular control over thermo-responsive systems with lower critical solution temperature behavior. Aggregate 2021, 2, 35–47. [Google Scholar] [CrossRef]
- Perdomo, F.A.; Khalit, S.H.; Adjiman, C.S.; Galindo, A.; Jackson, G. Description of the thermodynamic properties and fluid-phase behavior of aqueous solutions of linear, branched, and cyclic amines. AIChE J. 2021, 67, e17194. [Google Scholar] [CrossRef]
- Xu, W.; Gao, X.; Zheng, L.; Lu, F. Ionic-Liquid-Based Aqueous Two-Phase Systems Induced by Intra- and Intermolecular Hydrogen Bonds. Molecules 2022, 27, 5307. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.P.; Donate, F.A.; Frank, T.C.; LaTulip, R.J.; Wilson, L.C. Mutual Solubility and Lower Critical Solution Temperature for Water + Glycol Ether Systems. J. Chem. Eng. Data 2005, 50, 869–877. [Google Scholar] [CrossRef]
- Kuchierskaya, A.A.; Semenov, A.P.; Sayfutdinova, A.R.; Kopitsyn, D.S.; Vinokurov, V.A.; Anisimov, M.A.; Novikov, A.A. Interfacial tension and phase properties of water—Hydrotrope—Oil solutions: Water—2-butoxyethanol—Toluene. J. Mol. Liq. 2021, 344, 117683. [Google Scholar] [CrossRef]
- Steltenpohl, P.; Graczová, E. Application of extended NRTL equation for ternary liquid-liquid and vapor-liquid-liquid equilibria description. Chem. Pap. 2010, 64, 310–317. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s Chemical Engineers’ Handbook: Liquid-Liquid Extraction Operations and Equipment, 7th ed.; McGraw-Hill: New York, NY, USA, 1997; ISBN 0070498415. [Google Scholar]
- Illner, M.; Esche, E.; Repke, J.-U. Optimal Control of Surfactant Containing Multiphase Systems—Challenges and Solution Strategies for a Stable Mini-Plant Operation. In Proceedings of the 13th International Symposium on Process Systems Engineering (PSE 2018), San Diego, CA, USA, 1–5 July 2018; Elsevier: Amsterdam, The Netherlands, 2018; pp. 739–744, ISBN 9780444642417. [Google Scholar]
- Illner, M.; Kozachynskyi, V.; Esche, E.; Repke, J.-U. Fast-track realization of reactive microemulsion systems—Systematic system analysis and tailored application of PSE methods. Chem. Eng. Sci. 2022, 252, 117290. [Google Scholar] [CrossRef]
- Merchuk, J.C.; Andrews, B.A.; Asenjo, J.A. Aqueous two-phase systems for protein separation. Studies on phase inversion. J. Chromatogr. B Biomed. Sci. Appl. 1998, 711, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Filho, M.A.; Caldas, M.C.B.; Vasconcelos, L.T.C.D.P.; Ribeiro, V.T.; de Araújo, J.S.; de Padilha, C.E.; Sousa Junior, F.C.; dos Santos, E.S. Partitioning and recovery of an elongation factor (1-γ) of Leishmania infantum chagasi expressed in E. coli M15 with simultaneous endotoxin removal using aqueous two-phase system. Sep. Sci. Technol. 2020, 55, 1156–1166. [Google Scholar] [CrossRef]
- Silva, D.F.C.; Azevedo, A.M.; Fernandes, P.; Chu, V.; Conde, J.P.; Aires-Barros, M.R. Determination of aqueous two phase system binodal curves using a microfluidic device. J. Chromatogr. A 2014, 1370, 115–120. [Google Scholar] [CrossRef]
- Pedro, M.d.S.; Oliveira, L.A.F.; Padilha, C.E.d.A.; Santos, E.S.d.; de Oliveira, J.A.; Souza, D.F.d.S. Effect of flow patterns on bovine serum albumin and ampicillin partitioning using aqueous two-phase systems in microdevice. Sep. Purif. Technol. 2021, 254, 117592. [Google Scholar] [CrossRef]
- Kurnik, I.S.; Mussagy, C.U.; Pereira, J.F.B.; Lopes, A.M. Amphiphilic copolymer aqueous solutions with cholinium ionic liquids as adjuvants: New insights into determination of binodal curves and phase-separation mechanisms. J. Mol. Liq. 2020, 318, 114245. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Solvents; ChemTec: New York, NY, USA, 2001; ISBN 1895198240. [Google Scholar]
- Flick, E.W. Industrial Solvents Handbook, 5th ed.; Noyes Data Corp: Westwood, NJ, USA, 1998; ISBN 0815514131. [Google Scholar]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. Properties of Gases and Liquids, 5th ed.; McGraw-Hill Education; McGraw Hill: New York, NY, USA, 2020; ISBN 9780071499996. [Google Scholar]
- Leblanc, G.E.; Secco, R.A.; Kostic, M. Viscosity Measurement: Measurement, Instrumentation, and Sensors Handbook; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Pina-Martinez, A.; Privat, R.; Jaubert, J.-N.; Peng, D.-Y. Updated versions of the generalized Soave α-function suitable for the Redlich-Kwong and Peng-Robinson equations of state. Fluid Phase Equilibria 2019, 485, 264–269. [Google Scholar] [CrossRef]
- Arashiro, E.Y.; Demarquette, N.R. Use of the pendant drop method to measure interfacial tension between molten polymers. Mat. Res. 1999, 2, 23–32. [Google Scholar] [CrossRef]
- Berry, J.D.; Neeson, M.J.; Dagastine, R.R.; Chan, D.Y.C.; Tabor, R.F. Measurement of surface and interfacial tension using pendant drop tensiometry. J. Colloid Interface Sci. 2015, 454, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Kingery, W.D.; Humenik, M. Surface Tension at Elevated Temperatures. I. Furnace and Method for Use of the Sessile Drop Method; Surface Tension of Silicon, Iron and Nickel. J. Phys. Chem. 1953, 57, 359–363. [Google Scholar] [CrossRef]
- Staicopolus, D.N. The computation of surface tension and of contact angle by the sessile-drop method. J. Colloid Sci. 1962, 17, 439–447. [Google Scholar] [CrossRef]
- Bachmann, J.; Horton, R.; van der Ploeg, R.R.; Woche, S. Modified sessile drop method for assessing initial soil-water contact angle of sandy soil. Soil Sci. Soc. Am. J. 2000, 64, 564–567. [Google Scholar] [CrossRef]
- Zamora, J.M.; Marquez, R.; Forgiarini, A.M.; Langevin, D.; Salager, J.-L. Interfacial rheology of low interfacial tension systems using a new oscillating spinning drop method. J. Colloid Interface Sci. 2018, 519, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Drelich, J.; Fang, C.; White, C.L. Measurement of Interfacial Tension in Fluid-Fluid Systems. Encycl. Surf. Colloid Sci. 2002, 3, 3158–3163. [Google Scholar]
- Du Noüy, P.L. A new apparatus for measuring surface tension. J. Gen. Physiol. 1919, 1, 521–524. [Google Scholar] [CrossRef] [Green Version]
- Della Volpe, C.; Siboni, S. The Wilhelmy method: A critical and practical review. Surf. Innov. 2018, 6, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Gaonkar, A.G.; Neuman, R.D. The effect of wettability of wilhelmy plate and du Nou¨y ring on interfacial tension measurements in solvent extraction systems. J. Colloid Interface Sci. 1984, 98, 112–119. [Google Scholar] [CrossRef]
- Padday, J.F.; Russell, D.R. The measurement of the surface tension of pure liquids and solutions. J. Colloid Sci. 1960, 15, 503–511. [Google Scholar] [CrossRef]
- Bikerman, J.J. Physical Surfaces; Academic Press: New York, NY, USA, 1970; ISBN 9780323163040. [Google Scholar]
- Seeto, Y.; Puig, J.E.; Scriven, L.E.; Davis, H.T. Interfacial tensions in systems of three liquid phases. J. Colloid Interface Sci. 1983, 96, 360–372. [Google Scholar] [CrossRef]
- Messager, A.; Miracle-Sole, S.; Ruiz, J.; Shlosman, S. Interfaces in the Potts Model II: Antonov’s Rule and Rigidity of the Order Disorder Interface. Commun. Math. Phys. 1991, 140, 275–290. [Google Scholar] [CrossRef]
- Tadros, T.F. Emulsion Formation, Stability, and Rheology. In Emulsion Formation and Stability; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 1–75. [Google Scholar]
- Psillakis, E. Vortex-assisted liquid-liquid microextraction revisited. TrAC Trends Anal. Chem. 2019, 113, 332–339. [Google Scholar] [CrossRef]
- Pacek, A.W.; Man, C.C.; Nienow, A.W. On the Sauter mean diameter and size distributions in turbulent liquid/liquid dispersions in a stirred vessel. Chem. Eng. Sci. 1998, 53, 2005–2011. [Google Scholar] [CrossRef]
- Hernandez-Alvarado, F.; Kalaga, D.V.; Turney, D.; Banerjee, S.; Joshi, J.B.; Kawaji, M. Void fraction, bubble size and interfacial area measurements in co-current downflow bubble column reactor with microbubble dispersion. Chem. Eng. Sci. 2017, 168, 403–413. [Google Scholar] [CrossRef]
- Selker, A.H.; Sleicher, C.A. Factors affecting which phase will disperse when immiscible liquids are stirred together. Can. J. Chem. Eng. 1965, 43, 298–301. [Google Scholar] [CrossRef]
- Rousseau, R.W. Handbook of Separation Process Technology; Wiley: New York, NY, USA; Chichester, UK, 1987; ISBN 9780471895589. [Google Scholar]
- Zhou, H.; Tang, X.; Zhu, Z.; Li, S. 0D Homogeneous Simulation of Droplet Size Evolution in a Turbulent Stirred Tank. Ind. Eng. Chem. Res. 2022, 61, 5632–5641. [Google Scholar] [CrossRef]
- Simmons, M.J.H.; Azzopardi, B.J. Drop size distributions in dispersed liquid-liquid pipe flow. Int. J. Multiph. Flow 2001, 27, 843–859. [Google Scholar] [CrossRef]
- Seibert, A.F.; Fair, J.R. Hydrodynamics and mass transfer in spray and packed liquid-liquid extraction columns. Ind. Eng. Chem. Res. 1988, 27, 470–481. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Drzymala, J. Physical meaning of the Sauter mean diameter of spherical particulate matter. Part. Sci. Technol. 2016, 34, 645–647. [Google Scholar] [CrossRef]
- Khakpay, A.; Abolghasemi, H. The effects of impeller speed and holdup on mean drop size in a mixer settler with spiral-type impeller. Can. J. Chem. Eng. 2010, 88, 329–334. [Google Scholar] [CrossRef]
- Topuz, H.; Wilkinson, W.L. Droplet Size Correlation Modeling In Agitated Immiscible Liquid Systems. Int. J. Sci. Technol. Res. 2017, 6, 368–382. [Google Scholar]
- Hinze, J.O. Fundamentals of the hydrodynamic mechanism of splitting in dispersion processes. AIChE J. 1955, 1, 289–295. [Google Scholar] [CrossRef]
- Komogorov, A. On the breakage of drops in a turbulent flow. Dokl. Akad. Navk. SSSR 1949, 66, 825–828. [Google Scholar]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineers’ Handbook: Centrifugal Extractors, 8th ed.; McGraw-Hill: New York, NY, USA, 2008; ISBN 9780071422949. [Google Scholar]
- Angeli, P.; Hweitt, G.F. Drop size distributions in horizontal oil-water dispersed flows. Chem. Eng. Sci. 2000, 55, 3133–3143. [Google Scholar] [CrossRef]
- Wang, C.Y.; Calabrese, R.V. Drop breakup in turbulent stirred-tank contactors. Part II: Relative influence of viscosity and interfacial tension. AIChE J. 1986, 32, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.A.; DeVincentis, B.; Wutz, J.; Ricci, F. Predicting the diameters of droplets produced in turbulent liquid–liquid dispersion. AIChE J. 2022, 68, e17667. [Google Scholar] [CrossRef]
- White, F.M. Fluid Mechanics, 7th ed.; McGraw-Hill Education: New York, NY, USA, 2011; ISBN 9780073529349. [Google Scholar]
- Komar, P.D.; Reimers, C.E. Grain Shape Effects on Settling Rates. J. Geol. 1978, 86, 193–209. [Google Scholar] [CrossRef]
- Jeelani, S.A.K.; Hartland, S. Prediction of steady state dispersion height from batch settling data. AIChE J. 1985, 31, 711–720. [Google Scholar] [CrossRef]
- Meon, W.; Rommel, W.; Blass, E. Plate separators for dispersed liquid-liquid systems: Hydrodynamic coalescence model. Chem. Eng. Sci. 1993, 48, 159–168. [Google Scholar] [CrossRef]
- Meon, W.; Blass, E. Drop Coalescence on Inclined Plates in Liquids. Chem. Eng. Technol. 1991, 14, 11–19. [Google Scholar] [CrossRef]
- Li, J.; Gu, Y. Coalescence of oil-in-water emulsions in fibrous and granular beds. Sep. Purif. Technol. 2005, 42, 1–13. [Google Scholar] [CrossRef]
- Shin, C.; Chase, G.G. Water-in-oil coalescence in micro-nanofiber composite filters. AIChE J. 2004, 50, 343–350. [Google Scholar] [CrossRef]
- Ergun, S. Fluid Flow Through Packed Columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Koekemoer, A.; Luckos, A. Effect of material type and particle size distribution on pressure drop in packed beds of large particles: Extending the Ergun equation. Fuel 2015, 158, 232–238. [Google Scholar] [CrossRef]
- Bretney, E. Water Purifier. US Patent 453,105, 26 May 1891. [Google Scholar]
- Divakar, S.; Padaki, M.; Balakrishna, R.G. Review on Liquid-Liquid Separation by Membrane Filtration. ACS Omega 2022, 7, 44495–44506. [Google Scholar] [CrossRef]
- Kyotani, T.; Richter, H. Zeolite Membrane: From Microstructure to Separation Performance. Membranes 2022, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Abe, C.; Ikeda, A. Pervaporative Dehydration of Organic Solvents Using High-Silica CHA-Type Zeolite Membrane. Membranes 2021, 11, 229. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Matsuura, W.; Abe, C.; Ikeda, A. Influence of Organic Solvent Species on Dehydration Behaviors of NaA-Type Zeolite Membrane. Membranes 2021, 11, 347. [Google Scholar] [CrossRef]
- Mhatre, S.; Vivacqua, V.; Ghadiri, M.; Abdullah, A.M.; Al-Marri, M.J.; Hassanpour, A.; Hewakandamby, B.; Azzopardi, B.; Kermani, B. Electrostatic phase separation: A review. Chem. Eng. Res. Des. 2015, 96, 177–195. [Google Scholar] [CrossRef]
- Alexander, M. Biodegradation and Biomediation, 2nd ed.; Academic Press: San Diego, CA, USA, 1999; ISBN 9780120498611. [Google Scholar]
- Cláudio, A.F.M.; Marques, C.F.C.; Boal-Palheiros, I.; Freire, M.G.; Coutinho, J.A.P. Development of back-extraction and recyclability routes for ionic-liquid-based aqueous two-phase systems. Green Chem 2014, 16, 259–268. [Google Scholar] [CrossRef]
- Mai, N.L.; Ahn, K.; Koo, Y.-M. Methods for recovery of ionic liquids—A review. Process Biochem. 2014, 49, 872–881. [Google Scholar] [CrossRef]
- Rodríguez-Llorente, D.; Cañada-Barcala, A.; Álvarez-Torrellas, S.; Águeda, V.I.; García, J.; Larriba, M. A Review of the Use of Eutectic Solvents, Terpenes and Terpenoids in Liquid–liquid Extraction Processes. Processes 2020, 8, 1220. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Env. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Verdía Barbará, P.; Abouelela Rafat, A.; Hallett, J.P.; Brandt-Talbot, A. Purifying cellulose from major waste streams using ionic liquids and deep eutectic solvents. Curr. Opin. Green Sustain. Chem. 2023, 41, 100783. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Kumar, S.; Bahadur, I.; Singh, T.; Varma, R.S. Deep eutectic solvents as reusable catalysts and promoter for the greener syntheses of small molecules: Recent advances. J. Mol. Liq. 2023, 371, 121013. [Google Scholar] [CrossRef]
- Lee, K.M.; Quek, J.D.; Tey, W.Y.; Lim, S.; Kang, H.-S.; Quen, L.K.; Mahmood, W.A.W.; Jamaludin, S.I.S.; Teng, K.H.; Khoo, K.S. Biomass valorization by integrating ultrasonication and deep eutectic solvents: Delignification, cellulose digestibility and solvent reuse. Biochem. Eng. J. 2022, 187, 108587. [Google Scholar] [CrossRef]
- Hanada, T.; Goto, M. Cathode recycling of lithium-ion batteries based on reusable hydrophobic eutectic solvents. Green Chem 2022, 24, 5107–5115. [Google Scholar] [CrossRef]
- Panić, M.; Andlar, M.; Tišma, M.; Rezić, T.; Šibalić, D.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Natural deep eutectic solvent as a unique solvent for valorisation of orange peel waste by the integrated biorefinery approach. Waste Manag. 2021, 120, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, R.; Ludwig, M.; Webber, G.B.; Atkin, R.; Page, A.J. Nanostructure, hydrogen bonding and rheology in choline chloride deep eutectic solvents as a function of the hydrogen bond donor. Phys. Chem. Chem. Phys. 2017, 19, 3297–3306. [Google Scholar] [CrossRef]
- Del Mar Contreras-Gámez, M.; Galán-Martín, Á.; Seixas, N.; da Costa Lopes, A.M.; Silvestre, A.; Castro, E. Deep eutectic solvents for improved biomass pretreatment: Current status and future prospective towards sustainable processes. Bioresour. Technol. 2023, 369, 128396. [Google Scholar] [CrossRef] [PubMed]
- Rout, A.; Wellens, S.; Binnemans, K. Separation of rare earths and nickel by solvent extraction with two mutually immiscible ionic liquids. RSC Adv. 2014, 4, 5753. [Google Scholar] [CrossRef] [Green Version]
- Riaño, S.; Binnemans, K. Extraction and separation of neodymium and dysprosium from used NdFeB magnets: An application of ionic liquids in solvent extraction towards the recycling of magnets. Green Chem. 2015, 17, 2931–2942. [Google Scholar] [CrossRef]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological Evaluations of Rare Earths and Their Health Impacts to Workers: A Literature Review. Saf. Health Work 2013, 4, 12–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Liang, T. Accumulation and fractionation of rare earth elements in atmospheric particulates around a mine tailing in Baotou, China. Atmos. Environ. 2014, 88, 23–29. [Google Scholar] [CrossRef]


| Type | Shake Test Observations | Interfacial Tension * | Density Difference * | Viscosity of Each Phase * | Presence of Fine Solids or Surfactants * |
|---|---|---|---|---|---|
| I | Dispersion band collapses within 5 min with crystal-clear liquids on top and bottom | Moderate to high, 10−6 N/m or higher | Δρ > 100 kg/m³ | µ < 0.005 Ns/m2 | Negligible |
| II | Dispersion band collapses within 10 to 20 min with clear liquids on top and bottom | Moderate, ~10−6 N/m | Δρ > 100 kg/m³ | µ < 0.02 Ns/m2 | Negligible |
| III | Dispersion band collapses within 20 min but one or more phases remain cloudy | Low to moderate, (3–10)·10−7 N/m | Δρ > 50 kg/m³ | µ < 0.1 Ns/m2 | Might be present in low concentration |
| IVa | Stable dispersion is formed (dispersion band does not collapse within an hour or longer)—high viscosity | Low to high | Δρ > 100 kg/m³ | µ > 0.1 Ns/m2 in one of the phases | Negligible |
| IVb | Stable dispersion is formed—low interfacial tension | <3·10−7 N/m | Δρ > 100 kg/m³ | µ < 0.1 Ns/m2 | Negligible |
| IVc | Stable dispersion is formed—low density difference | Low to high | Δρ < 50 kg/m³ | µ < 0.1 Ns/m2 | Negligible |
| IVd | Stable dispersion is formed—stabilized by surface-active components or solids | Low | Δρ > 100 kg/m³ | µ < 0.1 Ns/m2 | Enough surfactant/solids to keep emulsion stable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrijević, D.; Bösenhofer, M.; Harasek, M. Liquid–Liquid Phase Separation of Two Non-Dissolving Liquids—A Mini Review. Processes 2023, 11, 1145. https://doi.org/10.3390/pr11041145
Dimitrijević D, Bösenhofer M, Harasek M. Liquid–Liquid Phase Separation of Two Non-Dissolving Liquids—A Mini Review. Processes. 2023; 11(4):1145. https://doi.org/10.3390/pr11041145
Chicago/Turabian StyleDimitrijević, Dragana, Markus Bösenhofer, and Michael Harasek. 2023. "Liquid–Liquid Phase Separation of Two Non-Dissolving Liquids—A Mini Review" Processes 11, no. 4: 1145. https://doi.org/10.3390/pr11041145
APA StyleDimitrijević, D., Bösenhofer, M., & Harasek, M. (2023). Liquid–Liquid Phase Separation of Two Non-Dissolving Liquids—A Mini Review. Processes, 11(4), 1145. https://doi.org/10.3390/pr11041145








