Mathematical Modeling of Microbial Electrolysis Cells for Enhanced Urban Wastewater Treatment and Hydrogen Generation
Abstract
1. Introduction
2. Materials and Methods
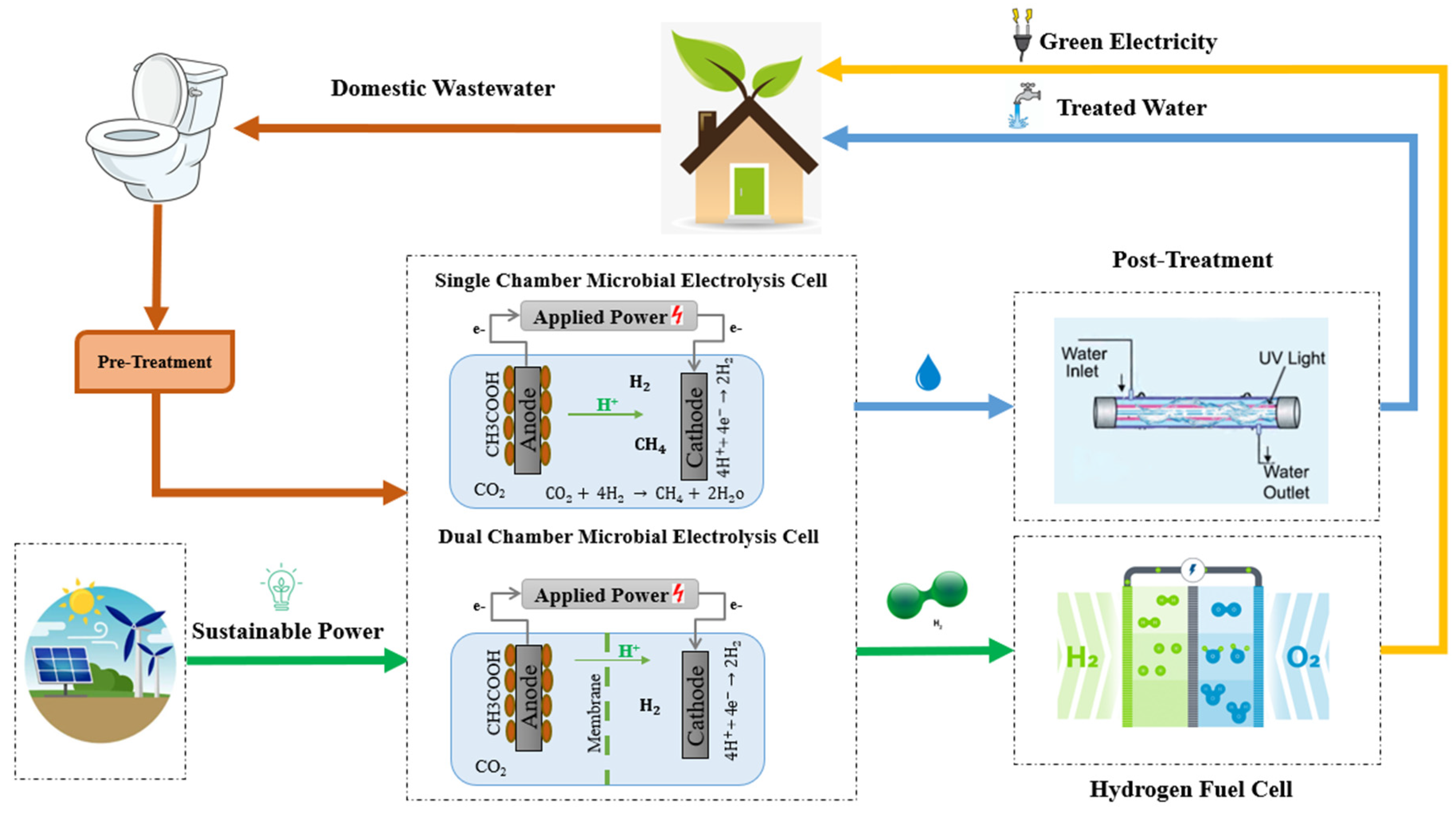
2.1. Model Description of Microbial Electrolysis Cells (MECs)
2.2. Equations for Modeling of SMEC and DMEC
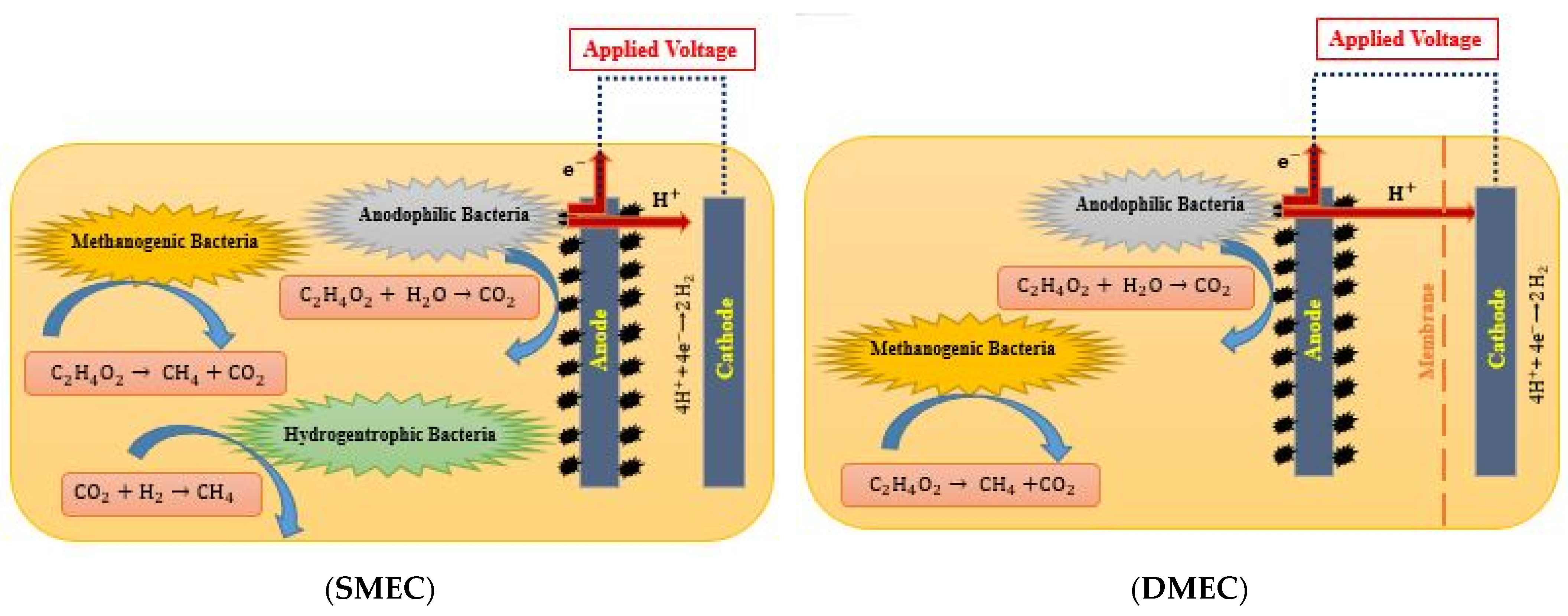
2.2.1. Chemical Reactions at Electrodes
- SMEC
- DMEC
2.2.2. Mass Balance Equations
2.2.3. Hydrogen Production Rate
- SMEC
- DMEC
2.2.4. Intracellular Mass Balance
2.2.5. Microbial Kinetic
2.2.6. Electrochemical Equations
2.3. Desing Parameters
3. Results and Discussion
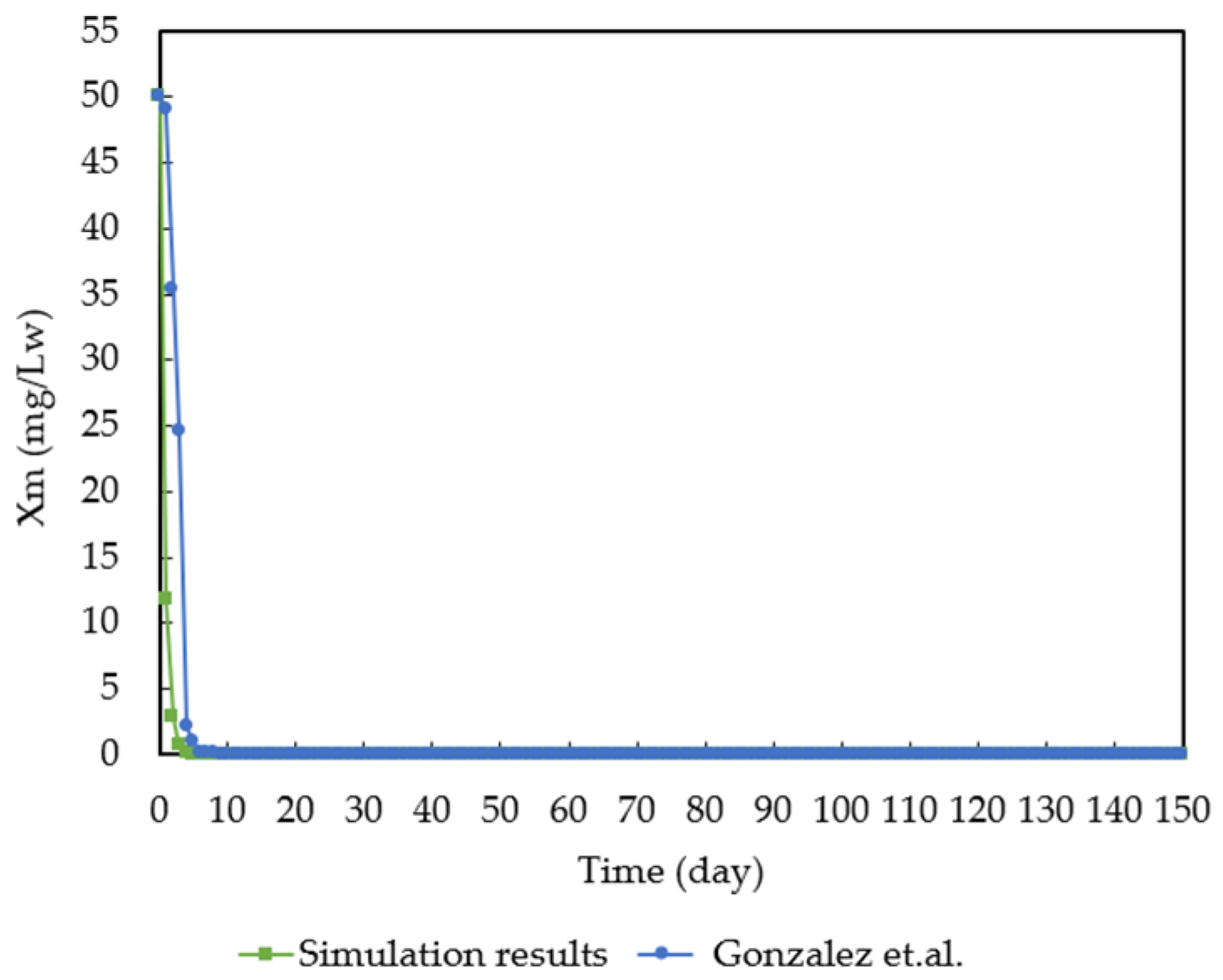
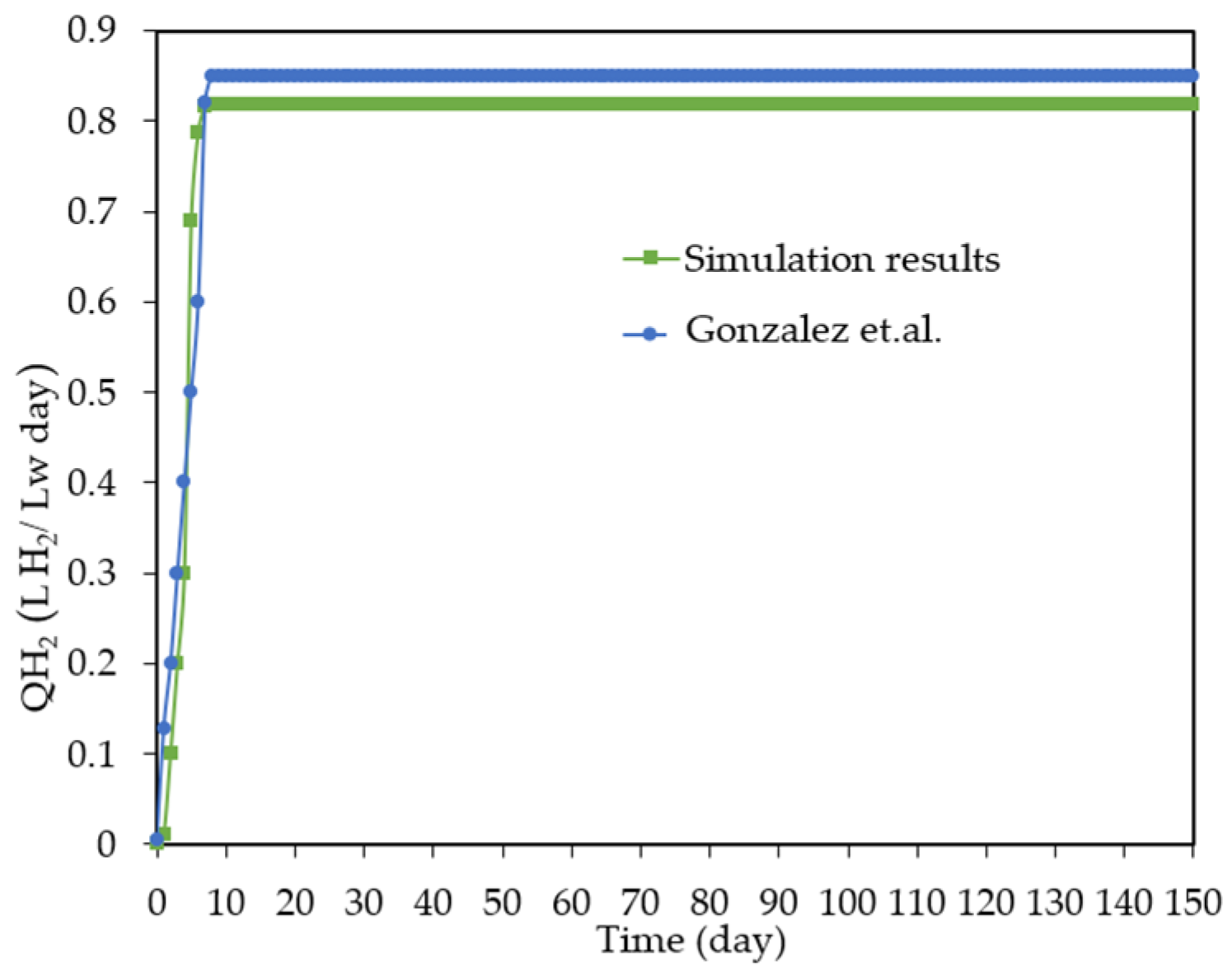
3.1. Model Validation
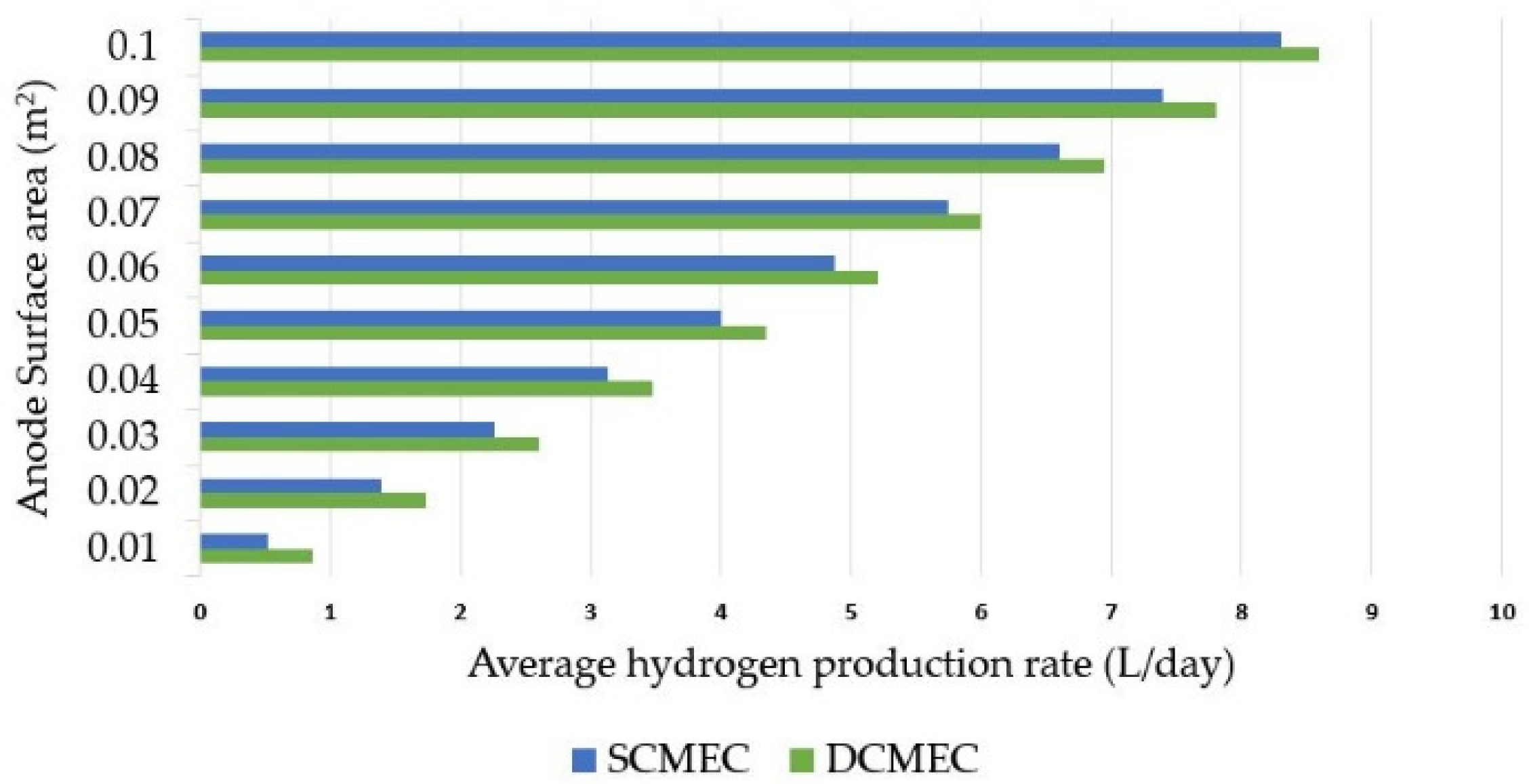
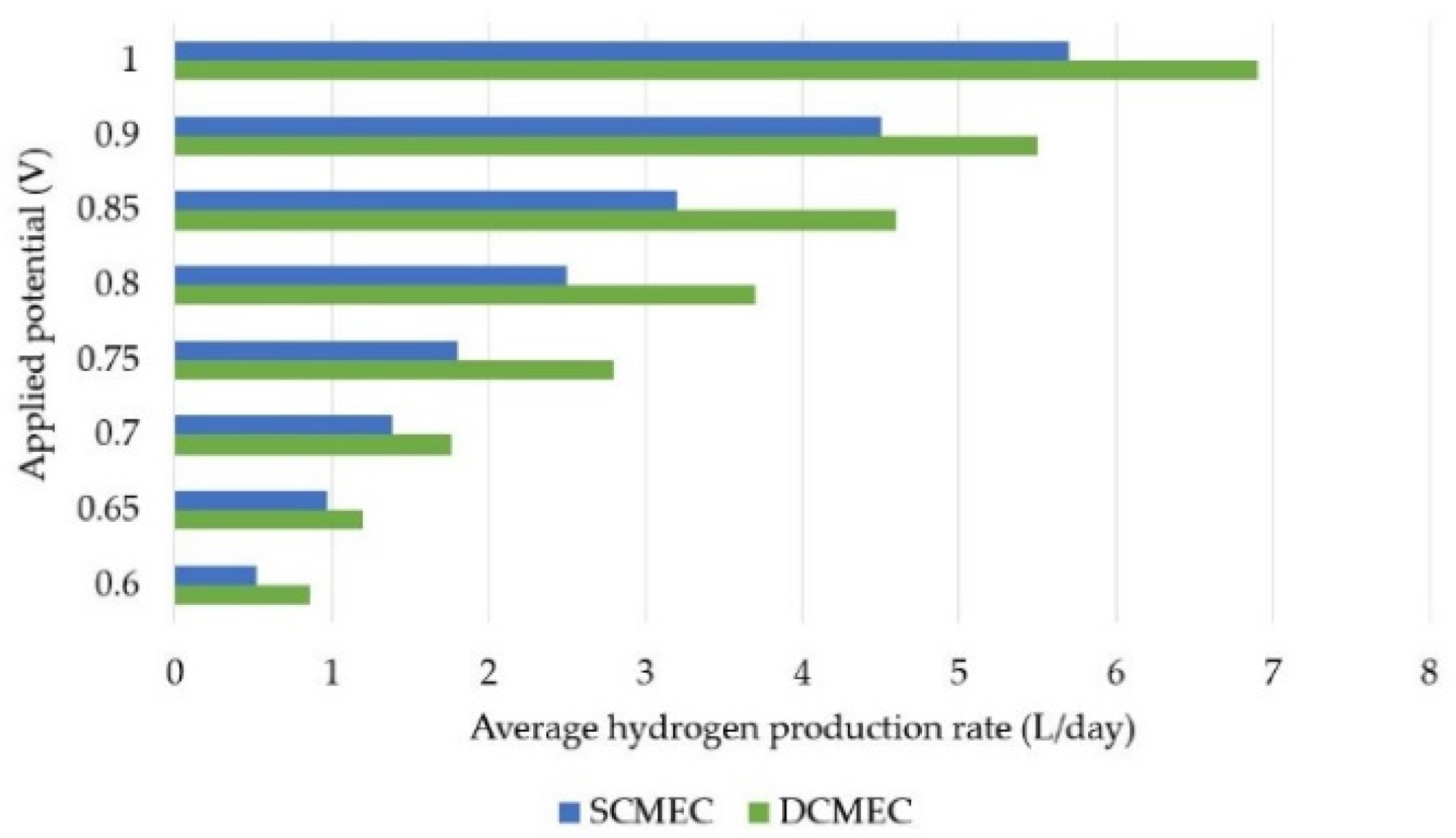
3.2. HPR in DMEC vs. SMEC
3.3. Sensitive Analysis
3.4. Case Study
4. CWTPs vs. MECs
5. WE vs. MECs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alanne, K.; Cao, S. An overview of the concept and technology of ubiquitous energy. Appl. Energy 2019, 238, 284–302. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, C.; Lai, Q.; Liu, W.; Wang, D.-W.; Aguey-Zinsou, K.-F. Tailoring magnesium based materials for hydrogen storage through synthesis: Current state of the art. Energy Storage Mater. 2018, 10, 168–198. [Google Scholar] [CrossRef]
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Sdanghi, G.; Maranzana, G.; Celzard, A.; Fierro, V. Review of the current technologies and performances of hydrogen compression for stationary and automotive applications. Renew. Sustain. Energy Rev. 2019, 102, 150–170. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strateg. Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Ursua, A.; Gandia, L.M.; Sanchis, P. Hydrogen production from water electrolysis: Current status and future trends. Proc. IEEE 2011, 100, 410–426. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; de Souza Candeo, E.; Medeiros, A.B.P.; de Carvalho, J.C.; de Andrade Tanobe, V.O.; Soccol, C.R.; Sydney, E.B. Hydrogen: Current advances and patented technologies of its renewable production. J. Clean. Prod. 2020, 286, 124970. [Google Scholar] [CrossRef]
- Saithong, N.; Authayanun, S.; Patcharavorachot, Y.; Arpornwichanop, A. Thermodynamic analysis of the novel chemical looping process for two-grade hydrogen production with CO2 capture. Energy Convers. Manag. 2019, 180, 325–337. [Google Scholar] [CrossRef]
- Westphal, M.I.; Zhou, L.; Satterthwaite, D.; Martin, S. Powering Cities in the Global South: How Energy Access for All Benefits the Economy and the Environment; World Resources Institute: Washington, DC, USA, 2017. [Google Scholar]
- Luo, C.; Posen, I.D.; Hoornweg, D.; MacLean, H.L. Modelling future patterns of urbanization, residential energy use and greenhouse gas emissions in Dar es Salaam with the Shared Socio-Economic Pathways. J. Clean. Prod. 2020, 254, 119998. [Google Scholar] [CrossRef]
- Gupta, S.; Srivastava, P.; Patil, S.A.; Yadav, A.K. A comprehensive review on emerging constructed wetland coupled microbial fuel cell technology: Potential applications and challenges. Bioresour. Technol. 2021, 320, 124376. [Google Scholar] [CrossRef] [PubMed]
- Yasri, N.; Roberts, E.P.L.; Gunasekaran, S. The electrochemical perspective of bioelectrocatalytic activities in microbial electrolysis and microbial fuel cells. Energy Rep. 2019, 5, 1116–1136. [Google Scholar] [CrossRef]
- Wilberforce, T.; Sayed, E.T.; Abdelkareem, M.A.; Elsaid, K.; Olabi, A.G. Value added products from wastewater using bioelectrochemical systems: Current trends and perspectives. J. Water Process Eng. 2021, 39, 101737. [Google Scholar] [CrossRef]
- Jafary, T.; Daud, W.R.W.; Ghasemi, M.; Bakar, M.H.A.; Sedighi, M.; Kim, B.H.; Carmona-Martínez, A.A.; Jahim, J.M.; Ismail, M. Clean hydrogen production in a full biological microbial electrolysis cell. Int. J. Hydrogen Energy 2019, 44, 30524–30531. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Source 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Cotterill, S.E.; Dolfing, J.; Jones, C.; Curtis, T.P.; Heidrich, E.S. Low Temperature Domestic Wastewater Treatment in a Microbial Electrolysis Cell with 1 m2 Anodes: Towards System Scale-Up. Fuel Cells 2017, 17, 584–592. [Google Scholar] [CrossRef]
- Baeza, J.A.; Martínez-Miró, À.; Guerrero, J.; Ruiz, Y.; Guisasola, A. Bioelectrochemical hydrogen production from urban wastewater on a pilot scale. J. Power Source 2017, 356, 500–509. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Dolfing, J.; Scott, K.; Edwards, S.R.; Jones, C.; Curtis, T.P. Production of hydrogen from domestic wastewater in a pilot-scale microbial electrolysis cell. Appl. Microbiol. Biotechnol. 2013, 97, 6979–6989. [Google Scholar] [CrossRef]
- Pinto, R.P.; Srinivasan, B.; Escapa, A.; Tartakovsky, B. Multi-population model of a microbial electrolysis cell. Environ. Sci. Technol. 2011, 45, 5039–5046. [Google Scholar] [CrossRef]
- Mardanpour, M.M.; Yaghmaei, S. Dynamical analysis of microfluidic microbial electrolysis cell via integrated experimental investigation and mathematical modeling. Electrochim. Acta 2017, 227, 317–329. [Google Scholar] [CrossRef]
- Flores-Estrella, R.A.; Rodríguez-Valenzuela, G.; Ramírez-Landeros, J.R.; Alcaraz-González, V.; González-Álvarez, V. A simple microbial electrochemical cell model and dynamic analysis towards control design. Chem. Eng. Commun. 2020, 207, 493–505. [Google Scholar] [CrossRef]
- Alcaraz, V.; Rodriguez, G.; Gomez, J.J.; Dotto, G.L.; Flores, R.A. Hydrogen production automatic control in continuous microbial electrolysis cells reactors used in wastewater treatment. J. Environ. Manag. 2021, 281, 111869. [Google Scholar] [CrossRef] [PubMed]
- Bienvenue sur Montréal.ca. Available online: https://montreal.ca/ (accessed on 15 June 2022).
- Enright, W.H. The Numerical Analysis of Ordinary Differential Equations: Runge-Kutta and General Linear Methods (JC Butcher). In Society for Industrial and Applied Mathematics; John Wiley: Chichester, UK, 1989. [Google Scholar]
- Hairer, E.; Wanner, G. Solving Ordinary Differential Equations II. In Springer Series in Computational Mathematics; Springer: Berlin/Heidelberg, Germany, 1996; Volume 14. [Google Scholar] [CrossRef]
- Maktabifard, M.; Zaborowska, E.; Makinia, J. Achieving energy neutrality in wastewater treatment plants through energy savings and enhancing renewable energy production. Rev. Environ. Sci. Bio/Technol. 2018, 17, 655–689. [Google Scholar] [CrossRef]


| Parameters | Description | Value and Units |
|---|---|---|
| [] | Dissolved hydrogen saturated concentration | 1.5 (mg/L) |
| Half–rate constant for hydrogenotrophic microorganisms | 0.001 (mg/L) | |
| Constant, which determines the slope of the curve in the equation | 0.024 (L/mg X) | |
| The decomposition rate of anodophilic microorganisms | 0.04 (1/d) | |
| The decomposition rate of hydrogenotrophic microorganisms | 0.01 (1/d) | |
| The decomposition rate of methanogenic microorganisms | 0.01 (1/d) | |
| Half–rate (Monod) constant for anodophilic microorganisms | 20 (mg S/L) | |
| Half–rate (Monod) constant for methanogenic microorganisms | 80 (mg S/L) | |
| Half–rate constant for the oxidized intracellular mediator | 0.01 (mg M/L) | |
| The yield rate for hydrogen-consuming methanogenic microorganisms | 0.05 (ml H2/mg X) | |
| Hydrogen yield | 0.9 | |
| The yield rate for the oxidized mediator | 3.3 (mg M/mg A) | |
| β | Oxidation transfer coefficient or reduction | 0.5 |
| γ | Mediator molar mass | 663,400 (mg M/mole M) |
| Treatment Method | Energy Requirement | Energy Content | Surplus Energy Outputs |
|---|---|---|---|
| CWTPs | 0.3 kWh/m3 | - | - |
| DMEC | 1.7 kWh/m3 | 2.58 kWh/m3 | 0.9 kWh/m3 |
| SMEC | 1.7 kWh/m3 | 1.56 kWh/m3 | −0.14 kWh/m3 |
| Technology | Energy Consumption for 1 kg H2 Production | Pure Water Requirement | Wastewater Requirement |
|---|---|---|---|
| WE | 51 kWh | 0.009 m3 | - |
| DMEC | 24.5 kWh | - | 14.5 m3 |
| SMEC | 30 kWh | - | 23 m3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahimi, N.; Eicker, U. Mathematical Modeling of Microbial Electrolysis Cells for Enhanced Urban Wastewater Treatment and Hydrogen Generation. Processes 2023, 11, 1157. https://doi.org/10.3390/pr11041157
Rahimi N, Eicker U. Mathematical Modeling of Microbial Electrolysis Cells for Enhanced Urban Wastewater Treatment and Hydrogen Generation. Processes. 2023; 11(4):1157. https://doi.org/10.3390/pr11041157
Chicago/Turabian StyleRahimi, Narges, and Ursula Eicker. 2023. "Mathematical Modeling of Microbial Electrolysis Cells for Enhanced Urban Wastewater Treatment and Hydrogen Generation" Processes 11, no. 4: 1157. https://doi.org/10.3390/pr11041157
APA StyleRahimi, N., & Eicker, U. (2023). Mathematical Modeling of Microbial Electrolysis Cells for Enhanced Urban Wastewater Treatment and Hydrogen Generation. Processes, 11(4), 1157. https://doi.org/10.3390/pr11041157








