Influence of Chemical Grafting Method on the Performance of SiO2 Nanocomposite Pour Point Depressant
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Prepartiom
2.2.1. Preparation of Octadecyl Methacrylate/Maleic Anhydride Copolymer (PM18)
2.2.2. Preparation of PM18/SiO2 Nanocomposite Pour Point Depressant
2.2.3. Preparation of Amino Modified Nanosilica
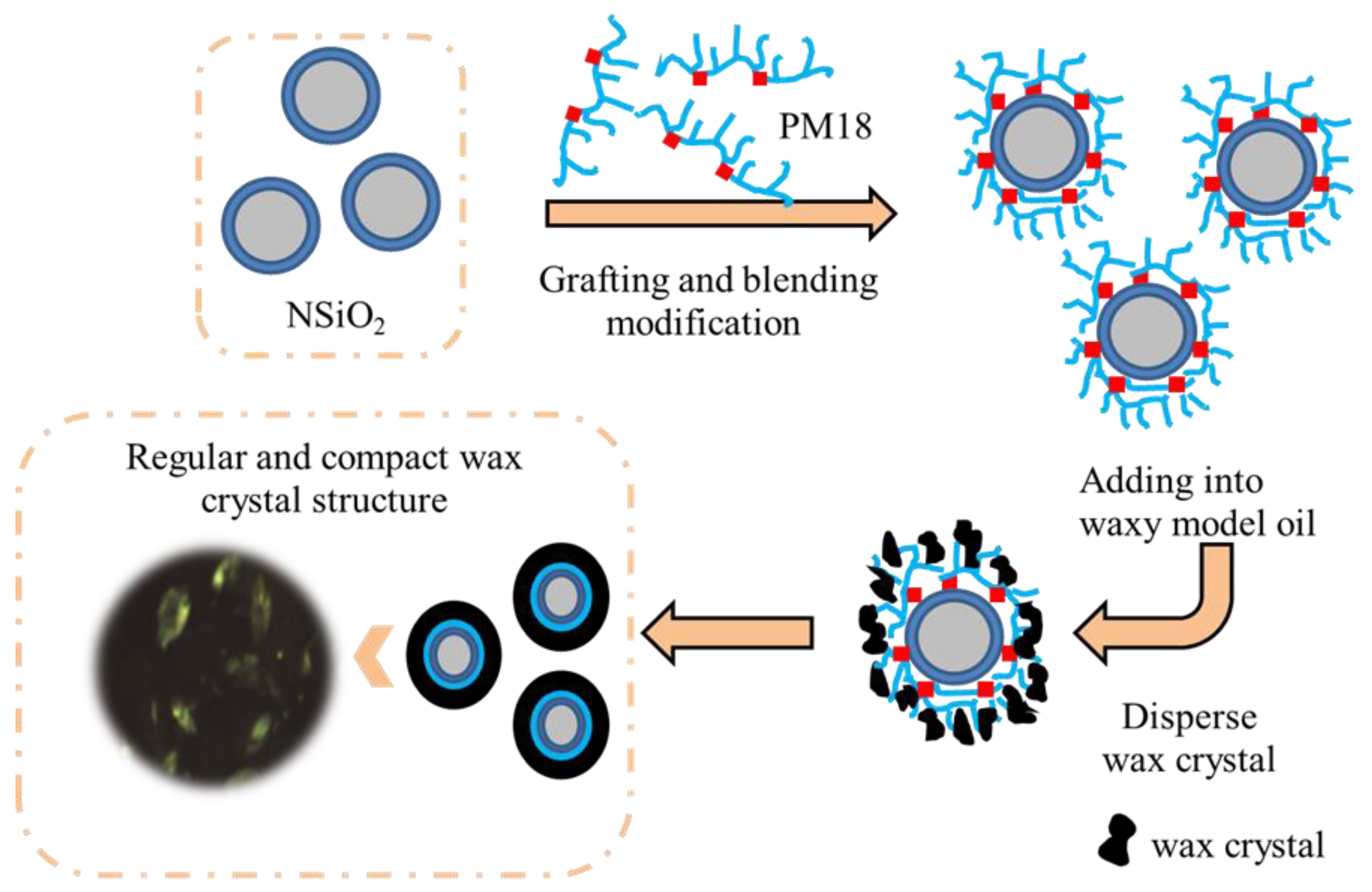
2.2.4. Preparation of PM18-g-NSiO2 Nanocomposite Pour Point Depressant
2.2.5. Preparation of Waxy Model Oil
2.3. Characterization
2.3.1. Nanocomposite Pour Point Depressant Properties Characterization
2.3.2. Model Oil Properties Characterization
Pour Point Test
Viscosity Curves Test
Polarized Optical Microscopy (POM)
3. Results
3.1. Characterization Analysis
3.1.1. FT-IR Analysis
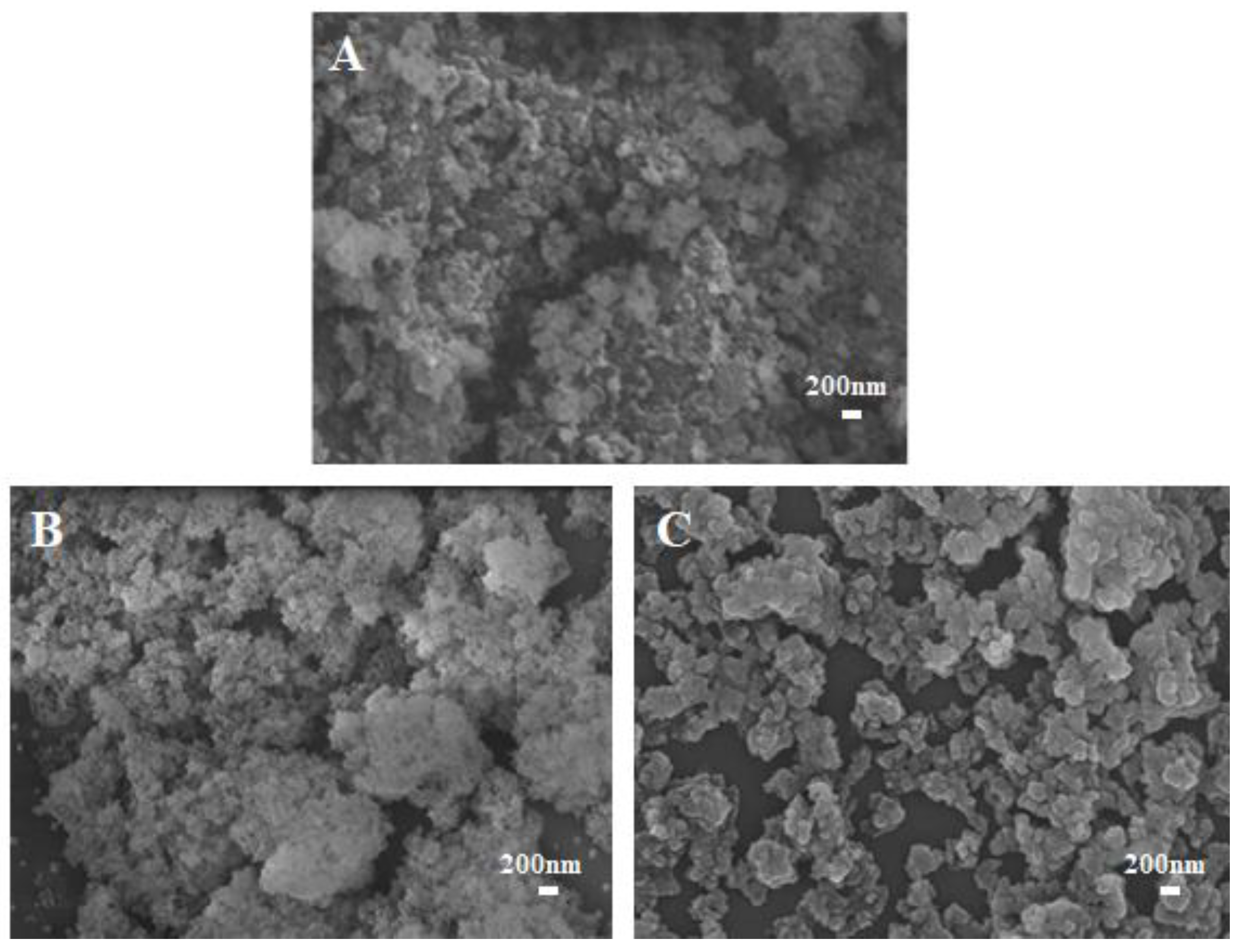
3.1.2. SEM Analysis
3.2. Effect Evaluation of Nano-SiO2 PPD Synthesized by Different Method on Model Oil
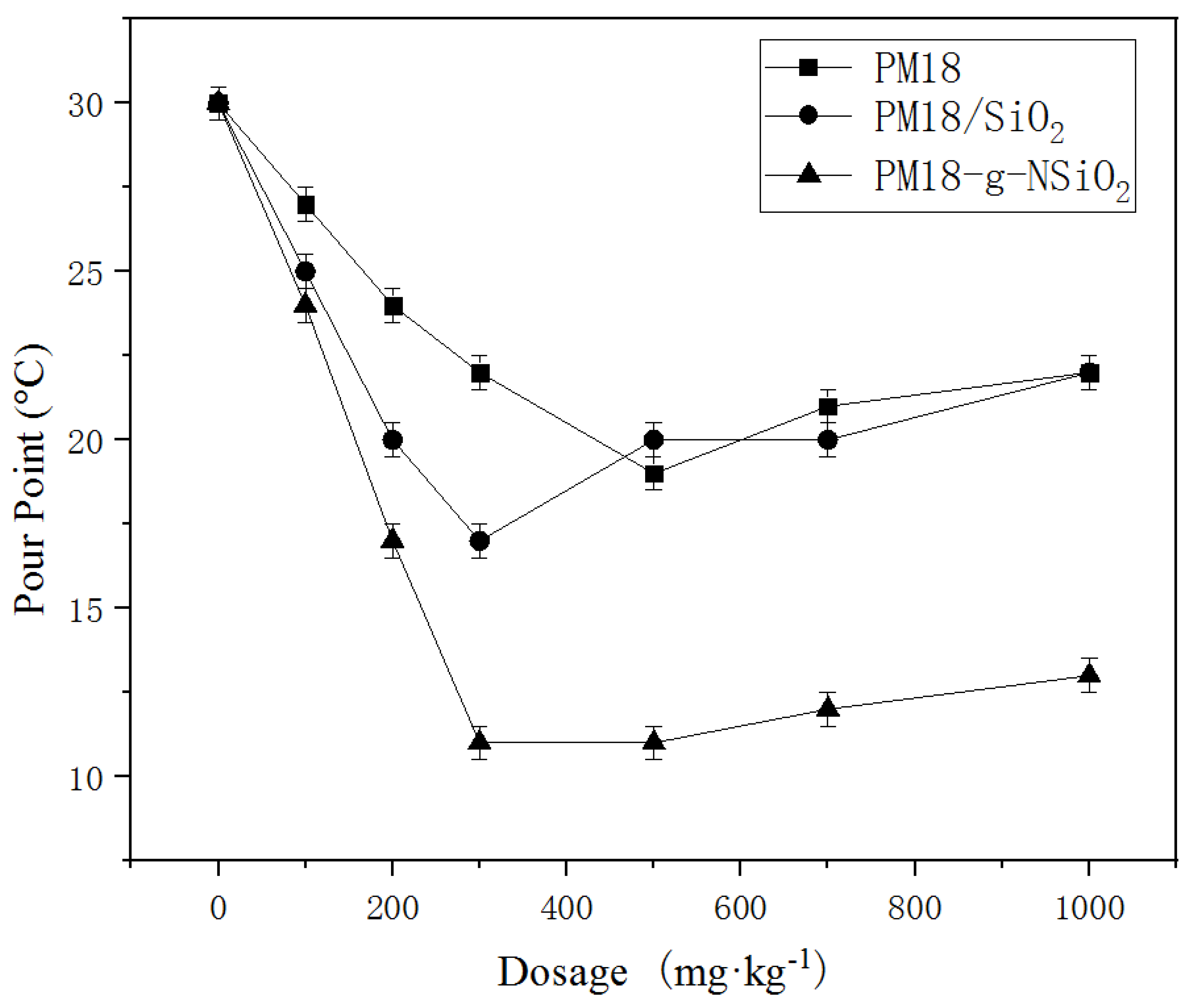
3.2.1. Pour Point
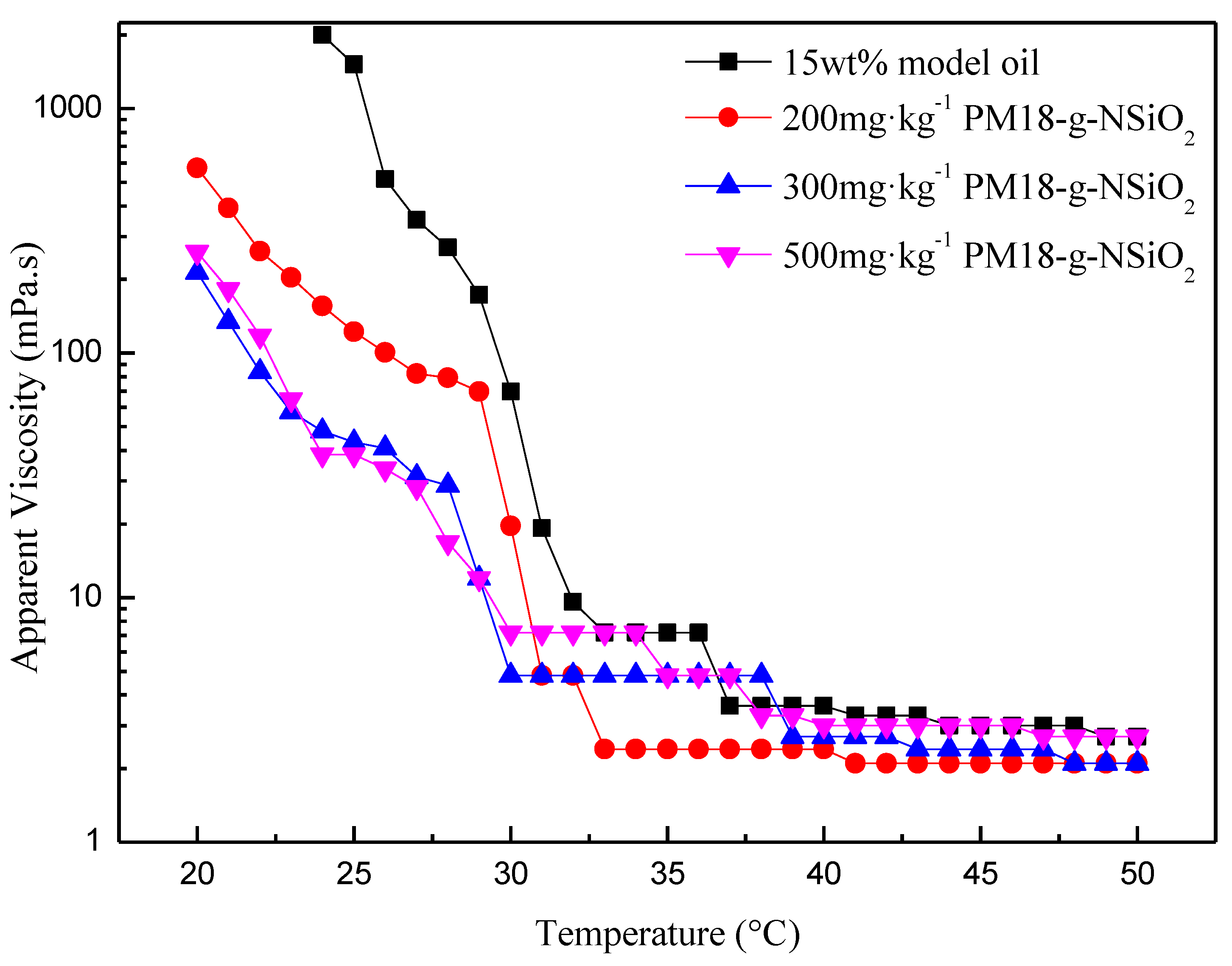
3.2.2. Rheology Properties
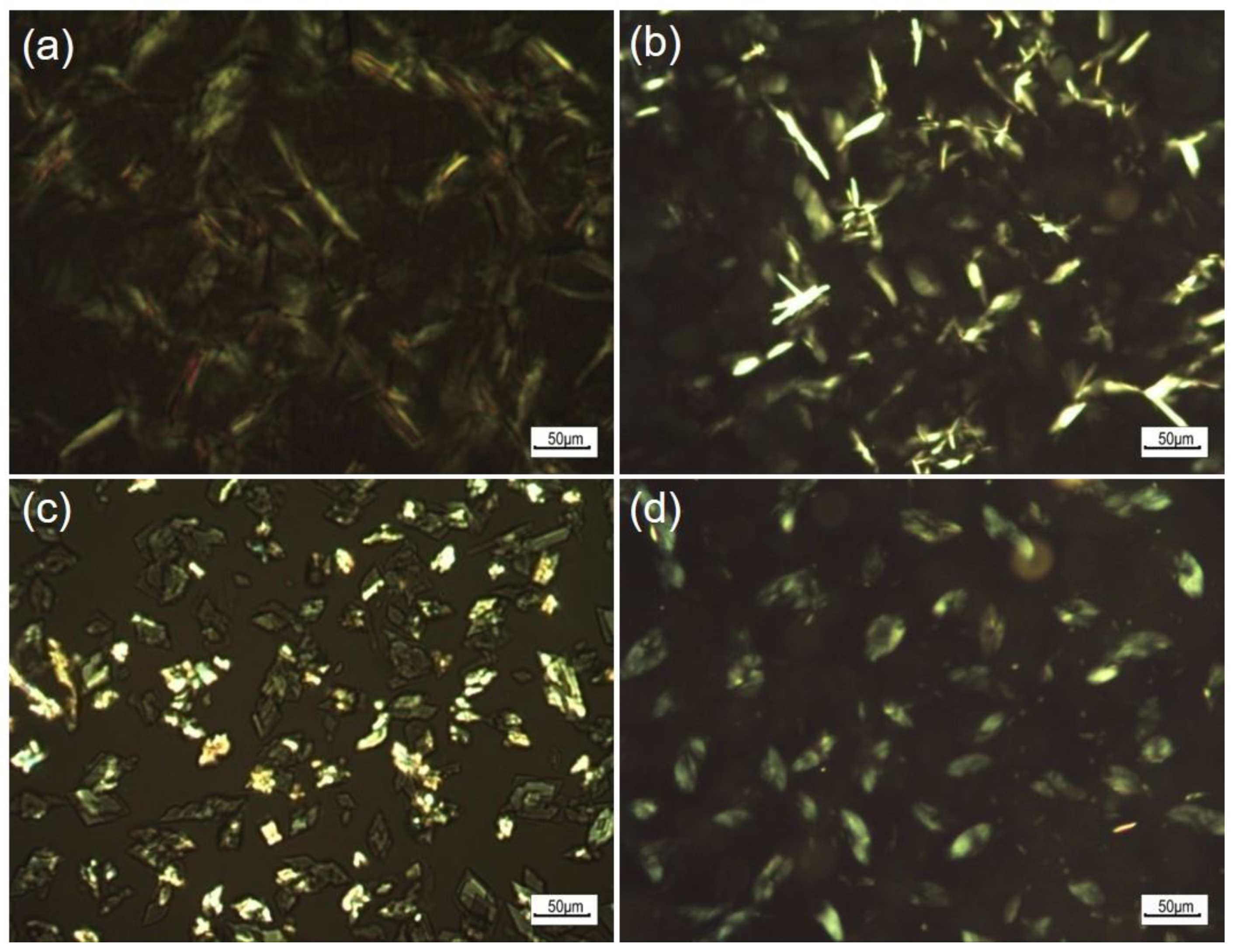
3.2.3. Microscopic Study
3.3. Proposed Mechanism
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kapustin, N.O.; Grushevenko, D.A. Global prospects of unconventional oil in the turbulent market: A long term outlook to 2040. Oil Gas Sci. Technol.—Rev. d’IFP Energ. Nouv. 2018, 73, 67. [Google Scholar] [CrossRef]
- Cao, J.; Liu, L.; Liu, C.; He, C. Phase transition mechanisms of paraffin in waxy crude oil in the absence and presence of pour point depressant. J. Mol. Liq. 2022, 345, 116989. [Google Scholar] [CrossRef]
- Ragunathan, T.; Husin, H.; Wood, C.D. Wax Formation Mechanisms, Wax Chemical Inhibitors and Factors Affecting Chemical Inhibition. Appl. Sci. 2020, 10, 479. [Google Scholar] [CrossRef]
- Olajire, A.A. Review of wax deposition in subsea oil pipeline systems and mitigation technologies in the petroleum industry. Chem. Eng. J. Adv. 2021, 6, 100104. [Google Scholar] [CrossRef]
- Guo, W.; Li, H.; Li, Z.; Chen, C. The effect of dynamic cooling on the flow properties of PPD-treated crude oil. Pet. Sci. Technol. 2018, 36, 1787–1793. [Google Scholar] [CrossRef]
- Xia, X.; Li, C.; Dai, S.; Duan, Z.; Lian, W.; Yao, B.; Sun, G.; Yang, F. Modification effect of macroporous comb-like polymeric pour point depressants on the flow behavior of model waxy oils. Fuel 2022, 314, 123113. [Google Scholar] [CrossRef]
- Yang, T.; Wu, J.; Yuan, M.; Li, X.; Yin, S.; Su, B.; Yan, J.; Lin, H.; Xue, Y.; Han, S. Influence of polar groups on the depressive effects of polymethacrylate polymers as cold flow improvers for diesel fuel. Fuel 2021, 290, 120035. [Google Scholar] [CrossRef]
- Oliveira, L.M.S.L.; Nunes, R.C.P.; Melo, I.C.; Ribeiro, Y.L.L.; Reis, L.G.; Dias, J.C.M.; Guimarães, R.C.L.; Lucas, E.F. Evaluation of the correlation between wax type and structure/behavior of the pour point depressant. Fuel Process. Technol. 2016, 149, 268–274. [Google Scholar] [CrossRef]
- Ren, F.; Lu, Y.; Sun, B.; Wang, C.; Yan, J.; Lin, H.; Xue, Y.; Han, S. Structure regulation and influence of comb copolymers as pour point depressants on low temperature fluidity of diesel fuel. Energy 2022, 254, 124438. [Google Scholar] [CrossRef]
- Sun, B.; Chen, F.; Lin, H.; Xue, Y.; Han, S. Influence of comb type terpolymers of methyl benzyl acrylate-co-hexadecene-maleic anhydride with tetradecyl pendant on cold flow properties of diesel fuel. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130636. [Google Scholar] [CrossRef]
- Yuan, M.; Li, X.; Xue, Y.; Lin, H.; Han, S. Methylcyclohexyl methacrylate–methacrylate copolymers: An effective cold flow improver for the biodiesel blends. Res. Chem. Intermed. 2022, 48, 2665–2681. [Google Scholar] [CrossRef]
- Jia, X.; Fu, M.; Xing, X.; Wei, L.; Song, Y.; Zhang, L.; Geng, X.; Guo, H. Submicron carbon-based hybrid nano-pour-point depressant with outstanding pour point depressant and excellent viscosity depressant. Arab. J. Chem. 2022, 15, 104157. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Betiha, M.A.; Osman, D.I.; Mahmoud, T. Synthesis and characterization of nanohybrid of poly(octadecylacrylates derivatives)/montmorillonite as pour point depressants and flow improver for waxy crude oil. J. Appl. Polym. Sci. 2019, 136, 47333. [Google Scholar] [CrossRef]
- Zhao, Z.-B.; Tai, L.; Zhang, D.-M.; Wang, Z.-F.; Jiang, Y. Preparation of poly (octadecyl methacrylate)/silica-(3-methacryloxypropyl trimethoxysilane)/silica multi-layer core-shell nanocomposite with thermostable hydrophobicity and good viscosity break property. Chem. Eng. J. 2017, 307, 891–896. [Google Scholar] [CrossRef]
- Li, N.; Mao, G.; Wu, W.; Liu, Y. Effect evaluation of ethylene vinyl acetate/nano-montmorillonite pour-point depressant on improving the flow properties of model oil. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 296–303. [Google Scholar] [CrossRef]
- Huang, H.; Wang, W.; Peng, Z.; Ding, Y.; Li, K.; Li, Q.; Gong, J. The influence of nanocomposite pour point depressant on the crystallization of waxy oil. Fuel 2018, 221, 257–268. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Z.N.; Jing, G.L.; Liu, S.; Yang, Y.H.; Xu, J.Q. Synthesis of chemical grafting pour point depressant EVAL-GO and its effect on the rheological properties of Daqing crude oil. Fuel Process. Technol. 2021, 223, 107000. [Google Scholar] [CrossRef]
- Mahmoud, T.; Betiha, M.A. Poly(octadecyl acrylate-co-vinyl neodecanoate)/Oleic Acid-Modified Nano-graphene Oxide as a Pour Point Depressant and an Enhancer of Waxy Oil Transportation. Energy Fuels 2021, 35, 6101–6112. [Google Scholar] [CrossRef]
- Yao, B.; Li, C.X.; Yang, F.; Zhang, Y.; Xiao, Z.Q.; Sun, G.Y. Structural properties of gelled Changqing waxy crude oil benefitted with nanocomposite pour point depressant. Fuel 2016, 184, 544–554. [Google Scholar] [CrossRef]
- Yao, B.; Li, C.X.; Yang, F.; Sjoblom, J.; Zhang, Y.; Norrman, J.; Paso, K.; Xiao, Z.Q. Organically modified nano-clay facilitates pour point depressing activity of polyoctadecylacrylate. Fuel 2016, 166, 96–105. [Google Scholar] [CrossRef]
- Zhu, H.; Li, C.; Xiu, Z.; Zhao, Z.; Mu, K.; Dai, H.; Wang, F.; Yang, F.; Yao, B. Effect of Ethylene-Vinyl Acetate Copolymer/Amino-Functionalized Polymethylsilsesquioxane Composite Wax Inhibitor on the Rheological and Wax Depositing Characteristics of Waxy Crude Oil. Energy Fuels 2020, 34, 8120–8128. [Google Scholar] [CrossRef]
- Yao, B.; Li, C.; Zhang, X.; Yang, F.; Sun, G.; Zhao, Y. Performance improvement of the ethylene-vinyl acetate copolymer (EVA) pour point depressant by small dosage of the amino-functionalized polymethylsilsesquioxane (PAMSQ) microsphere. Fuel 2018, 220, 167–176. [Google Scholar] [CrossRef]
- Jing, G.L.; Sun, Z.N.; Tu, Z.Y.; Bian, X.D.; Liang, Y. Influence of Different Vinyl Acetate Contents on the Properties of the Copolymer of Ethylene and Vinyl Acetate/Modified Nano-SiO2 Composite Pour-Point Depressant. Energy Fuels 2017, 31, 5854–5859. [Google Scholar] [CrossRef]
- Sun, Z.; Jing, G.; Tu, Z. Effect of modified nano-silica/EVA on flow behavior and wax crystallization of model oils with different wax contents. J. Dispers. Sci. Technol. 2017, 39, 71–76. [Google Scholar] [CrossRef]
- Li, N.; Mao, G.; Liu, Y. Effect of the Evaluation and Mechanism Analysis of a Novel Nanohybrid Pour Point Depressant on Facilitating Flow Properties of Crude Oil. Energy Fuels 2018, 32, 10563–10570. [Google Scholar] [CrossRef]
- Norrman, J.; Solberg, A.; Sjöblom, J.; Paso, K. Nanoparticles for Waxy Crudes: Effect of Polymer Coverage and the Effect on Wax Crystallization. Energy Fuels 2016, 30, 5108–5114. [Google Scholar] [CrossRef]
- Yang, F.; Paso, K.; Norrman, J.; Li, C.X.; Oschmann, H.; Sjoblom, J. Hydrophilic Nanoparticles Facilitate Wax Inhibition. Energy Fuels 2015, 29, 1368–1374. [Google Scholar] [CrossRef]
- Mao, J.; Kang, Z.; Yang, X.; Lin, C.; Zheng, L.; Zuo, M.; Mao, J.; Dai, S.; Xue, J.; Ouyang, D. Synthesis and Performance Evaluation of a Nanocomposite Pour-Point Depressant and Viscosity Reducer for High-Pour-Point Heavy Oil. Energy Fuels 2020, 34, 7965–7973. [Google Scholar] [CrossRef]
- El-Ghazawy, R.A.; Atta, A.M.; Kabel, K.I. Modified maleic anhydride-co-octadecene copolymers as flow improver for waxy Egyptian crude oil. J. Pet. Sci. Eng. 2014, 122, 411–419. [Google Scholar] [CrossRef]
- SY/T 0541-94; Test Method for Gel Point of Crude Oils. China Petroleum and Chemical Press: Beijing, China, 1994.
- Sharma, R.; Deka, B.; Mahto, V.; Barifcani, A.; Vuthaluru, H. Experimental investigation into the development and evaluation of ionic liquid and its graphene oxide nanocomposite as novel pour point depressants for waxy crude oil. J. Pet. Sci. Eng. 2022, 208, 109691. [Google Scholar] [CrossRef]
- Soldi, R.A.; Oliveira, A.R.S.; Barbosa, R.V.; César-Oliveira, M.A.F. Polymethacrylates: Pour point depressants in diesel oil. Eur. Polym. J. 2007, 43, 3671–3678. [Google Scholar] [CrossRef]
- Jie, Z.; Guo, Z.; Du, W.; Gu, X.; Wang, M.; Zhang, Z.; Ma, Y.; Chen, G. Preparation and Performance of Vegetable Oils Fatty Acids Hydroxylmethyl Triamides as Crude Oil Flow Improvers. Pet. Chem. 2018, 58, 1070–1075. [Google Scholar] [CrossRef]
- Tai, Y.; Qian, J.; Zhang, Y.; Huang, J. Study of surface modification of nano-SiO2 with macromolecular coupling agent (LMPB-g-MAH). Chem. Eng. J. 2008, 141, 354–361. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, J.; Zhu, X.; Jin, D.; Yang, T.; Yin, S.; Lin, H.; Han, S.; Xue, Y. Performance improvement of the benzyl methacrylate-methacrylate copolymers pour point depressant by hybrid with nano-silica for diesel fuels. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 1–9. [Google Scholar] [CrossRef]
- Ning, X.; Song, X.; Zhang, S.; Wang, Y.; Feng, Y. Insights into Flow Improving for Waxy Crude Oil Doped with EVA/SiO2 Nanohybrids. ACS Omega 2022, 7, 5853–5863. [Google Scholar] [CrossRef]
- Maleki, A.; Hosseini-Dastgerdi, Z.; Rashidi, A. Effect of nanoparticle modified polyacrylamide on wax deposition, crystallization and flow behavior of light and heavy crude oils. J. Dispers. Sci. Technol. 2021, 42, 1–11. [Google Scholar] [CrossRef]
- Zhao, Z.; Xue, Y.; Xu, G.; Zhou, J.; Lian, X.; Liu, P.; Chen, D.; Han, S.; Lin, H. Effect of the nano-hybrid pour point depressants on the cold flow properties of diesel fuel. Fuel 2017, 193, 65–71. [Google Scholar] [CrossRef]
- Vakili, S.; Mohammadi, S.; Mirzaei Derazi, A.; Mahmoudi Alemi, F.; Hayatizadeh, N.; Ghanbarpour, O.; Rashidi, F. Effect of metal oxide nanoparticles on wax formation, morphology, and rheological behavior in crude oil: An experimental study. J. Mol. Liq. 2021, 343, 117566. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Wei, Z.; Han, S.; Dai, B.; Lin, H. Synthesis of nano-hybrid polymethacrylate-carbon dots as pour point depressant and combined with ethylene-vinyl acetate resin to improve the cold flow properties of diesel fuels. Energy 2022, 253, 124186. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, F.; Sun, B.; Lin, H.; Dai, B.; Han, S. Effect of nanocomposite as pour point depressant on the cold flow properties and crystallization behavior of diesel fuel. Chin. Chem. Lett. 2022, 33, 2677–2680. [Google Scholar] [CrossRef]
- Sharma, R.; Mahto, V.; Vuthaluru, H. Synthesis of PMMA/modified graphene oxide nanocomposite pour point depressant and its effect on the flow properties of Indian waxy crude oil. Fuel 2019, 235, 1245–1259. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.L.; Liang, Y.; Sun, Z.N.; Liu, Y.; Jing, G.L. Influence of EVA/nano-sepiolite on the wax crystal and rheological property of Daqing crude oil. J. Dispers. Sci. Technol. 2021, 42, 1125–1131. [Google Scholar] [CrossRef]
- Huang, H.; Li, K.; Wang, D.; Liang, P.; Yang, J.; Ding, Y.; Gong, J. Further Discussion of Nanocomposite Pour Point Depressants on the Influence of Paraffin Wax Crystallization: A Novel Small Angle X-ray Diffraction Study. Cryst. Growth Des. 2022, 22, 6870–6878. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Yan, B.; Jing, G.; Yang, Y.; Li, H.; Zhang, F. Influence of Chemical Grafting Method on the Performance of SiO2 Nanocomposite Pour Point Depressant. Processes 2023, 11, 1159. https://doi.org/10.3390/pr11041159
Sun Z, Yan B, Jing G, Yang Y, Li H, Zhang F. Influence of Chemical Grafting Method on the Performance of SiO2 Nanocomposite Pour Point Depressant. Processes. 2023; 11(4):1159. https://doi.org/10.3390/pr11041159
Chicago/Turabian StyleSun, ZhengNan, Biao Yan, GuoLin Jing, YiHai Yang, HongJing Li, and FuNing Zhang. 2023. "Influence of Chemical Grafting Method on the Performance of SiO2 Nanocomposite Pour Point Depressant" Processes 11, no. 4: 1159. https://doi.org/10.3390/pr11041159
APA StyleSun, Z., Yan, B., Jing, G., Yang, Y., Li, H., & Zhang, F. (2023). Influence of Chemical Grafting Method on the Performance of SiO2 Nanocomposite Pour Point Depressant. Processes, 11(4), 1159. https://doi.org/10.3390/pr11041159





