A Novel Eco-Friendly Circular Approach to Comprehensive Utilizing Bittern Waste and Oyster Shell
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Method
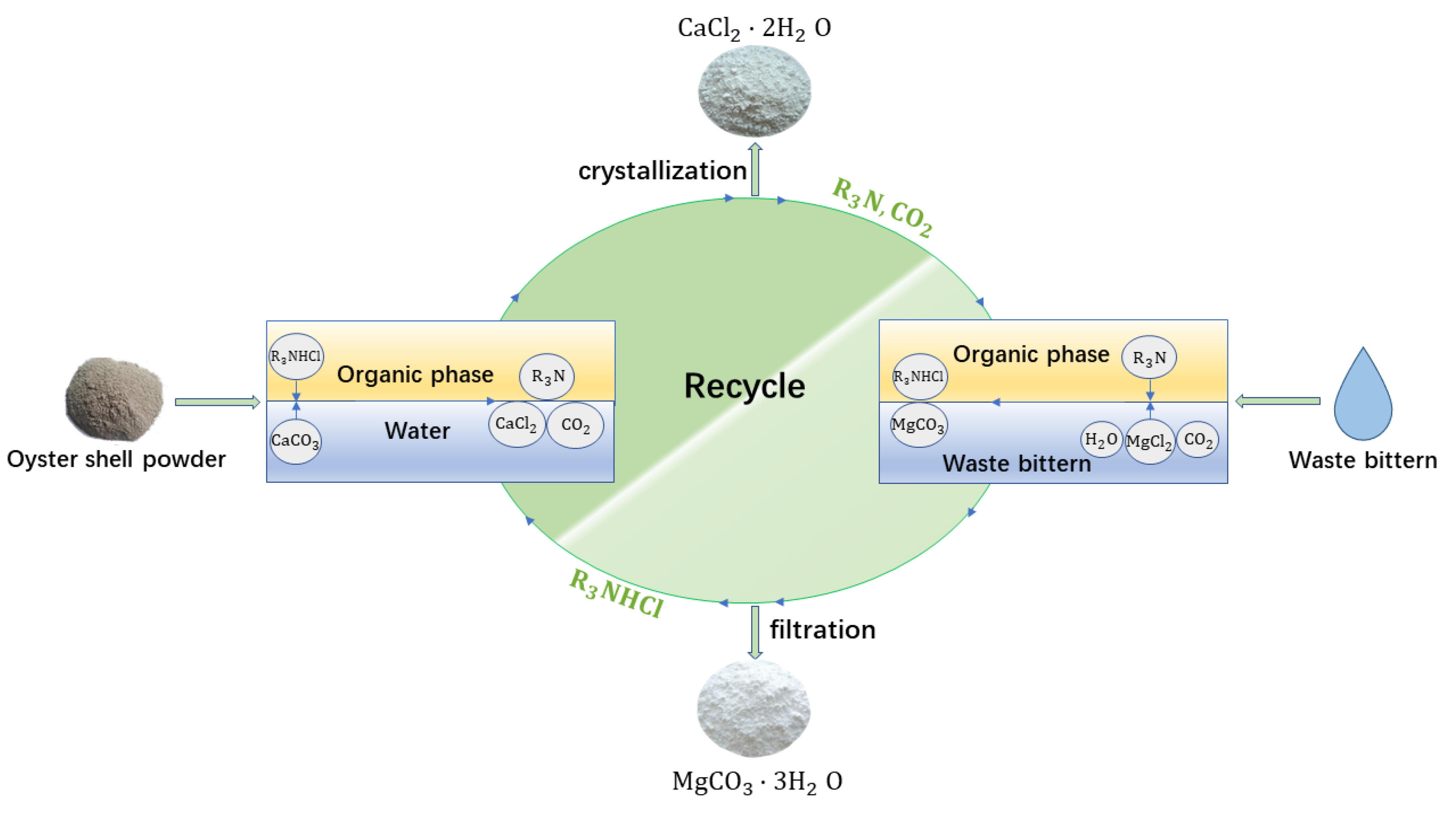
2.2.1. Waste Bittern Utilization
2.2.2. Oyster Shell Utilization
3. Results and Discussion
3.1. Effects of the Operating Conditions
3.1.1. Effect of Phase Ratio of Reactants on the Yield of MgCO3·3H2O
3.1.2. Effect of Reaction Time and Stirring Speed on the Yield of MgCO3·3H2O
3.1.3. Effect of Phase Ratio of the Aqueous and Oil Phase on R3N Regeneration Rate
3.1.4. Effect of Reaction Temperature on R3N Regeneration Rate
3.2. Thermodynamic Function Calculation and Reaction Mechanism of the R3N Regeneration Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Characterization Methods
Appendix A.2. Characterization Results
Appendix A.2.1. The Fourier Transform Infrared Spectroscopy
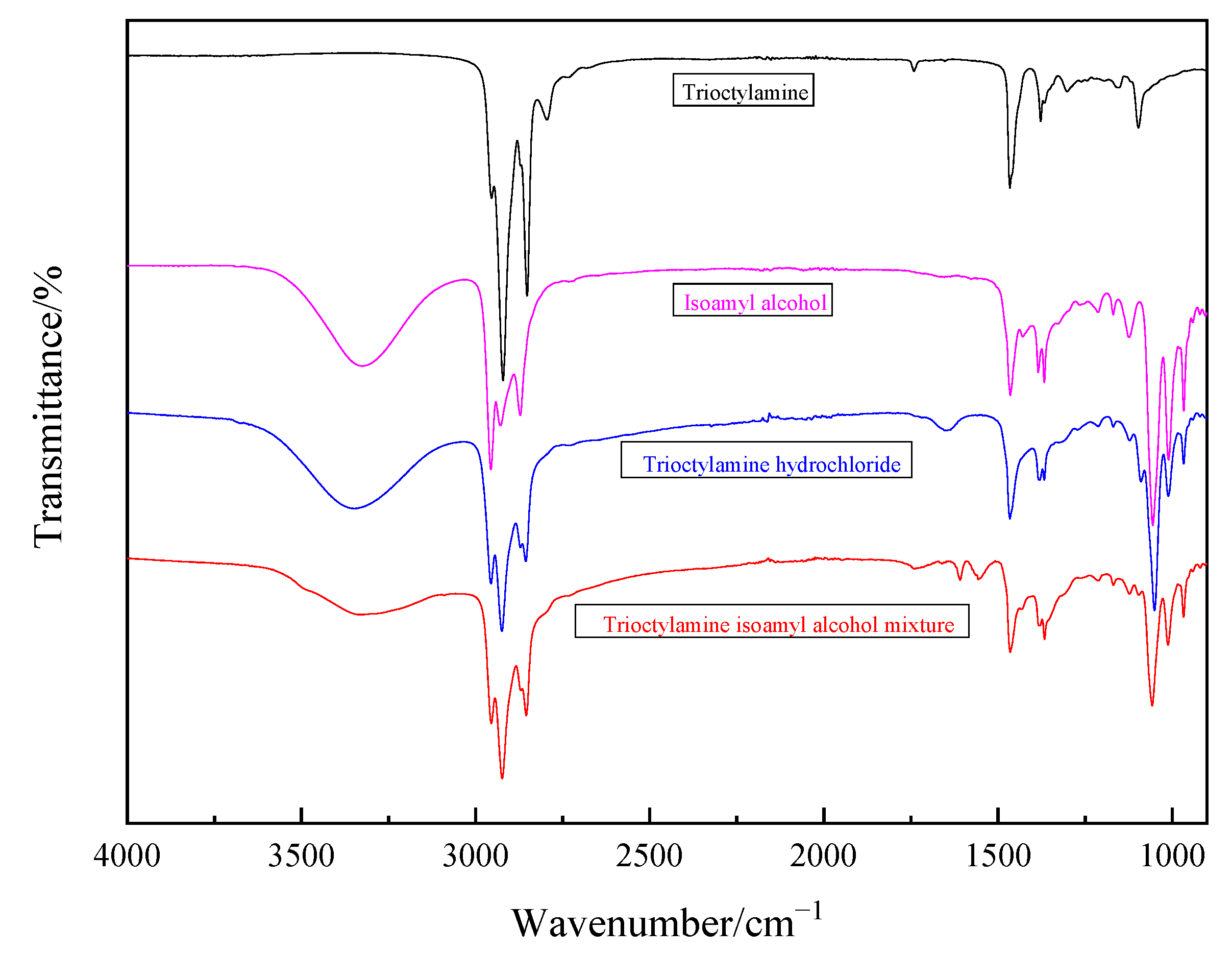
Appendix A.2.2. The X-ray Diffraction Spectra for Products (MgCO3·3H2O and CaCl2·2H2O)

Appendix B
| Sequence of Experiments | Water Sample Volume/mL | EDTA Volume /mL | Magnesium Ions’ Concentration/g/L |
|---|---|---|---|
| 1 | 50.00 | 54.55 | 56.49 |
| 2 | 50.00 | 55.20 | 57.17 |
| 3 | 50.00 | 55.15 | 57.11 |
| Average value | 50.00 | 54.97 | 56.92 |
References
- Alammar, A.; Hardian, R.; Szekely, G. Upcycling agricultural waste into membranes: From date seed biomass to oil and solvent-resistant nanofiltration. Green Chem. 2022, 24, 365–374. [Google Scholar] [CrossRef]
- Kita, Y.; Amao, Y. Visible-light-driven 3-hydroxybutyrate production from acetone and low concentrations of CO2 with a system of hybridized photocatalytic NADH regeneration and multi-biocatalysts. Green Chem. 2023, 25, 2699–2710. [Google Scholar] [CrossRef]
- Cavalcante, J.; Hardian, R.; Szekely, G. Antipathogenic upcycling of face mask waste into separation materials using green solvents. SM&T 2022, 32, e00448. [Google Scholar] [CrossRef]
- Zhang, N.; Moment, A. Upcycling Construction and Demolition Waste into Calcium Carbonates: Characterization of Leaching Kinetics and Carbon Mineralization Conditions. ACS Sustain. Chem. Eng. 2023, 11, 866–879. [Google Scholar] [CrossRef]
- Mustafa, J.; Mourad, A.A.H.I.; Al-Marzouqi, A.H.; El-Naas, M.H. Simultaneous treatment of reject brine and capture of carbon dioxide: A comprehensive review. Desalination 2020, 483, 114386. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.J.; Loizidou, M. Desalination brine disposal methods and treatment technologies—A review. Sci. Total. Environ. 2019, 693, 133545. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dong, Y.; Kong, C. Manufacture of sodium-free lithium chloride from salt lake brine. Sep. Purif. Technol. 2014, 136, 309–313. [Google Scholar] [CrossRef]
- Guo, X.F.; Li, D.; Liu, J.L.; Wang, Z.R.; Wang, J.; Zhao, Y.Y.; Yuan, J.S. Separation of sodium and potassium using adsorption—Elution/crystallization scheme from bittern. Chem. Eng. Res. Des. 2020, 161, 72–81. [Google Scholar] [CrossRef]
- Pan, X.J.; Dou, Z.H.; Zhang, T.A.; Meng, D.L.; Fan, Y.Y. Separation of metal ions and resource utilization of magnesium from saline lake brine by membrane electrolysis. Sep. Purif. Technol. 2020, 251, 117316. [Google Scholar] [CrossRef]
- Yu, X.; Cui, J.; Liu, C.; Yuan, F.; Guo, Y.; Deng, T. Separation of magnesium from high Mg/Li ratio brine by extraction with an organic system containing ionic liquid. Chem. Eng. Sci. 2020, 229, 116019. [Google Scholar] [CrossRef]
- Virolainen, S.; Fallah Fini, M.; Miettinen, V.; Laitinen, A.; Haapalainen, M.; Sainio, T. Removal of calcium and magnesium from lithium brine concentrate via continuous counter-current solvent extraction. Hydrometallurgy 2016, 162, 9–15. [Google Scholar] [CrossRef]
- Mohammad, A.F.; El-Naas, M.H.; Al-Marzouqi, A.H.; Suleiman, M.I.; Al Musharfy, M. Optimization of magnesium recovery from reject brine for reuse in desalination post-treatment. J. Water Process. Eng. 2019, 31, 100810. [Google Scholar] [CrossRef]
- Bang, J.H.; Chae, S.C.; Lee, S.W.; Kim, J.W.; Song, K.; Kim, J.; Kim, W. Sequential carbonate mineralization of desalination brine for CO2 emission reduction. J. CO2 Util. 2019, 33, 427–433. [Google Scholar] [CrossRef]
- Wang, J.; Ye, X.; Zhang, Z.; Ye, Z.L.; Chen, S. Selection of cost-effective magnesium sources for fluidized struvite crystallization. J. Environ. Sci. 2018, 70, 144–153. [Google Scholar] [CrossRef]
- Freitas de Alvarenga, R.A.; Galindro, B.M.; Helpa, C.F.; Soares, S.R. The recycling of oyster shells: An environmental analysis using life cycle assessment. J. Environ. Manag. 2012, 106, 102–109. [Google Scholar] [CrossRef]
- Hamester, M.R.R.; Balzer, P.S.; Becker, D. Characterization of calcium carbonate obtained from oyster and mussel shells and incorporation in polypropylene. Mater. Res. 2012, 15, 204–208. [Google Scholar] [CrossRef] [Green Version]
- Tongamp, W.; Kano, J.; Zhang, Q.; Saito, F. Simultaneous treatment of PVC and oyster-shell wastes by mechanochemical means. Waste Manag. 2008, 28, 484–488. [Google Scholar] [CrossRef]
- Yoon, G.L.; Kim, B.T.; Kim, B.O.; Han, S.H. Chemical–mechanical characteristics of crushed oyster-shell. Waste Manag. 2003, 23, 825–834. [Google Scholar] [CrossRef]
- Lertwattanaruk, P.; Makul, N.; Siripattarapravat, C. Utilization of ground waste seashells in cement mortars for masonry and plastering. J. Environ. Manag. 2012, 111, 133–141. [Google Scholar] [CrossRef]
- Lertcumfu, N.; Jaita, P.; Manotham, S.; Jarupoom, P.; Eitssayeam, S.; Pengpat, K.; Rujijanagul, G. Properties of calcium phosphates ceramic composites derived from natural materials. Ceram. Int. 2016, 42, 10638–10644. [Google Scholar] [CrossRef]
- Chen, B.; Peng, X.; Wang, J.G.; Wu, X. Laminated microstructure of Bivalva shell and research of biomimetic ceramic/polymer composite. Ceram. Int. 2004, 30, 2011–2014. [Google Scholar] [CrossRef]
- Hoque, M.E. Processing and Characterization of Cockle Shell Calcium Carbonate (CaCO3) Bioceramic for Potential Application in Bone Tissue Engineering. J. Mater. Sci. 2013, 2, 132. [Google Scholar] [CrossRef]
- Jung, S.; Heo, N.S.; Kim, E.J.; Oh, S.Y.; Lee, H.U.; Kim, I.T.; Hur, J.; Lee, G.W.; Lee, Y.C.; Huh, Y.S. Feasibility test of waste oyster shell powder for water treatment. Process Saf. Environ. 2016, 102, 129–139. [Google Scholar] [CrossRef]
- Kwon, H.B.; Lee, C.W.; Jun, B.S.; Yun, J.d.; Weon, S.Y.; Koopman, B. Recycling waste oyster shells for eutrophication control. Resour. Conserv. Recycl. 2004, 41, 75–82. [Google Scholar] [CrossRef]
- Ma, K.W.; Teng, H. CaO Powders from Oyster Shells for Efficient CO2 Capture in Multiple Carbonation Cycles. J. Am.Ceram. Soc. 2010, 93, 221–227. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhao, C.S.; Chen, H.C.; Duan, L.B.; Chen, X.P. CO2 Capture Behavior of Shell during Calcination/Carbonation Cycles. Chem. Eng. Technol. 2009, 32, 1176–1182. [Google Scholar] [CrossRef]
- Viriya-Empikul, N.; Krasae, P.; Puttasawat, B.; Yoosuk, B.; Chollacoop, N.; Faungnawakij, K. Waste shells of mollusk and egg as biodiesel production catalysts. Bioresour. Technol. 2010, 101, 3765–3767. [Google Scholar] [CrossRef] [PubMed]
- Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Worawanitchaphong, P.; Trongyong, S. Calcium oxide derived from waste shells of mussel, cockle, and scallop as the heterogeneous catalyst for biodiesel production. Sci. World J. 2013, 2013, 460923. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, M.; Liu, X.; Wang, P.; Xi, Z. Experiment and Optimization for Simultaneous Carbonation of Ca(2+) and Mg(2+) in A Two-phase System of Insoluble Diisobutylamine and Aqueous Solution. Sci. Rep. 2015, 5, 10862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wang, W.; Wang, M.; Wang, P. Experimental Study of CO2 Mineralization in Ca2+-Rich Aqueous Solutions Using Tributylamine as an Enhancing Medium. Energy Fuels 2014, 28, 2047–2053. [Google Scholar] [CrossRef]
- Zhou, Z.; Liang, F.; Qin, W.; Fei, W. Coupled reaction and solvent extraction process to form Li2CO3: Mechanism and product characterization. AIChE J. 2014, 60, 282–288. [Google Scholar] [CrossRef]
- Chen, G.; Song, X.; Dong, C.; Sun, S.; Sun, Z.; Yu, J. Mineralizing CO2 as MgCO3·3H2O Using Abandoned MgCl2 Based on a Coupled Reaction–Extraction–Alcohol Precipitation Process. Energy Fuels 2016, 30, 7551–7559. [Google Scholar] [CrossRef]
- Li, Y.; Song, X.; Chen, G.; Sun, S.; Xu, Y.; Yu, J. Extraction of hydrogen chloride by a coupled reaction-solvent extraction process. Front. Chem. Sci. Eng. 2015, 9, 479–487. [Google Scholar] [CrossRef]





| Element | Cl | Mg | Na | S | K | Al | Br |
|---|---|---|---|---|---|---|---|
| Mass fraction/% | 18.24 | 4.76 | 4.15 | 2.71 | 1.35 | 0.54 | 0.25 |
| Element | Ca | Na | S | Cl | Mg | Si | Al |
|---|---|---|---|---|---|---|---|
| Mass fraction/% | 21.29 | 0.64 | 0.20 | 0.42 | 0.21 | 0.09 | 0.05 |
| Compound | Mg | Ca | Si | S | Al |
|---|---|---|---|---|---|
| Wt% | 50.13 | 0.996 | 0.059 | 0.035 | 0.042 |
| Compound | Cl | Ca | Mg | Na | S |
|---|---|---|---|---|---|
| Wt% | 52.79 | 31.38 | 0.694 | 0.622 | 0.217 |
| T/(K) | c(R3N·H+)(o)/(mol/L) | c(CaCl2)(w)/(mol /L) | c(R3N)(o)/(mol/L) | ln K |
|---|---|---|---|---|
| 353.15 | 0.032 | 0.603 | 2.065 | 7.798 |
| 343.15 | 0.046 | 0.594 | 2.052 | 7.081 |
| 333.15 | 0.066 | 0.579 | 2.034 | 6.313 |
| 323.15 | 0.086 | 0.564 | 2.012 | 5.736 |
| 318.15 | 0.106 | 0.549 | 1.992 | 5.270 |
| 302.15 | 0.139 | 0.524 | 1.959 | 4.642 |
| Thermodynamic Functions (298.15 K) | CaCO3 | HCl | CaCl2 | H2O | CO2 |
|---|---|---|---|---|---|
| ΔfHm (KJ/mol) | −1206.92 | −92.307 | −795.4 | −285.83 | −393.509 |
| ΔfGm (KJ/mol) | −1128.79 | −95.299 | −748.8 | −237.129 | −394.359 |
| Sm (J/mol/K) | 92.9 | 186.908 | 108.4 | 69.91 | 213.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, W.; Yang, Y.; Yang, D.; Arowo, M.; Wu, S.; He, Y.; Zeng, Q. A Novel Eco-Friendly Circular Approach to Comprehensive Utilizing Bittern Waste and Oyster Shell. Processes 2023, 11, 1209. https://doi.org/10.3390/pr11041209
Pan W, Yang Y, Yang D, Arowo M, Wu S, He Y, Zeng Q. A Novel Eco-Friendly Circular Approach to Comprehensive Utilizing Bittern Waste and Oyster Shell. Processes. 2023; 11(4):1209. https://doi.org/10.3390/pr11041209
Chicago/Turabian StylePan, Wei, Yucheng Yang, Daomao Yang, Moses Arowo, Shuai Wu, Yingjie He, and Qingyou Zeng. 2023. "A Novel Eco-Friendly Circular Approach to Comprehensive Utilizing Bittern Waste and Oyster Shell" Processes 11, no. 4: 1209. https://doi.org/10.3390/pr11041209
APA StylePan, W., Yang, Y., Yang, D., Arowo, M., Wu, S., He, Y., & Zeng, Q. (2023). A Novel Eco-Friendly Circular Approach to Comprehensive Utilizing Bittern Waste and Oyster Shell. Processes, 11(4), 1209. https://doi.org/10.3390/pr11041209





