Thermal Processing of Acidified Vegetables: Effect on Process Time-Temperature, Color and Texture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing Treatments
2.2.1. Preparation
2.2.2. Acidification
2.2.3. Measurement of pH and Acidity
2.2.4. Process Establishment
- Sterilization process:Sterilization process lethality, Fo (low-acid vegetable) = ∫ 10((T−121.1)/z) dt
- Pasteurization process:Pasteurization process lethality, Po (acidified vegetable) = ∫ 10((T−90.0)/z) dt
2.3. Color
2.4. Texture
2.5. Post Process De-Acidification Treatment (Radish)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Commercial Sterilization and Pasteurization Process Times
3.2. Texture
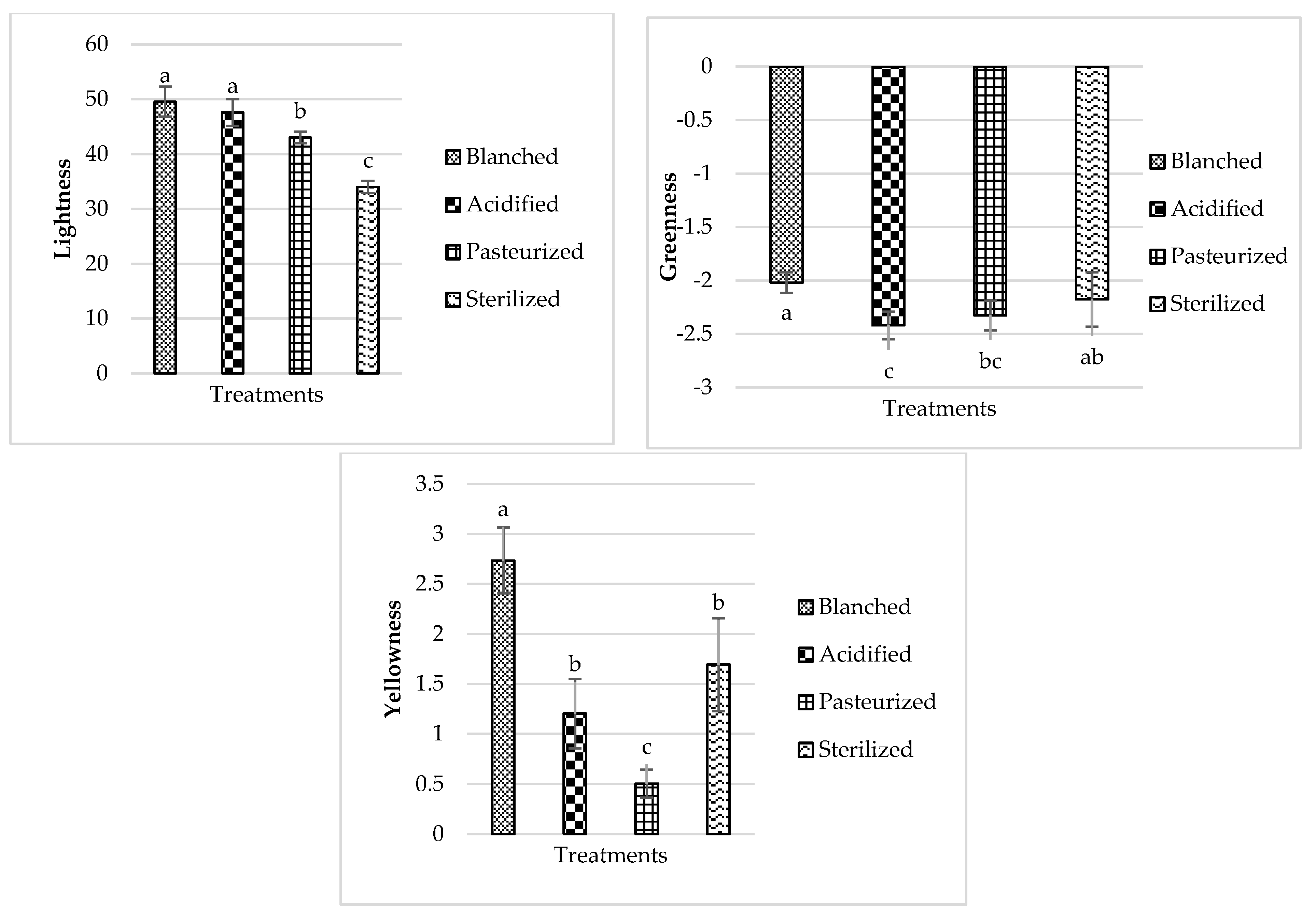
3.3. Color
3.3.1. Beets
3.3.2. Carrot
3.3.3. Turnip
3.3.4. Radish
3.4. De-Acidification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ramaswamy, H.S.; Marcotte, M. Food Processing: Principles and Applications, 1st ed.; Taylor and Francis: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2005; p. 440. [Google Scholar]
- Chen, C.R.; Ramaswamy, H.S. Modeling and optimization of constant retort temperature (CRT) thermal processing using coupled neural network and genetic algorithm. J. Food Process Eng. 2002, 25, 351–379. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.P.; Ramaswamy, H.S. Effect of Processing Conditions on Quality of Green Beans Subjected to Reciprocating Agitation Thermal Processing. Food Res. Int. 2015, 78, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, H.S.; Grabowski, S. Influence of entrapped air on the heating behavior of a model food packaged in semi-rigid plastic containers during thermal processing. LWT Food Sci. Technol. 1996, 29, 82–93. [Google Scholar] [CrossRef]
- Ramaswamy, H.S.; Awuah, G.B.; Simpson, B.K. Heat transfer and lethality considerations in aseptic processing of liquid particle mixtures: A review. Crit. Rev. Food Technol. 1997, 37, 253–286. [Google Scholar] [CrossRef]
- Breidt, F.; Kay, K.; Osborne, J.; Ingham, B.; Arritt, F. Thermal processing of acidified foods with pH 4.1 to pH 4.6. Food Prot. Trends 2014, 34, 132–138. [Google Scholar]
- Odlaug, T.E.; Pflug, I.J. Thermal destruction of Clostridium botulinum spores suspended in tomato juice in aluminum thermal death time tubes. Appl. Environ. Microbiol. 1977, 34, 23–29. [Google Scholar] [CrossRef]
- Ramaswamy, H.S. Novel food technologies for enhancing food security and food safety. Ethiop. J. Appl. Sci. Technol. 2013, 23, 17–25. [Google Scholar]
- Rattan, N.S.; Ramaswamy, H.S. Comparison of free/bi-axial, fixed axial, end-over-end and static thermal processing effects on process lethality and quality changes in canned potatoes. LWT Food Sci. Technol. 2014, 58, 150–157. [Google Scholar] [CrossRef]
- Tola, Y.B.; Ramaswamy, H.S. Thermal destruction kinetics of Bacillus licheniformis spores in carrot juice extract as influenced by pH, type of acidifying agent and heating method. LWT Food Sci. Technol. 2014, 56, 131–137. [Google Scholar] [CrossRef]
- Tola, Y.B.; Ramaswamy, H.S. Combined effects of high pressure, moderate heat and pH on the inactivation kinetics of Bacillus licheniformis spores in carrot juice. Food Res. International. 2014, 62, 50–58. [Google Scholar] [CrossRef]
- Azizi, A.; Ranganna, S. Thermal processing of acidified vegetables. J. Food Sci. Technol. 1993, 30, 422–428. [Google Scholar]
- Tola, Y.B.; Ramaswamy, H.S. Microbiological Design and Validation of Thermal and High Pressure Processing of Acidified Carrots and Assessment of Product Quality. J. Food Process. Preserv. 2015, 39, 2991–3004. [Google Scholar] [CrossRef]
- Tola, Y.B.; Ramaswamy, H.S. Novel processing methods: Updates on acidified vegetables in thermal processing. Curr. Opin. Food Sci. 2018, 23, 64–69. [Google Scholar] [CrossRef]
- Smith, A.; Stratton, J. Acidified foods: Food processing for entrepreneurs series. In Institute of Agricultural and Natural Resources; University of Nebraska Lincoln: Lincoln, NE, USA, 2007. [Google Scholar]
- Juliao, P.C.; Maslanka, S.; Dykes, J.; Gaul, L.; Bagdure, S.; Granzow-Kibiger, L.; Barzilay, E.J. National outbreak of type a foodborne botulism associated with a widely distributed commercially canned hot dog chili sauce. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 56, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Tola, Y.B.; Ramaswamy, H.S. Evaluation of high pressure (HP) treatment for rapid and uniform pH reduction in carrots. J. Food Eng. 2013, 116, 900–909. [Google Scholar] [CrossRef]
- Barron, F.H.; Fraser, A.M. Acidified foods: Food safety considerations for food processors. Food Ind. 2013, 231–239. [Google Scholar]
- García-Pérez, J.V.; Cárcel, J.A.; Benedito, J.; Mulet, A. Power Ultrasound Mass Transfer Enhancement in Food Drying. Food Bioprod. Process. 2007, 85, 247–254. [Google Scholar] [CrossRef]
- Rojas, M.L.; Alvim, I.D.; Augusto, P.E.D. Incorporation of microencapsulated hydrophilic and lipophilic nutrients into foods by using ultrasound as a pre-treatment for drying: A prospective study. Ultrason. Sonochem. 2019, 54, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Shi, S.; Wan, X. Impact of ultrasonic-assisted extraction on the chemical and sensory quality of tea infusion. J. Food Eng. 2006, 74, 557–560. [Google Scholar] [CrossRef]
- Mashkour, M.; Maghsoudlou, Y.; Kashaninejad, M.; Aalami, M. Effect of ultrasound pretreatment on iron fortification of potato using vacuum impregnation. J. Food Process. Preserv. 2018, 42, e13590. [Google Scholar] [CrossRef]
- Ashokkumar, M. Theoretical and Experimental Sonochemistry Involving Inorganic Systems; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Miano, A.C.; Rojas, M.L.; Augusto, P.E.D. Structural changes caused by ultrasound pretreatment: Direct and indirect demonstration in potato cylinders. Ultrason. Sonochem. 2019, 52, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.C.; Parker, M.L.; Smith, A.C.; Waldron, K.W. Pectin Distribution at the Surface of Potato Parenchyma Cells in Relation to Cell−Cell Adhesion. J. Agric. Food Chem. 2001, 49, 4364–4371. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Lei, R.; Ryan, J.; Arrutia Rodriguez, F.; Rastall, B.; Chatzifragkou, A.; Binner, E. Understanding the influence of processing conditions on the extraction of rhamnogalacturonan-I “hairy” pectin from sugar beet pulp. Food Chem. X 2019, 2, 100026. [Google Scholar] [CrossRef] [PubMed]
- Sila, D.N.; Smout, C.; Vu, S.T.; Van Loey, A.; Hendrickx, M. Influence of pretreatment conditions on the texture and cell wall components of carrots during thermal processing. J. Food Sci. 2005, 70, E85–E91. [Google Scholar] [CrossRef]
- Finotti, E.; Bertone, A.; Vivanti, V. Balance between nutrients and anti-nutrients in nine Italian potato cultivars. Food Chem. 2006, 99, 698–701. [Google Scholar] [CrossRef]
- Moreira, L.A.; Oliveira, F.A.R.; Oliveira, J.C.; Singh, R.P. Textural changes in vegetables during thermal processing. II. Effects of acidification and selected pretreatments on texture of turnips. J. Food Process. Preserv. 1994, 18, 497–508. [Google Scholar] [CrossRef]
- Paciulli, M.; Medina-Meza, I.G.; Chiavaro, E.; Barbosa-Cánovas, G.V. Impact of thermal and high pressure processing on quality parameters of beetroot (Beta vulgaris L.). LWT-Food Sci. Technol. 2016, 68, 98–104. [Google Scholar] [CrossRef]
- Park, S.-O.; Kim, W.-K.; Park, D.-J.; Lee, S.-J. Effect of blanching time on the quality characteristics of elderly-friendly kkakdugi. Food Sci. Biotechnol. 2017, 26, 419–425. [Google Scholar] [CrossRef]
- Tola, Y.B.; Ramaswamy, H.S. Thermal processing principles. In Food Biochemistry and Food Processing, 2nd ed.; Simpson, B.K., Ed.; Willey-Blackwell: Hoboken, NJ, USA, 2012; pp. 725–745. [Google Scholar]
- Ramaswamy, H.S.; Abbatemarco, C.A.; Sablani, S.S. Heat transfer rates in a canned food model as influenced by processing in an end-over-end rotary steam/air retort. J. Food Proc. Preserv. 1993, 17, 269–286. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Peng, Z.; Zhao, Y.; Wu, K.; Zhou, N.; Bai, W. The impact of ultrasonic treatment on blueberry wine anthocyanin color and its In-vitro anti-oxidant capacity. Food Chem. 2020, 333, 127455. [Google Scholar] [CrossRef]
- Chen, C.; Marcotte, M.; Taherian, A. Kinetic modeling of texture properties of Bologna sausage under cooking conditions. Int. J. Food Prop. 2009, 12, 252–260. [Google Scholar] [CrossRef]
- Vervoort, L.; Van der Plancken, I.; Grauwet, T.; Verlinde, P.; Matser, A.; Hendrickx, M.; Van Loey, A. Thermal versus high pressure processing of carrots: A comparative pilot-scale study on equivalent basis. Innov. Food Sci. Emerg. Technol. 2012, 15, 1–13. [Google Scholar] [CrossRef]
- Zivanovic, S.; Buescher, R.; Kim, S. Mushroom texture, cell wall composition, color, and ultrastructure as affected by pH and temperature. J. Food Sci. 2003, 68, 1860–1865. [Google Scholar] [CrossRef]
- Fraeye, I.; De Roeck, A.; Duvetter, T.; Verlent, I.; Hendrickx, M.; Van Loey, A. Influence of pectin properties and processing conditions on thermal pectin degradation. Food Chem. 2007, 105, 555–563. [Google Scholar] [CrossRef]
- Herbach, K.; Stintzing, F.; Carle, R. Impact of thermal treatment on color and pigment pattern of red beet (Beta vulgaris L.) preparations. J. Food Sci. 2004, 69, C491–C49842. [Google Scholar] [CrossRef]
- Manchali, S.; Murthy, K.N.C.; Nagaraju, S.; Neelwarne, B. Stability of Betalain Pigments of Red Beet. In Red Beet Biotechnology: Food and Pharmaceutical Applications; Neelwarne, B., Ed.; Springer: Boston, MA, USA, 2012; pp. 55–74. [Google Scholar]
- Latorre, M.E.; Bonelli, P.R.; Rojas, A.M.; Gerschenson, L.N. Microwave inactivation of red beet (Beta vulgaris L. var. conditiva) peroxidase and polyphenoloxidase and the effect of radiation on vegetable tissue quality. J. Food Eng. 2012, 109, 676–684. [Google Scholar] [CrossRef]
- Eyarkai Nambi, V.; Gupta, R.K.; Kumar, S.; Sharma, P.C. Degradation kinetics of bioactive components, antioxidant activity, colour and textural properties of selected vegetables during blanching. J. Food Sci. Technol. 2016, 53, 3073–3082. [Google Scholar] [CrossRef]
- Prieto-Santiago, V.; Cavia, M.M.; Alonso-Torre, S.R.; Carrillo, C. Relationship between color and betalain content in different thermally treated beetroot products. J. Food Sci. Technol. 2020, 57, 3305–3313. [Google Scholar] [CrossRef]
- Gonçalves, E.; Pinheiro, J.; Abreu, M.; Brandão, T.; Silva, C. Carrot (Daucus carota L.) peroxidase inactivation, phenolic content and physical changes kinetics due to blanching. J. Food Eng. 2010, 97, 574–581. [Google Scholar] [CrossRef]

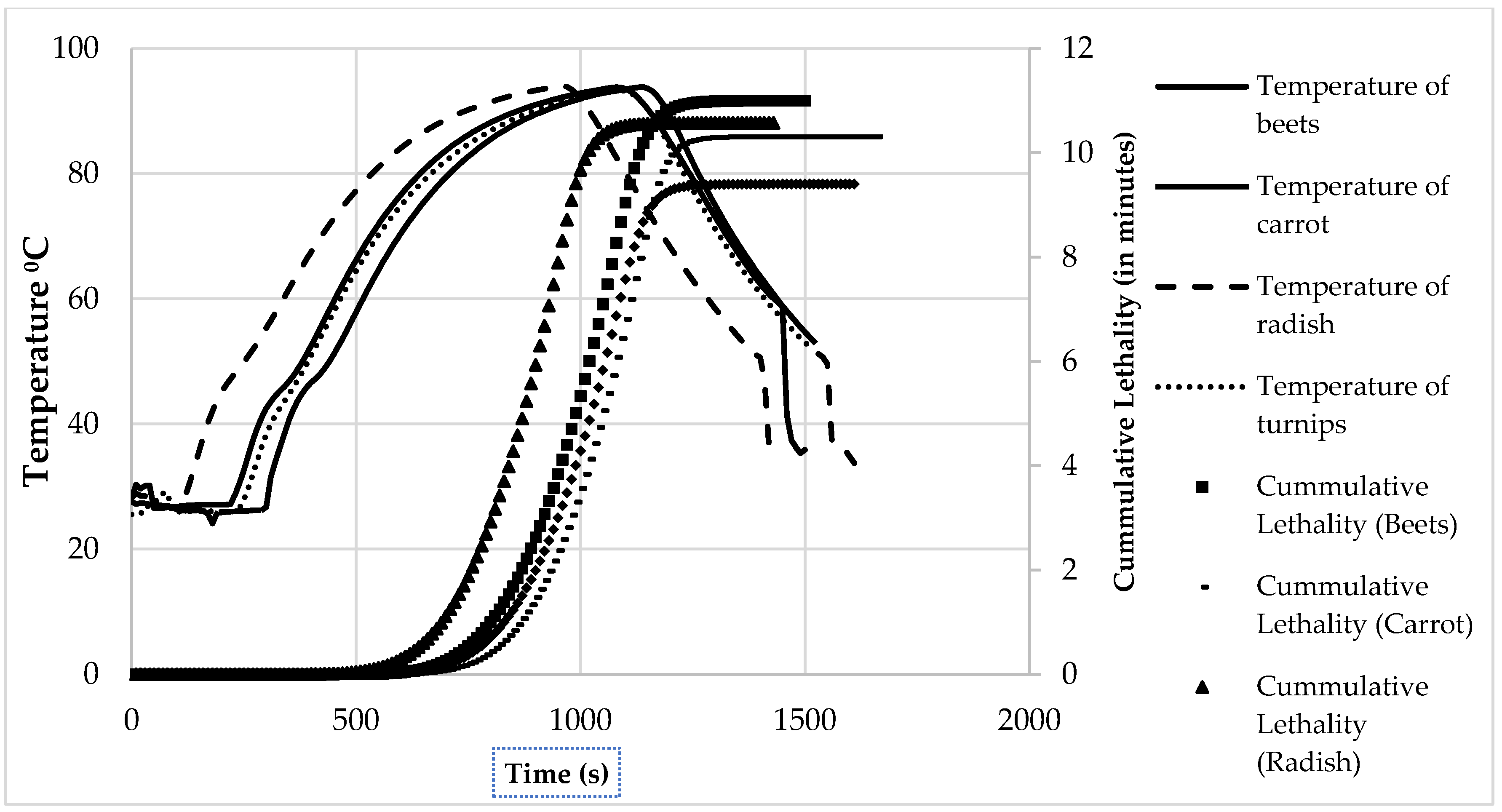
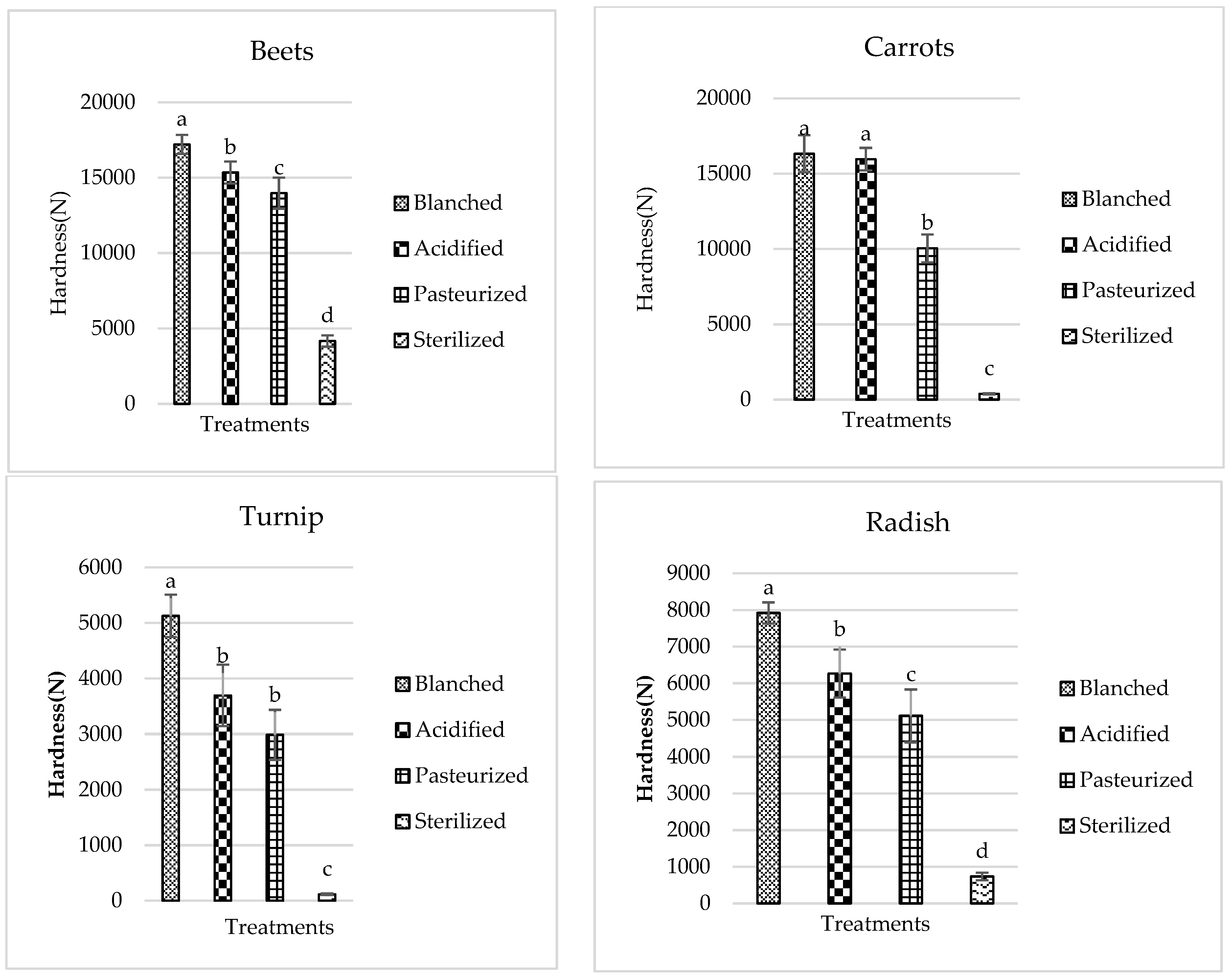
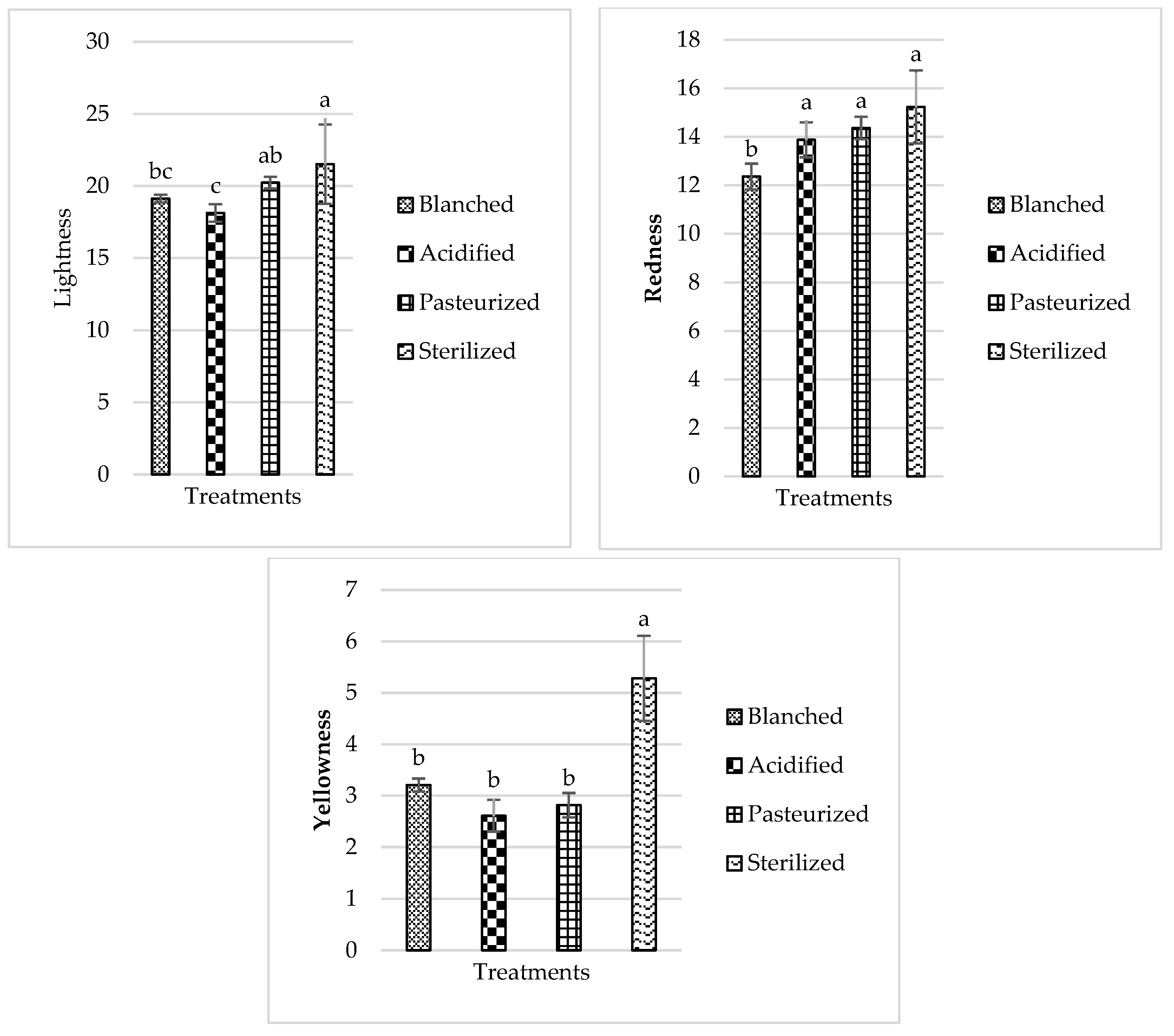
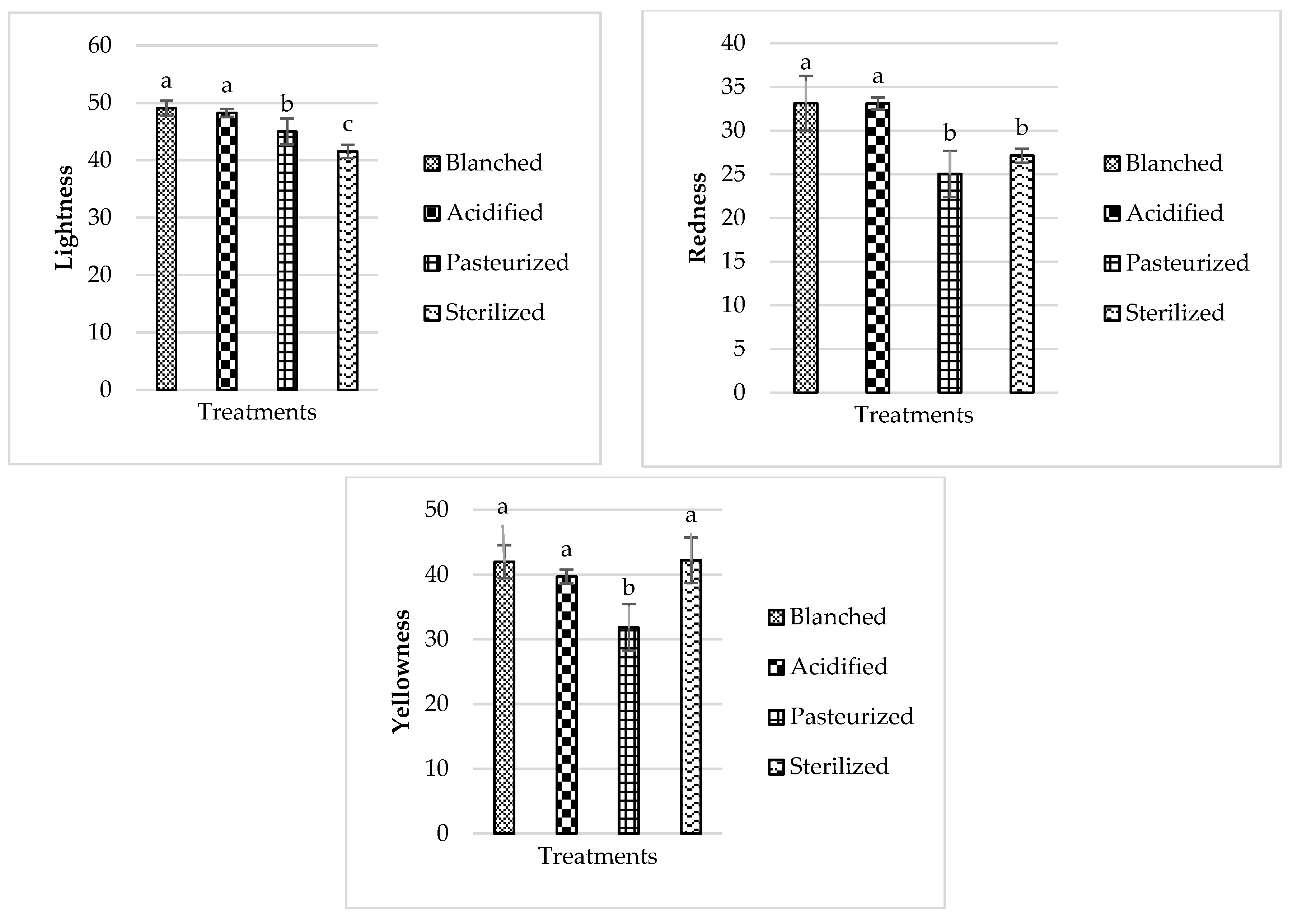

| Vegetable | Pasteurization Cook Time at 110 °C (min) | Sterilization Cook Time at 120 °C (min) |
|---|---|---|
| Carrots | 13.3 ± 1.06 | 11.9 ± 0.12 |
| Radishes | 11.7 ± 0.56 | 12.2 ± 0.16 |
| Beets | 12.7 ± 0.09 | 12.1 ± 0.07 |
| Turnip | 12.9 ± 0.35 | 12.1 ± 0.35 |
| Vegetable | L Value Mean | Std. Dev. | a Value Mean | Std. Dev. | b Value Mean | Std. Dev. | Hardness (N) Mean | Std. Dev. |
|---|---|---|---|---|---|---|---|---|
| Carrots | 52.9 | 4.10 | 39.0 | 4.37 | 49.5 | 4.68 | 32,800 | 1074 |
| Radish | 70.4 | 1.17 | –0.99 | 0.04 | 6.21 | 0.55 | 15,560 | 786 |
| Beets | 15.3 | 0.26 | 19.4 | 1.74 | 6.23 | 0.91 | 31,720 | 1237 |
| Turnip | 85.0 | 2.40 | –0.50 | 0.08 | 11.0 | 0.63 | 21,530 | 1171 |
| Time (min) | Acidity % | pH |
|---|---|---|
| 0 | 0.150 | 4.50 |
| 5.0 | 0.085 | 4.80 |
| 10 | 0.068 | 4.85 |
| 15 | 0.065 | 5.00 |
| 20 | 0.062 | 5.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, H.; Ramaswamy, H.S. Thermal Processing of Acidified Vegetables: Effect on Process Time-Temperature, Color and Texture. Processes 2023, 11, 1272. https://doi.org/10.3390/pr11041272
Singh H, Ramaswamy HS. Thermal Processing of Acidified Vegetables: Effect on Process Time-Temperature, Color and Texture. Processes. 2023; 11(4):1272. https://doi.org/10.3390/pr11041272
Chicago/Turabian StyleSingh, Harsimar, and Hosahalli S. Ramaswamy. 2023. "Thermal Processing of Acidified Vegetables: Effect on Process Time-Temperature, Color and Texture" Processes 11, no. 4: 1272. https://doi.org/10.3390/pr11041272
APA StyleSingh, H., & Ramaswamy, H. S. (2023). Thermal Processing of Acidified Vegetables: Effect on Process Time-Temperature, Color and Texture. Processes, 11(4), 1272. https://doi.org/10.3390/pr11041272











