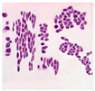
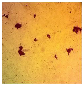
Abstract
The extract of P. amygdalus var. amara is known for its health benefits, which include lowering diabetes and heart disease risks. In eight human tumor cell lines, P. amygdalus var. amara demonstrated potent anti-cancer activity, including NB4, Huh-7, A-549, SKOV-3, PC-3, T-24, U937, and Hep-2. There was a notable change in the morphology of nearly all cancer cell lines, and cancer cells continued to exist. Incubation for 12 h, 24 h, 48 h, or 72 h resulted in the lowest viable cell concentration at 48 h, which was 34.65% lower than that of the non-treated cells. During exposure to the extract, the majority of cells lost their typical morphology and shrank in size. According to the cell viability data, the P. amygdalus var. amara treatment significantly decreased the cancer cells’ growth in most cancer cell lines when doses and time were taken into consideration.
1. Introduction
Prunus amygdalus (P. amygdalus) is a prevailing plant imported from all over the world and is cultivated in extensive areas in China [1]. It is one of the main varieties of almonds that are distinguished by their oil, which is rich in essential fatty acids such as linoleic acid and non-essential oleic acid. Moreover, it comprises numerous water- and fat-soluble vitamins, including vitamin A, thiamine, riboflavin, pyridoxal, and tocopherols [2,3,4]. Among its diverse health benefits, P. amygdalus var. amara reduces glucose homeostasis, oxidative stress, and inflammation so as to alleviate modifiable cardiovascular risk [5,6,7,8]. Although it contains amygdalin, an enzyme that breaks it down into deleterious components, along with subservient components such as hydrocyanic acid (HCN) and benzaldehyde when it is crushed or chewed, its application hasn’t been effective [9].
Amygdalin was first isolated from P. amygdalus var. amara seeds in 1830. Laetrile is a particular synonym of the amygdalin extract, which exists naturally in Prunus fruits’ pips such as apricots, peaches, plums, P. amygdalus var. amara, and raw nuts [10].
Typically, HCN and its salts’ derivatives are renowned as deleterious ingredients with strong adverse effects on living organisms [11,12], as a high dose of cyanide is lethal within minutes [13]. The known lethal dose of HCN is 1 mg/kg (by body weight), and several countries have enforced its restricted use in food and drink [14]. Hence, a convenient modification and treatment are imposed before utilization. Ultrasonic therapies are the most efficient and optimal treatment, with a 98.4% loss of cyanide when 700 W of ultrasonic power is used for 50 min, and there is no significant effect on the physicochemical properties [15].
Hypotheses concerning the importance of amygdalin as an anti-cancerous substance are controversial. Several theories suggest that neoplastic cells comprise plenteous β-glucosidases, which liberate hydrogen cyanide from vitamin B17 via hydrolysis. Natural tissues are apparently unaffected, as they contain low levels of β-glucosidase enzyme, as well as typically high levels of the converting enzyme rhodanese, which converts HCN into less poisonous thiocyanic acid salts. However, it was later reported that all neoplastic and normal cells contain traces of β-glucosidases and exact amounts of rhodanese [16].
Despite this pessimistic report on amygdalin, some studies reported that amygdalin has an anti-cancerous potential [15] that prompts programmed death along with prostate cancer [16], human cervical carcinoma [17], lowered effectiveness of kidney fibroblasts, as well as interstitial fibrosis [18], and inhibited bladder cancer cell development by the down-modulation of cell cycle regulatory proteins cdk2 and cyclin A [10,17,19].
Typically, bisphosphonates and phosphonates are steady analogues of pyrophosphates and phosphates, which appear to be a significant denomination of some chemicals used in pharmacognosy and by medicinal chemists [20].
Pyrophosphates are identified by carrying a P-O-P radix, whereas bisphosphonates possess the P-C-P radix, which is significantly more resistant to breakdown. Prodrugs of pyrophosphates had been more or less uniquely utilized as nucleoside analogues. The anti-cancer and antiviral potential of these prodrugs is due to the fact that these nucleoside analogues undergo sequential phosphorylation by the action of specific kinases, yielding nucleoside triphosphate metabolites that suppress the action of nucleic acid polymerases [21]. There is strong evidence that bisphosphonates have either forthright or circuitous anti-cancer effects on patients suffering from early breast cancer, prostate cancer, or typical multiple myeloma, demonstrating considerable survival benefits. Conveniently, adjuvant protocols employing N-containing bisphosphonates in integration therapy led to an augmented antitumor potential in breast tumors [22].
Prunasin is a cyanogenic glycoside related to amygdalin and belongs to the genus prunus. It is the glucoside of (R)-mandelonitrile in chemistry. Therefore, prunasin has been identified in species belonging to the genus prunus, including P. maximowiczii, Prunus japonica, as well as bitter almonds. Researchers discovered that the accumulation or lack of prunasin, as well as amygdalin, in the almond kernel, is capable of producing sweet and bitter genotypes [23].
Following the creation of (R)-prunasin, the product is additionally glycosylated into amygdalin using either isoform UGT94AF2 or UGT94AF1 [1]. The expression of UGTAF1/2 and prunasin hydrolases are found in a low concentration of (R)-prunasin in almond tissues. Moreover, it is essential to note that an α-glucosidase or prunasin hydrolase could convert (R)-prunasin to mandelonitrile, its precursor, which is able to be spontaneously or enzymatically hydrolyzed to benzaldehyde, as well as HCN [23,24]. Prunus mume extracts exhibit hepatoprotective, anti-inflammatory, antioxidant, antibacterial, and anti-cancerous properties. A survey of the antitumor actions of MK615 and other Prunus mume extracts was conducted, and information has been provided regarding the natural products observed in the extracts, such as ursolic acid and oleanic acid, and the action mechanisms of these extracts. MK615 was found to inhibit proliferation and induce apoptotic death in various types of cancer cells from all solid and hematological tumors [25]. This study was mainly conducted to reveal the in vitro assessment of the antiproliferative effect of alcoholic seed extractions on eight cell cultures of cancer cell lines, namely, NB4 (acute promyelocytic leukemia (APL)), Huh-7 (liver cancer), A-549 (non-small-cell lung cancer), SKOV-3 (ovarian cancer), PC-3 (prostate cancer), T-24 (urinary bladder cancer), U937 (lymphoma), and Hep-2 (head and neck cancer) using an MTT assay.
2. Materials and Methods
2.1. Collection and Extraction of P. amygdalus var. amara
The plant was obtained, identified, and matched to the coded voucher specimen (Eg-N. S42217) of the Cairo University Herbarium, Egypt. The plant material was typically collected in a 100 mL conical flask, and absolute ethanol was added in a 1:25 (m/v) solid/liquid ratio. The extraction was performed at a temperature of 34.4 °C using a water bath via reflux. The evaporation of the liquid extract was carried out at 50 °C to remove the excess ethanol remaining through the vacuum rotary evaporator. A desiccator was utilized to keep the extract inside until it was completely dry [26].
2.2. Cell Cultures
All carcinoma cells were obtained from the cell bank (Cell Biology Center—Cairo, Egypt). Eight cell cultures have been tested: NB4, Huh-7, A-549, SKOV-3, PC-3, T-24, U937, and Hep-2. Cells were cultured in DMEM medium supplemented with 100 mg/mL streptomycin and 100 units/mL penicillin, as well as 10% heat-inactivated fetal bovine serum, at 37 °C in a humidified, 5% (v/v) CO2 atmosphere [27].
2.3. Cell Viability
The MTT assay was used to determine whether plant extracts, as well as fractions of P. amygdalus var. amara, inhibited cancer cell proliferation. MDA-MB231 and MCF-7 cells that were exponentially expanding were seeded into 96-well plates (104 cells/mL per well) along with 100 µL of media [27] and enabled to attach for 24 h. After being exposed to different concentrations of P. amygdalus var. amara alcoholic seed extraction, cells were allowed to continue to incubate for 48 h. Cells in the control group only received media containing a maximum dose of 0.1% DMSO. The tested chemical medium was taken out, washed with 200 mL of phosphate-buffered saline (PBS), and then 20 mL of tetrazolium dye (MTT) reagent solution (5 mg/mL of MTT in PBS) was added. The mixture was then incubated for 4 h at 37 °C.
A LABTECH-FLUOstar Omega microplate reader (Ortenberg, Germany) was used to measure the absorbance after the medium was removed and 100 L of DMSO was added [28].
2.4. Percentage of Cell Viability
Given that cell viability is the percentage of living, healthy cells in a community, the cell viability percentage of a particular sample can be determined as follows: Cell proliferation refers to a cell’s capacity for reproducing itself through cell division, or cytokinesis. In accordance with the following equation, only healthy cells will split and proliferate, whereas damaged, dying, or dead cells will not [27].
where Ao (is the absorbance of cells treated with 0.1% DMSO medium), and At (is the absorbance of cells treated with the P. amygdalus var. amara extract). Moreover, 0.1% (v/v) DMSO in the medium was utilized as a negative control. Thus, every treatment was carried out three times. As a control, doxorubicin is utilized. IC50 numbers were determined in a GraphPad prism utilizing dose–response inhibition curves [27].
Cell viability% = [(Ao − At)/Ao) × 100] − 100
2.5. Statistical Analysis
IC50 values are presented as mean ± standard error of the mean (SEM) Statistical significance was considered when the p-value was ≤ 0.05.
3. Results
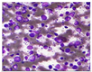
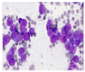
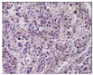
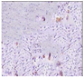
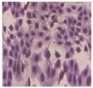
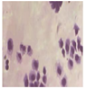
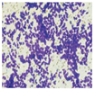
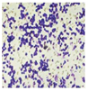
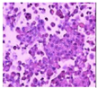
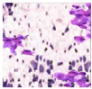
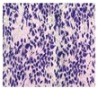
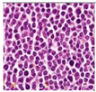
All plant samples were examined against eight cancer cell lines, and the anti-cancer impacts, as well as microscopic examination-stained cell line results, are summarized in Table 1, Table 2 and Table 3 and Figure 1 and Figure 2.

Table 1.
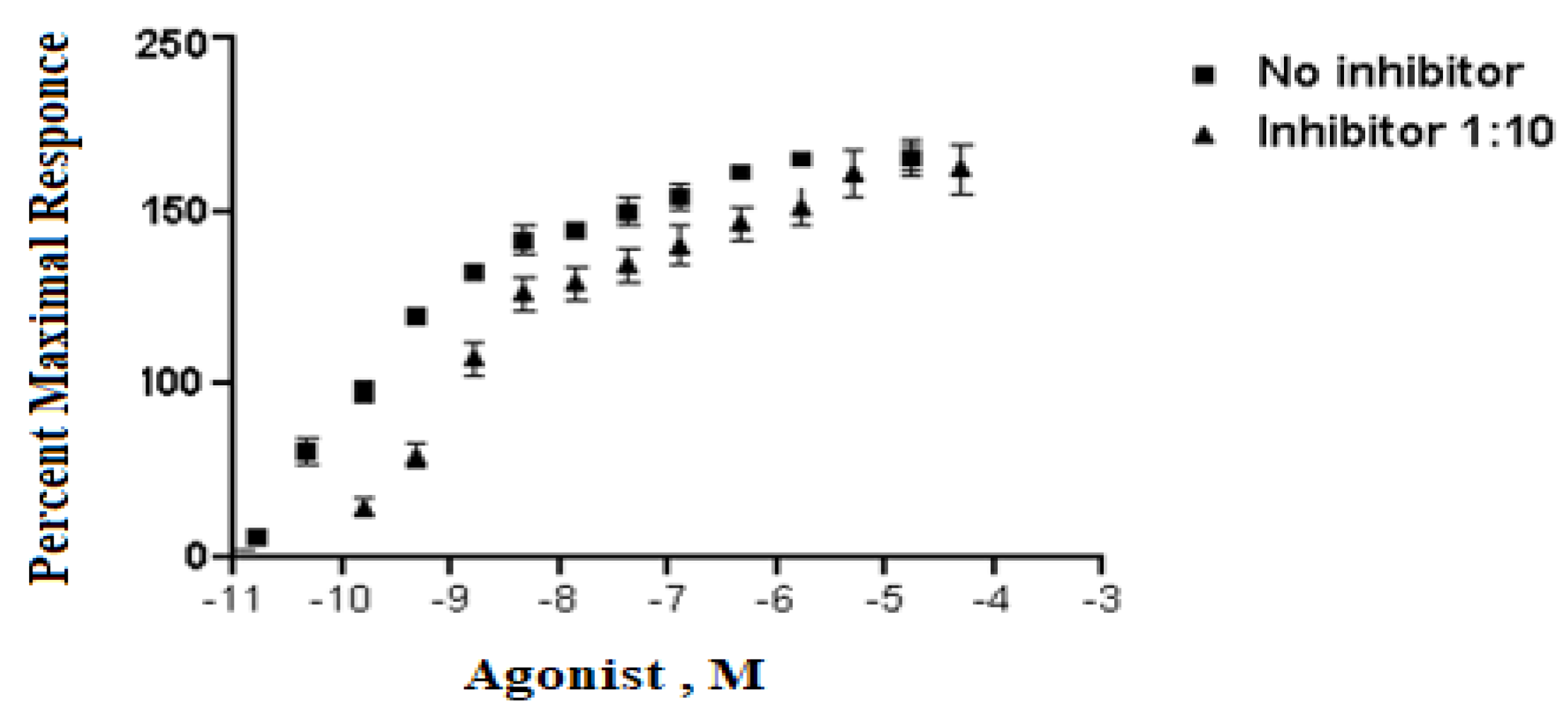
Analyzing dose–response data of P. amygdalus var. amara at various concentrations (5–300 µg/mL). A means measuring dose value without inhibitor, while B means measuring dose with inhibitor percent at 1:10. The X values mean agonist dose, and they are given in molar concentration, expressed in exponential notation (Figure 1). The Y values are responses in arbitrary units from Y1 to Y3 (replicates).

Table 2.
Microscopic examination-stained cell line study of P. amygdalus alcoholic seed extractions.

Table 3.
Anti-cancer impacts of P. amygdalus var. amara alcoholic seed extractions.
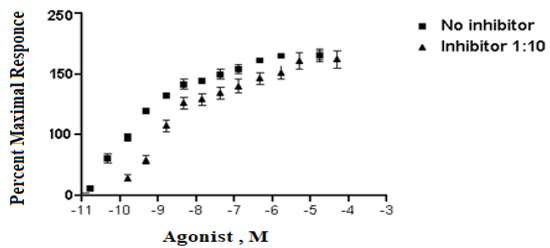
Figure 1.
The inhibitory effect curve of P. amygdalus var. amara at various concentrations (5–300 µg/mL).
Figure 2.
The inhibitory effect prism of P. amygdalus var. amara at different concentrations (5–300 µg/mL).
Table 3 and Figure 3 show the inhibitory impact of P. amygdalus var. amara on right cancer cell lines at several concentrations (5–300 µg/mL).
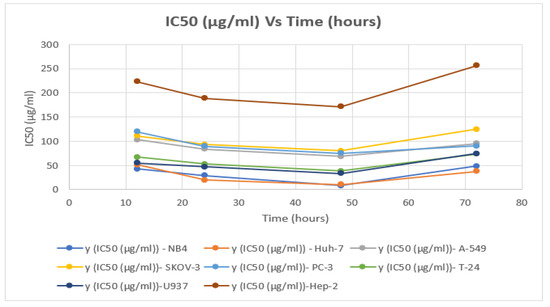
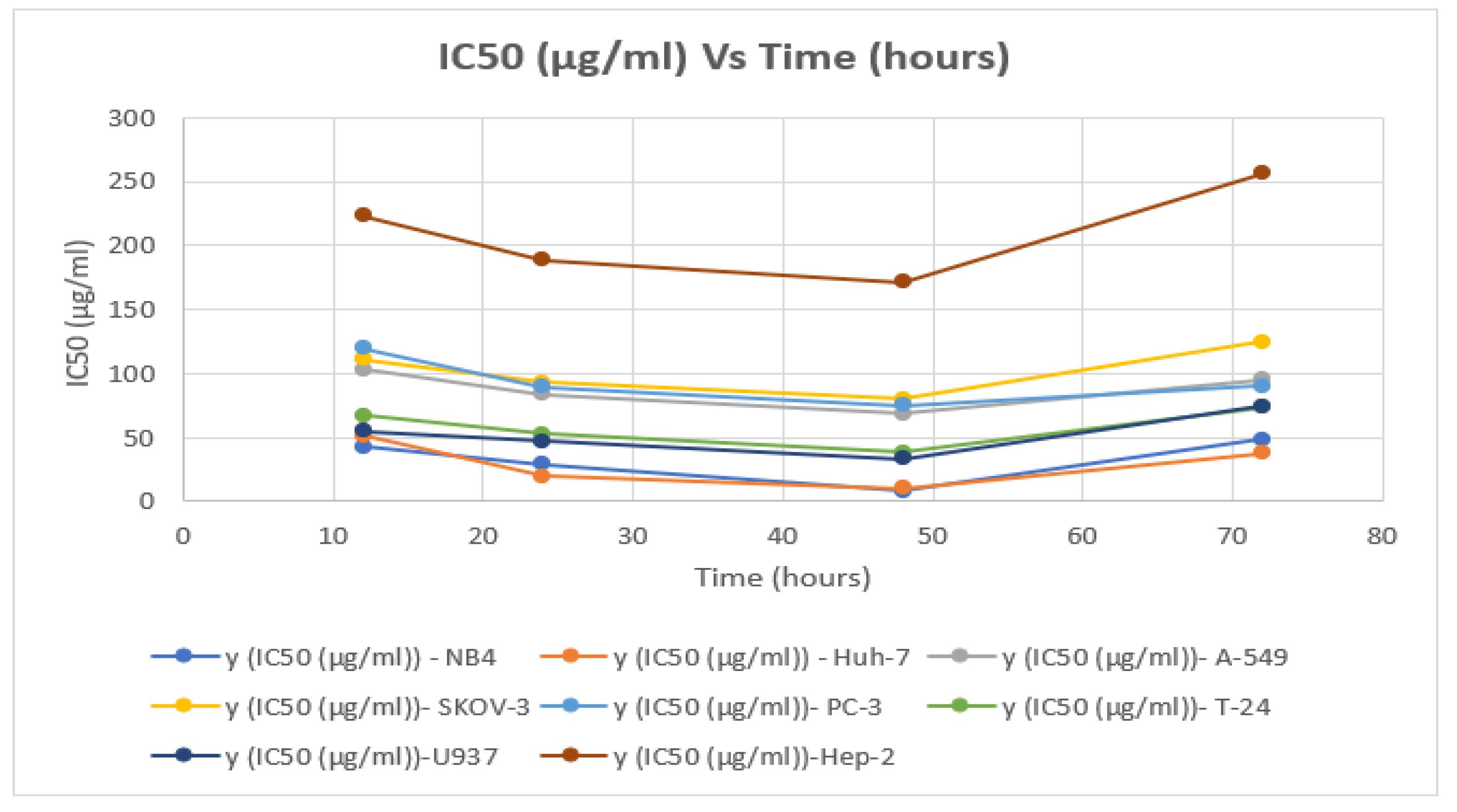
Figure 3.
Findings IC50 (5–300 µg/mL) of P. amygdalus var. amara with time (h) for 8 cancer cell lines.
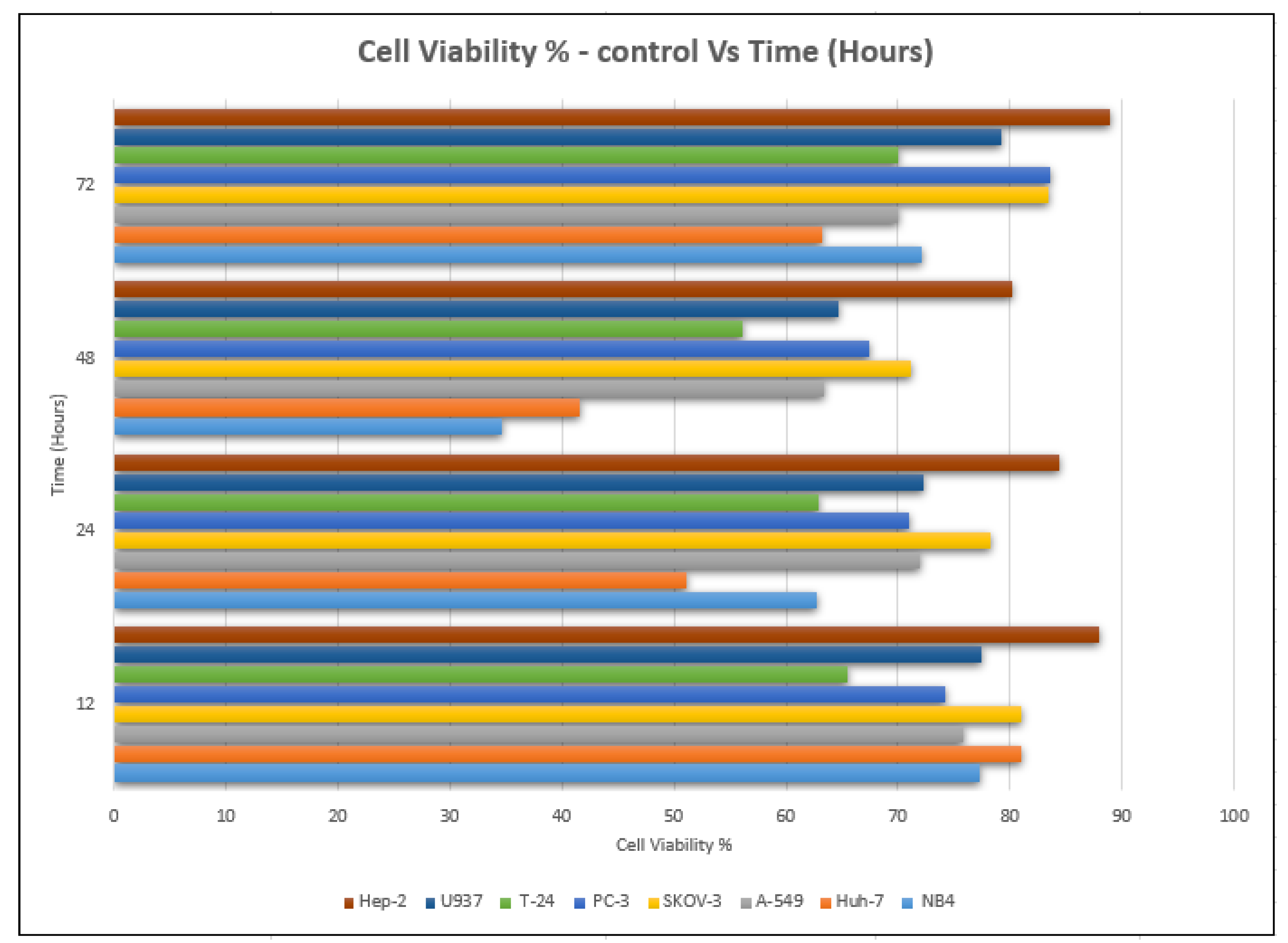
The lowest value of cell viability was recorded at an IC50 concentration of 8.22 ± 0.04 µg/mL, and it was 34.65%, which was compared to that of cells not treated with the extract (98.34%) after 48 h of the incubation period (Table 2 and Table 3). Moreover, it decreased after 24 h of incubation, as the value of cell viability decreased from 98.59% to 62.76% at 29.06 ± 0.141 µg/mL (Figure 4).
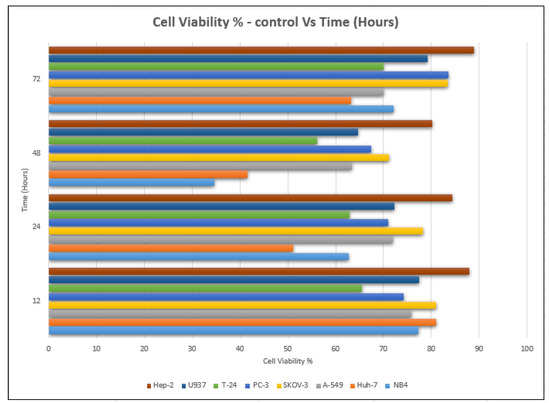
Figure 4.
Cell viability % with time (h) of P. amygdalus var. amara for 8 cancer cell lines.
Morphological changes in Huh-7 (hepatocellular carcinoma) were studied after being treated with P. amygdalus. The morphological alterations observed in Huh-7 cells are shown in Table 2. Changes in morphology were found to be concentration-dependent. Cells incubated with P. amygdalus for different exposure times showed reduced cell viability, which had a normal morphology; in the case of incubating liver cells with P. amygdalus for 48 h, the cells lost 48.94% of their viability compared to that of the controls: 98.11%. Cell adhesion capacity and cell viability were reduced to 58.35% as compared to that of the control cells (Table 3). The proportion of Huh-7 was found to be 98.34%. In general, most of the cells exposed to P. amygdalus lost their typical morphology and appeared to be smaller in size. P. amygdalus extracts showed very good IC50 values, indicating the potential of P. amygdalus to inhibit the expansion of these types of cancer cell lines (Table 3).
A significant decrease in the number of Huh-7 cells was not observed in aqueous P. amygdalus at all the tested concentrations (5–275 µg/mL). P. amygdalus extracts had an IC50 that showed very good potential to inhibit the expansion of this cancer cell line. A significant decrease in the number of Huh-7 cells was identified by aqueous P. amygdalus at all the examined concentrations (5–275 μg/mL). Microscopic studies (Table 2) showed that the extract in the concentrations used has a strong and significant (p < 0.05) effect on reducing the growth of Huh-7 cells during incubation periods of the cells with the extract. After 48 h of incubation, the IC50 was 10.21 ± 0.05 g/mL, and the cells lost 58.35% of their viability. After 24 h of incubation (IC50 has been determined to be 19.42 ± 0.09 g/mL), the viability dropped to 51.06% when it was compared to that of the control cells (Table 3).
The viability of cancer cells decreased in all cases after treatment, but it reached its lowest point after 48 h of incubation, where it was documented at 63.43%, as opposed to that of the untreated cells (98.94%) (Figure 4). According to the cell viability data, the P. amygdalus treatment significantly decreased the growth of cancer cells in a dose- and time-dependent manner. Using a phase-contrast microscope and different incubation times of 12, 24, 48, and 72 h, the researchers evaluated the cytotoxic activity of P. amygdalus against the human A-549 large-cell lung cancer at various concentrations (5–275 g/mL). The scale bar was set at 100 g/mL.
It was discovered that the viability of both P. amygdalus alcoholic seed extraction-treated large-cell lung cancer (A-549) cells decreased with an increase in treatment and incubation time (12, 24, 48, and 72 h) (Table 2).
The IC50 of P. amygdalus on A-549 large lung cancer cells at 24 h was found to be 103.06 ± 0.50 µg/mL, whereas the IC50 of P. amygdalus at 48 h was found to be 68.54 ± 0.32 µg/mL; at this value of IC50, 36.57% of the cells were found to be dead (Table 3).
The effect of plant extract on the SKOV-3 cell line showed direct anti-proliferative activity after four hours of incubation. Since the degree of the deficiency was not significant across all the experiment’s incubation times, there was no discernible impact on cell viability. Anti-cancer activity after 12 h was found to decrease in cell viability from 98.068% to 81% (a decrease of only 17.068%) at an IC50 concentration of 111.35 ± 0.55 μg/mL after a 12 h incubation period between an ovarian cancer cell line and the plant extract (Table 3). The best result was obtained at IC50 = 80.32 ± 0.40 μg/mL, after an incubation period of 48 h, where the decrease in cell viability was 26.87% (from 97.98 % to 71.11%). The results showed that increasing the incubation period to 72 h at a concentration curve of IC50 equal to 90.3 ± 0.43 µg/mL under the same conditions in the experiment did not decrease the vitality of the cells, but rather it increased its value, which confirms to us that increasing the incubation period to this period is not effective, as evidenced by the anti-cancer activity of the botanical extract of plant fruit seeds (Table 3). Light microscopic examination of the cells in the figures (Table 2) showed an antiproliferative effect on PC-3, which was greater than 67.46% at 75.22 ± 0.35 µg/mL of biological competence for cancer cell viability (Table 3).
The concentration value (75.22 µg/m of IC50) recorded the best value as an anti-cancer cell effect based on the viability of the cells, and it was 67.46% after an incubation period of the extract for 48 h. While the smallest effect occurred after an incubation period of 72 h, when the extract had a negative effect on the inhibition of cancer cells (Figure 3 and Figure 4).
Microscopic examination of T-24 (transitional cell carcinoma) was used to evaluate the anti-proliferative activity. Morphological differences observed in vitro in T-24 carcinomas are illustrated in Table 2. There were changes in the morphology of cells incubated with plant extract at different times. The capacity of the cancer cells was found to be reduced in viability to 36.57% compared with their viability in the first incubation period of 12 h.
Cancer cell growth and survival and the development of ablation-resistant prostate tumors were evaluated. As presented in Table 1 and Table 2, the plant extract had anti-cancer effects on the viability of T-24.
The proliferation of cancer cells decreased to 64.67% within 48 h and 72.32% as compared to that of the untreated cells. The MTT assay showed IC50 values with the effect of P. amygdalus extract on the viability of the U937 cell line. Table 2 and Figure 2 and Figure 3 indicate the logarithmic effect of different concentrations of P. amygdalus on cells as determined by Prism in the dose-response analysis. The treatment with P. amygdalus extracts on the proliferation of U937 cells showed a moderate effect; the IC50 values were 33.05 ± 0.16 µg/mL after 48 h of incubation and 47.3 ± 0.22 µg/mL after 24 h, but the IC50 increased with 72 h of incubation time as shown in Table 3. This also led to an increase in the viability of lymphoma cells, and thus the efficiency of the extract in treating U937 human lymphoma cancer cells decreased at an IC50 of 74.35 ± 0.36 µg/mL (Table 3).
P. amygdalus was added to Hep-2 cells, and the number of cell viability decreased with increasing incubation treatment times (12, 24 and 48 h), but there was an increase in it after incubation for 72 h (Table 3).
P. amygdalus did not give strong evidence for its use as an inhibitor of cancerous cells in Hep-2 tumors; the viability of cells was reduced to the lowest value after incubation for 48 h (to 80.14%) compared to that of the control cells (98.61%) (Figure 3 and Figure 4). A test conducted in vitro on the antiproliferative activity of P. amygdalus was performed using the MTT assay for the assessment of the cytotoxic influence of P. amygdalus on human Hep-2. The IC50 for P. amygdalus was discovered to influence Hep-2 cells by reducing the anti-proliferative action at various concentrations (5–275 g/mL), as well as durations of incubation (12, 24, 48, and 72 h) (Figure 1). Based on microscopic examination (Table 2 and Table 3), the proliferative activity was decreased, and the IC50 value was determined to be 171.05 ± 0.83 µg/mL, with 19.86% of cancer cells identified as dead after only 48 hours of incubation.
4. Discussion
The incidence of cancer is constantly rising due to contemporary changes in nutritional habits and lifestyle. Hence, there is a pressing need for the identification of novel plant extracts and therapeutics that could specifically act on cancer cells without harming normal cells [29].
As it is much safer, less toxic, and easily accessible, herbal medicine has become a source of anti-cancer constituents that fight carcinogenic cells by different mechanisms [30].
The anti-tumor influence of P. amygdalus var. amara was evaluated in the present investigation. The extract has been examined on eight cancer cell lines at different doses as well as responses. We examined the dose-response data to figure out the effect of the herbal remedies substance’s concentration on cancer cell viability across different concentrations (5–300 µg/mL) as well as incubation times. The photomicrographs of the plant extract in our study demonstrate a strong anti-cancer impact on human tumor cell lines. The study used various concentrations of 5, 10, 20, 25, 50, 75, 100, 150, 250, and 275 μg/mL. There are significant microscopic morphology variations and effects on the survival of cancer cells in almost all the stained cancer cell lines.
The extract used in the current study gave clear positive indicators for NB4 cancer cells using the MTT assay. As the experiment’s overall incubation times increased, the standard concentration had a significant impact on the cell viability percentage.
It is abundantly clear from the P. amygdalus extract’s cytotoxic effect on PC-3, which yields a variety of effects and values, that the plant extract is effective at getting rid of or lessening the number of cancer cells. Different effects and values give a clear indication that the plant extract has a positive effect on eliminating or reducing the number of cancer cells.
Additionally, the MTT assay was used to examine the extract’s cytotoxicity toward the T-24 cell line. Generally, the outcomes showed that the cytotoxicity of the plant material extract against T-24 cells evidences some of the cytotoxic compounds expressed by IC50. The most significant level of cytotoxic activity of plant extract was found to be 38.39 ± 0.18 µg/mL (IC50). These results indicated a decrease in the vitality of the cells by 56.09% after 48 h of incubation (Table 3). Furthermore, it showed a clear cytotoxic effect due to the treatment by increasing the incubation time, except for the last period of incubation (72 h).
The MTT assay was used to investigate the cytotoxic effect of P. amygdalus on human U937. Regardless of the concentration, a decrease in cell viability occurred in a time-dependent manner for concentrations that showed the extract’s efficacy as compared to those of the untreated controls (Figure 4). The observations would imply that P. amygdalus performs more action on U937 cells (Table 3). The viability showed a significant decrease at most of the incubation times (12, 24, and 48 h) with different concentrations of P. amygdalus, but there were no significant decreases in cell viability after 72 h of incubation with P. amygdalus.
Our study showed promising results, and P. amygdalus var. amara, which is similar to most of the prunus species in terms of anti-tumor influence due to various ingredients and via different mechanisms.
Recently, prunus species extracts have been reported to have a potential anti-cancer influence. The active component, gallic acid, which is extracted from P. macrocarpa fruit, typically revealed a significant role in colon adenocarcinoma, lung cancer, and leukemia cell lines by the induction of apoptosis [31,32].
Prunus armeniaca seeds were found to contain several cyanogenic glycosides that have immune-stimulant properties, antioxidant efficacy, and anti-cancer efficacy and could be utilized to suppress many types of cancers. Vitamin B17, in this respect, showed great potential to treat prostate cancer [32,33,34].
Comparably, in a rat model experiment, Verdi et al. displayed a robust protective efficacy of apricots (prunus species) against oxidative intestinal damage [35].
The potential inhibitory impact of the plant extract could be due to its active phytochemical ingredients, such as amygdalin, prunasin, hydroxybenzoic acid, polyphenolic compounds, flavanol glycosides, pyrophosphate derivatives, amino acids, fatty acids, and sterols. These phytochemical compounds may cause attenuation of the growth and proliferation in a dose-dependent pattern by inhibiting the expression of some oncogenic genes affecting the cancer cell cycle, modulating some apoptosis-related signaling molecules and proteins, or by having a direct cytotoxic effect [36,37,38].
5. Conclusions
In this study, photomicrographs of P. amygdalus extract at various concentrations demonstrated significant anti-cancer activity against human tumor cell lines: NB4, Huh-7, A-549, SKOV-3, PC-3, T-24, U937, and Hep-2. Furthermore, it had a significant morphological impact on the survival of cancer cells in almost all cancer cell lines. The lowest value of cell viability was recorded at the IC50 concentration of 8.22 ± 0.04 µg/mL, and it was 34.65% compared to cells that were not treated with the extract (98.34%) after a 48 h incubation period. Most of the cells exposed to P. amygdalus lost their typical morphology and appeared to be smaller in size. The cell viability results recommended that P. amygdalus treatment significantly restricted cancer cell growth in most of all cancer cell lines with respect to the dose and time.
Author Contributions
Conceptualization, M.H.F.S. and G.M.A.-M.; methodology, G.M.A.-M. and M.H.F.S.; software, G.M.A.-M.; validation, A.A.A. and A.F.A.; writing—original draft preparation, M.H.F.S. and G.M.A.-M.; writing—review and editing, M.A.; project administration, M.H.F.S.; funding acquisition, A.A.A., A.F.A. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by Institutional Fund Projects under the no. (IFP-A-2022-2-5-21). Therefore, authors gratefully acknowledge technical and financial support from the Ministry of Education and University of Hafr Al Batin, Saudi Arabia.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garita-Cambronero, J.; Palacio-Bielsa, A.; Cubero, J. Xanthomonas arboricola pv. pruni, causal agent of bacterial spot of stone fruits and almond: Its genomic and phenotypic characteristics in the X. arboricola species context. Mol. Plant Pathol. 2018, 19, 2053–2065. [Google Scholar] [CrossRef]
- Brennan, R. Introduction to Fruit Crops; Rieger, M., Ed.; Haworth Press: New York, NY, USA, 2007; Experimental Agriculture; Volume 43, p. 406. [Google Scholar]
- Gallier, S.; Gordon, K.C.; Singh, H. Chemical and structural characterisation of almond oil bodies and bovine milk fat globules. Food Chem. 2012, 132, 1996–2006. [Google Scholar]
- Jin, F.; Wang, J.; Regenstein, J.M.; Wang, F. Effect of Roasting Temperatures on the Properties of Bitter Apricot (Armeniaca sibirica L.) Kernel Oil. J. Oleo Sci. 2018, 67, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Kamil, A.; Chen, C.Y.O. Health Benefits of Almonds beyond Cholesterol Reduction. J. Agric. Food Chem. 2012, 60, 6694–6702. [Google Scholar] [CrossRef] [PubMed]
- Grundy, M.M.; Lapsley, K.; Ellis, P.R. A review of the impact of processing on nutrient bioaccessibility and digestion of almonds. Int. J. Food Sci. Technol. 2016, 51, 1937–1946. [Google Scholar] [CrossRef]
- Martínez, M.L.; Marín, M.A.; Gili, R.D.; Penci, M.C.; Ribotta, P.D. Effect of defatted almond flour on cooking, chemical and sensorial properties of gluten-free fresh pasta. Int. J. Food Sci. Technol. 2017, 52, 2148–2155. [Google Scholar] [CrossRef]
- Staugler, J.M.; Babin, M.C.; Matthews, M.C.; Brittain, M.K.; Perry, M.R. Development of a hydrogen cyanide inhalation exposure system and determination of the inhaled median lethal dose in the swine model. Inhal. Toxicol. 2018, 30, 195–202. [Google Scholar] [CrossRef]
- Karsavuran, N.; Charehsaz, M.; Celik, H.; Asma, B.M.; Yakıncı, C.; Aydin, A.; Aydın, A. Amygdalin in bitter and sweet seeds of apricots. Toxicol. Environ. Chem. 2014, 96, 1564–1570. [Google Scholar] [CrossRef]
- Shalayel, M.H.F. Beyond Laetrile (Vitamin B-17) Controversy-Antitumor Illusion or Revolution. Br. Biomed. Bull. 2017, 5, 296. [Google Scholar]
- Stamyr, K.; Mörk, A.K.; Johanson, G. Physiologically based pharmacokinetic modeling of hydrogen cyanide levels in human breath. Arch. Toxicol. 2015, 89, 1287–1296. [Google Scholar] [CrossRef]
- Brückner, A.; Raspotnig, G.; Wehner, K.; Meusinger, R.; Norton, R.A.; Heethoff, M. Storage and release of hydrogen cyanide in a chelicerate (Oribatula tibialis). Proc. Natl. Acad. Sci. USA 2017, 114, 3469–3472. [Google Scholar] [CrossRef]
- Kashala-Abotnes, E.; Sombo, M.-T.; Okitundu, D.L.; Kunyu, M.; Makila-Mabe, G.B.; Tylleskär, T.; Sikorskii, A.; Banea, J.-P.; Ngoyi, D.M.; Tshala-Katumbay, D.; et al. Dietary cyanogen exposure and early child neurodevelopment: An observational study from the Democratic Republic of Congo. PLoS ONE 2018, 13, e0193261. [Google Scholar] [CrossRef]
- Lounglawan, P.; Khungaew, M.; Suksombat, W. Silage Production from Cassava Peel and Cassava Pulp as Energy Source in Cattle Diets. J. Anim. Vet. Adv. 2011, 10, 1007–1011. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, M.; Chen, J.; Wang, M.; Yu, X. Reduction of cyanide content of bitter almond and its oil using different treatments. Int. J. Food Sci. Technol. 2019, 54, 3083–3090. [Google Scholar] [CrossRef]
- Duke, J.A. CRC Handbook of Medicinal Spices, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Fukuda, T.; Ito, H.; Mukainaka, T.; Tokuda, H.; Nishino, H.; Yoshida, T. Anti-tumor promoting effect of glycosides from Prunus persica seeds. Biol. Pharm. Bull. 2003, 26, 271–273. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Wang, F.; Hu, J.; Cui, A.; Wei, C.; Yang, Q.; Li, F. Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol. Immunotoxicol. 2013, 35, 43–51. [Google Scholar] [CrossRef]
- Makarević, J.; Rutz, J.; Juengel, E.; Kaulfuss, S.; Reiter, M.; Tsaur, I.; Bartsch, G.; Haferkamp, A.; Blaheta, R.A. Amygdalin blocks bladder cancer cell growth in vitro by diminishing cyclin A and cdk2. PLoS ONE 2014, 9, e105590. [Google Scholar] [CrossRef]
- Ebetino, F.H.; Sun, S.; Cherian, P.; Roshandel, S.; Neighbors, J.D.; Hu, E.; Dunford, J.E.; Sedghizadeh, P.P.; McKenna, C.E.; Srinivasan, V.; et al. Bisphosphonates: The role of chemistry in understanding their biological actions and structure-activity relationships, and new directions for their therapeutic use. Bone 2022, 156, 116289. [Google Scholar] [CrossRef]
- Rudge, E.S.; Chan, A.H.Y.; Leeper, F.J. Prodrugs of pyrophosphates and bisphosphonates: Disguising phosphorus oxyanions. RSC Med. Chem. 2022, 13, 375–391. [Google Scholar] [CrossRef]
- Shalayel, M.H.F.; Alsareii, S.A.; Elbashir, A.M. Bisphosphonates: From bone anti-resorptive to anti-cancer drugs. J. Med. Oncol. Ther. 2017, 2, 20–23. [Google Scholar]
- Sánchez-Pérez, R.; Belmonte, F.S.; Borch, J.; Dicenta, F.; Møller, B.L.; Jørgensen, K. Prunasin hydrolases during fruit development in sweet and bitter almonds. Plant Physiol. 2012, 158, 1916–1932.24. [Google Scholar] [CrossRef]
- Zhou, J.; Hartmann, S.; Shepherd, B.K.; Poulton, J.E. Investigation of the microheterogeneity and aglycone specificity-conferring residues of black cherry prunasin hydrolases. Plant Physiol. 2002, 129, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Anticancer properties of Prunus mume extracts (Chinese plum, Japanese apricot). J. Ethnopharmacol. 2020, 246, 112215. [Google Scholar] [CrossRef] [PubMed]
- Savić, I.; Nikolić, V.D.; Savić-Gajić, I.; Kundaković, T.; Stanojković, T.; Najman, S.J. Chemical composition and biological activity of the plum seed extract. Adv. Technol. 2016, 5, 38–45. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sodde, V.K.; Lobo, R.; Kumar, N.; Maheshwari, R.; Shreedhara, C.S. Cytotoxic activity of Macrosolen parasiticus (L.) Danser on the growth of breast cancer cell line (MCF-7). Pharmacogn. Mag. 2015, 11 (Suppl. 1), S156–S160. [Google Scholar]
- Nelson, V.K.; Sahoo, N.K.; Sahu, M.; Sudhan, H.H.; Pullaiah, C.P.; Muralikrishna, K.S. In Vitro anticancer activity of Eclipta alba whole plant extract on colon cancer cell HCT-116. BMC Compl. Med. 2020, 20, 355. [Google Scholar] [CrossRef]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer Plants: A Review of the Active Phytochemicals, Applications in Animal Models, and Regulatory Aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef]
- Sohi, K.K.; Mittal, N.; Hundal, M.K.; Khanduja, K.L. Gallic acid, an antioxidant, exhibits antiapoptotic potential in normal human lymphocytes: A Bcl-2 independent mechanism. J. Nutr. Sci. Vitaminol. 2003, 49, 221–227. [Google Scholar] [CrossRef]
- Yan, J.; Tong, S.; Li, J.; Lou, J. Preparative isolation and purification of amygdalin from Prunus armeniaca L. with high recovery by high-speed countercurrent chromatography. J. Liq. Chromatog. Rela. Technol. 2006, 29, 1271–1279. [Google Scholar] [CrossRef]
- Gomaa, E.Z. In vitro antioxidant, antimicrobial, and antitumor activities of bitter almond and sweet apricot (Prunus armeniaca L.) kernels. Food Sci. Biotechnol. 2013, 22, 455–463. [Google Scholar] [CrossRef]
- Madrau, M.A.; Piscopo, A.; Sanguinetti, A.M.; Del Caro, A.; Poiana, M.; Romeo, F.V.; Piga, A. Effect of drying temperature on polyphenolic content and antioxidant activity of apricots. Eur. Food Res. Technol. 2009, 228, 441. [Google Scholar] [CrossRef]
- Vardi, N.; Parlakpinar, H.; Ozturk, F.; Ates, B.; Gul, M.; Cetin, A.; Erdogan, A.; Otlu, A. Potent protective effect of apricot and β-carotene on methotrexate-induced intestinal oxidative damage in rats. Food Chem. Toxicol. 2008, 46, 3015–3022. [Google Scholar] [CrossRef]
- Park, H.-J.; Yoon, S.-H.; Han, L.-S.; Zheng, L.-T.; Jung, K.-H.; Uhm, Y.-K.; Lee, J.-H.; Jeong, J.-S.; Joo, W.-S.; Yim, S.-V.; et al. Amygdalin inhibits genes related to cell cycle in SNU-C4 human colon cancer cells. World J. Gastroenterol. 2005, 11, 5156–5161. [Google Scholar]
- Lee, H.M.; Moon, A. Amygdalin Regulates Apoptosis and Adhesion in Hs578T Triple-Negative Breast Cancer Cells. Biomol. Ther. 2016, 24, 62–66. [Google Scholar] [CrossRef]
- Singh, R.; Hasan, S.M.; Ved, A.; Prakash, O. An update on phytochemicals, nutritional composition, and pharmacological significance of prunus amygdalus batsch: A comprehensive review. Int. J. Pharm. Sci. Res. 2022, 13, 1463–1478. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).