Fundamentals and Manipulation of Bare Droplets and Liquid Marbles as Open Microfluidic Platforms
Abstract
1. Introduction
2. Fundamentals of Bare Droplets and Liquid Marbles
2.1. Bare Droplets on Solid Substrates
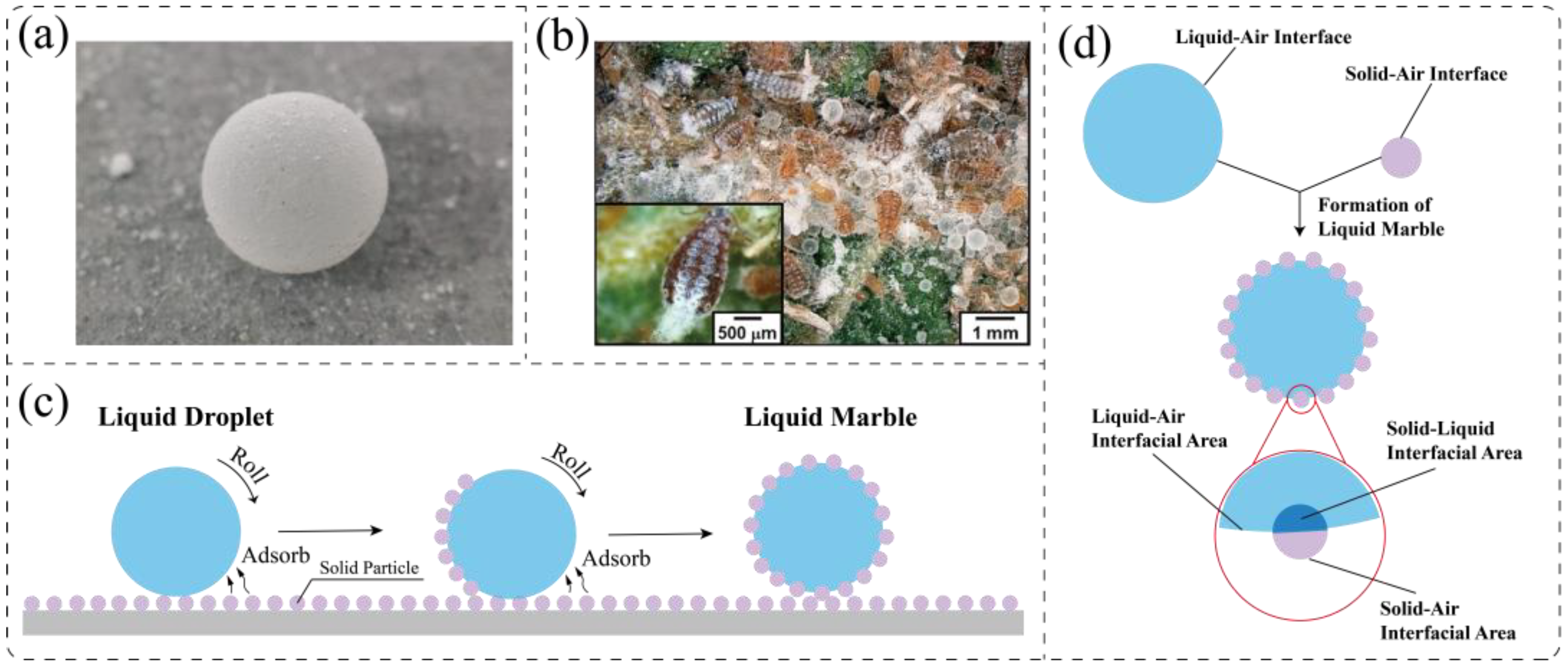
2.2. Liquid Marbles from Nature to Lab
2.2.1. Formation Mechanism
2.2.2. Particle Effects on Marble Performance
2.3. Friction Obstacles in Driving Small-Volume Liquid
2.4. Actuation Principles of Small-Volume Liquid
2.4.1. Wettability Gradients
2.4.2. Body Forces
2.4.3. Other Schemes
3. Transportation of Bare Droplets and Liquid Marbles
3.1. Spontaneous Motions
3.1.1. Surface Textures
3.1.2. Surface Chemistry
3.1.3. Marangoni Flow
3.1.4. Substrate Structures
3.2. Motions with External Assistance
3.2.1. Electric Field
3.2.2. Magnetic Field
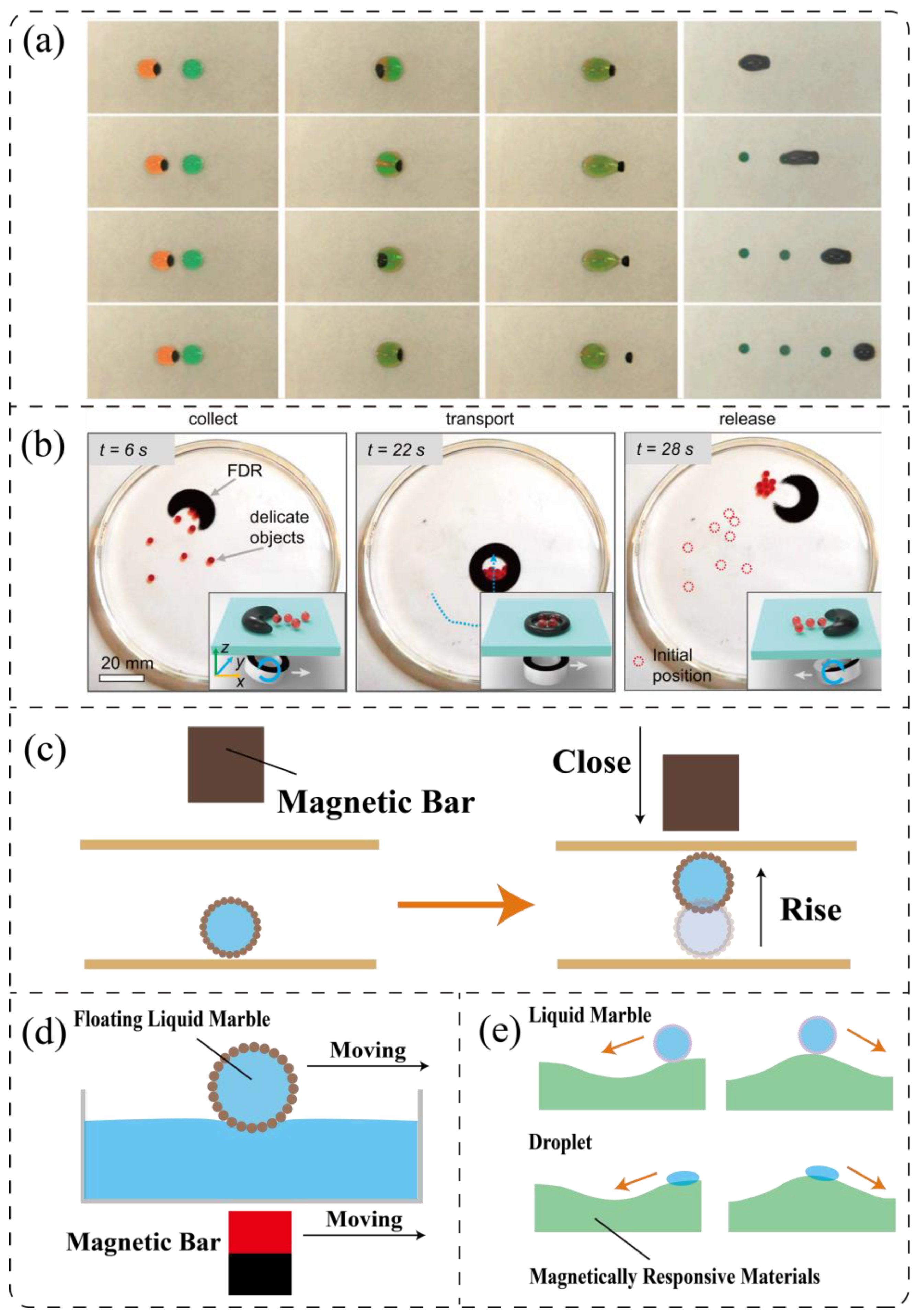
3.2.3. Acoustic Field
3.2.4. Optical Field
4. Other Manipulation Tasks of Bare Droplets and Liquid Marbles
4.1. Coalescence

4.2. Mixing
4.3. Splitting
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manz, A.; Graber, N.; Widmer, H. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuators B Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Woolley, A.T.; Mathies, R.A. Ultra-High-Speed DNA Sequencing Using Capillary Electrophoresis Chips. Anal. Chem. 1995, 67, 3676–3680. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, K.R.; Ooi, C.H.; Jin, J.; Dao, D.V.; Nguyen, N.-T. An automated on-demand liquid marble generator based on electrohydrodynamic pulling. Rev. Sci. Instrum. 2019, 90, 055102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, C.; Qi, B.; Wei, J.; Zhang, Y. Study of droplet self-migration on silicon surface with radial micro-fin structures. Exp. Therm. Fluid Sci. 2020, 114, 110075. [Google Scholar] [CrossRef]
- Lv, J.-A.; Liu, Y.; Wei, J.; Chen, E.; Qin, L.; Yu, Y. Photocontrol of fluid slugs in liquid crystal polymer microactuators. Nature 2016, 537, 179–184. [Google Scholar] [CrossRef]
- Lei, W.; Hou, G.; Liu, M.; Rong, Q.; Xu, Y.; Tian, Y.; Jiang, L. High-speed transport of liquid droplets in magnetic tubular microactuators. Sci. Adv. 2018, 4, eaau8767. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Elvira, K.S.; i Solvas, X.C.; Wootton, R.C.; Demello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, Y.; Xu, Y.; Li, Z.; Chen, J. Fabrication of antigen-containing nanoparticles using microfluidics with Tesla structure. Electrophoresis 2020, 41, 902–908. [Google Scholar] [CrossRef]
- Sreejith, K.R.; Gorgannezhad, L.; Jin, J.; Ooi, C.H.; Stratton, H.; Dao, D.V.; Nguyen, N.-T. Liquid marbles as biochemical reactors for the polymerase chain reaction. Lab Chip 2019, 19, 3220–3227. [Google Scholar] [CrossRef]
- Sreejith, K.R.; Gorgannezhad, L.; Jin, J.; Ooi, C.H.; Takei, T.; Hayase, G.; Stratton, H.; Lamb, K.; Shiddiky, M.; Dao, D.V.; et al. Core-Shell Beads Made by Composite Liquid Marble Technology as A Versatile Microreactor for Polymerase Chain Reaction. Micromachines 2020, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.-T.; Hejazian, M.; Ooi, C.H.; Kashaninejad, N. Recent Advances and Future Perspectives on Microfluidic Liquid Handling. Micromachines 2017, 8, 186. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.K.; Ooi, C.H.; Singha, P.; Jin, J.; Sreejith, K.R.; Phan, H.P.; Nguyen, N.T. Liquid Marbles as Miniature Reactors for Chemical and Biological Applications. Processes 2020, 8, 793. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, S.; Lin, L.; Mao, S.; Lin, J.-M. Emerging open microfluidics for cell manipulation. Chem. Soc. Rev. 2021, 50, 5333–5348. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shah, M.P.; Shelper, T.B.; Nazareth, L.; Barker, M.; Velasquez, J.T.; Ekberg, J.A.K.; Vial, M.-L.; John, J.A.S. Naked Liquid Marbles: A Robust Three-Dimensional Low-Volume Cell-Culturing System. ACS Appl. Mater. Interfaces 2019, 11, 9814–9823. [Google Scholar] [CrossRef] [PubMed]
- Efremov, A.N.; Stanganello, E.; Welle, A.; Scholpp, S.; Levkin, P.A. Micropatterned superhydrophobic structures for the simultaneous culture of multiple cell types and the study of cell–cell communication. Biomaterials 2013, 34, 1757–1763. [Google Scholar] [CrossRef]
- Song, W.; Lima, A.C.; Mano, J.F. Bioinspired methodology to fabricate hydrogel spheres for multi-applications using superhydrophobic substrates. Soft Matter 2010, 6, 5868–5871. [Google Scholar] [CrossRef]
- Li, L.; Tian, J.; Li, M.; Shen, W. Superhydrophobic surface supported bioassay—An application in blood typing. Colloids Surf. B Biointerfaces 2013, 106, 176–180. [Google Scholar] [CrossRef]
- Lima, A.C.; Batista, P.; Valente, T.A.; Silva, A.S.; Correia, I.J.; Mano, J.F. Novel Methodology Based on Biomimetic Superhydrophobic Substrates to Immobilize Cells and Proteins in Hydrogel Spheres for Applications in Bone Regeneration. Tissue Eng. Part A 2013, 19, 1175–1187. [Google Scholar] [CrossRef]
- Dandan, M.; Erbil, H.Y. Evaporation Rate of Graphite Liquid Marbles: Comparison with Water Droplets. Langmuir 2009, 25, 8362–8367. [Google Scholar] [CrossRef] [PubMed]
- Tosun, A.; Erbil, H.Y. Evaporation rate of PTFE liquid marbles. Appl. Surf. Sci. 2009, 256, 1278–1283. [Google Scholar] [CrossRef]
- Ooi, C.H.; Vadivelu, R.; Jin, J.; Sreejith, K.R.; Singha, P.; Nguyen, N.-K.; Nguyen, N.-T. Liquid marble-based digital microfluidics—Fundamentals and applications. Lab Chip 2021, 21, 1199–1216. [Google Scholar] [CrossRef] [PubMed]
- Polwaththe-Gallage, H.-N.; Ooi, C.H.; Jin, J.; Sauret, E.; Nguyen, N.-T.; Li, Z.; Gu, Y. The stress-strain relationship of liquid marbles under compression. Appl. Phys. Lett. 2019, 114, 043701. [Google Scholar] [CrossRef]
- Paven, M.; Mayama, H.; Sekido, T.; Butt, H.-J.; Nakamura, Y.; Fujii, S. Light-Driven Delivery and Release of Materials Using Liquid Marbles. Adv. Funct. Mater. 2016, 26, 3199–3206. [Google Scholar] [CrossRef]
- Chu, Y.; Liu, F.; Qin, L.; Pan, Q. Remote Manipulation of a Microdroplet in Water by Near-Infrared Laser. ACS Appl. Mater. Interfaces 2016, 8, 1273–1279. [Google Scholar] [CrossRef]
- Ooi, C.H.; Plackowski, C.; Nguyen, A.V.; Vadivelu, R.K.; John, J.A.S.; Dao, D.V.; Nguyen, N.-T. Floating mechanism of a small liquid marble. Sci. Rep. 2016, 6, 21777. [Google Scholar] [CrossRef]
- Yukioka, S.; Fujiwara, J.; Okada, M.; Fujii, S.; Nakamura, Y.; Yusa, S.I. CO2-Gas-Responsive Liquid Marble. Langmuir 2020, 36, 6971–6976. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Paulis, M.; Yuan, J.; Mecerreyes, D. Magnetic Poly(Ionic Liquid) Microcapsules for Oil Capture and Recovery. Part. Part. Syst. Charact. 2016, 33, 734–739. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Ren, L.; Xiang, B.; Li, J. Facile preparation of colorful liquid marbles and liquid marbles used in water pollutant detection. J. Adhes. Sci. Technol. 2016, 31, 1125–1132. [Google Scholar] [CrossRef]
- Malinowski, R.; Parkin, I.P.; Volpe, G. Advances towards programmable droplet transport on solid surfaces and its applications. Chem. Soc. Rev. 2020, 49, 7879–7892. [Google Scholar] [CrossRef] [PubMed]
- Bonn, D.; Eggers, J.; Indekeu, J.; Meunier, J.; Rolley, E. Wetting and spreading. Rev. Mod. Phys. 2009, 81, 739–805. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, W. Biomimic from the superhydrophobic plant leaves in nature: Binary structure and unitary structure. Plant Sci. 2007, 172, 1103–1112. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, L.; Zhang, Z.; Wu, S.; Li, G.; Wu, P.; Hu, Y.; Li, J.; Chu, J.; Wu, D. Biomimetic surfaces with anisotropic sliding wetting by energy-modulation femtosecond laser irradiation for enhanced water collection. RSC Adv. 2017, 7, 11170–11179. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, P.; Zhang, L.; Liu, H.; Jiang, Y.; Zhang, D.; Han, Z.; Jiang, L. Continuous directional water transport on the peristome surface of Nepenthes alata. Nature 2016, 532, 85–89. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Öner, D.; McCarthy, T.J. Ultrahydrophobic surfaces. Effects of topography length scales on wettability. Langmuir 2000, 16, 7777–7782. [Google Scholar] [CrossRef]
- Lei, L.; Wang, Q.; Xu, S.; Wang, N.; Zheng, X. Fabrication of superhydrophobic concrete used in marine environment with anti-corrosion and stable mechanical properties. Constr. Build. Mater. 2020, 251, 118946. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, D.; Deng, Y.; Shi, Y.; Wang, K. Mechanically Robust Superhydrophobic Steel Surface with Anti-Icing, UV-Durability, and Corrosion Resistance Properties. ACS Appl. Mater. Interfaces 2015, 7, 6260–6272. [Google Scholar] [CrossRef]
- Han, H.; Lee, J.S.; Kim, H.; Shin, S.; Lee, J.; Kim, J.; Hou, X.; Cho, S.W.; Seo, J.; Lee, T. Single-Droplet Multiplex Bioassay on a Robust and Stretchable Extreme Wetting Substrate through Vacuum-Based Droplet Manipulation. ACS Nano 2018, 12, 932–941. [Google Scholar] [CrossRef]
- Gross, M.; Varnik, F.; Raabe, D.; Steinbach, I. Small droplets on superhydrophobic substrates. Phys. Rev. E 2010, 81, 051606. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Kasahara, M.; Akimoto, S.-I.; Hariyama, T.; Takaku, Y.; Yusa, S.-I.; Okada, S.; Nakajima, K.; Hirai, T.; Mayama, H.; Okada, S.; et al. Liquid Marbles in Nature: Craft of Aphids for Survival. Langmuir 2019, 35, 6169–6178. [Google Scholar] [CrossRef]
- Aussillous, P.; Quéré, D. Liquid marbles. Nature 2001, 411, 924–927. [Google Scholar] [CrossRef]
- Oliveira, N.M.; Zhang, Y.S.; Ju, J.; Chen, A.-Z.; Chen, Y.; Sonkusale, S.R.; Dokmeci, M.R.; Reis, R.L.; Mano, J.F.; Khademhosseini, A. Hydrophobic Hydrogels: Toward Construction of Floating (Bio)microdevices. Chem. Mater. 2016, 28, 3641–3648. [Google Scholar] [CrossRef]
- Pennarossa, G.; Manzoni, E.F.M.; Ledda, S.; Deeguileor, M.; Gandolfi, F.; Brevini, T.A.L. Use of a PTFE Micro-Bioreactor to Promote 3D Cell Rearrangement and Maintain High Plasticity in Epigenetically Erased Fibroblasts. Stem Cell Rev. Rep. 2018, 15, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Fu, N.; Chen, X.D.; Shen, W. Respirable liquid marble for the cultivation of microorganisms. Colloids Surf. B Biointerfaces 2013, 106, 187–190. [Google Scholar] [CrossRef]
- Vadivelu, R.K.; Ooi, C.H.; Yao, R.-Q.; Velasquez, J.T.; Pastrana, E.; Diaz-Nido, J.; Lim, F.; Ekberg, J.A.K.; Nguyen, N.-T.; John, J.A.S. Generation of three-dimensional multiple spheroid model of olfactory ensheathing cells using floating liquid marbles. Sci. Rep. 2015, 5, 15083. [Google Scholar] [CrossRef]
- Sreejith, K.R.; Ooi, C.H.; Dao, D.V.; Nguyen, N.-T. Evaporation dynamics of liquid marbles at elevated temperatures. RSC Adv. 2018, 8, 15436–15443. [Google Scholar] [CrossRef]
- McHale, G.; Newton, M.I. Liquid marbles: Principles and applications. Soft Matter 2011, 7, 5473–5481. [Google Scholar] [CrossRef]
- Saczek, J.; Yao, X.; Zivkovic, V.; Mamlouk, M.; Wang, D.; Pramana, S.S.; Wang, S. Long-Lived Liquid Marbles for Green Applications. Adv. Funct. Mater. 2021, 31, 2011198. [Google Scholar] [CrossRef]
- Zang, D.; Chen, Z.; Zhang, Y.; Lin, K.; Geng, X.; Binks, B.P. Effect of particle hydrophobicity on the properties of liquid water marbles. Soft Matter 2013, 9, 5067–5073. [Google Scholar] [CrossRef]
- Dickinson, E.; Ettelaie, R.; Kostakis, T.; Murray, B.S. Factors Controlling the Formation and Stability of Air Bubbles Stabilized by Partially Hydrophobic Silica Nanoparticles. Langmuir 2004, 20, 8517–8525. [Google Scholar] [CrossRef]
- Cengiz, U.; Erbil, H.Y. The lifetime of floating liquid marbles: The influence of particle size and effective surface tension. Soft Matter 2013, 9, 8980–8991. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Chen, C.; Yang, T.; Wang, J.; Guo, L.; Liu, P.; Kong, T. Larger Stabilizing Particles Make Stronger Liquid Marble. Small 2018, 15, e1804549. [Google Scholar] [CrossRef]
- Fullarton, C.; Draper, T.C.; Phillips, N.; Mayne, R.; Costello, B.P.J.D.L.; Adamatzky, A. Evaporation, Lifetime, and Robustness Studies of Liquid Marbles for Collision-Based Computing. Langmuir 2018, 34, 2573–2580. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Hapgood, K.; Shen, W. Observation of the liquid marble morphology using confocal microscopy. Chem. Eng. J. 2010, 162, 396–405. [Google Scholar] [CrossRef]
- Asaumi, Y.; Rey, M.; Oyama, K.; Vogel, N.; Hirai, T.; Nakamura, Y.; Fujii, S. Effect of Stabilizing Particle Size on the Structure and Properties of Liquid Marbles. Langmuir 2020, 36, 13274–13284. [Google Scholar] [CrossRef]
- Singha, P.; Nguyen, N.K.; Nguyen, V.T.; Sreejith, K.R.; Tran, D.T.; Nguyen, A.V.; Nguyen, N.T.; Ooi, C.H. Investigation of liquid marble shell using X-ray: Shell thickness and effective surface tension. ChemNanoMat 2021, 8, e202100423. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Shi, H.; Li, X. Mechanical robustness of monolayer nanoparticle-covered liquid marbles. Soft Matter 2020, 16, 4632–4639. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, H.; Wang, Y.; Wang, H.; Huang, J.; Duan, M. Liquid marbles from soot films. Soft Matter 2020, 16, 4512–4519. [Google Scholar] [CrossRef] [PubMed]
- Laborie, B.; Lachaussée, F.; Lorenceau, E.; Rouyer, F. How coatings with hydrophobic particles may change the drying of water droplets: Incompressible surface versus porous media effects. Soft Matter 2013, 9, 4822–4830. [Google Scholar] [CrossRef]
- Gao, N.; Geyer, F.; Pilat, D.W.; Wooh, S.; Vollmer, D.; Butt, H.-J.; Berger, R. How drops start sliding over solid surfaces. Nat. Phys. 2017, 14, 191–196. [Google Scholar] [CrossRef]
- Mahadevan, L.; Pomeau, Y. Rolling droplets. Phys. Fluids 1999, 11, 2449–2453. [Google Scholar] [CrossRef]
- Olin, P.; Lindström, S.B.; Pettersson, T.; Wågberg, L. Water Drop Friction on Superhydrophobic Surfaces. Langmuir 2013, 29, 9079–9089. [Google Scholar] [CrossRef]
- Backholm, M.; Molpeceres, D.; Vuckovac, M.; Nurmi, H.; Hokkanen, M.J.; Jokinen, V.; Timonen, J.V.I.; Ras, R.H.A. Water droplet friction and rolling dynamics on superhydrophobic surfaces. Commun. Mater. 2020, 1, 1–8. [Google Scholar] [CrossRef]
- Extrand, C.W.; Kumagai, Y. Liquid Drops on an Inclined Plane: The Relation between Contact Angles, Drop Shape, and Retentive Force. J. Colloid Interface Sci. 1995, 170, 515–521. [Google Scholar] [CrossRef]
- Bormashenko, E.; Bormashenko, Y.; Oleg, G. On the Nature of the Friction between Nonstick Droplets and Solid Substrates. Langmuir 2010, 26, 12479–12482. [Google Scholar] [CrossRef]
- Aussillous, P.; Quéré, D. Properties of liquid marbles. Proc. R. Soc. A Math. Phys. Eng. Sci. 2006, 462, 973–999. [Google Scholar] [CrossRef]
- Ooi, C.H.; Jin, J.; Sreejith, K.R.; Nguyen, A.V.; Evans, G.M.; Nguyen, N.-T. Manipulation of a floating liquid marble using dielectrophoresis. Lab Chip 2018, 18, 3770–3779. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.H.; Nguyen, A.V.; Evans, G.M.; Dao, D.V.; Nguyen, N.T. Measuring the Coefficient of Friction of a Small Floating Liquid Marble. Sci. Rep. 2016, 6, 38346. [Google Scholar] [CrossRef] [PubMed]
- Brochard, F. Motions of droplets on solid surfaces induced by chemical or thermal gradients. Langmuir 1989, 5, 432–438. [Google Scholar] [CrossRef]
- Shastry, A.; Case, M.J.; Böhringer, K.F. Directing Droplets Using Microstructured Surfaces. Langmuir 2006, 22, 6161–6167. [Google Scholar] [CrossRef] [PubMed]
- Berge, B. Electrocapillarity and wetting of insulator films by water. Comptes Rendus De L Acad. Des Sci. Ser. Ii 1993, 317, 157–163. [Google Scholar]
- Mugele, F.; Baret, J.-C. Electrowetting: From basics to applications. J. Phys. Condens. Matter 2005, 17, R705–R774. [Google Scholar] [CrossRef]
- Newton, M.I.; Herbertson, D.L.; Elliott, S.J.; Shirtcliffe, N.J.; McHale, G. Electrowetting of liquid marbles. J. Phys. D Appl. Phys. 2007, 40, 20–24. [Google Scholar] [CrossRef]
- Baird, E.; Young, P.; Mohseni, K. Electrostatic force calculation for an EWOD-actuated droplet. Microfluid. Nanofluidics 2007, 3, 635–644. [Google Scholar] [CrossRef]
- Barman, J.; Shao, W.; Tang, B.; Yuan, D.; Groenewold, J.; Zhou, G. Wettability Manipulation by Interface-Localized Liquid Dielectrophoresis: Fundamentals and Applications. Micromachines 2019, 10, 329. [Google Scholar] [CrossRef]
- Pethig, R. Review article-dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 2010, 4, 022811. [Google Scholar] [CrossRef]
- Long, Z.; Shetty, A.M.; Solomon, M.; Larson, R.G. Fundamentals of magnet-actuated droplet manipulation on an open hydrophobic surface. Lab Chip 2009, 9, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Friend, J.; Yeo, L.Y. Microscale acoustofluidics: Microfluidics driven via acoustics and ultrasonics. Rev. Mod. Phys. 2011, 83, 647–704. [Google Scholar] [CrossRef]
- Sudeepthi, A.; Nath, A.; Yeo, L.Y.; Sen, A.K. Coalescence of Droplets in a Microwell Driven by Surface Acoustic Waves. Langmuir 2021, 37, 1578–1587. [Google Scholar] [CrossRef]
- Andrade, M.A.B.; Camargo, T.S.A.; Marzo, A. Automatic contactless injection, transportation, merging, and ejection of droplets with a multifocal point acoustic levitator. Rev. Sci. Instrum. 2018, 89, 125105. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Marrone, B.L. Droplet translocation by focused surface acoustic waves. Microfluid. Nanofluidics 2012, 13, 715–722. [Google Scholar] [CrossRef]
- Yeo, L.Y.; Friend, J.R. Surface Acoustic Wave Microfluidics. Annu. Rev. Fluid Mech. 2014, 46, 379–406. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z. Spontaneous directional transportations of water droplets on surfaces driven by gradient structures. Nanoscale 2018, 10, 13814–13831. [Google Scholar] [CrossRef]
- Brzoska, J.B.; Brochard-Wyart, F.; Rondelez, F. Motions of droplets on hydrophobic model surfaces induced by thermal gradients. Langmuir 1993, 9, 2220–2224. [Google Scholar] [CrossRef]
- Cira, N.J.; Benusiglio, A.; Prakash, M. Vapour-mediated sensing and motility in two-component droplets. Nature 2015, 519, 446–450. [Google Scholar] [CrossRef]
- Bormashenko, E.; Bormashenko, Y.; Grynyov, R.; Aharoni, H.; Whyman, G.; Binks, B.P. Self-Propulsion of Liquid Marbles: Leidenfrost-like Levitation Driven by Marangoni Flow. J. Phys. Chem. C 2015, 119, 9910–9915. [Google Scholar] [CrossRef]
- Higuera, F.J.; Medina, A.; Liñán, A. Capillary rise of a liquid between two vertical plates making a small angle. Phys. Fluids 2008, 20, 102102. [Google Scholar] [CrossRef]
- Ju, J.; Xiao, K.; Yao, X.; Bai, H.; Jiang, L. Bioinspired Conical Copper Wire with Gradient Wettability for Continuous and Efficient Fog Collection. Adv. Mater. 2013, 25, 5937–5942. [Google Scholar] [CrossRef]
- Alosaimi, F.K.; Tung, T.T.; Dao, V.-D.; Huyen, N.K.; Nine, J.; Hassan, K.; Ma, J.; Losic, D. Graphene-based multifunctional surface and structure gradients engineered by atmospheric plasma. Appl. Mater. Today 2022, 27, 101486. [Google Scholar] [CrossRef]
- Hernández, S.C.; Bennett, C.J.C.; Junkermeier, C.; Tsoi, S.D.; Bezares, F.J.; Stine, R.; Robinson, J.; Lock, E.; Boris, D.R.; Pate, B.D.; et al. Chemical Gradients on Graphene to Drive Droplet Motion. ACS Nano 2013, 7, 4746–4755. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, J.; Cheng, J.; Xu, S.; Pi, P.; Wen, X. Enhanced Movement of Two-Component Droplets on a Wedge-Shaped Ag/Cu Surface by a Wettability Gradient. ACS Appl. Mater. Interfaces 2021, 13, 15857–15865. [Google Scholar] [CrossRef]
- Aliabadi, M.; Zarkesh, A.; Siampour, H.; Abbasian, S.; Mahdavinejad, M.; Moshaii, A. Bioinspired Azimuthally Varying Nanoscale Cu Columns on Acupuncture Needles for Fog Collection. ACS Appl. Nano Mater. 2021, 4, 8733–8743. [Google Scholar] [CrossRef]
- Bormashenko, E.; Pogreb, R.; Whyman, G.; Musin, A. Jetting liquid marbles: Study of the Taylor instability in immersed marbles. Colloid Polym. Sci. 2013, 291, 1535–1539. [Google Scholar] [CrossRef]
- McHale, G.; Herbertson, D.L.; Elliott, S.J.; Shirtcliffe, N.J.; Newton, M.I. Electrowetting of Nonwetting Liquids and Liquid Marbles. Langmuir 2006, 23, 918–924. [Google Scholar] [CrossRef]
- Khaw, M.K.; Ooi, C.H.; Mohd-Yasin, F.; Vadivelu, R.; John, J.S.; Nguyen, N.-T. Digital microfluidics with a magnetically actuated floating liquid marble. Lab Chip 2016, 16, 2211–2218. [Google Scholar] [CrossRef]
- Li, A.; Li, H.; Li, Z.; Zhao, Z.; Li, K.; Li, M.; Song, Y. Programmable droplet manipulation by a magnetic-actuated robot. Sci. Adv. 2020, 6, eaay5808. [Google Scholar] [CrossRef]
- Timonen, J.V.I.; Latikka, M.; Leibler, L.; Ras, R.H.A.; Ikkala, O. Switchable Static and Dynamic Self-Assembly of Magnetic Droplets on Superhydrophobic Surfaces. Science 2013, 341, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Wixforth, A.; Strobl, C.; Gauer, C.; Toegl, A.; Scriba, J.; von Guttenberg, Z. Acoustic manipulation of small droplets. Anal. Bioanal. Chem. 2004, 379, 982–991. [Google Scholar] [CrossRef]
- Diguet, A.; Guillermic, R.-M.; Magome, N.; Saint-Jalmes, A.; Chen, Y.; Yoshikawa, K.; Baigl, D. Photomanipulation of a Droplet by the Chromocapillary Effect. Angew. Chem. Int. Ed. 2009, 48, 9281–9284. [Google Scholar] [CrossRef]
- Greenspan, H.P. On the motion of a small viscous droplet that wets a surface. J. Fluid Mech. 1978, 84, 125. [Google Scholar] [CrossRef]
- Chaudhury, M.K.; Whitesides, G.M. How to Make Water Run Uphill. Science 1992, 256, 1539–1541. [Google Scholar] [CrossRef] [PubMed]
- van Assenbergh, P.; Meinders, E.; Geraedts, J.; Dodou, D. Nanostructure and Microstructure Fabrication: From Desired Properties to Suitable Processes. Small 2018, 14, e1703401. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cheng, J.; Zhou, C.; Xing, H.; Wen, X.; Pi, P.; Xu, S. Droplet Motion on a Shape Gradient Surface. Langmuir 2017, 33, 4172–4177. [Google Scholar] [CrossRef]
- Han, X.; Wang, L.; Wang, X. Fabrication of Chemical Gradient Using Space Limited Plasma Oxidation and its Application for Droplet Motion. Adv. Funct. Mater. 2012, 22, 4533–4538. [Google Scholar] [CrossRef]
- Washizu, M. Electrostatic actuation of liquid droplets for micro-reactor applications. IEEE Trans. Ind. Appl. 1998, 34, 732–737. [Google Scholar] [CrossRef]
- Bormashenko, E.; Pogreb, R.; Balter, R.; Gendelman, O.; Aurbach, D. Composite non-stick droplets and their actuation with electric field. Appl. Phys. Lett. 2012, 100, 151601. [Google Scholar] [CrossRef]
- Ooi, C.H.; Jin, J.; Nguyen, A.V.; Evans, G.M.; Nguyen, N.-T. Picking up and placing a liquid marble using dielectrophoresis. Microfluid. Nanofluidics 2018, 22, 142. [Google Scholar] [CrossRef]
- Jin, J.; Ooi, C.H.; Sreejith, K.R.; Dao, D.V.; Nguyen, N.-T. Dielectrophoretic Trapping of a Floating Liquid Marble. Phys. Rev. Appl. 2019, 11, 044059. [Google Scholar] [CrossRef]
- Jin, J.; Ooi, C.H.; Sreejith, K.R.; Zhang, J.; Nguyen, A.V.; Evans, G.M.; Dao, D.V.; Nguyen, N.-T. Accurate dielectrophoretic positioning of a floating liquid marble with a two-electrode configuration. Microfluid. Nanofluidics 2019, 23, 85. [Google Scholar] [CrossRef]
- Lee, J.; Moon, H.; Fowler, J.; Schoellhammer, T.; Kim, C.-J. Electrowetting and electrowetting-on-dielectric for microscale liquid handling. Sens. Actuators A Phys. 2002, 95, 259–268. [Google Scholar] [CrossRef]
- Cooney, C.G.; Chen, C.-Y.; Emerling, M.R.; Nadim, A.; Sterling, J.D. Electrowetting droplet microfluidics on a single planar surface. Microfluid. Nanofluidics 2006, 2, 435–446. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.-H. Full-Range Magnetic Manipulation of Droplets via Surface Energy Traps Enables Complex Bioassays. Adv. Mater. 2013, 25, 2903–2908. [Google Scholar] [CrossRef]
- Yang, C.; Ning, Y.; Ku, X.; Zhuang, G.; Li, G. Automatic magnetic manipulation of droplets on an open surface using a superhydrophobic electromagnet needle. Sens. Actuators B Chem. 2018, 257, 409–418. [Google Scholar] [CrossRef]
- Damodara, S.; Sen, A. Magnetic field assisted droplet manipulation on a soot-wax coated superhydrophobic surface of a PDMS-iron particle composite substrate. Sens. Actuators B Chem. 2017, 239, 816–823. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Z.; Li, G. Programmable droplet manipulation by combining a superhydrophobic magnetic film and an electromagnetic pillar array. Sens. Actuators B Chem. 2018, 262, 892–901. [Google Scholar] [CrossRef]
- Fan, X.; Dong, X.; Karacakol, A.C.; Xie, H.; Sitti, M. Reconfigurable multifunctional ferrofluid droplet robots. Proc. Natl. Acad. Sci. USA 2020, 117, 27916–27926. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, J.; Wang, H.; Wang, X.; Lin, T. Magnetic liquid marbles: Manipulation of liquid droplets using highly hydrophobic Fe3O4 nanoparticles. Adv. Mater. 2010, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wang, H.; Zhao, Y.; Dai, L.; Feng, L.; Wang, X.; Lin, T. Magnetic liquid marbles: A “precise” miniature reactor. Adv. Mater. 2010, 22, 4814–4818. [Google Scholar] [CrossRef] [PubMed]
- Vialetto, J.; Hayakawa, M.; Kavokine, N.; Takinoue, M.; Varanakkottu, S.N.; Rudiuk, S.; Anyfantakis, M.; Morel, M.; Baigl, D. Magnetic Actuation of Drops and Liquid Marbles Using a Deformable Paramagnetic Liquid Substrate. Angew. Chem. Int. Ed. 2017, 56, 16565–16570. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, Y.; Reboud, J.; Wilson, R.; Cooper, J.M. Tuneable surface acoustic waves for fluid and particle manipulations on disposable chips. Lab Chip 2010, 10, 1898–1901. [Google Scholar] [CrossRef]
- Yang, T.-H.; Yang, H.-C.; Chang, C.-H.; Prabhu, G.R.D.; Urban, P.L. Microanalysis Using Acoustically Actuated Droplets Pinned Onto a Thread. IEEE Access 2019, 7, 154743–154749. [Google Scholar] [CrossRef]
- Zang, D.; Li, J.; Chen, Z.; Zhai, Z.; Geng, X.; Binks, B.P. Switchable Opening and Closing of a Liquid Marble via Ultrasonic Levitation. Langmuir 2015, 31, 11502–11507. [Google Scholar] [CrossRef]
- Chen, Z.; Zang, D.; Zhao, L.; Qu, M.; Li, X.; Li, X.; Li, L.; Geng, X. Liquid Marble Coalescence and Triggered Microreaction Driven by Acoustic Levitation. Langmuir 2017, 33, 6232–6239. [Google Scholar] [CrossRef]
- Ichimura, K.; Oh, S.-K.; Nakagawa, M. Light-Driven Motion of Liquids on a Photoresponsive Surface. Science 2000, 288, 1624–1626. [Google Scholar] [CrossRef]
- Hwang, H.; Papadopoulos, P.; Fujii, S.; Wooh, S. Driving Droplets on Liquid Repellent Surfaces via Light-Driven Marangoni Propulsion. Adv. Funct. Mater. 2022, 32, 2111311. [Google Scholar] [CrossRef]
- Kavokine, N.; Anyfantakis, M.; Morel, M.; Rudiuk, S.; Bickel, T.; Baigl, D. Light-Driven Transport of a Liquid Marble with and against Surface Flows. Angew. Chem. Int. Ed. 2016, 55, 11183–11187. [Google Scholar] [CrossRef]
- Jin, J.; Ooi, C.H.; Dao, D.V.; Nguyen, N.-T. Coalescence Processes of Droplets and Liquid Marbles. Micromachines 2017, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, J.D.; Burton, J.C.; Nagel, S.R.; Appathurai, S.; Harris, M.T.; Basaran, O.A. The inexorable resistance of inertia determines the initial regime of drop coalescence. Proc. Natl. Acad. Sci. USA 2012, 109, 6857–6861. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fu, X.; Binks, B.P.; Shum, H.C. Coalescence of electrically charged liquid marbles. Soft Matter 2016, 13, 119–124. [Google Scholar] [CrossRef]
- Jin, J.; Ooi, C.H.; Dao, D.V.; Nguyen, N.-T. Liquid marble coalescence via vertical collision. Soft Matter 2018, 14, 4160–4168. [Google Scholar] [CrossRef] [PubMed]
- Rozynek, Z.; Khobaib, K.; Mikkelsen, A. Opening and Closing of Particle Shells on Droplets via Electric Fields and Its Applications. ACS Appl. Mater. Interfaces 2019, 11, 22840–22850. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Singha, P.; An, H.; Phan, H.P.; Nguyen, N.T.; Ooi, C.H. Electrostatically excited liquid marble as a micromixer. React. Chem. Eng. 2021, 6, 1386–1394. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Singha, P.; Dai, Y.; Sreejith, K.R.; Phan, H.P.; Nguyen, N.T.; Ooi, C.H. Controllable high-performance liquid marble micromixer. Lab Chip 2022, 22, 1508–1518. [Google Scholar] [CrossRef]
- Wang, E.N.; Bucaro, M.A.; Taylor, J.A.; Kolodner, P.; Aizenberg, J.; Krupenkin, T. Droplet mixing using electrically tunable superhydrophobic nanostructured surfaces. Microfluid. Nanofluidics 2008, 7, 137–140. [Google Scholar] [CrossRef]
- Won, T.; Lee, K.Y.; Chung, S.K. Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; IEEE: Manhattan, NY, USA, 2019; pp. 65–67. [Google Scholar]
- Liu, Z.; Yang, T.; Huang, Y.; Liu, Y.; Chen, L.; Deng, L.; Shum, H.C.; Kong, T. Electrocontrolled Liquid Marbles for Rapid Miniaturized Organic Reactions. Adv. Funct. Mater. 2019, 29, 1901101. [Google Scholar] [CrossRef]
- Pollack, M.G.; Shenderov, A.D.; Fair, R.B. Electrowetting-based actuation of droplets for integrated microfluidics. Lab Chip 2002, 2, 96–101. [Google Scholar] [CrossRef]
- Mertaniemi, H.; Jokinen, V.; Sainiemi, L.; Franssila, S.; Marmur, A.; Ikkala, O.; Ras, R.H.A. Superhydrophobic Tracks for Low-Friction, Guided Transport of Water Droplets. Adv. Mater. 2011, 23, 2911–2914. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Song, B.; Hu, H.; Du, X.; Zhou, F. Selectively splitting a droplet using superhydrophobic stripes on hydrophilic surfaces. Phys. Chem. Chem. Phys. 2015, 17, 13800–13803. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chan, K.F.; Ji, F.; Wang, Q.; Chiu, P.W.Y.; Guo, Z.; Zhang, L. On-Demand Coalescence and Splitting of Liquid Marbles and Their Bioapplications. Adv. Sci. 2019, 6, 1802033. [Google Scholar] [CrossRef]
- Liu, J.; Zuo, P. Wetting and elasto-plasticity based sculpture of liquid marbles. Eur. Phys. J. E 2016, 39, 17. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, R.; Zhu, X.; Ye, D.; Yang, Y.; Li, W.; Li, D.; Li, H.; Liao, Q. Micro-object manipulation by decanol liquid lenses. Lab Chip 2022, 22, 2844–2852. [Google Scholar] [CrossRef] [PubMed]













| Motion Forms | Approaches | Driving Force | Principles | |
|---|---|---|---|---|
| Spontaneous motions | surface textures | ○○ | surface tension | surface texture gradient |
| surface chemistry | ○○ | surface chemistry gradient | ||
| Marangoni flow | ○/◎ | surface tension | temperature gradient | |
| concentration gradient | ||||
| wedge corner | ○ | capillary force | gradient structures | |
| conical structure | ○ | Laplace pressure | ||
| Motions with external assistance | electric field | ○○○/◎◎◎ | electrostatic force | electrostatic effect |
| dielectrophoretic force | dielectrophoresis | |||
| surface tension | electrowetting | |||
| magnetic field | ○○/◎◎◎ | magnetic force | magnetic field action | |
| acoustic field | ○○/◎◎ | acoustic force | acoustic wave action | |
| optical field | ○/◎◎ | surface tension | optical-induced temperature gradient | |
| optical-induced molecular structure change | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Xie, Y.; Chen, H.; Yu, Z.; Shi, L.; Jin, J. Fundamentals and Manipulation of Bare Droplets and Liquid Marbles as Open Microfluidic Platforms. Processes 2023, 11, 983. https://doi.org/10.3390/pr11040983
Huang Z, Xie Y, Chen H, Yu Z, Shi L, Jin J. Fundamentals and Manipulation of Bare Droplets and Liquid Marbles as Open Microfluidic Platforms. Processes. 2023; 11(4):983. https://doi.org/10.3390/pr11040983
Chicago/Turabian StyleHuang, Zheng, Yuanhao Xie, Huaying Chen, Zhihang Yu, Liuyong Shi, and Jing Jin. 2023. "Fundamentals and Manipulation of Bare Droplets and Liquid Marbles as Open Microfluidic Platforms" Processes 11, no. 4: 983. https://doi.org/10.3390/pr11040983
APA StyleHuang, Z., Xie, Y., Chen, H., Yu, Z., Shi, L., & Jin, J. (2023). Fundamentals and Manipulation of Bare Droplets and Liquid Marbles as Open Microfluidic Platforms. Processes, 11(4), 983. https://doi.org/10.3390/pr11040983








