Sustainable Routes for Wool Grease Removal Using Green Solvent Cyclopentyl Methyl Ether in Solvent Extraction and Biosurfactant Wool Protein Hydrolyzate in Scouring
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Wool Grease Solvent Extraction
2.1.2. Wool Scouring
2.2. Methods
2.2.1. Wool Grease Solvent Extraction
2.2.2. Wool Scouring
Preparation of WPH Biosurfactant
Wool Scouring
2.3. Characterisation
2.3.1. Wool Grease Solvent Extraction
FT-IR Analysis
DSC Analysis
GC-FID Analysis
- Lanolin saponification
- Derivatization of fatty acids
- Preparation of standard compounds for calibration graph
- GC-FID analysis
2.3.2. Wool Scouring
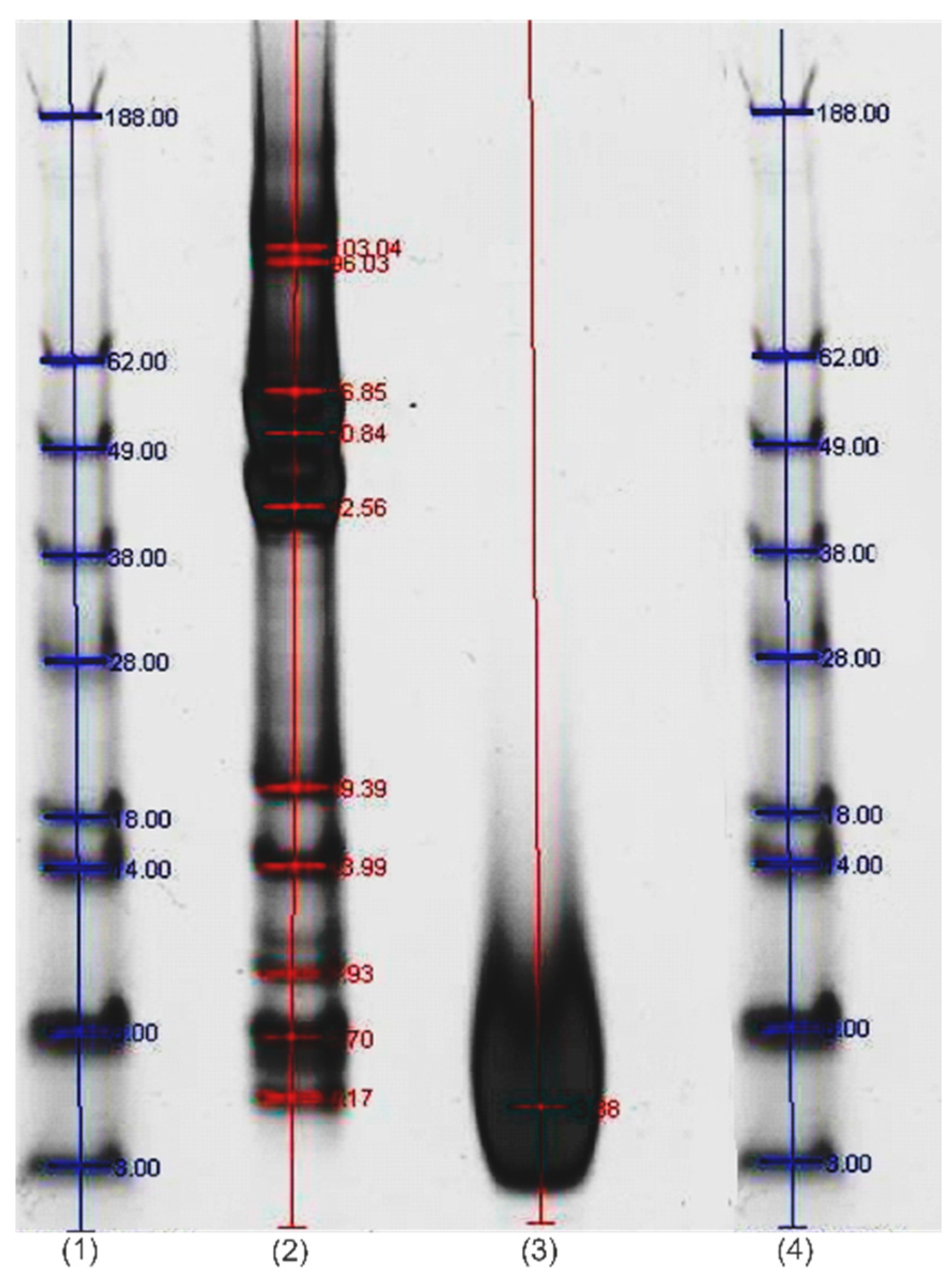
Molecular Weight Distribution of Wool Protein Hydrolyzate
Scanning Electron Microscopy
Washing Yield
Color Measurement
3. Results and Discussion
3.1. Wool Grease Extraction
3.1.1. Amount of Grease Extracted
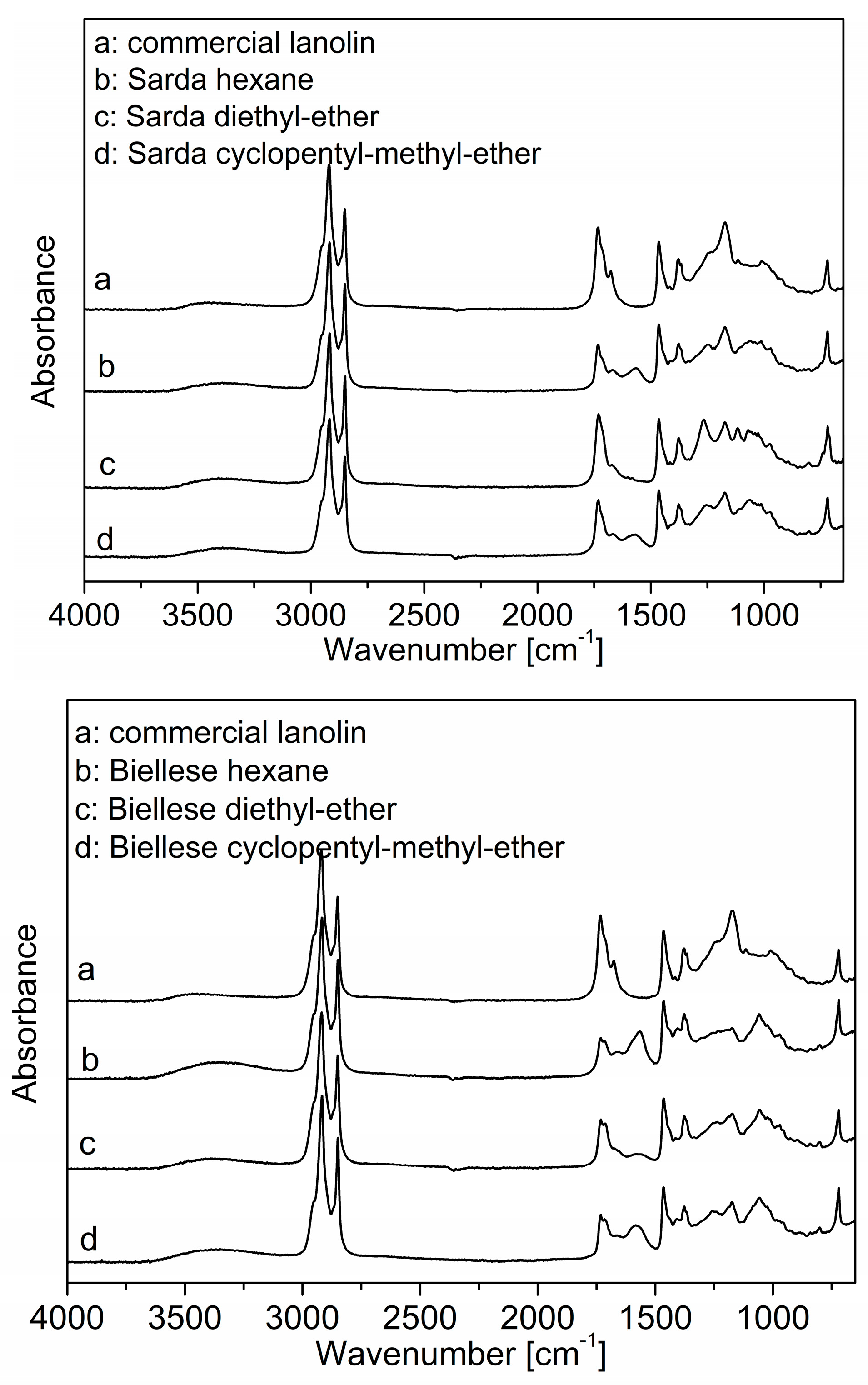
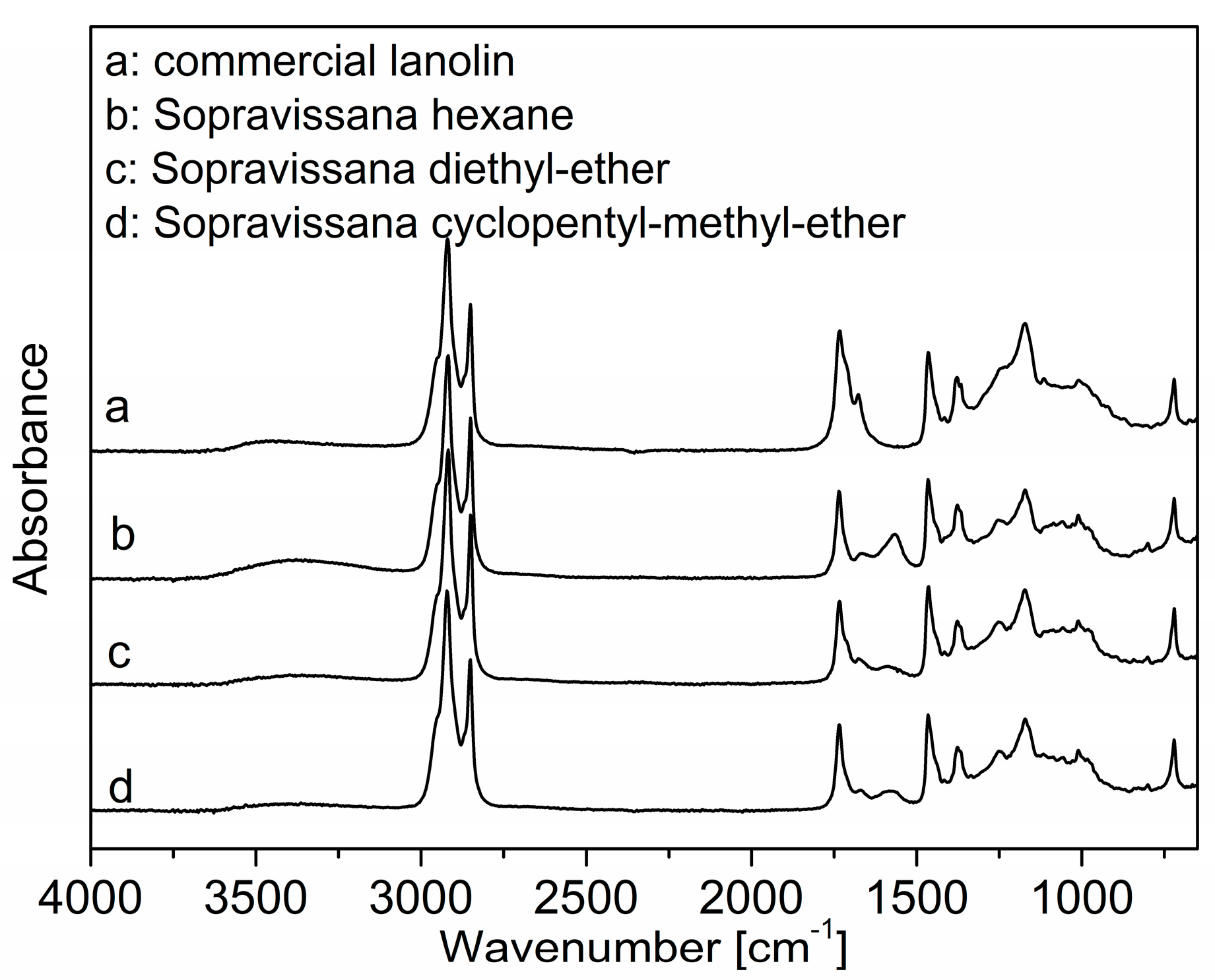
3.1.2. FT-IR Analysis
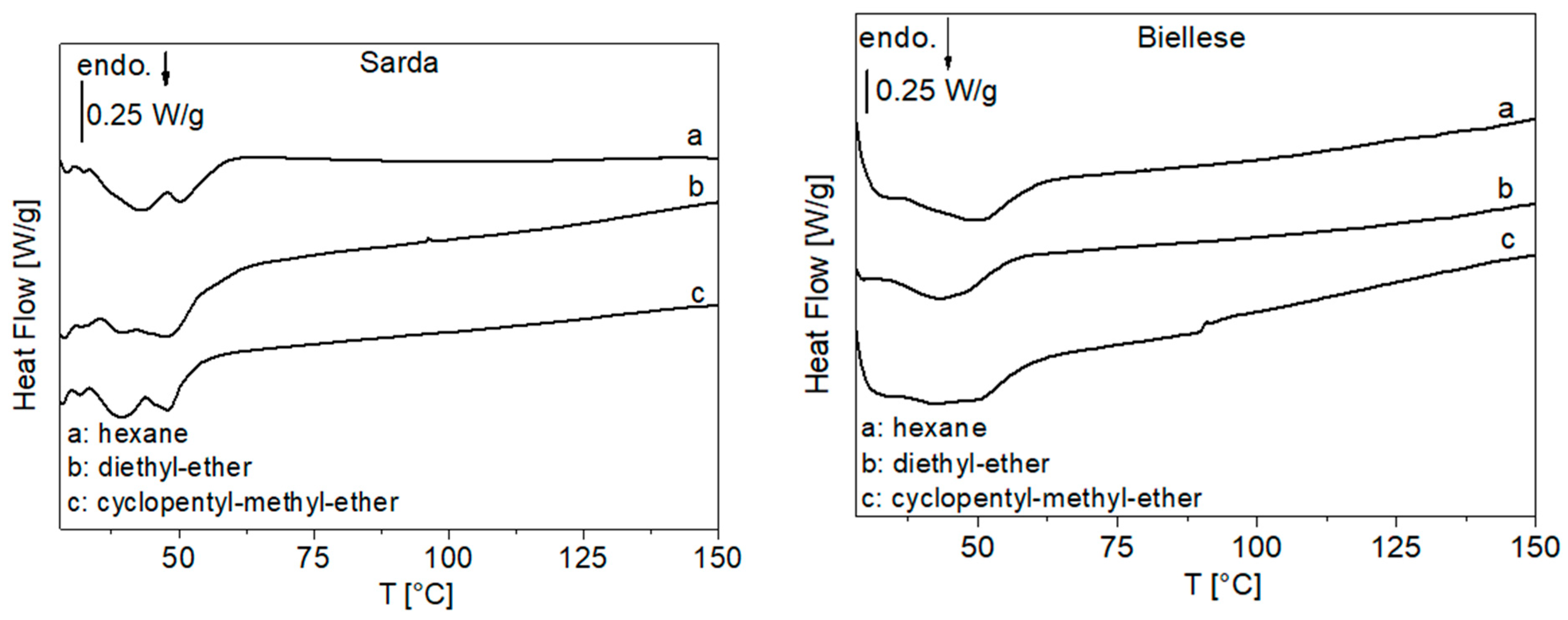
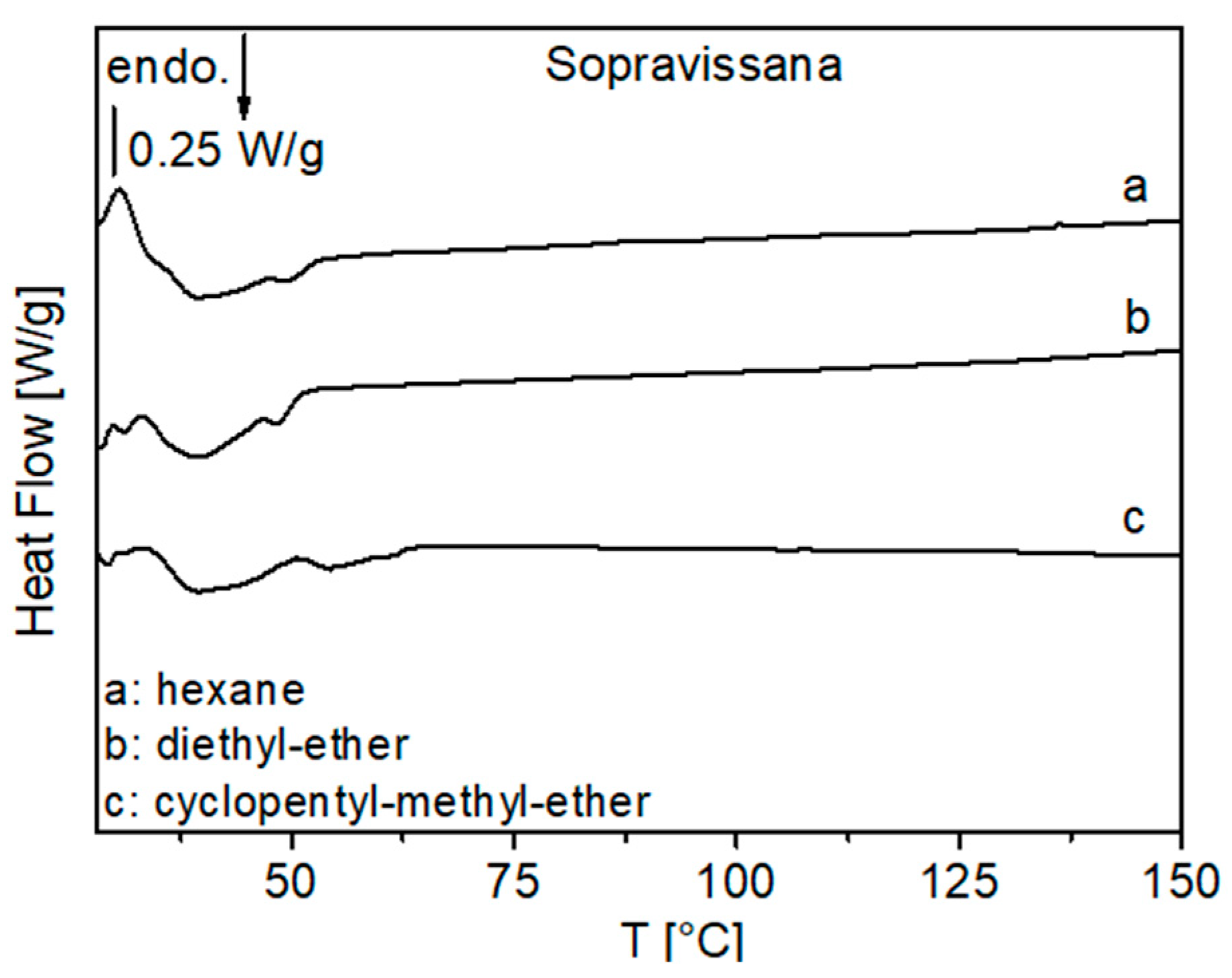
3.1.3. DSC Analysis
3.1.4. GC-FID Analysis
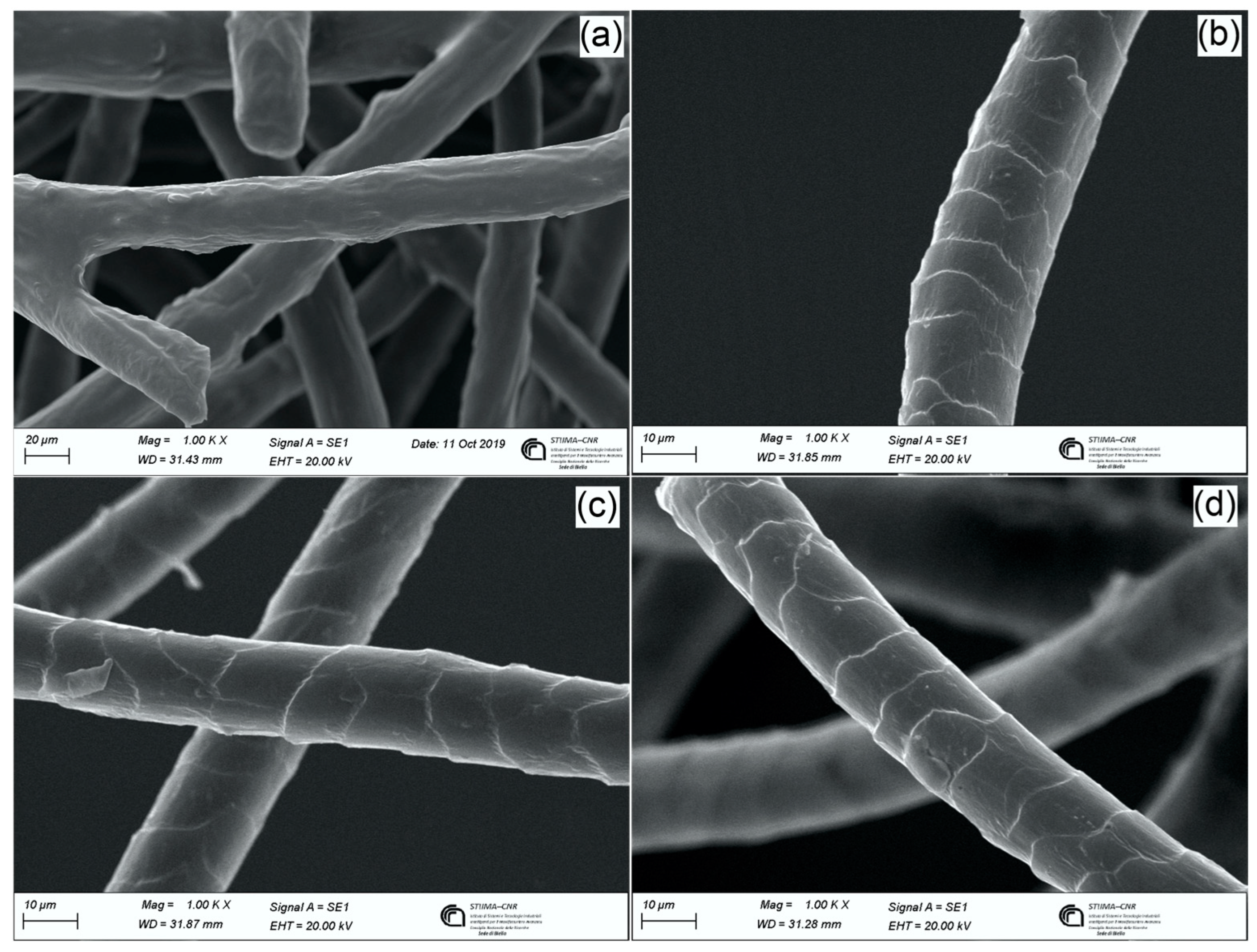
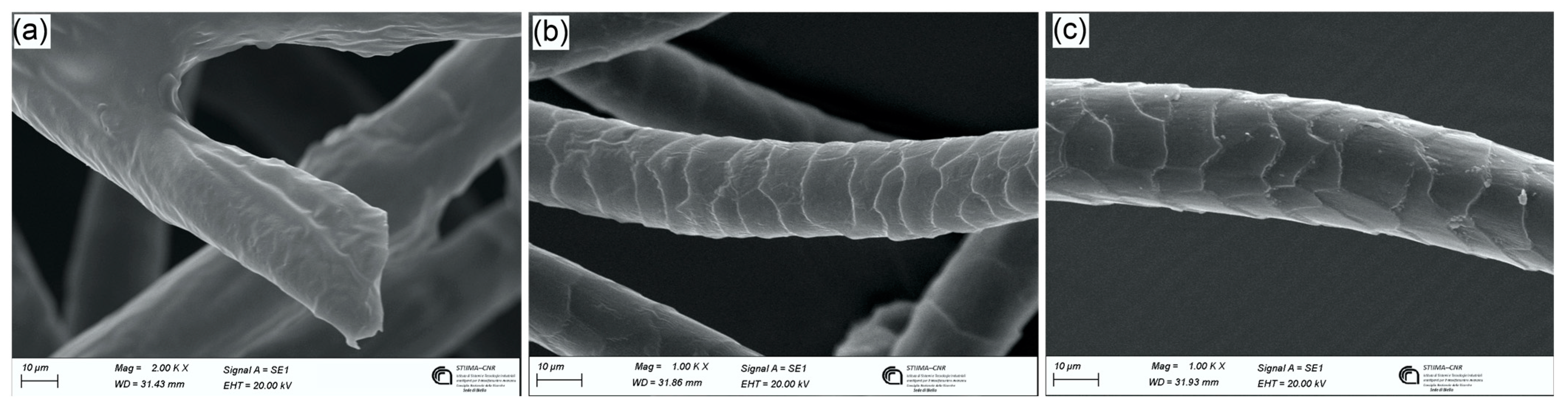
3.1.5. Morphological Characterization by SEM Analysis
3.2. Wool Scouring Using WPH as Biosurfactant
3.2.1. Molecular Weight Distribution
3.2.2. Optimization of Wool Scouring
Effect of Temperature
Material to Liquor Ratio
WPH Concentration
3.2.3. Morphological Characterization by SEM Analysis
3.3. Cost-Effectiveness
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korjenic, A.; Klarić, S.; Hadžić, A.; Korjenic, S. Sheep Wool as a Construction Material for Energy Efficiency Improvement. Energies 2015, 8, 5765–5781. [Google Scholar] [CrossRef]
- Schlossman, M.L.; McCarthy, J.P. Lanolin and Its Derivatives. J. Am. Oil Chem. Soc. 1978, 55, 447–450. [Google Scholar] [CrossRef]
- Sengupta, A.; Behera, J. Comprehensive View on Chemistry, Manufacturing & Applications of Lanolin Extracted from Wool Pretreatment. Am. J. Eng. Res. 2014, 3, 33–43. [Google Scholar]
- Hussien, A. Utilization of Lanolin in Microwave-Assisted Pigment Printing of Textiles. Egypt. J. Chem. 2020, 63, 3259–3269. [Google Scholar] [CrossRef]
- Bertolini, V.; Pallavicini, M.; Tibhe, G.; Roda, G.; Arnoldi, S.; Monguzzi, L.; Zoccola, M.; Di Nardo, G.; Gilardi, G.; Bolchi, C. Synthesis of α-Hydroxy Fatty Acids from Fatty Acids by Intermediate α-Chlorination with TCCA under Solvent-Free Conditions: A Way to Valorization of Waste Fat Biomasses. ACS Omega 2021, 6, 31901–31906. [Google Scholar] [CrossRef]
- Thewlis, J. Lanolin for Cosmetics Application. Agro Food Ind. Hi-Tech 1997, 8(5), 37–40. [Google Scholar]
- Thewlis, J. Lanolin for Cosmetics Application. Agro Food Ind. Hi-Tech 1997, 8(3), 14–20. [Google Scholar]
- Thewlis, J. Lanolin for Cosmetics Application. Agro Food Ind. Hi-Tech 1997, 8(4), 10–15. [Google Scholar]
- Taleb, M.A.; El-Sayed, H. Preparation and Characterization of Lanolin-Based Condensate and Its Utilization as a Nonionic Softener for Wool Fabric Surface. J. Appl. Res. Technol. 2021, 19, 508–520. [Google Scholar] [CrossRef]
- Khattab, T.A.; Mowafi, S.; El-Sayed, H. Development of Mechanically Durable Hydrophobic Lanolin/Silicone Rubber Coating on Viscose Fibers. Cellulose 2019, 26, 9361–9371. [Google Scholar] [CrossRef]
- Stewart, R.G. Woolscouring and Allied Technology; WRONZ: Christchurch, New Zealand, 1985; ISBN 978-0-908699-03-2. [Google Scholar]
- López-Mesas, M.; Carrillo, F.; Gutiérrez, M.C.; Crespi, M. Alternative Methods for the Wool Wax Extraction from Wool Scouring Wastes. Grasas Aceites 2007, 58, 402–407. [Google Scholar] [CrossRef]
- Choudhury, A.K.R. Green Chemistry and Textile Industry. J. Text. Eng. Fash. Technol. 2017, 2, 1–12. [Google Scholar] [CrossRef]
- Kassim, M.A.; Meng, T.K.; Serri, N.A.; Yusoff, S.B.; Shahrin, N.A.M.; Seng, K.Y.; Bakar, M.H.A.; Keong, L.C. Sustainable Biorefinery Concept for Industrial Bioprocessing. In Biorefinery Production Technologies for Chemicals and Energy; Kuila, A., Mukhopadhyay, M., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 15–39. ISBN 978-1-119-59142-9. [Google Scholar]
- Rapinel, V.; Claux, O.; Abert-Vian, M.; McAlinden, C.; Bartier, M.; Patouillard, N.; Jacques, L.; Chemat, F. 2-Methyloxolane (2-MeOx) as Sustainable Lipophilic Solvent to Substitute Hexane for Green Extraction of Natural Products. Properties, Applications, and Perspectives. Molecules 2020, 25, 3417. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K. The Toxicological Assessment of Cyclopentyl Methyl Ether (CPME) as a Green Solvent. Molecules 2013, 18, 3183–3194. [Google Scholar] [CrossRef] [PubMed]
- Azzena, U.; Carraro, M.; Pisano, L.; Monticelli, S.; Bartolotta, R.; Pace, V. Cyclopentyl Methyl Ether: An Elective Ecofriendly Ethereal Solvent in Classical and Modern Organic Chemistry. ChemSusChem 2019, 12, 40–70. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s Solvent Selection Guide—Embedding Sustainability into Solvent Selection Starting at Medicinal Chemistry. Green Chem. 2011, 13, 854. [Google Scholar] [CrossRef]
- Watanabe, K.; Yamagiwa, N.; Torisawa, Y. Cyclopentyl Methyl Ether as a New and Alternative Process Solvent. Org. Process Res. Dev. 2007, 11, 251–258. [Google Scholar] [CrossRef]
- Watanabe, K.; Goto, K. Cyclopentyl Methyl Ether. J. Synth. Org. Chem. Jpn. 2003, 61, 806–808. [Google Scholar] [CrossRef]
- de Gonzalo, G.; Alcántara, A.R.; Domínguez de María, P. Cyclopentyl Methyl Ether (CPME): A Versatile Eco-Friendly Solvent for Applications in Biotechnology and Biorefineries. ChemSusChem 2019, 12, 2083–2097. [Google Scholar] [CrossRef]
- de Jesus, S.S.; Filho, R.M. Recent Advances in Lipid Extraction Using Green Solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289. [Google Scholar] [CrossRef]
- Anastas, P.T.; Kirchhoff, M.M. Origins, Current Status, and Future Challenges of Green Chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef]
- Mendonca, G.; Mattos, M. Green Chlorination of Organic Compounds Using Trichloroisocyanuric Acid (TCCA). Curr. Org. Synth. 2014, 10, 820–836. [Google Scholar] [CrossRef]
- Bolchi, C.; Valoti, E.; Straniero, V.; Ruggeri, P.; Pallavicini, M. From 2-Aminomethyl-1,4-Benzodioxane Enantiomers to Unichiral 2-Cyano- and 2-Carbonyl-Substituted Benzodioxanes via Dichloroamine. J. Org. Chem. 2014, 79, 6732–6737. [Google Scholar] [CrossRef]
- Pallavicini, M.; Bolchi, C.; Fumagalli, L.; Piccolo, O.; Valoti, E. Highly Efficient Racemisation of a Key Intermediate of the Antibiotic Moxifloxacin. Tetrahedron Asymmetry 2011, 22, 379–380. [Google Scholar] [CrossRef]
- Hassan, M.M.; Shao, J.Z. Chemical Processing of Wool: Sustainability Considerations. Key Eng. Mater. 2015, 671, 32–39. [Google Scholar] [CrossRef]
- Bhavsar, P.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Mossotti, R.; Rovero, G.; Giansetti, M.; Tonin, C. Superheated Water Hydrolysis of Waste Wool in a Semi-Industrial Reactor to Obtain Nitrogen Fertilizers. ACS Sustain. Chem. Eng. 2016, 4, 6722–6731. [Google Scholar] [CrossRef]
- Halliday, L.A. Woolscouring, Carbonising and Effluent Treatment. In Wool; Elsevier: Amsterdam, The Netherlands, 2002; pp. 21–59. ISBN 978-1-85573-574-3. [Google Scholar]
- Yordanov, D.; Betcheva, R.; Yotova, L. Biotechnological Treatment of Effluent from the Combined Enzymatic-Ultrasound Scouring of Raw Wool. Eur. J. Chem. 2010, 1, 12–14. [Google Scholar] [CrossRef]
- Priac, A.; Morin-Crini, N.; Druart, C.; Gavoille, S.; Bradu, C.; Lagarrigue, C.; Torri, G.; Winterton, P.; Crini, G. Alkylphenol and Alkylphenol Polyethoxylates in Water and Wastewater: A Review of Options for Their Elimination. Arab. J. Chem. 2017, 10, S3749–S3773. [Google Scholar] [CrossRef]
- Maguire, R.J. Review of the Persistence of Nonylphenol and Nonylphenol Ethoxylates in Aquatic Environments. Water Qual. Res. J. 1999, 34, 37–78. [Google Scholar] [CrossRef]
- Talmage, S.S. Environmental and Human Safety of Major Surfactants: Alcohol Ethoxylates and Alkylphenol Ethoxylates; Lewis Publishers: Boca Raton, FL, USA, 1994; ISBN 978-1-56670-017-7. [Google Scholar]
- Pan, Y.; Wang, W.; Gong, K.; Hurren, C.J.; Li, Q. Ultrasonic Scouring as a Pretreatment of Wool and Its Application in Low-Temperature Dyeing. Text. Res. J. 2019, 89, 1975–1982. [Google Scholar] [CrossRef]
- Das, T.; Ramaswamy, G.N. Enzyme Treatment of Wool and Specialty Hair Fibers. Text. Res. J. 2006, 76, 126–133. [Google Scholar] [CrossRef]
- López-Mesas, M.; Carrillo, F.; Crespi, M. Microwave Enhanced Extraction of Wool Wax from Solid Wool Scour Wastes. Anal. Chim. Acta 2003, 494, 255–260. [Google Scholar] [CrossRef]
- Bahtiyari, M.İ.; Duran, K. A Study on the Usability of Ultrasound in Scouring of Raw Wool. J. Clean. Prod. 2013, 41, 283–290. [Google Scholar] [CrossRef]
- Bozaci, E. Investigation of Biosurfactant Usage in Raw Wool Scouring by Response Surface Methodology. Tekst. Ve Konfeksiyon 2017, 27, 382–392. [Google Scholar]
- Leighs, S.J.; McNeil, S.J.; Ranford, S.L. The Application of Biosurfactants for Scouring Wool. Color. Technol. 2019, 135, 48–52. [Google Scholar] [CrossRef]
- Dimitrijev Dwyer, M.; Brech, M.; Yu, L.; Middelberg, A.P.J. Intensified Expression and Purification of a Recombinant Biosurfactant Protein. Chem. Eng. Sci. 2014, 105, 12–21. [Google Scholar] [CrossRef]
- Xia, J. Protein-Based Surfactants; CRC Press: Boca Raton, FL, USA, 2001; ISBN 978-1-4822-6971-0. [Google Scholar]
- Takehara, M. Properties and Applications of Amino Acid Based Surfactants. Colloids Surf. 1989, 38, 149–167. [Google Scholar] [CrossRef]
- Zoccola, M.; Aluigi, A.; Patrucco, A.; Tonin, C. Extraction, Processing and Applications of Wool Keratin. In Keratin Structure, Properties and Applications; Series: Protein Biochemistry, Synthesis, Structure, and Cellular Functions Series; Nova Publishers: New York, NY, USA, 2012; ISBN 1-62100-392-2. [Google Scholar]
- Hoshino, M. Foam Fire Extinguishing Agent. J. Jpn. Oil Chem. Soc. 1993, 42, 856–867. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Z.; Liu, Y.; Wang, J.; Yang, K.; Chen, D.; Liang, Z.; Li, Z. Antibacterial and Biodegradable Keratin-Based Quaternary Ammonium Salt Surfactant Potential as Hair Care Additive. J. Dispers. Sci. Technol. 2022, 1–10. [Google Scholar] [CrossRef]
- Bhavsar, P.S.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Mossotti, R.; Giansetti, M.; Rovero, G.; Maier, S.S.; Muresan, A.; Tonin, C. Superheated Water Hydrolyzed Keratin: A New Application as a Foaming Agent in Foam Dyeing of Cotton and Wool Fabrics. ACS Sustain. Chem. Eng. 2017, 5, 9150–9159. [Google Scholar] [CrossRef]
- Peiravi-Rivash, O.; Mashreghi, M.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Producing Bacterial Nano-Cellulose and Keratin from Wastes to Synthesize Keratin/Cellulose Nanobiocomposite for Removal of Dyes and Heavy Metal Ions from Waters and Wastewaters. Colloids Surf. Physicochem. Eng. Asp. 2023, 656, 130355. [Google Scholar] [CrossRef]
- Tonin, C.; Zoccola, M.; Aluigi, A.; Varesano, A.; Montarsolo, A.; Vineis, C.; Zimbardi, F. Study on the Conversion of Wool Keratin by Steam Explosion. Biomacromolecules 2006, 7, 3499–3504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Li, J.; Zhao, Z.; Liu, X.; Li, Z.; Han, Y.; Hu, J.; Chen, A. Isolation and Characterization of Biofunctional Keratin Particles Extracted from Wool Wastes. Powder Technol. 2013, 246, 356–362. [Google Scholar] [CrossRef]
- Berechet, M.D.; Niculescu, M.D.; Gaidau, C.; Ignat, M.; Epure, D.G. Alkaline-Enzymatic Hydrolysis of Wool Waste for Different Applications. Rev. Chim. 2018, 69, 1649–1654. [Google Scholar] [CrossRef]
- Dias, G.J.; Haththotuwa, T.N.; Rowlands, D.S.; Gram, M.; Bekhit, A.E.-D.A. Wool Keratin—A Novel Dietary Protein Source: Nutritional Value and Toxicological Assessment. Food Chem. 2022, 383, 132436. [Google Scholar] [CrossRef]
- Tonin, C.; Aluigi, A.; Vineis, C.; Varesano, A.; Montarsolo, A.; Ferrero, F. Thermal and Structural Characterization of Poly(Ethylene-Oxide)/Keratin Blend Films. J. Therm. Anal. Calorim. 2007, 89, 601–608. [Google Scholar] [CrossRef]
- Okoro, O.V.; Jafari, H.; Hobbi, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Enhanced Keratin Extraction from Wool Waste Using a Deep Eutectic Solvent. Chem. Pap. 2022, 76, 2637–2648. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Bhavsar, P.S.; Kannan, S.; Pinjari, D.V.; Pandit, A.B. Acoustic Cavitation Assisted Alkaline Hydrolysis of Wool Based Keratins to Produce Organic Amendment Fertilizers. ACS Sustain. Chem. Eng. 2016, 4, 2789–2796. [Google Scholar] [CrossRef]
- Yin, J.; Rastogi, S.; Terry, A.E.; Popescu, C. Self-Organization of Oligopeptides Obtained on Dissolution of Feather Keratins in Superheated Water. Biomacromolecules 2007, 8, 800–806. [Google Scholar] [CrossRef]
- Bhavsar, P.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Rovero, G.; Tonin, C. Comparative Study on the Effects of Superheated Water and High Temperature Alkaline Hydrolysis on Wool Keratin. Text. Res. J. 2017, 87, 1696–1705. [Google Scholar] [CrossRef]
- Innocenti, R.; Zoccola, M. Near Infrared Reflectance Spectroscopy as a Tool for the Determination of Dichloromethane Extractable Matter and Moisture Content in Combed Wool Slivers. J. Infrared Spectrosc. 2003, 11, 333–340. [Google Scholar] [CrossRef]
- Method for the Determination of Dichloromethane Soluble Matter in Combed Wool and Commercially Scoured or Carbonised Wool; Test Method IWTO-10; International Wool Textile Organization: Brussels, Belgium.
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Zoccola, M.; Aluigi, A.; Tonin, C. Characterisation of Keratin Biomass from Butchery and Wool Industry Wastes. J. Mol. Struct. 2009, 938, 35–40. [Google Scholar] [CrossRef]
- Method for the Measurement of the Colour of Raw Wool; Test Method IWTO-56; International Wool Textile Organization: Brussels, Belgium.
- Kaur, D.; Singh, O.P. Fleece Composition of Wool of Different Breeds of Sheep. Text. Trends 2005, 48, 39–43. [Google Scholar]
- Sagiri, S.S.; Behera, B.; Pal, K.; Basak, P. Lanolin-Based Organogels as a Matrix for Topical Drug Delivery. J. Appl. Polym. Sci. 2013, 128, 3831–3839. [Google Scholar] [CrossRef]
- Shanmugavel, M.; Lakshmi, J.N.; Vasantharaj, S.; Anu, C.; Paul, L.E.; Kumar, R.P.; Gnanamani, A. Wealth from Waste: Recovery of the Commercially Important Waxy Ester from Enzymatic Dehaired Sheep Wool. Biocatal. Agric. Biotechnol. 2019, 20, 101255. [Google Scholar] [CrossRef]
- Okpuwhara, R.O.; Oboirien, B.O.; Sadiku, E.R. The Lanolin-based Oil Plasticized Polylactide: Thermal and Chemical Characteristics. Polym. Eng. Sci. 2022, 62, 1571–1581. [Google Scholar] [CrossRef]
- Barba Albanell, C.; Carrer, V.; Marti, M.; Iglesias, J.; Iglesias, J.; Coderch, L. Solvent-Extracted Wool Wax: Thermotropic Properties and Skin Efficacy. Skin Pharmacol. Physiol. 2018, 31, 198–205. [Google Scholar] [CrossRef]
- e Silva, N.M.P.R.; Meira, H.M.; Almeida, F.C.G.; de Silva, R.d.C.F.S.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Natural Surfactants and Their Applications for Heavy Oil Removal in Industry. Sep. Purif. Rev. 2019, 48, 267–281. [Google Scholar] [CrossRef]
- Das, D.; Das, S. Wool Structure and Morphology. In Wool Fiber Reinforced Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 13–32. ISBN 978-0-12-824056-4. [Google Scholar]
- Valverde, A.; Recasens, F. Extraction of Solid Lanoline from Raw Wool with Near-Critical Ethanol-Modified CO2—A Mass Transfer Model. J. Supercrit. Fluids 2019, 145, 151–161. [Google Scholar] [CrossRef]
- Morán, M.C.; Pinazo, A.; Pérez, L.; Clapés, P.; Angelet, M.; García, M.T.; Vinardell, M.P.; Infante, M.R. “Green” Amino Acid-Based Surfactants. Green Chem 2004, 6, 233–240. [Google Scholar] [CrossRef]
- Madani, M.; Zargar, G.; Takassi, M.A.; Daryasafar, A.; Wood, D.A.; Zhang, Z. Fundamental Investigation of an Environmentally-Friendly Surfactant Agent for Chemical Enhanced Oil Recovery. Fuel 2019, 238, 186–197. [Google Scholar] [CrossRef]
- Rahman, M.; Nur, M.G. Golam Nur Feasible Application of Modern Eco-Friendly Treatment of Wool Fabric before Coloration. Int. J. Sci. Res. Publ. 2014, 4, 1–7. [Google Scholar]
- Li, Q.; Hurren, C.J.; Wang, X. Ultrasonic Assisted Industrial Wool Scouring. Procedia Eng. 2017, 200, 39–44. [Google Scholar] [CrossRef]
- Vujasinović, E.; Tarbuk, A.; Pušić, T.; Dekanić, T. Bio-Innovative Pretreatment of Coarse Wool Fibers. Processes 2022, 11, 103. [Google Scholar] [CrossRef]
- de Jesus, S.S.; Ferreira, G.F.; Moreira, L.S.; Wolf Maciel, M.R.; Maciel Filho, R. Comparison of Several Methods for Effective Lipid Extraction from Wet Microalgae Using Green Solvents. Renew. Energy 2019, 143, 130–141. [Google Scholar] [CrossRef]
- Bijoy, R.; Agarwala, P.; Roy, L.; Thorat, B.N. Unconventional Ethereal Solvents in Organic Chemistry: A Perspective on Applications of 2-Methyltetrahydrofuran, Cyclopentyl Methyl Ether, and 4-Methyltetrahydropyran. Org. Process Res. Dev. 2022, 26, 480–492. [Google Scholar] [CrossRef]
- de Jesus, S.S.; Ferreira, G.F.; Wolf Maciel, M.R.; Maciel Filho, R. Biodiesel Purification by Column Chromatography and Liquid-Liquid Extraction Using Green Solvents. Fuel 2019, 235, 1123–1130. [Google Scholar] [CrossRef]
| Wool | Characteristics | Diameter (μm) | Use |
|---|---|---|---|
| Sarda | long and coarse | 38 | breed for milk production |
| Biellese | very course and short | 36 | breed for meat production |
| Sopravissana | fine | 22 | breed for wool and meat |
| Apparatus | LAUDA H20S-Hydro Shaking Water Baths |
| Material | Sopravissana wool |
| Process | Batch scouring |
| Surfactant | Wool protein hydrolyzate (1–5%), commercial Biotex AL (2 g/L) |
| Bath ratio | 1:10–1:50 w/w |
| Temperature | 35–65 °C |
| Sample | Sample Replicates (% w/w) | Mean (% w/w) | s.d. (% w/w) | ||
|---|---|---|---|---|---|
| Sarda—hexane | 2.83 | 4.32 | 3.51 | 3.55 | 0.74 |
| Sarda—diethyl ether | 6.12 | 1.63 | 3.95 | 3.90 | 2.24 |
| Sarda—cyclopentyl methyl ether | 3.22 | 4.26 | 6.25 | 4.57 | 1.53 |
| Biellese—hexane | 3.64 | 3.07 | 4.31 | 3.67 | 0.62 |
| Biellese—diethyl ether | 3.96 | 1.90 | 3.68 | 3.18 | 1.11 |
| Biellese—cyclopentyl methyl ether | 4.55 | 5.91 | 6.15 | 5.53 | 0.86 |
| Sopravissana—hexane | 14.97 | 16.66 | 20.4 | 17.34 | 2.77 |
| Sopravissana—diethyl ether | 25.42 | 21.85 | 24.09 | 23.78 | 1.80 |
| Sopravissana—Cyclopentyl methyl ether | 25.61 | 23.87 | 27.79 | 25.75 | 1.96 |
| Properties | CPME | Diethyl Ether | Hexane |
|---|---|---|---|
| Boiling point [°C] | 106 | 34.5 | 68.7 |
| Dipole moment [D] | 1.27 | 1.12 | 0.08 |
| Solubility in water [g/100 g] | 1.1 | 6.5 | 0.001 |
| Solubility of water in the solvent [g/100 g] | 0.3 | 1.2 | 0.01 |
| LogPow | 1.59 | 0.89 | 3.90 |
| Sample | FA | mg | % (w/w) |
|---|---|---|---|
| LA | C14 | 0.868 | 0.87 |
| C16 | 1.480 | 1.48 | |
| C18 | 0.874 | 0.87 | |
| 2-OH C16 | 16.594 | 16.6 | |
| Total weight % | 19.82 | ||
| LB | C14 | 1.850 | 1.85 |
| C16 | 2.668 | 2.67 | |
| C18 | 0.933 | 0.93 | |
| 2-OH C16 | 12.366 | 12.37 | |
| Total weight % | 17.82 | ||
| LC | C14 | 0.976 | 0.98 |
| C16 | 1.959 | 1.96 | |
| C18 | 0.795 | 0.79 | |
| 2-OH C16 | 11.791 | 11.79 | |
| Total weight% | 15.52 | ||
| Temperature °C | Grease Content (%) | Whiteness Index | Yellowness Index | Washing Yield (%) |
|---|---|---|---|---|
| 35 | 1.53 ± 0.08 | 71.92 | 13.89 | 42.64 ± 2.22 |
| 45 | 1.69 ± 0.11 | 73.41 | 13.42 | 53.92 ± 1.21 |
| 55 | 1.98 ± 0.13 | 73.42 | 14.98 | 49.03 ± 3.25 |
| 65 | 4.03 ± 0.22 | 76.86 | 14.06 | 50.66 ± 0.12 |
| Material-to-Liquor Ratio | Grease Content (%) | Whiteness Index | Yellowness Index | Washing Yield (%) |
|---|---|---|---|---|
| 1:10 | 2.16 ± 0.12 | 67.96 | 11.88 | 52.57 ± 1.18 |
| 1:20 | 0.74 ± 0.13 | 69 | 12.03 | 50.32 ± 2.03 |
| 1:30 | 2.02 ± 0.20 | 68.1 | 11.93 | 51.80 ± 1.45 |
| 1:40 | 0.86 ± 0.12 | 67.31 | 11.59 | 51.74 ± 0.54 |
| 1:50 | 1.32 ± 0.08 | 69.31 | 12.23 | 51.87 ± 1.78 |
| WPH (%) | Grease Content (%) | Whiteness Index | Yellowness Index | Washing Yield (%) |
|---|---|---|---|---|
| Biotex AL (2 g/L) | 0.38 ± 0.14 | 68.44 | 4.45 | 50.38 ± 1.84 |
| 1 | 2.97 ± 0.11 | 71.46 | 4.2 | 50.90 ± 1.33 |
| 2 | 0.31 ± 0.09 | 73.7 | 4.63 | 48.91 ± 2.43 |
| 3 | 0.36 ± 0.13 | 71.86 | 3.63 | 50.80 ± 0.59 |
| 4 | 0.30 ± 0.06 | 68.02 | 4.41 | 52.60 ± 2.09 |
| 5 | 0.72 ± 0.11 | 70.32 | 5.67 | 51.40 ± 1.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhavsar, P.; Zoccola, M.; Dalla Fontana, G.; Pallavicini, M.; Roda, G.; Bolchi, C. Sustainable Routes for Wool Grease Removal Using Green Solvent Cyclopentyl Methyl Ether in Solvent Extraction and Biosurfactant Wool Protein Hydrolyzate in Scouring. Processes 2023, 11, 1309. https://doi.org/10.3390/pr11051309
Bhavsar P, Zoccola M, Dalla Fontana G, Pallavicini M, Roda G, Bolchi C. Sustainable Routes for Wool Grease Removal Using Green Solvent Cyclopentyl Methyl Ether in Solvent Extraction and Biosurfactant Wool Protein Hydrolyzate in Scouring. Processes. 2023; 11(5):1309. https://doi.org/10.3390/pr11051309
Chicago/Turabian StyleBhavsar, Parag, Marina Zoccola, Giulia Dalla Fontana, Marco Pallavicini, Gabriella Roda, and Cristiano Bolchi. 2023. "Sustainable Routes for Wool Grease Removal Using Green Solvent Cyclopentyl Methyl Ether in Solvent Extraction and Biosurfactant Wool Protein Hydrolyzate in Scouring" Processes 11, no. 5: 1309. https://doi.org/10.3390/pr11051309
APA StyleBhavsar, P., Zoccola, M., Dalla Fontana, G., Pallavicini, M., Roda, G., & Bolchi, C. (2023). Sustainable Routes for Wool Grease Removal Using Green Solvent Cyclopentyl Methyl Ether in Solvent Extraction and Biosurfactant Wool Protein Hydrolyzate in Scouring. Processes, 11(5), 1309. https://doi.org/10.3390/pr11051309






