Effect of 450 nm Visible Blue Light from Light-Emitting Diode on Escherichia coli O157:H7 in Agar Gels: Optimizing the Lighting Array and Quantitative Microbial Exposure Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Inoculum
2.2. Sample Preparation
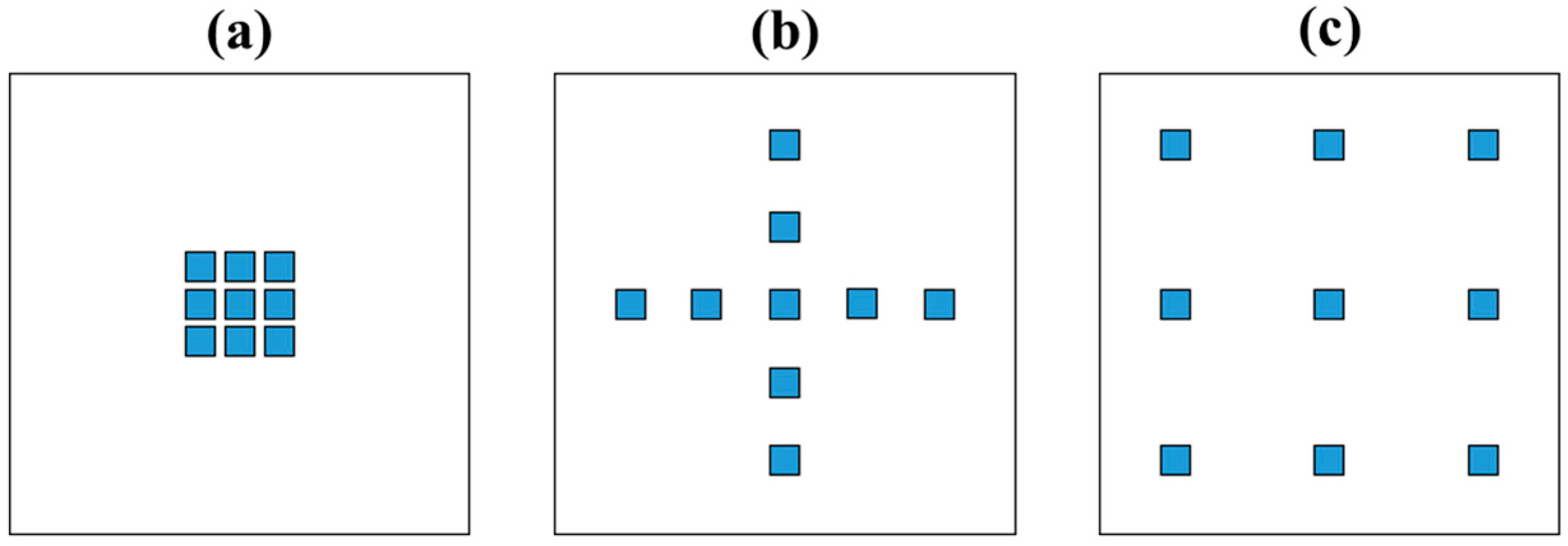
2.3. Design of LED Arrays
2.4. Bacterial Enumeration
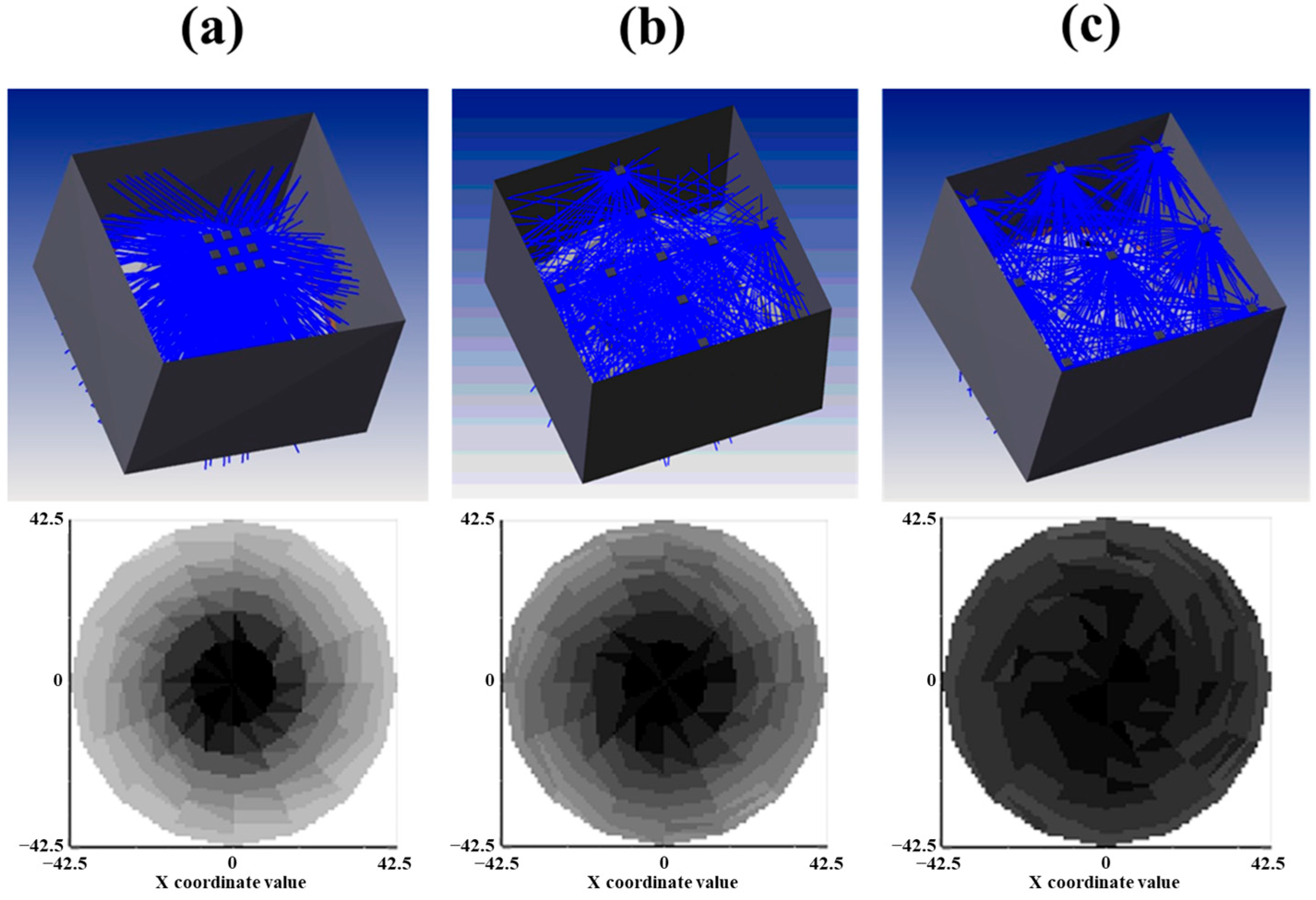
2.5. Optical Simulation for Ray Tracing
2.6. Exposure Assessment
2.7. Growth Model
2.8. Monte Carlo Simulation for Exposure Assessment
2.9. Statistical Analysis
3. Results and Discussion
3.1. Influence of the LED Arrangement on the Solid Model Systems
3.2. Photoinactivation Effect of Blue Light Treatment on E. coli O157:H7
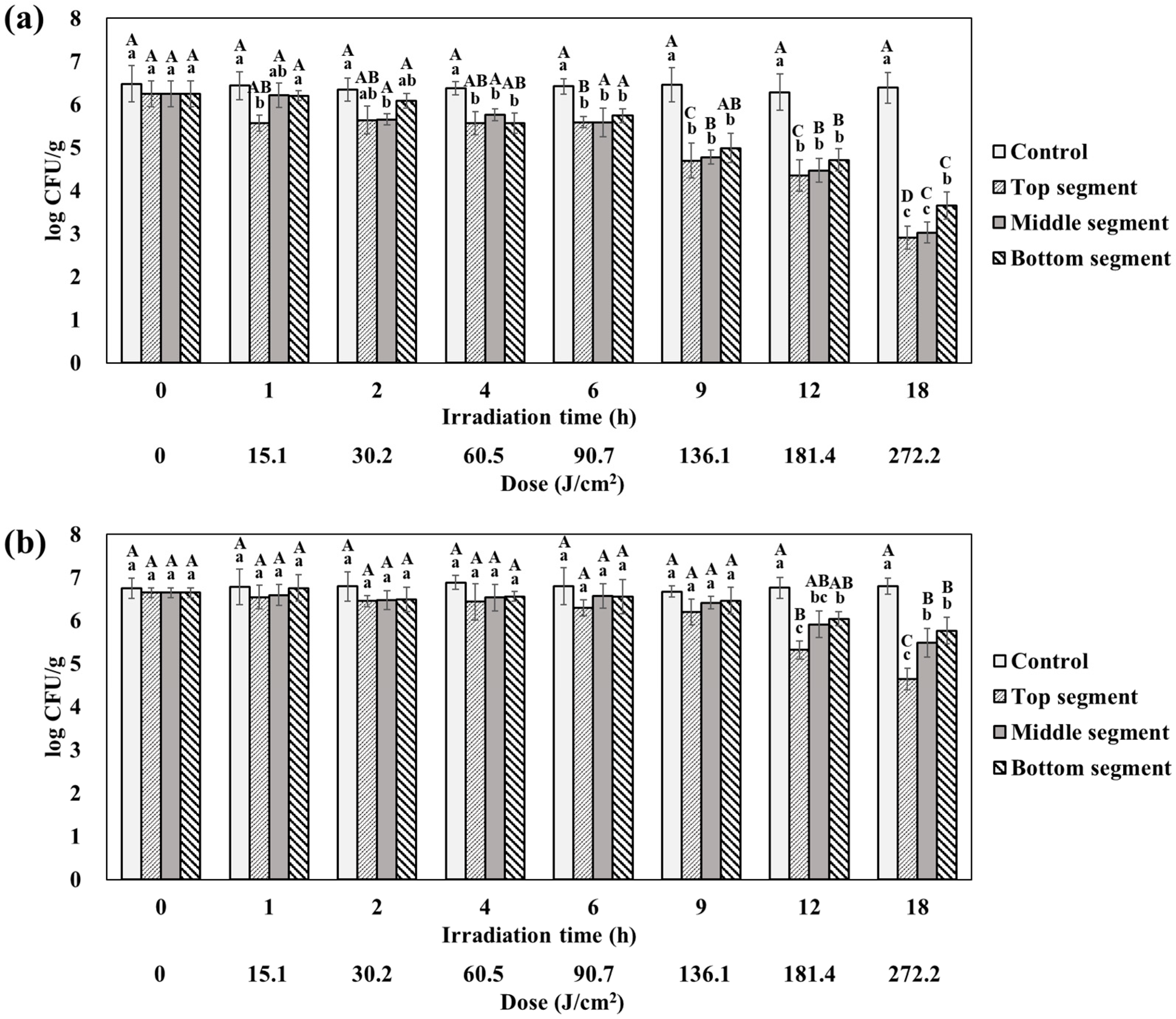
3.3. Height-Dependence of Blue Light Treatment on a Solid Model System
3.4. Exposure Assessment of Agar Gels after LED Inactivation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Toole, M.; Diamond, D. Absorbance based light emitting diode optical sensors and sensing devices. Sensors 2008, 8, 2453–2479. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.C.; Huang, H.J.; Chao, C.C.; Huang, M.C. Design and analysis of a high-intensity LED lighting module for underwater illumination. Appl. Ocean Res. 2013, 39, 89–96. [Google Scholar] [CrossRef]
- Prasad, A.; Du, L.; Zubair, M.; Subedi, S.; Ullah, A.; Roopesh, M.S. Applications of Light-Emitting Diodes (LEDs) in food processing and water treatment. Food Eng. Rev. 2020, 12, 268–289. [Google Scholar] [CrossRef]
- Kim, D.K.; Kang, D.H. Efficacy of light-emitting diodes emitting 395, 405, 415, and 425 nm blue light for bacterial inactivation and the microbicidal mechanism. Food Res. Int. 2021, 141, 110105. [Google Scholar] [CrossRef]
- Soukos, N.S.; Ximenez-Fyvie, L.A.; Hamblin, M.R.; Socransky, S.S.; Hasan, T. Targeted antimicrobial photochemotherapy. Antimicrob. Agents Chemother. 1998, 42, 2595–2601. [Google Scholar] [CrossRef]
- Endarko, E.; Maclean, M.; Timoshkin, I.V.; MacGregor, S.J.; Anderson, J.G. High-intensity 405 nm light inactivation of Listeria monocytogenes. Photochem. Photobiol. 2012, 88, 1280–1286. [Google Scholar] [CrossRef]
- Ganz, R.A.; Viveiros, J.; Ahmad, A.; Ahmadi, A.; Khalil, A.; Tolkoff, M.J.; Nishioka, N.S.; Hamblin, M.R. Helicobacter pylori in patients can be killed by visible light. Lasers Surg. Med. 2005, 36, 260–265. [Google Scholar] [CrossRef]
- Luksienė, Z.; Zukauskas, A. Prospects of photosensitization in control of pathogenic and harmful micro-organisms. J. Appl. Microbiol. 2009, 107, 1415–1424. [Google Scholar] [CrossRef]
- D’Souza, C.; Yuk, H.; Khoo, G.H.; Zhou, W. Application of light-emitting diodes in food production, postharvest preservation, and microbiological food safety. Compr. Rev. Food Sci. Food Saf. 2015, 14, 719–740. [Google Scholar] [CrossRef]
- Ghate, V.; Kumar, A.; Zhou, W.; Yuk, H. Irradiance and temperature influence the bactericidal effect of 460-nanometer light-emitting diodes on Salmonella in orange juice. J. Food Prot. 2016, 79, 553–560. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Shewale, S.R.; Rajoriya, D.; Hebbar, H.U. Impact of blue LED illumination and natural photosensitizer on bacterial pathogens, enzyme activity and quality attributes of fresh-cut pineapple slices. Food Bioprocess Tech. 2021, 14, 362–372. [Google Scholar] [CrossRef]
- Guo, D.; Bai, Y.; Fei, S.; Yang, Y.; Li, J.; Yang, B.; Lü, X.; Xia, X.; Shi, C. Effects of 405 ± 5-nm LED Illumination on Environmental Stress Tolerance of Salmonella Typhimurium in Sliced Beef. Foods 2022, 11, 136. [Google Scholar] [CrossRef]
- Panisello, P.J.; Rooney, R.; Quantick, P.C.; Stanwell-Smith, R. Application of foodborne disease outbreak data in the development and maintenance of HACCP systems. Int. J. Food Microbiol. 2000, 59, 221–234. [Google Scholar] [CrossRef]
- Trinetta, V.; Vaidya, N.; Linton, R.; Morgan, M. A comparative study on the effectiveness of chlorine dioxide gas, ozone gas and e-beam irradiation treatments for inactivation of pathogens inoculated onto tomato, cantaloupe and lettuce seeds. Int. J. Food Microbiol. 2011, 146, 203–206. [Google Scholar] [CrossRef]
- Hernandez-Carmona, G.; Freile-Pelegrín, Y.; Hernández-Garibay, E. Conventional and alternative technologies for the extraction of algal polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals, 1st ed.; Dominguez, H., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 475–516. [Google Scholar] [CrossRef]
- Xiao, Q.; Weng, H.; Ni, H.; Hong, Q.; Lin, K.; Xiao, A. Physicochemical and gel properties of agar extracted by enzyme and enzyme-assisted methods. Food Hydrocoll. 2019, 87, 530–540. [Google Scholar] [CrossRef]
- Padmesh, S.; Singh, A. Agars: Properties and Applications. In Polysaccharides: Properties and Applications; Inamuddin, I., Ahamed, M.I., Boddula, R., Altalhi, T.A., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 75–93. [Google Scholar] [CrossRef]
- Huang, P.; Cheng, Y.; Lu, W.; Li, P. Optimization of Concentration-Time, Agar, and Sugar Concentration for Sweet Gelatinized Adzuki-Bean Jelly Cake (Yokan) by Response Surface Methodology. Gels 2022, 8, 540. [Google Scholar] [CrossRef]
- Riedel, R.; Böhme, B.; Rohm, H. Development of formulations for reduced-sugar and sugar-free agar-based fruit jellies. Int. J. Food Sci. Tech. 2015, 50, 1338–1344. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Kamińska-Dwórznicka, A. Effect of addition of chokeberry juice concentrate and foaming agent on the physical properties of agar gel. Gels 2021, 7, 137. [Google Scholar] [CrossRef]
- Nauta, M.J. Separation of uncertainty and variability in quantitative microbial risk assessment models. Int. J. Food Microbiol. 2000, 57, 9–18. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Hebbar, H.U. Sono-photodynamic inactivation of Escherichia coli and Staphylococcus aureus in orange juice. Ultrason. Sonochem. 2019, 57, 108–115. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J. Effects of 405 nm light-emitting diode treatment on microbial community on fresh-cut pakchoi and antimicrobial action against Pseudomonas reinekei and Pseudomonas palleroniana. J. Food Saf. 2021, 41, e12920. [Google Scholar] [CrossRef]
- Saxena, R.; Vasudevan, S.; Patil, D.; Ashoura, N.; Grimwade, J.E.; Crooke, E. Nucleotide-induced conformational changes in Escherichia coli DnaA protein are required for bacterial ORC to pre-RC conversion at the chromosomal origin. Int. J. Mol. Sci. 2015, 16, 27897–27911. [Google Scholar] [CrossRef] [PubMed]
- Hirt, B.; Fiege, J.; Cvetkova, S.; Gräf, V.; Scharfenberger-Schmeer, M.; Durner, D.; Stahl, M. Comparison and prediction of UV-C inactivation kinetics of S. cerevisiae in model wine systems dependent on flow type and absorbance. LWT 2022, 169, 114062. [Google Scholar] [CrossRef]
- Palmer, J.M. Radiometry and photometry: Units and conversions. In Handbook of Optics, 3rd ed.; McGraw-Hill, Inc.: New York, NY, USA, 2010; pp. 7–11. [Google Scholar]
- Bolton, J.R.; Linden, K.G. Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments. J. Environ. Eng. 2003, 129, 209–215. [Google Scholar] [CrossRef]
- Park, H.W.; Yoon, W.B. A quantitative microbiological exposure assessment model for Bacillus cereus in pasteurized rice cakes using computational fluid dynamics and Monte Carlo simulation. Food Res. Int. 2019, 125, 108562. [Google Scholar] [CrossRef]
- Mataragas, M.; Zwietering, M.H.; Skandamis, P.N.; Drosinos, E.H. Quantitative microbiological risk assessment as a tool to obtain useful information for risk managers—Specific application to Listeria monocytogenes and ready-to-eat meat products. Int. J. Food Microbiol. 2010, 141, S170–S179. [Google Scholar] [CrossRef]
- Ding, T.; Iwahori, J.; Kasuga, F.; Wang, J.; Forghani, F.; Park, M.; Oh, D. Risk assessment for Listeria monocytogenes on lettuce from farm to table in Korea. Food Control 2013, 30, 190–199. [Google Scholar] [CrossRef]
- Park, M.; Bahk, G. Current state for temperature management of cold and frozen food transportation vehicles in Jeonbuk province. J. Food Hyg. Saf. 2017, 32, 107–113. [Google Scholar] [CrossRef]
- Jung, H. Consumer Survey and Hazard Analysis for the Improvement of Food Hygiene and Safety in Purchase. Master’s Thesis, Korea University, Seoul, Republic of Korea, 2011. [Google Scholar]
- Kennedy, J.; Jackson, V.; Blair, I.S.; McDowell, D.A.; Cowan, C.; Bolton, D.J. Food safety knowledge of consumers and the microbiological and temperature status of their refrigerators. J. Food Prot. 2005, 68, 1421–1430. [Google Scholar] [CrossRef]
- Oh, D.; Ding, T.; Jin, Y. A new secondary model developed for the growth rate of Escherichia coli O157: H7 in broth. Indian J. Microbiol. 2012, 52, 99–101. [Google Scholar] [CrossRef]
- Xie, T.; Yuan, X.; Wen, D.; Shi, H. Growth and thermal inactivation of Listeria monocytogenes and Escherichia coli O157: H7 in four kinds of traditionally non-fermented soya bean products. Int. J. Food Sci. Tech. 2021, 56, 4062–4069. [Google Scholar] [CrossRef]
- Lee, S.; Han, A.; Yoon, J.; Lee, S. Growth evaluation of Escherichia coli O157: H7, Salmonella typhimurium, and Listeria monocytogenes in fresh fruit and vegetable juices via predictive modeling. LWT 2022, 162, 113485. [Google Scholar] [CrossRef]
- Juneja, V.K.; Melendres, M.V.; Huang, L.; Subbiah, J.; Thippareddi, H. Mathematical modeling of growth of Salmonella in raw ground beef under isothermal conditions from 10 to 45 C. Int. J. Food Microbiol. 2009, 131, 106–111. [Google Scholar] [CrossRef]
- Zwietering, M.H.; De Koos, J.T.; Hasenack, B.E.; De Witt, J.C.; Van’t Riet, K. Modeling of bacterial growth as a function of temperature. Appl. Environ. Microbiol. 1991, 57, 1094–1101. [Google Scholar] [CrossRef]
- Bowker, C.; Sain, A.; Shatalov, M.; Ducoste, J. Microbial UV fluence-response assessment using a novel UV-LED collimated beam system. Water Res. 2011, 45, 2011–2019. [Google Scholar] [CrossRef]
- Moreno, I.; Sun, C. Modeling the radiation pattern of LEDs. Opt. Express 2008, 16, 1808–1819. [Google Scholar] [CrossRef]
- Simons, R.; Gabbai, U.E.; Moram, M.A. Optical fluence modelling for ultraviolet light emitting diode-based water treatment systems. Water Res. 2014, 66, 338–349. [Google Scholar] [CrossRef]
- Jung, H.; Yuk, H.; Yoon, W.B. Effect of LED light on the inactivation of Bacillus cereus for extending shelf-life of extruded rice cake and simulation of the patterns of LED irradiation by various arrays of LEDs. J. Appl. Biol. Chem. 2019, 62, 181–186. [Google Scholar] [CrossRef]
- Kumar, A.; Ghate, V.; Kim, M.J.; Zhou, W.; Khoo, G.H.; Yuk, H.G. Inactivation and changes in metabolic profile of selected foodborne bacteria by 460 nm LED illumination. Food Microbiol. 2017, 63, 12–21. [Google Scholar] [CrossRef]
- Ghate, V.S.; Ng, K.S.; Zhou, W.; Yang, H.; Khoo, G.H.; Yoon, W.B.; Yuk, H.G. Antibacterial effect of light emitting diodes of visible wavelengths on selected foodborne pathogens at different illumination temperatures. Int. J. Food Microbiol. 2013, 166, 399–406. [Google Scholar] [CrossRef]
- Hyun, J.E.; Moon, S.K.; Lee, S.Y. Antibacterial activity and mechanism of 460–470 nm light-emitting diodes against pathogenic bacteria and spoilage bacteria at different temperatures. Food Control 2021, 123, 107721. [Google Scholar] [CrossRef]
- Bialka, K.L.; Demirci, A.; Walker, P.N.; Puri, V.M. Pulsed UV-light penetration of characterization and the inactivation of Escherichia coli K12 in solid model systems. Trans. ASABE 2008, 51, 195–204. [Google Scholar] [CrossRef]
- Kim, M.; Yuk, H. Antibacterial mechanism of 405-nanometer light-emitting diode against Salmonella at refrigeration temperature. Appl. Environ. Microbiol. 2017, 83, 2582. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lianto, D.K.; Koo, G.H.; Yuk, H. Antibacterial mechanism of riboflavin-mediated 460 nm light emitting diode illumination against Listeria monocytogenes in phosphate-buffered saline and on smoked salmon. Food Control 2021, 124, 107930. [Google Scholar] [CrossRef]



| Inputs | Implementations | Units | Sources/Note |
|---|---|---|---|
| Transportation from manufacturing facility | |||
| Temperature | Pert (6, 12, 20) | °C | [21] |
| Time | Pert (2, 4, 12) | h | [21] |
| Retail storage | |||
| Temperature | Normal (5.44, 2.32) | °C | [22] |
| Time | Pert (1, 8, 13) | h | Retailer info |
| Transportation from retail shop to home | |||
| Temperature | Pert (2, Uniform (4, 8.32), Uniform (15, 25)) | °C | [23] |
| Time | Pert (19.5, 59, 98.6) | min | [25] |
| Home storage | |||
| Temperature | Normal (5.85, 2.49) | °C | [26] |
| Time | Uniform (0, 21) | day | Retailer info |
| Petri Factor | LED Arrangement | ||
|---|---|---|---|
| Centered Array | Cross Array | Evenly Spaced Array | |
| Measured | 0.67 ± 0.09 c | 0.84 ± 0.05 b | 0.99 ± 0.06 a |
| Simulated | 0.54 | 0.70 | 0.85 |
| Inputs | Implementations | Units | Sources/Note |
|---|---|---|---|
| Initial level of Escherichia coli O157:H7 | |||
| Agar gels without dye | |||
| Top segment | Normal (2.90, 0.27) | Log CFU/g | Measured |
| Middle segment | Normal (3.02, 0.24) | Log CFU/g | Measured |
| Bottom segment | Normal (3.65, 0.31) | Log CFU/g | Measured |
| Agar gels with dye | |||
| Top segment | Normal (4.64, 0.25) | Log CFU/g | Measured |
| Middle segment | Normal (5.48, 0.33) | Log CFU/g | Measured |
| Bottom segment | Normal (5.76, 0.31) | Log CFU/g | Measured |
| Model | Parameter | Value |
|---|---|---|
| Modified Ratkowsky model | Tmax | 58.24 |
| Tmin | 0.31 | |
| b | 0.030 | |
| c | 0.046 | |
| Hyperbolic model | p | 105.50 |
| q | −18.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.; Yoon, W.B. Effect of 450 nm Visible Blue Light from Light-Emitting Diode on Escherichia coli O157:H7 in Agar Gels: Optimizing the Lighting Array and Quantitative Microbial Exposure Assessment. Processes 2023, 11, 1331. https://doi.org/10.3390/pr11051331
Jung H, Yoon WB. Effect of 450 nm Visible Blue Light from Light-Emitting Diode on Escherichia coli O157:H7 in Agar Gels: Optimizing the Lighting Array and Quantitative Microbial Exposure Assessment. Processes. 2023; 11(5):1331. https://doi.org/10.3390/pr11051331
Chicago/Turabian StyleJung, Hwabin, and Won Byong Yoon. 2023. "Effect of 450 nm Visible Blue Light from Light-Emitting Diode on Escherichia coli O157:H7 in Agar Gels: Optimizing the Lighting Array and Quantitative Microbial Exposure Assessment" Processes 11, no. 5: 1331. https://doi.org/10.3390/pr11051331






