Biowax Production from the Hydrotreatment of Refined Palm Oil (RPO)
Abstract
1. Introduction
2. Materials and Methods
2.1. Feed and Reagents
2.2. Catalysts
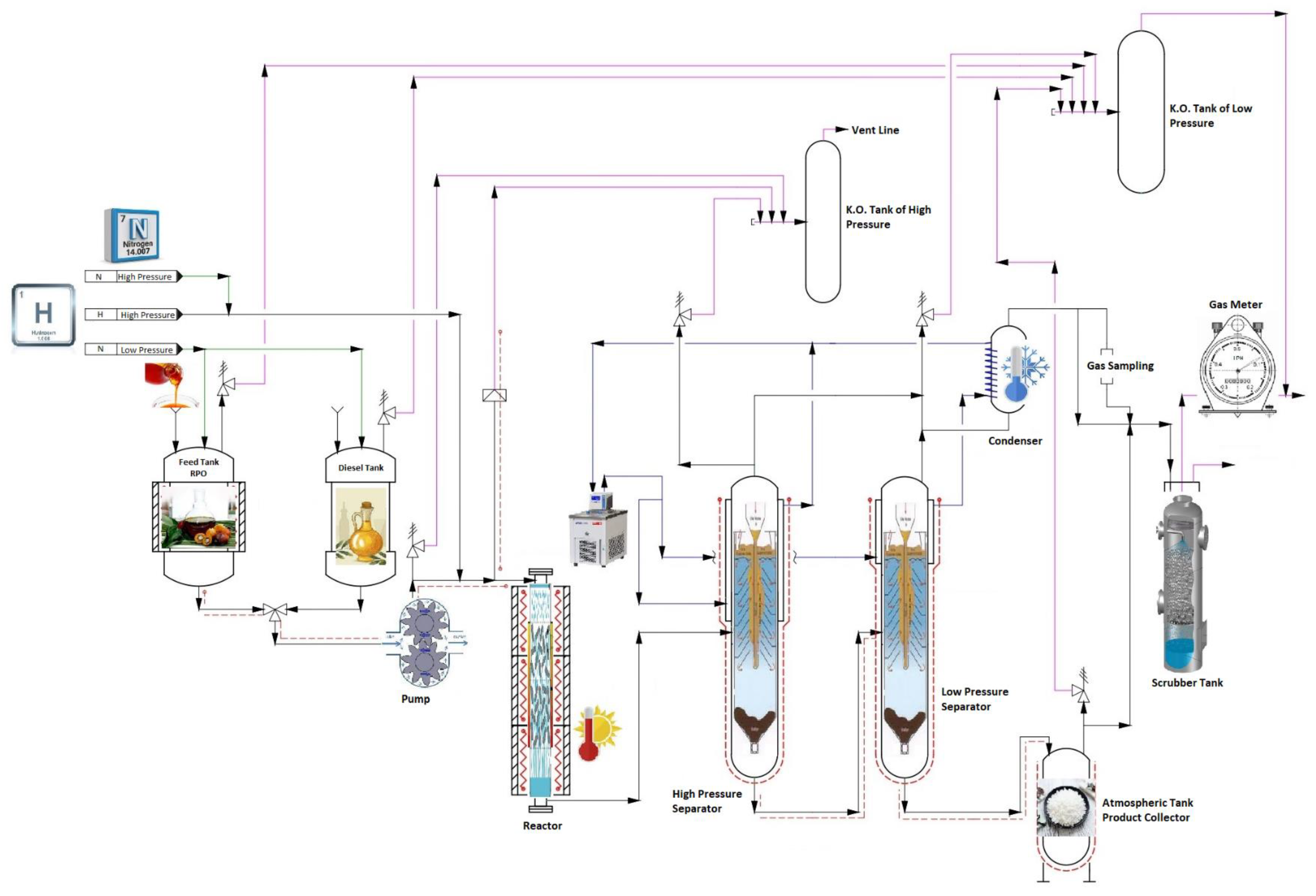
2.3. Experimental Set-Up
2.4. Catalytic Evaluation
2.5. Chromatographic Analysis
3. Results and Discussion
3.1. Operational Conditions Test for Reference 1 Catalyst (NiMoB/Al2O3)
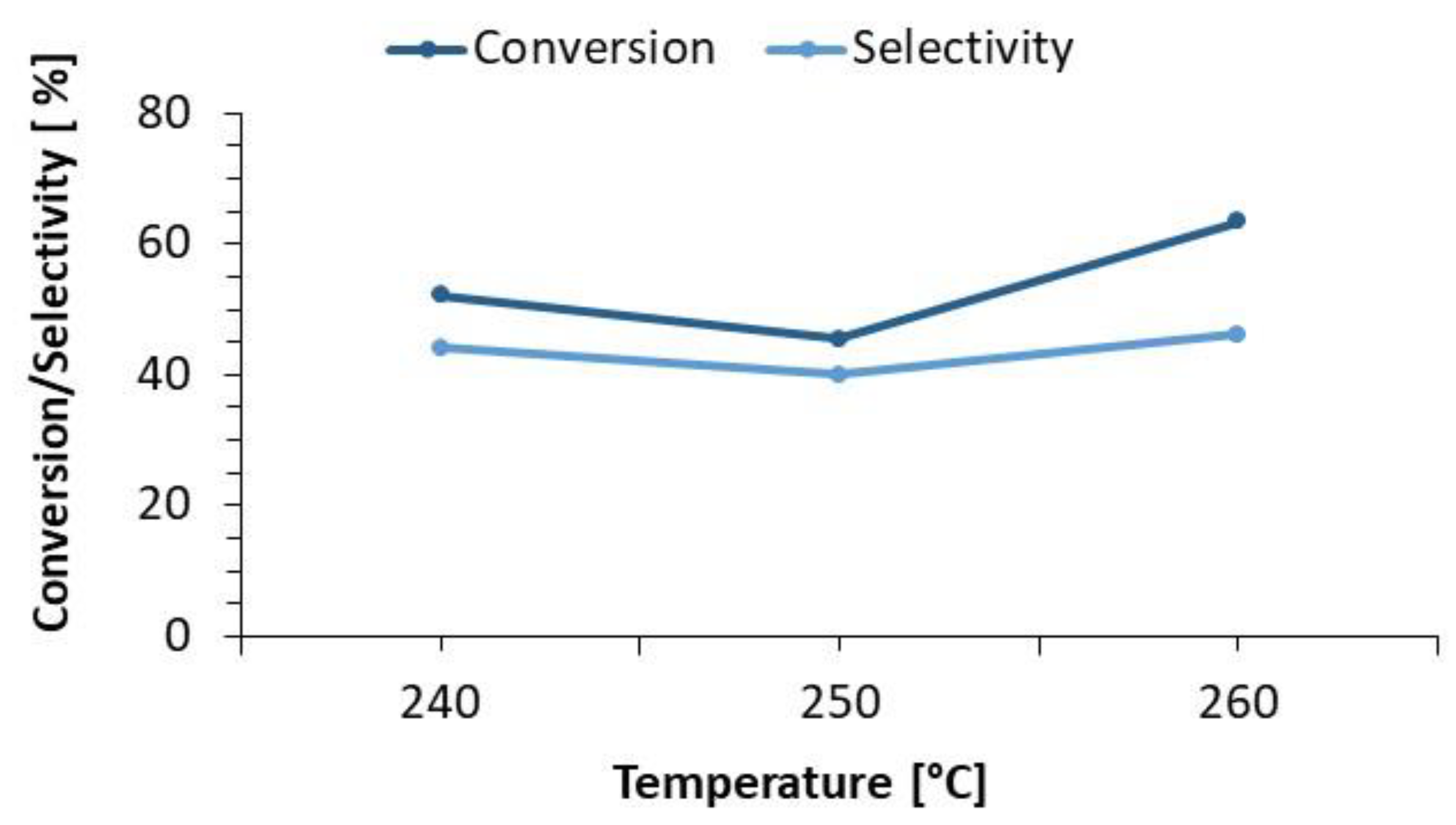
3.1.1. Temperature Effect on the Hydrotreatment of Vegetable Oils
3.1.2. Pressure Effect on the Hydrotreatment of Vegetable Oils
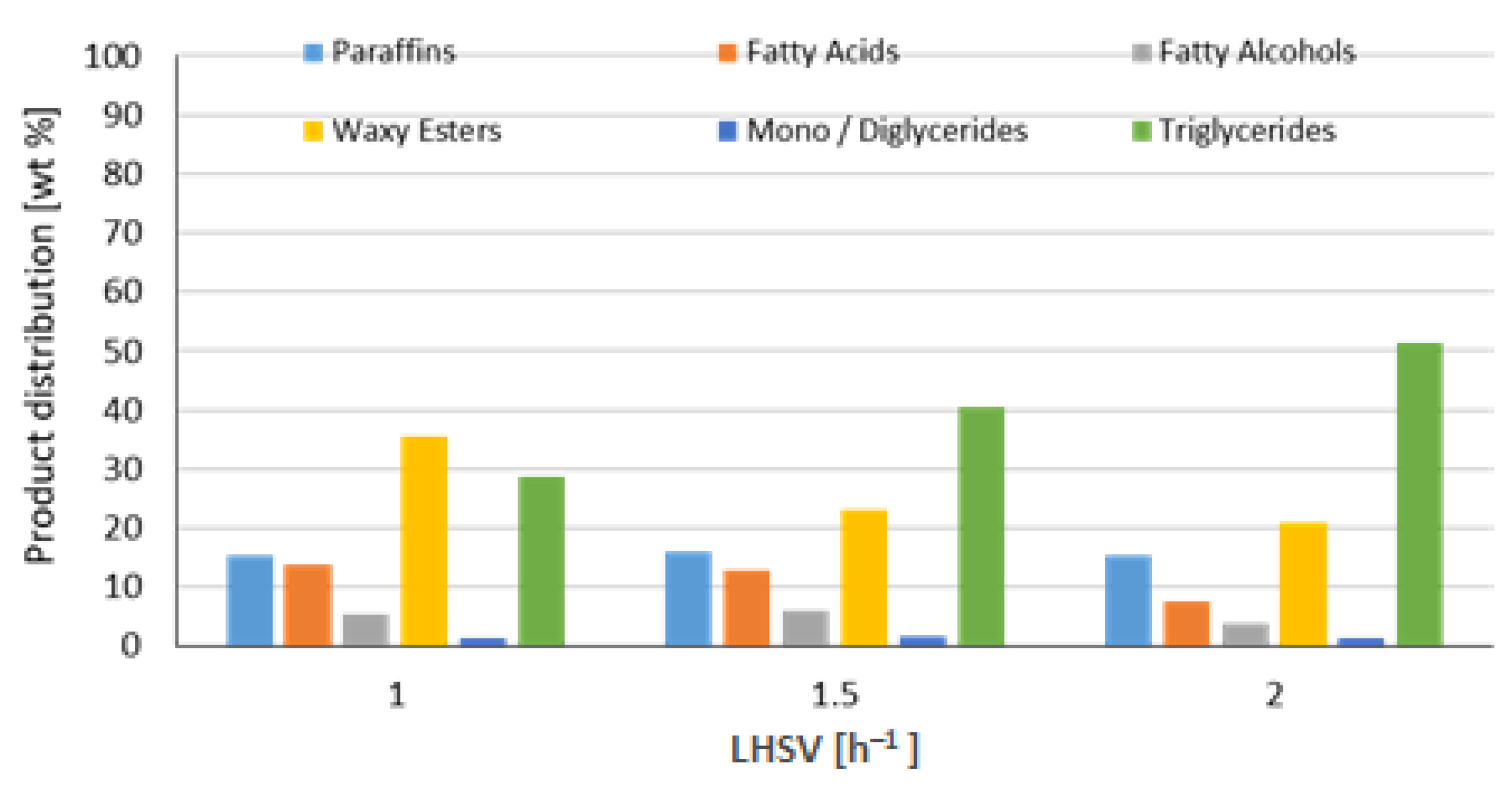
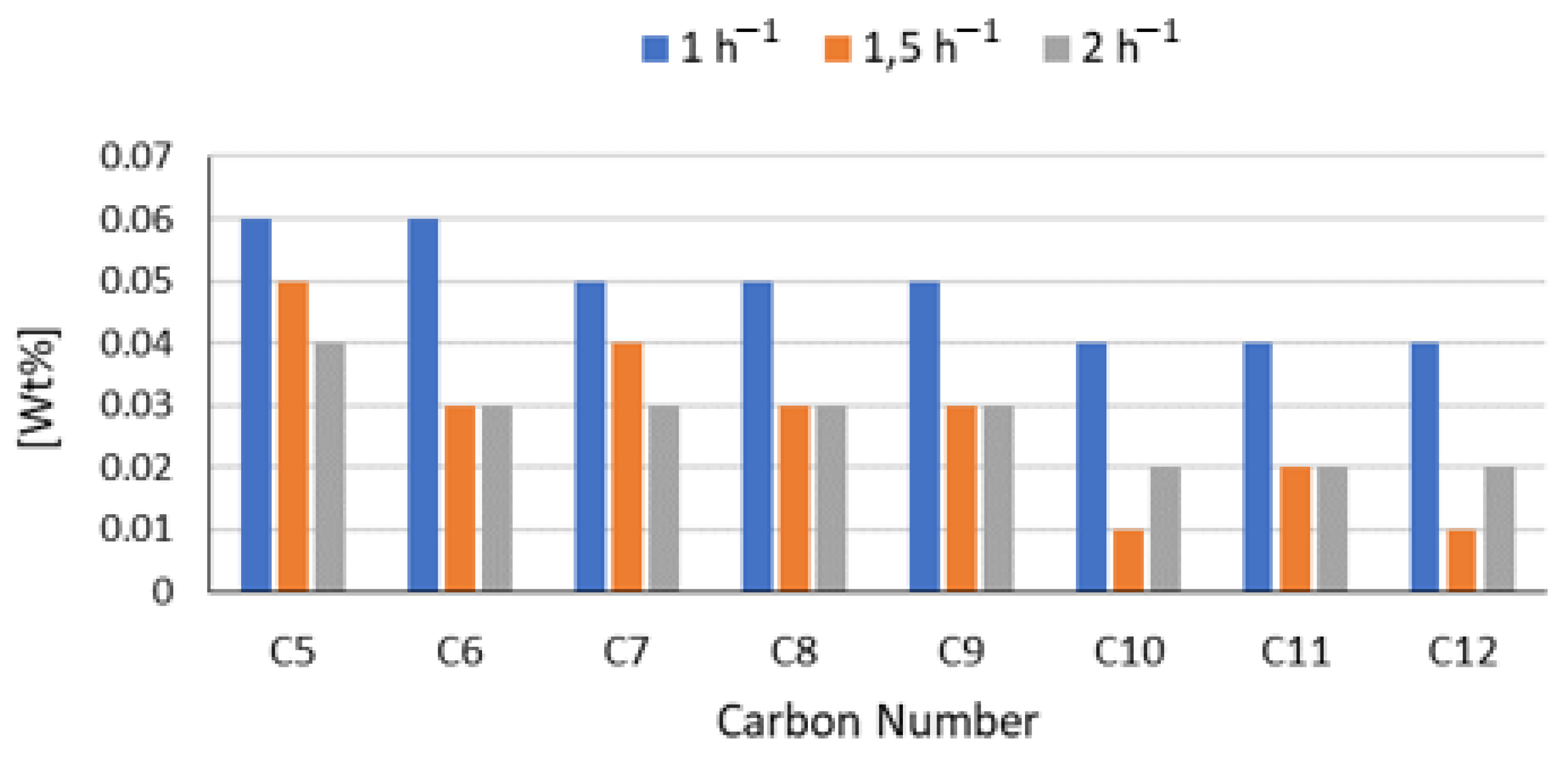
3.1.3. Liquid Hourly Space Velocity Effect on the Hydrotreatment of Vegetable Oils
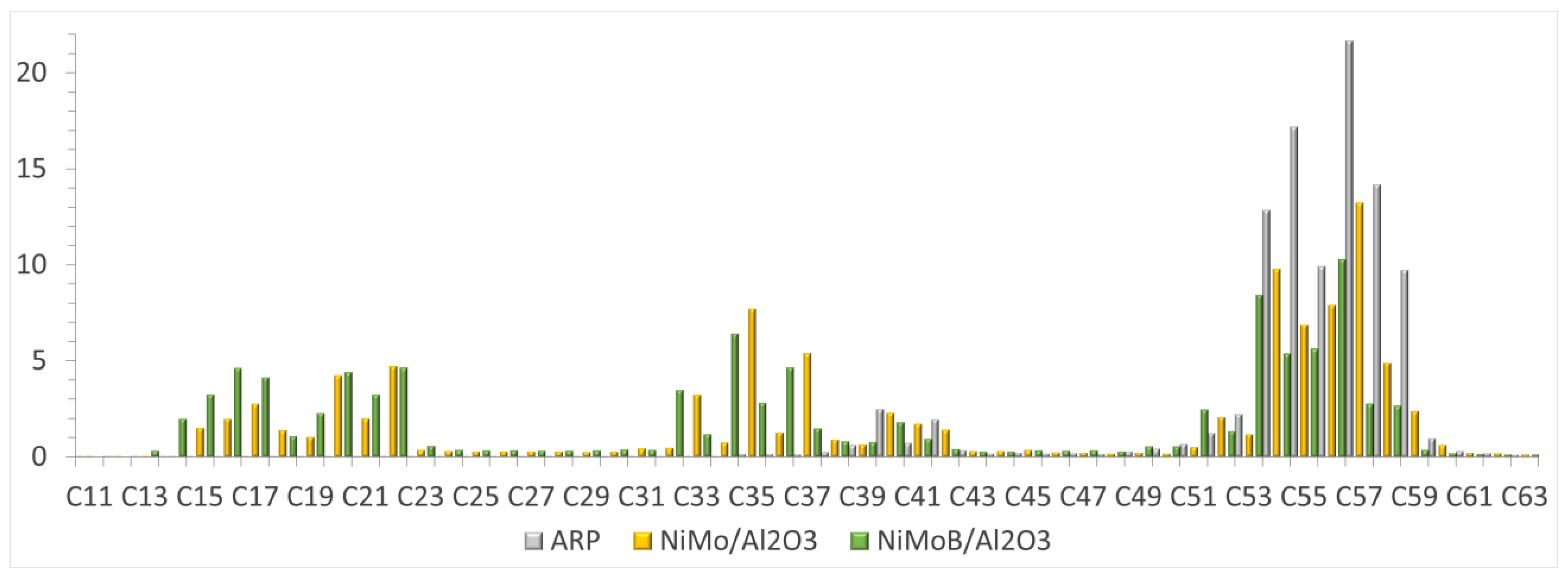
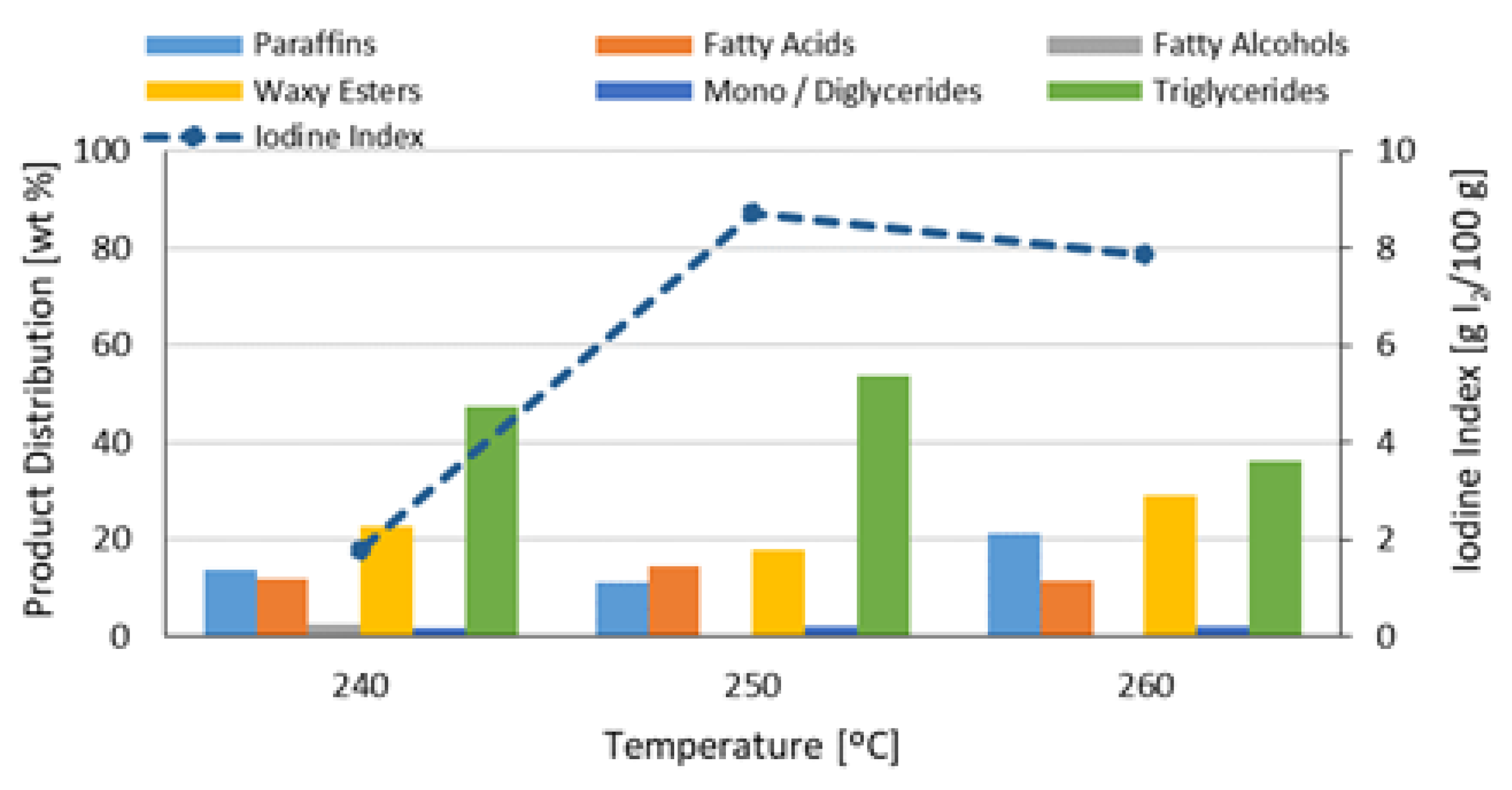
3.2. Effect Test of the Selected Operational Conditions in the Reference 2 Catalyst (NiMoB/Al2O3)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, H. Industrial Waxes; Chemical Pub Co.: New York, NY, USA, 1975. [Google Scholar]
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Aguieiras, E.C.; Papadaki, A.; Mallouchos, A.; Mandala, I.; Sousa, H.; Freire, D.M.; Koutinas, A.A. Enzymatic synthesis of bio-based wax esters from palm and soybean fatty acids using crude lipases produced on agricultural residues. Ind. Crops Prod. 2019, 139, 111499. [Google Scholar] [CrossRef]
- Khatun, R.; Reza, M.I.H.; Moniruzzaman, M.; Yaakob, Z. Sustainable oil palm industry: The possibilities. Renew. Sustain. Energy Rev. 2017, 76, 608–619. [Google Scholar] [CrossRef]
- List, G.R. 2—Oilseed Composition and Modification for Health and Nutrition. In Functional Dietary Lipids; Sanders, T.A.B., Ed.; Woodhead Publishing: Soston, UK, 2016; pp. 23–46. [Google Scholar] [CrossRef]
- Papadaki, A.; Mallouchos, A.; Efthymiou, M.-N.; Gardeli, C.; Kopsahelis, N.; Aguieiras, E.C.G.; Freire, D.M.G.; Papanikolaou, S.; Koutinas, A.A. Production of wax esters via microbial oil synthesis from food industry waste and by-product streams. Bioresour. Technol. 2017, 245, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, A.; Bouzidi, L.; Narine, S.S. Waxes derived from self-metathesis modified plant oil blends—A case for exploiting oligomerization to mitigate low molecular mass and unsaturation. Ind. Crops Prod. 2020, 143, 111936. [Google Scholar] [CrossRef]
- Ungcharoenwiwat, P.; Canyuk, B.; H-Kittikun, A. Synthesis of jatropha oil based wax esters using an immobilized lipase from Burkholderia sp. EQ3 and Lipozyme RM IM. Process Biochem. 2016, 51, 392–398. [Google Scholar] [CrossRef]
- Guzman, A.; Torres, J.E.; Prada, L.P.; Nuñez, M.L. Hydroprocessing of crude palm oil at pilot plant scale. Catal. Today 2010, 156, 38–43. [Google Scholar] [CrossRef]
- Guzmán, M.; Kafarov, V.; Guzmán, A.; Garzón, L. Influence of temperature during crude palm oil hydrotreating over NiMo/γ-Al2O3 catalysts. Ion 2013, 26, 7–14. [Google Scholar]
- Nuñez, M.; Prada, L. Proceso para la Obtención de Compuestos Parafínicos Sólidos por Hidrotratamiento de Aceites Vegetales. Organización Mundial de la Propiedad Intelectual WO2010067164A1, 17 June 2010. p. 29. [Google Scholar]
- Malins, K. Synthesis of renewable hydrocarbons from vegetable oil feedstock by hydrotreatment over selective sulfur-free SiO2-Al2O3 supported monometallic Pd, Pt, Ru, Ni, Mo and bimetallic NiMo catalysts. Fuel 2021, 285, 119129. [Google Scholar] [CrossRef]
- Kordulis, C.; Bourikas, K.; Gousi, M.; Kordouli, E.; Lycourghiotis, A. Development of nickel based catalysts for the transformation of natural triglycerides and related compounds into green diesel: A critical review. Appl. Catal. B Environ. 2016, 181, 156–196. [Google Scholar] [CrossRef]
- Afshar Taromi, A.; Kaliaguine, S. Green diesel production via continuous hydrotreatment of triglycerides over mesostructured γ-alumina supported NiMo/CoMo catalysts. Fuel Process. Technol. 2018, 171, 20–30. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Jiang, J.; Liu, P.; Li, F.; Ye, J.; Zhou, M. Hydrotreatment of vegetable oil for green diesel over activated carbon supported molybdenum carbide catalyst. Fuel 2018, 216, 738–746. [Google Scholar] [CrossRef]
- Plazas-González, M.; Guerrero-Fajardo, C.A.; Sodré, J.R. Modelling and simulation of hydrotreating of palm oil components to obtain green diesel. J. Clean. Prod. 2018, 184, 301–308. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Yusup, S.; Ramli, A.; Shahbaz, M.; Aqsha, A. Process optimization of green diesel selectivity and understanding of reaction intermediates. Renew. Energy 2020, 149, 1092–1106. [Google Scholar] [CrossRef]
- Papageridis, K.N.; Charisiou, N.D.; Douvartzides, S.L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKhoori, S.; Polychronopoulou, K.; Goula, M.A. Effect of operating parameters on the selective catalytic deoxygenation of palm oil to produce renewable diesel over Ni supported on Al2O3, ZrO2 and SiO2 catalysts. Fuel Process. Technol. 2020, 209, 106547. [Google Scholar] [CrossRef]
- Gutiérrez-Antonio, C.; Soria Ornelas, M.L.; Gómez-Castro, F.I.; Hernández, S. Intensification of the hydrotreating process to produce renewable aviation fuel through reactive distillation. Chem. Eng. Process.-Process Intensif. 2018, 124, 122–130. [Google Scholar] [CrossRef]
- Chu, P.L.; Vanderghem, C.; MacLean, H.L.; Saville, B.A. Process modeling of hydrodeoxygenation to produce renewable jet fuel and other hydrocarbon fuels. Fuel 2017, 196, 298–305. [Google Scholar] [CrossRef]
- Yang, J.; Xin, Z.; He, Q.; Corscadden, K.; Niu, H. An overview on performance characteristics of bio-jet fuels. Fuel 2019, 237, 916–936. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chen, Y.-K.; Wang, W.-C. The production of bio-jet fuel from palm oil derived alkanes. Fuel 2020, 260, 116345. [Google Scholar] [CrossRef]
- Dujjanutat, P.; Kaewkannetra, P. Production of bio-hydrogenated kerosene by catalytic hydrocracking from refined bleached deodorised palm/ palm kernel oils. Renew. Energy 2020, 147, 464–472. [Google Scholar] [CrossRef]
- Olarte, G.; Giraldo, S.; Garzón, L. Influencia de la temperatura y el tiempo de reacción en la hidroisomerización de diésel renovable. Ingenio Magno 2016, 6, 114–123. [Google Scholar]
- Gruber, H.; Lindner, L.; Arlt, S.; Reichhold, A.; Rauch, R.; Weber, G.; Trimbach, J.; Hofbauer, H. A novel production route and process optimization of biomass-derived paraffin wax for pharmaceutical application. J. Clean. Prod. 2020, 275, 124135. [Google Scholar] [CrossRef]
- Fei, T.; Wang, T. A review of recent development of sustainable waxes derived from vegetable oils. Curr. Opin. Food Sci. 2017, 16, 7–14. [Google Scholar] [CrossRef]
- Krendlinger, E.; Wolfmeier, U.; Schmidt, H.; Heinrichs, F.L.; Michalczyk, G.; Payer, W.; Dietsche, W.; Boehlke, K.; Hohner, G.; Wildgruber, J. Waxes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Chichester, UK, 2000; pp. 1–63. [Google Scholar] [CrossRef]
- Kubička, D.; Černý, R. Upgrading of Fischer–Tropsch Waxes by Fluid Catalytic Cracking. Ind. Eng. Chem. Res. 2012, 51, 8849–8857. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, G.V.; Serna-Saldívar, S.O.; Pérez-Carrillo, E.; Jordânia Silva, T.; Barrera-Arellano, D.; Buitimea-Cantúa, N.E. Effect of quality of carnauba wax (Copernica cerífera) on microstructure, textural, and rheological properties of soybean oil-based organogels. LWT 2021, 136, 110267. [Google Scholar] [CrossRef]
- Khelil, R.; Jardé, E.; Cabello-Hurtado, F.; Ould-el-Hadj Khelil, A.; Esnault, M.-A. Structure and composition of the wax of the date palm, Phoenix dactylifera L., from the septentrional Sahara. Sci. Hortic. 2016, 201, 238–246. [Google Scholar] [CrossRef]
- Robertson, D.; van Reenen, A.; Duveskog, H. A comprehensive investigation into the structure-property relationship of wax and how it influences the properties of hot melt adhesives. Int. J. Adhes. Adhes. 2020, 99, 102559. [Google Scholar] [CrossRef]
- Rogowski, I.; Gauvrit, J.-Y.; Léonard, D.; Lanteri, P. Typology of the gliding waxes in cross-country skiing: Comparison between classifications based on the chemical composition and those based on the physical and physicochemical properties. Cold Reg. Sci. Technol. 2005, 43, 140–149. [Google Scholar] [CrossRef]
- Anzenberger, C.; Li, S.; Bouzidi, L.; Narine, S.S. Synthesis of waxes from vegetable oil derived self-metathesized aliphatic esters. Ind. Crops Prod. 2016, 89, 368–375. [Google Scholar] [CrossRef]
- Dujjanutat, P.; Neramittagapong, A.; Kaewkannetra, P. Optimization of Bio-Hydrogenated Kerosene from Refined Palm Oil by Catalytic Hydrocracking. Energies 2019, 12, 3196. [Google Scholar] [CrossRef]
- Murphy, T.; Doucette, M.; House, N.; Richards, M. Triacylglycerol-Based Alternative to Paraffin Wax. US8202329B2, 19 June 2012. p. 12. [Google Scholar]
- Hassan, A.; Borsinger, G.; Rayford, A.; Hassan, A. High Shear Hydrogenation of Wax and Oil Mixtures. US8491777B2, 23 July 2013. Corporation, H.R.D., Ed., p. 17. [Google Scholar]
- Murphy, T. Triacylglycerol Based Wax for Use in Candles. US20040088908A1, 10 August 2004. p. 11. [Google Scholar]
- Hamilton, R.J.; Yaquob Raie, M.; Miwa, T.K. Structure of the alcohols derived from wax esters in jojoba oil. Chem. Phys. Lipids 1975, 14, 92–96. [Google Scholar] [CrossRef]
- Liu, Z.-K.; Ågren, J.; Hillert, M. Application of the Le Chatelier principle on gas reactions. Fluid Phase Equilibria 1996, 121, 167–177. [Google Scholar] [CrossRef]
- Krebs, E.; Silvi, B.; Raybaud, P. Mixed sites and promoter segregation: A DFT study of the manifestation of Le Chatelier’s principle for the Co(Ni)MoS active phase in reaction conditions. Catal. Today 2008, 130, 160–169. [Google Scholar] [CrossRef]
- Kirumakki, S.R.; Shpeizer, B.G.; Sagar, G.V.; Chary, K.V.R.; Clearfield, A. Hydrogenation of Naphthalene over NiO/SiO2–Al2O3 catalysts: Structure–activity correlation. J. Catal. 2006, 242, 319–331. [Google Scholar] [CrossRef]
- Hancsók, J.; Kasza, T.; Kovács, S.; Solymosi, P.; Holló, A. Production of bioparaffins by the catalytic hydrogenation of natural triglycerides. J. Clean. Prod. 2012, 34, 76–81. [Google Scholar] [CrossRef]
- Jahromi, H.; Agblevor, F.A. Hydrotreating of guaiacol: A comparative study of Red mud-supported nickel and commercial Ni/SiO2-Al2O3 catalysts. Appl. Catal. A Gen. 2018, 558, 109–121. [Google Scholar] [CrossRef]
- Mohamed, L.K.; El Kady, F.Y.; Shaban, S.A. The Effect of Boron on the Activity of Hydrotreating Co-Mo/Al2O3 Catalyst. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 35, 659–670. [Google Scholar] [CrossRef]
- Vatutina, Y.V.; Nadeina, K.A.; Klimov, O.V.; Kazakov, M.O.; Danilova, I.G.; Cherepanova, S.V.; Khabibulin, D.F.; Gerasimov, E.Y.; Prosvirin, I.P.; Dik, P.P.; et al. Peptization of alumina by ammonia to adjust catalytic properties of NiMo/B-Al2O3 hydrotreating catalysts. Catal. Today 2020, 375, 377–392. [Google Scholar] [CrossRef]
- Maity, S.K.; Lemus, M.; Ancheyta, J. Effect of Preparation Methods and Content of Boron on Hydrotreating Catalytic Activity. Energy Fuels 2011, 25, 3100–3107. [Google Scholar] [CrossRef]
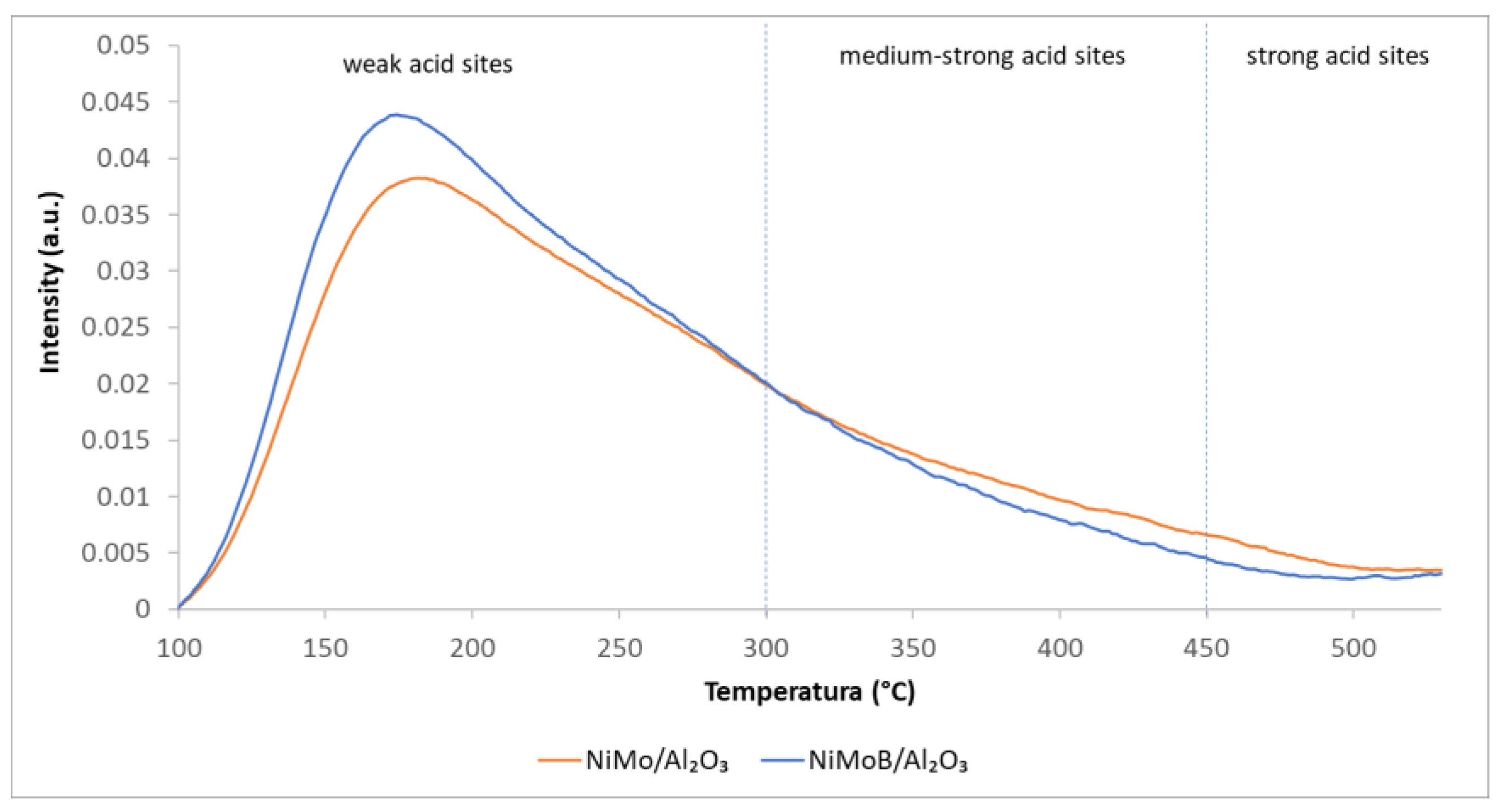



| Reactions | Reaction Type | ΔH0Rxn [kJ/mol] |
|---|---|---|
| TAG + 3H2 → FFA + C3H8 | Hydrogenolysis/Hydrogenation (Initial reactions) | ≈ −220 to −331 |
| FFA + 2H2 → FA + H2O | ≈ −41 | |
| FFA + H2/2H2 → HFFA | Hydrogenation | ≈ −110 * |
| FFA/HFFA →PC−1 + CO2 | Decarboxylation (DCO2) | ≈ −7 to −10 * |
| HFFA/FFA + H2/2H2 →PC−1 + H2O | Decarbonylation (DCO) | ≈ 32 to 35 * |
| HFFA/FFA + H2/2H2 →P + H2O | Hydrodeoxygenation | ≈ 107 |
| FFA/HFFA + FA → WE + H2O | Esterification | ≈ −15 |
| Characteristic | Analytical Method | Units | Refined Palm Oil (RPO) |
|---|---|---|---|
| Density (15 °C) | ASTM D 1298 | g/mL | 0.8944 |
| Molecular weight | g/mol | 659.5 | |
| Melting Point | NTC 213 | °C | 26.2 |
| Acid Number | ASTM D 664 | mg KOH/g | 0.18 |
| Iodine Value | NTC 283 | g I2/100 g | 49.7 |
| Fe Content | ICP-OES | ppm | <3.33 |
| P Content | ICP-OES | ppm | <5.47 |
| Na Content | ICP-OES | ppm | <5.07 |
| K Content | ICP-OES | ppm | <1.27 |
| Mg Content | ICP-OES | ppm | <0.17 |
| Ca Content | ICP-OES | ppm | <4.95 |
| Catalyst | Catalyst Bed [cm3] | Feed | Temperature [°C] | Pressure [bar] | LHSV [h−1] | Volumetric Ratio ® [LN/L] |
|---|---|---|---|---|---|---|
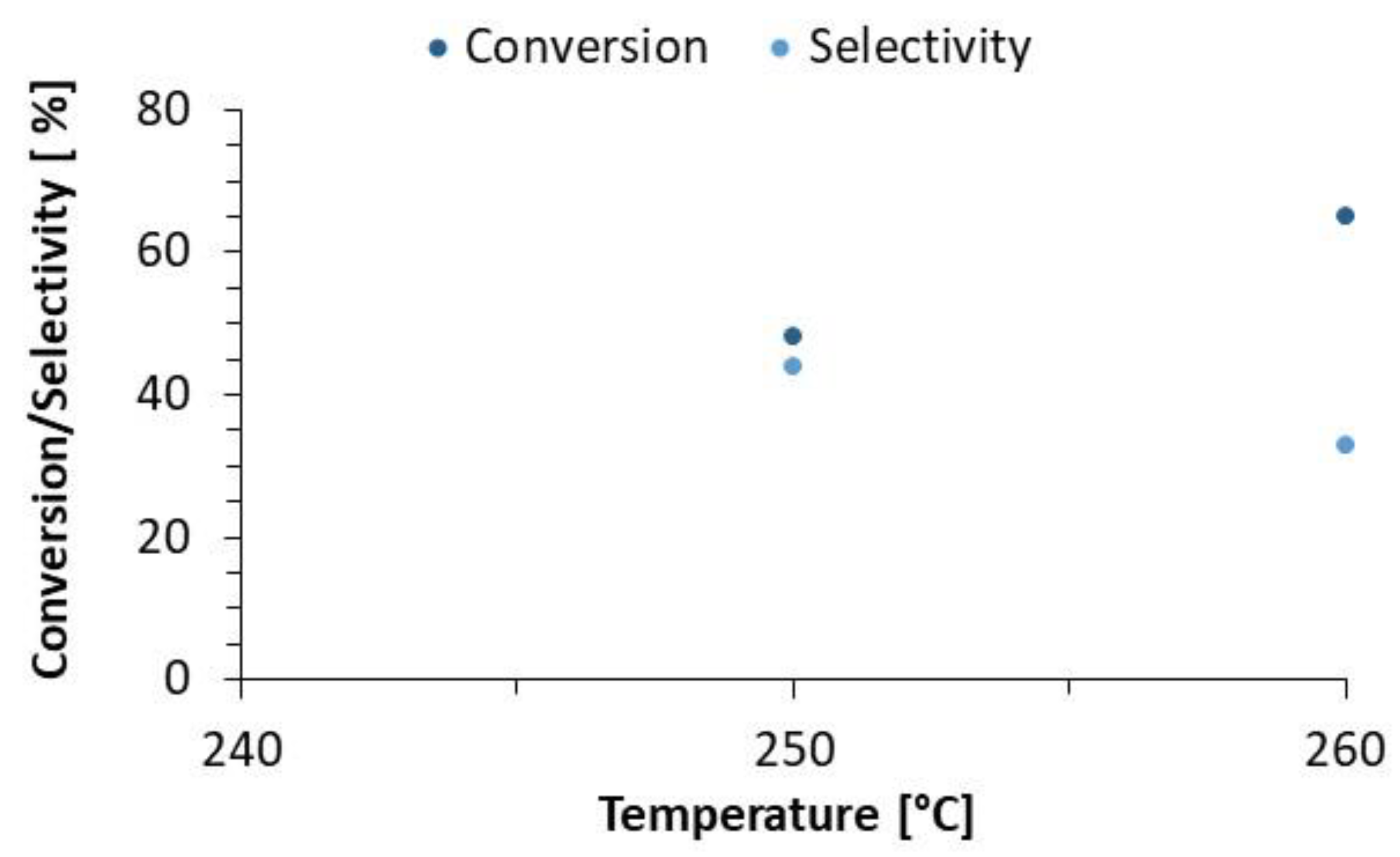
| Reference 1 (NiMo/Al2O3) | 90 | Refined Palm Oil (RPO) | 250 260 | 55 | 2 | 472 |
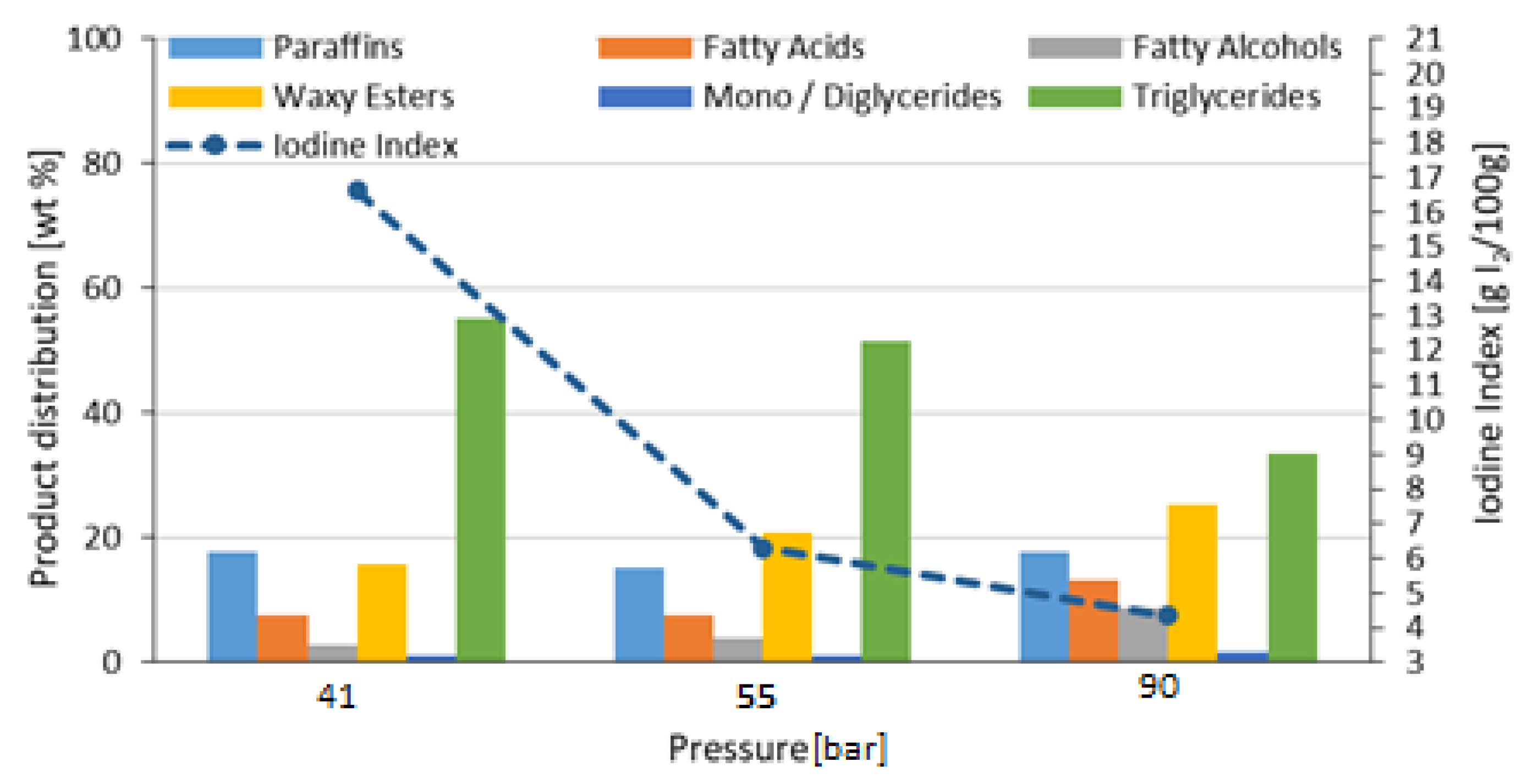
| Reference 1 (NiMo/Al2O3) | 90 | Refined Palm Oil (RPO) | 250 | 41 55 90 | 2 | 472 |
| Reference 1 (NiMo/Al2O3) | 90 | Refined Palm Oil (RPO) | 250 | 55 | 1 1.5 2 | 472 |
| Reference 2 (NiMoB/Al2O3) | 90 | Refined Palm Oil (RPO) | 240 250 260 | 55 | 1,5 | 472 |
| Temperature [°C] | CO2 [Vol. Molar%] | CO [Vol. Molar%] |
|---|---|---|
| 250 | 10.63 | 1.0 |
| 260 | 12.04 | 1.3 |
| Pressure [bar] | CO2 [Vol. Molar%] | CO [Vol. Molar%] |
|---|---|---|
| 41 | 9.40 | 1.01 |
| 55 | 10.63 | 1.00 |
| 90 | 11.32 | 1.10 |
| LHSV [h−1] | CO2 [Vol. Molar%] | CO [Vol. Molar%] |
|---|---|---|
| 1.0 | 14.61 | 1.37 |
| 1.5 | 10.86 | 1.16 |
| 2.0 | 10.63 | 1.00 |
| Temperature [°C] | CO2 [Vol. Molar%] | CO [Vol. Molar%] |
|---|---|---|
| 240 | 13.80 | 1.38 |
| 250 | 18.62 | 1.55 |
| 260 | 34.00 | 1.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olarte, G.; Garzón, L.; Sarmiento, J.; López-Giraldo, L.J.; Vivas-Báez, J.C. Biowax Production from the Hydrotreatment of Refined Palm Oil (RPO). Processes 2023, 11, 1372. https://doi.org/10.3390/pr11051372
Olarte G, Garzón L, Sarmiento J, López-Giraldo LJ, Vivas-Báez JC. Biowax Production from the Hydrotreatment of Refined Palm Oil (RPO). Processes. 2023; 11(5):1372. https://doi.org/10.3390/pr11051372
Chicago/Turabian StyleOlarte, Giovanny, Laura Garzón, José Sarmiento, Luis Javier López-Giraldo, and July C. Vivas-Báez. 2023. "Biowax Production from the Hydrotreatment of Refined Palm Oil (RPO)" Processes 11, no. 5: 1372. https://doi.org/10.3390/pr11051372
APA StyleOlarte, G., Garzón, L., Sarmiento, J., López-Giraldo, L. J., & Vivas-Báez, J. C. (2023). Biowax Production from the Hydrotreatment of Refined Palm Oil (RPO). Processes, 11(5), 1372. https://doi.org/10.3390/pr11051372






