Phenolic Profile, Inhibition of α-Amylase and α-Glucosidase Enzymes, and Antioxidant Properties of Solanum elaeagnifolium Cav. (Solanaceae): In Vitro and In Silico Investigations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Extracts
2.2. HPLC-DAD Analysis
2.3. Antioxidant Activity Assay
2.3.1. DPPH Assay
2.3.2. TAC Assay
2.4. Antidiabetic Activity Assay
2.4.1. α-Amylase Inhibition Assay
2.4.2. α-Glucosidase Inhibition Assay
2.5. In Silico Studies
2.6. Statistical Analysis
3. Results and Discussion
3.1. HPLC Analysis
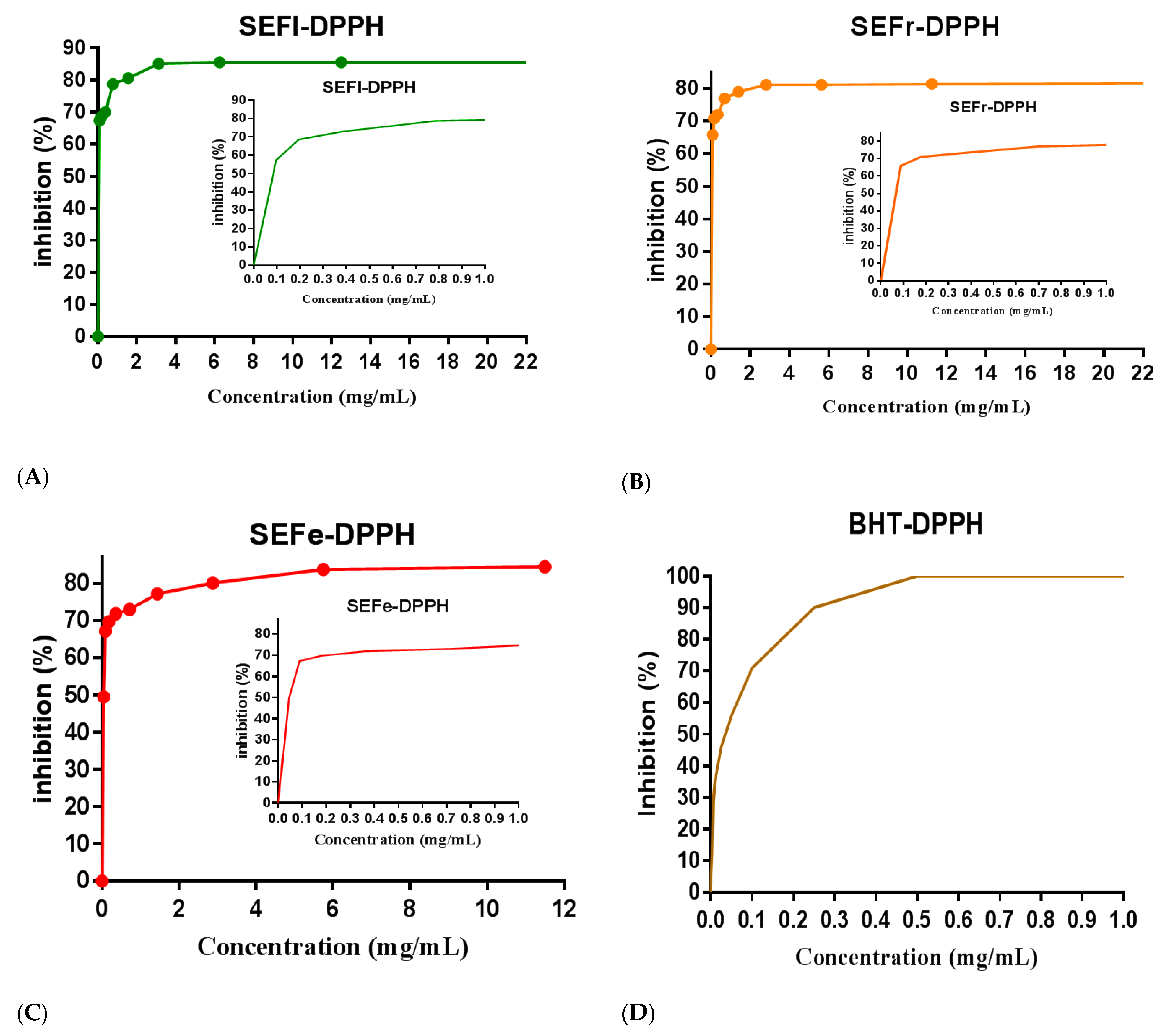
3.2. Antioxidant Activity
3.3. In Vitro Antidiabetic Activity
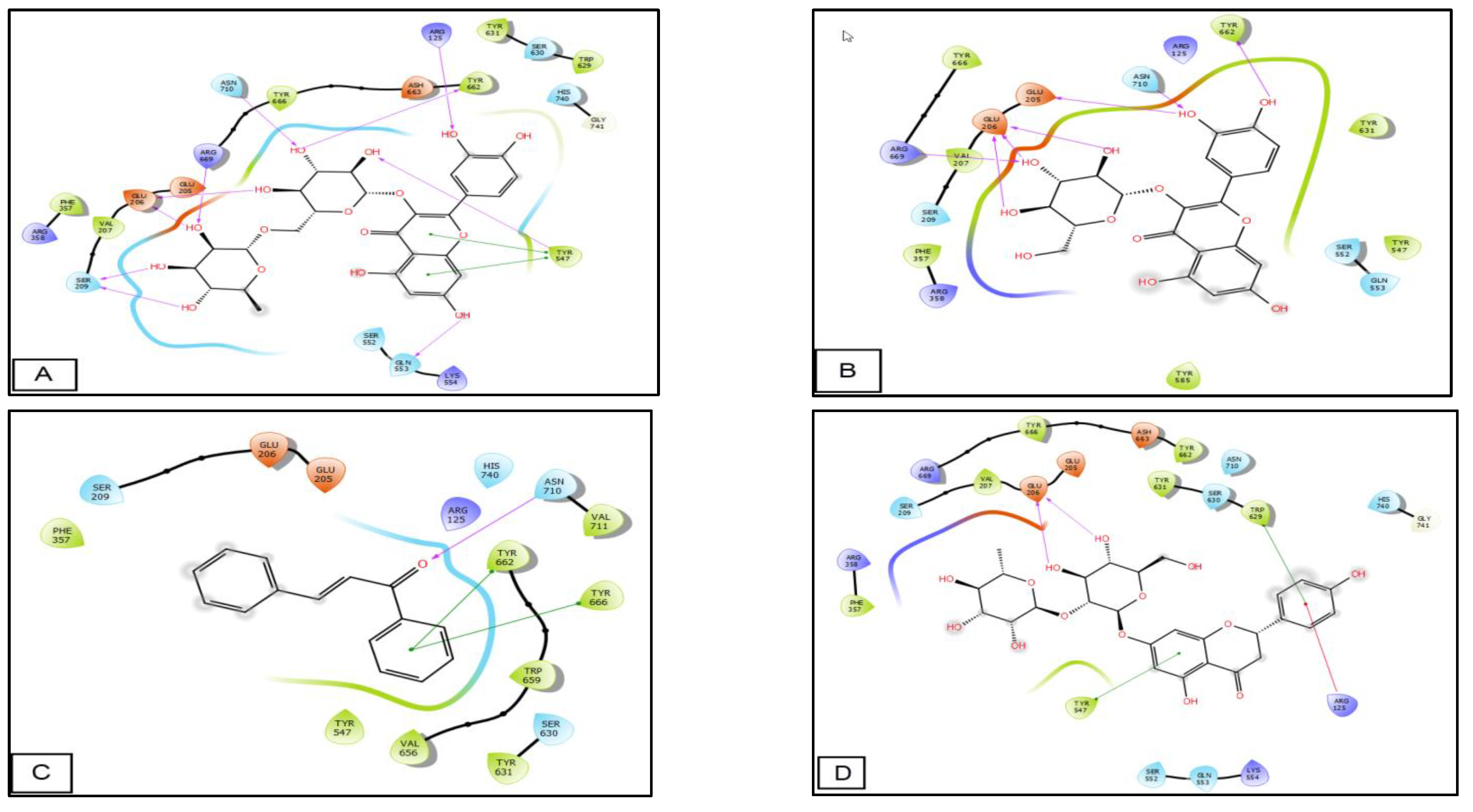
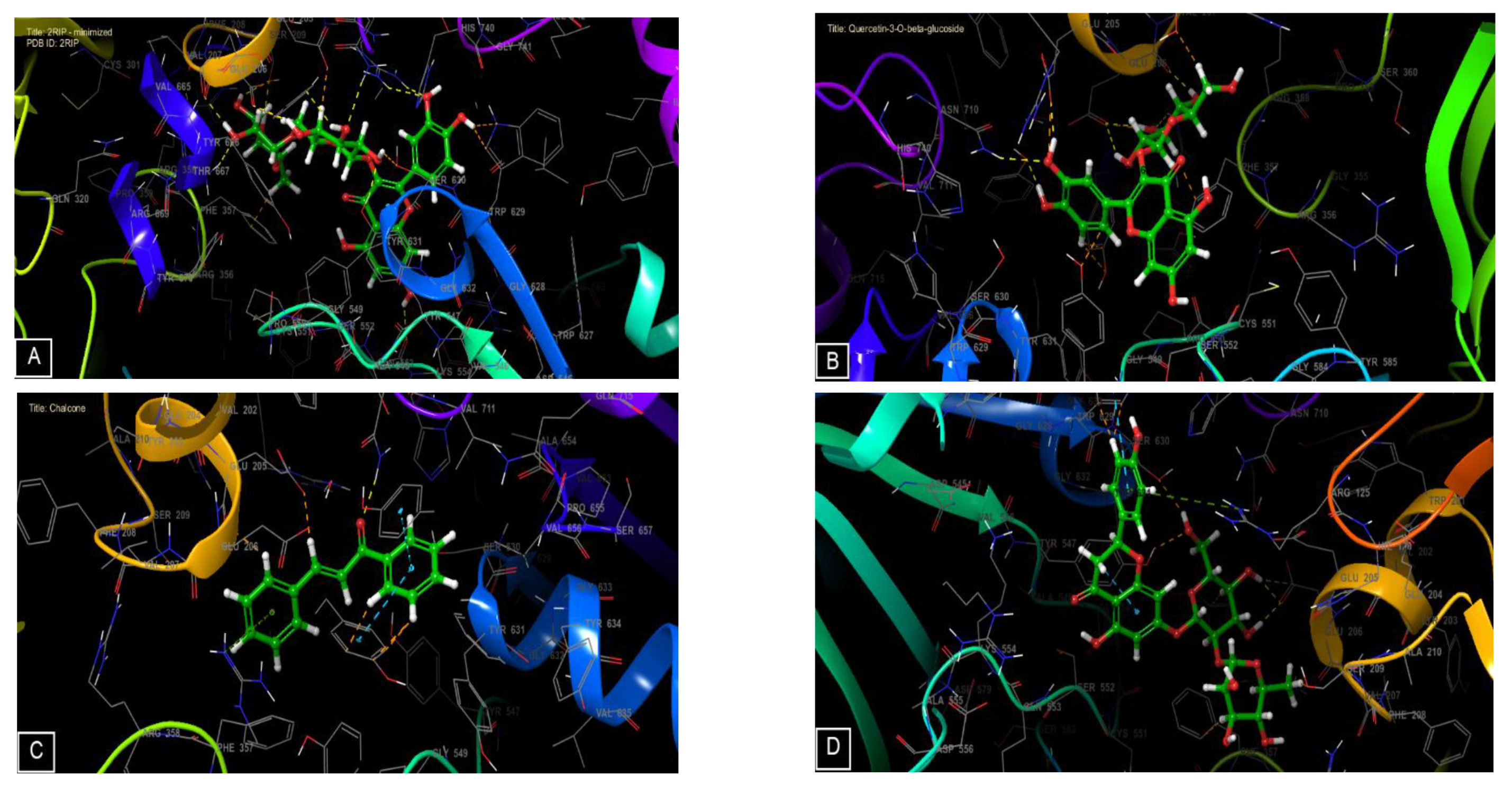
3.4. In Silico Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Ghouizi, A.; Ousaaid, D.; Laaroussi, H.; Bakour, M.; Aboulghazi, A.; Soutien, R.; Hano, C.; Badiaa, L. Ficus Carica (Linn.) Leaf and Bud Extracts and Their Combination Attenuates Type-1 Diabetes and Its Complications via the Inhibition of Oxidative Stress. Foods 2023, 12, 759. [Google Scholar] [CrossRef] [PubMed]
- CHETOUI, A.; Kaoutar, K.; Kardoudi, A.; Boutahar, K.; Chigr, F. Epidemiology of Diabetes in Morocco: Review of Data, Analysis and Perspectives. Int. J. Sci. Eng. Res. 2018, 9, 1310–1316. [Google Scholar]
- Houguig, K.; Ouzennou, N.; Rayadi, M.; Rkha, S. Observance of Hygiene and Dietary Rules and the Associated Factors among Diabetic Subjects in Essaouira Province, Morocco: A Cross-Sectional Study. Pan Afr. Med. J. 2022, 41, 22. [Google Scholar] [CrossRef]
- Xiong, W.-T.; Gu, L.; Wang, C.; Sun, H.-X.; Liu, X. Anti-Hyperglycemic and Hypolipidemic Effects of Cistanche Tubulosa in Type 2 Diabetic Db/Db Mice. J. Ethnopharmacol. 2013, 150, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, N.; Chinni, S.; Ramachawolran, G.; Sunitha, P.; Sankar, A.; Muthuvenkatachalam, B. Antidiabetic Activity of Solanum Torvum Fruit Extract in Streptozotocin-Induced Diabetic Rats. Front. Nutr. 2022, 9, 987552. [Google Scholar] [CrossRef] [PubMed]
- Laaraj, N.; Bouhrim, M.; Kharchoufa, L.; Tiji, S.; Bendaha, H.; Addi, M.; Drouet, S.; Hano, C.; Lorenzo, J.M.; Bnouham, M.; et al. Phytochemical Analysis, α-Glucosidase and α-Amylase Inhibitory Activities and Acute Toxicity Studies of Extracts from Pomegranate (Punica Granatum) Bark, a Valuable Agro-Industrial By-Product. Foods 2022, 11, 1353. [Google Scholar] [CrossRef]
- Benayad, O.; Bouhrim, M.; Tiji, S.; Kharchoufa, L.; Addi, M.; Drouet, S.; Hano, C.; Lorenzo, J.M.; Bendaha, H.; Bnouham, M.; et al. Phytochemical Profile, α-Glucosidase, and α-Amylase Inhibition Potential and Toxicity Evaluation of Extracts from Citrus Aurantium (L) Peel, a Valuable By-Product from Northeastern Morocco. Biomolecules 2021, 11, 1555. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary Polyphenols Modulate Starch Digestion and Glycaemic Level: A Review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1–15. [Google Scholar] [CrossRef]
- Hbika, A.; Daoudi, N.E.; Bouyanzer, A.; Bouhrim, M.; Mohti, H.; Loukili, E.H.; Mechchate, H.; Al-salahi, R.; Nasr, F.; Bnouham, M.; et al. Artemisia Absinthium L. Aqueous and Ethyl Acetate Extracts: Antioxidant Effect and Potential Activity In Vitro and In Vivo against Pancreatic α-Amylase and Intestinal α-Glucosidase. Pharmaceutics 2022, 14, 481. [Google Scholar] [CrossRef]
- Loukili, E.H.; Bouchal, B.; Bouhrim, M.; Abrigach, F.; Genva, M.; Kahina, Z.; Bnouham, M.; Bellaoui, M.; Hammouti, B.; Addi, M.; et al. Chemical Composition, Antibacterial, Antifungal and Antidiabetic Activities of Ethanolic Extracts of Opuntia Dillenii Fruits Collected from Morocco. J. Food Qual. 2022, 2022, 15. [Google Scholar] [CrossRef]
- Lachkar, N.; Lamchouri, F.; Bouabid, K.; Boulfia, M.; Senhaji, S.; Stitou, M.; Toufik, H. Mineral Composition, Phenolic Content, and In Vitro Antidiabetic and Antioxidant Properties of Aqueous and Organic Extracts of Haloxylon Scoparium Aerial Parts. Evid. -Based Complement. Altern. Med. 2021, 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Laaroussi, H.; Santos, P.; Genisheva, Z.; Bakour, M.; Ousaaid, D.; Teixeira, J.; Badiaa, L. Unraveling the Chemical Composition, Antioxidant, α-Amylase and α-Glucosidase Inhibition of Moroccan Propolis. Food Biosci. 2021, 42, 101160. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Nayak, R.; Behera, C.; Dash, S.; Nayak, S.; Sahu, B.; Bhutia, S.; Jena, M. Multifunctional Role of Fucoidan, Sulfated Polysaccharides in Human Health and Disease: A Journey under the Sea in Pursuit of Potent Therapeutic Agents. Int. J. Biol. Macromol. 2020, 164, 4263–4278. [Google Scholar] [CrossRef]
- Errajraji, A.; Ouhdouch, F.; El-Anssari, N. Usage des plantes médicinales dans le traitement du diabète de type 2 au Maroc: Use of medicinal plants for type 2 diabetes treatment, in Morocco. Médecine Des Mal. Métaboliques 2010, 4, 301–304. [Google Scholar] [CrossRef]
- Barkaoui, M.; Katiri, A.; Boubaker, H.; Msanda, F. Ethnobotanical Survey of Medicinal Plants Used in the Traditional Treatment of Diabetes in Chtouka Ait Baha and Tiznit (Western Anti-Atlas), Morocco. J. Ethnopharmacol. 2017, 198, 338–350. [Google Scholar] [CrossRef]
- Sarr, O.; Fall, A.; Gueye, R.; Diop, A.S.; Diatta, K.; Diop, N.; Diop, Y. Etude de l’activité Antioxydante Des Extraits Des Feuilles de Vitex Doniana (Verbenacea). Int. J. Biol. Chem. Sci. 2015, 9, 1263. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes Mellitus and Oxidative Stress—A Concise Review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Serigne, D.; Fall, A.; Diatta, K.; Sarr, A.; Sene, M.; Sene, M.; Mbaye, A.; Diatta, W.; Bassene, E. Evaluation de l’activité Antioxydante Des Extraits Hydro-Ethanoliques Des Feuilles et Écorces de Piliostigma Thonningii Schumach. Int. J. Biol. Chem. Sci. 2017, 11, 768. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects – A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Degreef, J. Botanique Systématique. Une Perspective Phylogénétique by Judd W.S.; Campbell C.S.; Kellogg E.A.; Stevens P.; Bouharmont J.; Evrard C.-M. Belg. J. Bot. 2002, 135, 133–134. [Google Scholar] [CrossRef]
- Brunel, S. Pest Risk Analysis for Solanum elaeagnifolium and International Management Measures Proposed. EPPO Bull. 2011, 41, 232–242. [Google Scholar] [CrossRef]
- Al-hamaideh, K.; Dmour, I.; El-Elimat, T.; Afifi, F. UPLC-MS Profile and Anti-Proliferative Activity of the Berries of an Aggressive Wild-Growing Weed: Solanum elaeagnifolium Cav. (Solanaceae). Trop. J. Nat. Prod. Res. 2020, 4, 1131–1138. [Google Scholar] [CrossRef]
- Badawy, A.; Zayed, R.; Ahmed, S.; Hassanean, H. Phytochemical and Pharmacological Studies of Solanum elaeagnifolium Growing in Egypt. J. Nat. Prod. 2013, 6, 156–167. [Google Scholar]
- Radwan, M.; Badawy, A.; Zayed, R.; Hassanin, H.; Elsohly, M.; Ahmed, S. Cytotoxic Flavone Glycosides from Solanum elaeagnifolium. Med. Chem. Res. 2014, 24, 1326–1330. [Google Scholar] [CrossRef]
- Njeh, F.; Feki, H.; Koubaa, I.; Hamed, N.; Mohamed, D.; Ayadi, A.; Hammami, H.; Jarraya, R. Molluscicidal Activity of Solanum Elaeagnifolium Seeds against Galba Truncatula Intermediate Host of Fasciola Hepatica: Identification of β -Solamarine. Pharm. Biol. 2015, 54, 1–6. [Google Scholar] [CrossRef]
- Bouslamti, M.; El Barnossi, A.; Kara, M.; Alotaibi, B.; Kamaly, O.; Assouguem, A.; Badiaa, L.; Benjelloun, A. Total Polyphenols Content, Antioxidant and Antimicrobial Activities of Leaves of Solanum elaeagnifolium Cav. from Morocco. Molecules 2022, 27, 4322. [Google Scholar] [CrossRef]
- Feki, H.; Koubaa, I.; Damak, M. Secondary Metabolites and Antioxidant Activity of Seed Extracts from Solanum elaeagnifolium Cav. Mediterr. J. Chem. 2014, 2, 639–647. [Google Scholar] [CrossRef]
- Bouslamti, M.; Chelouati, T.; El Moussaoui, A.; El Barnossi, A.; Badiaa, L.; Benjelloun, A. Solanum Elaeagnifolium Var. Obtusifolium (Dunal) Dunal: Antioxidant, Antibacterial, and Antifungal Activities of Polyphenol-Rich Extracts Chemically Characterized by Use of In Vitro and In Silico Approaches. Molecules 2022, 27, 8688. [Google Scholar] [CrossRef]
- Hawas, U.W.; Soliman, G.M.; Abou El-Kassem, L.T.; Farrag, A.R.H.; Mahmoud, K.; León, F. A New Flavonoid C-Glycoside from Solanum elaeagnifolium with Hepatoprotective and Curative Activities against Paracetamol-Induced Liver Injury in Mice. Z. Nat. C J. Biosci. 2013, 68, 19–28. [Google Scholar]
- Sakah, J.; Zhang, Y.-J. The Genus Solanum: An Ethnopharmacological, Phytochemical and Biological Properties Review. Nat. Prod. Bioprospect. 2019, 9, 77–137. [Google Scholar] [CrossRef]
- Contreras, L.; Emus-Medina, A.; Gutiérrez-Grijalva, E.; Perez, D.; Romero, C.A.; Heredia, J.B. Pharmacological Potential of Solanum Genus. In Solanum: An Overview. Plant Science Research and Practices; Nova Science: New York, NY, USA, 2020; pp. 1–199. ISBN 978-1-5361-8278-1. [Google Scholar]
- Romero, C.A.; Montoya-Inzunza, L.; Contreras, L.; Heredia, J.B.; Gutiérrez-Grijalva, E. Solanum Fruits: Phytochemicals, Bioaccessibility and Bioavailability, and Their Relationship With Their Health-Promoting Effects. Front. Nutr. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Gürbüz Çolak, N.; Uluışık, S.; Frary, A.; Frary, A.; Doganlar, S. Health Benefits and Bioactive Compounds of Eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Scorsatto, M.; Rosa, G.; Luiz, R.; Mulder, A.; Teodoro, A.; De Oliveira, J.M. Effect of Eggplant Flour (Solanum Melongena L.) Associated with Hypoenergetic Diet on Antioxidant Status in Overweight Women—a Randomised Clinical Trial. Int. J. Food Sci. Technol. 2019, 54, 2182–2189. [Google Scholar] [CrossRef]
- Mssillou, I.; Agour, A.; Slighoua, M.; Chebaibi, M.; Amrati, F.E.-Z.; Alshawwa, S.; Kamaly, O.; El Moussaoui, A.; Badiaa, L.; Derwich, E. Ointment-Based Combination of Dittrichia Viscosa L. and Marrubium Vulgare L. Accelerate Burn Wound Healing. Pharmaceuticals 2022, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Dalli, M.; Daoudi, N.E.; Azizi, S.-E.; Benouda, H.; Bnouham, M.; Gseyra, N. Chemical Composition Analysis Using HPLC-UV/GC-MS and Inhibitory Activity of Different Nigella Sativa Fractions on Pancreatic α-Amylase and Intestinal Glucose Absorption. BioMed Res. Int. 2021, 2021, 9979419. [Google Scholar] [CrossRef]
- Chebbac, K.; Ghneim, H.; El Moussaoui, A.; Bourhia, M.; El Barnossi, A.; Ouaritini, Z.; Salamatullah, A.; Alzahrani, A.; Aboul-Soud, M.; Giesy, J.; et al. Antioxidant and Antimicrobial Activities of Chemically-Characterized Essential Oil from Artemisia Aragonensis Lam. against Drug-Resistant Microbes. Molecules 2022, 27, 1136. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Fatima Zahra, J.; Bourhia, M.; Maliki, I.; Sounni, F.; Mothana, R.; Bousta, D.; Bari, A. Withania Frutescens: Chemical Characterization, Analgesic, Anti-Inflammatory, and Healing Activities. Open Chem. 2020. [Google Scholar] [CrossRef]
- Kara, M.; Assouguem, A.; Benmessaoud, S.; El Fadili, M.; Alshawwa, S.; Kamaly, O.; Saghrouchni, H.; Zerhouni, R.; Bahhou, J. Contribution to the Evaluation of Physicochemical Properties, Total Phenolic Content, Antioxidant Potential, and Antimicrobial Activity of Vinegar Commercialized in Morocco. Molecules 2022, 27, 770. [Google Scholar] [CrossRef]
- Ouattar, H.; Zouirech, O.; Kara, M.; Assouguem, A.; Almutairi, S.M.; Al-Hemaid, F.M.; Rasheed, R.A.; Ullah, R.; Abbasi, A.M.; Aouane, M.; et al. In Vitro Study of the Phytochemical Composition and Antioxidant, Immunostimulant, and Hemolytic Activities of Nigella sativa (Ranunculaceae) and Lepidium sativum Seeds. Molecules 2022, 27, 5946. [Google Scholar] [CrossRef]
- Hmamou, A.; Eloutassi, N.; Alshawwa, S.; kamaly, O.; Kara, M.; Bendaoud, A.; El-Assri, E.-M.; Tlemcani, S.; Mostafa, E.K.; Lahkimi, A. Total Phenolic Content and Antioxidant and Antimicrobial Activities of Papaver rhoeas L. Organ Extracts Growing in Taounate Region, Morocco. Molecules 2022, 27, 854. [Google Scholar] [CrossRef]
- Rajalakshmi, R.; Lalitha, P.; Sharma, S.C.; Rajiv, A.; Chithambharan, A.; Ponnusamy, A. In Silico Studies: Physicochemical Properties, Drug Score, Toxicity Predictions and Molecular Docking of Organosulphur Compounds against Diabetes Mellitus. J. Mol. Recognit. 2021, 34, e2925. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Soud, M.A.M.; Ennaji, H.; Kumar, A.; Alfhili, M.A.; Bari, A.; Ahamed, M.; Chebaibi, M.; Bourhia, M.; Khallouki, F.; Alghamdi, K.M.; et al. Antioxidant, Anti-Proliferative Activity and Chemical Fingerprinting of Centaurea Calcitrapa against Breast Cancer Cells and Molecular Docking of Caspase-3. Antioxidants 2022, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- Amrati, F.E.-Z.; Elmadbouh, O.; Chebaibi, M.; Soufi, B.; Conte, R.; Slighoua, M.; Saleh, A.; Kamaly, O.; Aziz, D.; Zair, T.; et al. Evaluation of the Toxicity of Caralluma europaea (C.E) Extracts and Their Effects on Apoptosis and Chemoresistance in Pancreatic Cancer Cells. J. Biomol. Struct. Dyn. 2022. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Zhang, X.; Caner, S.; Tysoe, C.; Nguyen, N.; Wicki, J.; Williams, D.; Coleman, J.; McNeill, J.; Yuen, V.; et al. The Amylase Inhibitor Montbretin A Reveals a New Glycosidase Inhibition Motif. Nat. Chem. Biol. 2015, 11, 691–696. [Google Scholar] [CrossRef]
- Mssillou, I.; Agour, A.; Hamamouch, N.; Badiaa, L.; Derwich, E. Chemical Composition and In Vitro Antioxidant and Antimicrobial Activities of Marrubium vulgare L. Sci. World J. 2021, 2021, 7011493. [Google Scholar] [CrossRef]
- Rezouki, S.; Allali, A.; Bouchra, L.; Eloutassi, N.; Fadli, M. The Impact of the Harvesting Period and Drying Conditions on the Essential Oil Yield of Rosmarinus Officinalis, Thymus Satureioides and Origanum Compactum from the Taza-Taounate Region. Asian J. Agric. Biol. 2021, 3, 202004251. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. Oxid. Med. Cell. Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef]
- Alonso, Á.; Castro-Mejías, R.; Rodrı́guez, M.C.; Guillén, D.; Barroso, C. Study of the Antioxidant Power of Brandies and Vinegars Derived from Sherry Wines and Correlation with Their Content in Polyphenols. Food Res. Int. 2004, 37, 715–721. [Google Scholar] [CrossRef]
- Khiya, Z.; Oualcadi, Y.; Gamar, A.; Berrekhis, F.; Zair, T.; Hilali, F.E. Correlation of Total Polyphenolic Content with Antioxidant Activity of Hydromethanolic Extract and Their Fractions of the Salvia Officinalis Leaves from Different Regions of Morocco. J. Chem. 2021, 2021, e8585313. [Google Scholar] [CrossRef]
- Rajalakshmi, P.; Pugalenthi, M. Phytochemical Screening and In Vitro Antioxidant Activity of Lantana Camara L. and Solanum Elaeagnifolium C. Int. J. Bot. Stud. 2016, 1, 26–29. [Google Scholar]
- Farag, R.S.; Abdel-Latif, M.S.; Abd El Baky, H.H.; Tawfeek, L.S. Phytochemical Screening and Antioxidant Activity of Some Medicinal Plants’ Crude Juices. Biotechnol. Rep. 2020, 28, e00536. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Le, K.; Chiu, F.; Ng, K. Identification and Quantification of Antioxidants in Fructus Lycii. Food Chem. 2007, 105, 353. [Google Scholar] [CrossRef]
- Laoufi, H.; Benariba, N.; Adjdir, S.; Djaziri, R. In Vitro α-Amylase and α-Glucosidase Inhibitory Activity of Ononis Angustissima Extracts. J. Appl. Pharm. Sci. 2017, 7, 191–198. [Google Scholar]
- Boulfia, M.; Lamchouri, F.; Senhaji, S.; Lachkar, N.; Bouabid, K.; Toufik, H. Mineral Content, Chemical Analysis, In Vitro Antidiabetic and Antioxidant Activities, and Antibacterial Power of Aqueous and Organic Extracts of Moroccan Leopoldia comosa (L.) Parl. Bulbs. Evid. -Based Complement. Altern. Med. 2021, 2021, 1–17. [Google Scholar] [CrossRef]
- Saraswathi, K.; Bharkavi, R.; Khusro, A.; Sivaraj, C.; Arumugam, P.; Alghamdi, S.; Dablool, A.; Almehmadi, M.; Bannunah, A.; Sahibzada, M. Assessment on in Vitro Medicinal Properties and Chemical Composition Analysis of Solanum Virginianum Dried Fruits. Arab. J. Chem. 2021, 14, 103442. [Google Scholar] [CrossRef]
- Malviya, N.; Jain, S.; Malviya, S. Antidiabetic Potential of Medicinal Plants. Acta Pol. Pharm. 2010, 67, 113–118. [Google Scholar]
- Nagarajan, Y. In Vitro Evaluation of Antidiabetic Potential of Leaf and Stem Extracts of Solanum Xanthocarpum and Solanum Nigrum. Int. J. Adv. Res. Biol. Sci. 2016, 3, 191–195. [Google Scholar]
- Kumar, P. A Review on the Pharmaceutical Activity of Solanum Surattense. GSC Adv. Res. Rev. 2021, 7, 038–044. [Google Scholar] [CrossRef]
- Sridevi, M.; Kalaiarasi, P.; Pugalendi, K. Antihyperlipidemic Activity of Alcoholic Leaf Extract of Solanum Surattense in Streptozotocin-Diabetic Rats. Asian Pac. J. Trop. Biomed. 2011, 1, S276–S280. [Google Scholar] [CrossRef]
- Al-Ishaq, R.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Sahnoun, M.; Trabelsi, S.; Bejar, S. Citrus Flavonoids Collectively Dominate the α-Amylase and α-Glucosidase Inhibitions. Biologia 2017, 72, 764–773. [Google Scholar] [CrossRef]
- Ahmed, O.; Mahmoud, A.; Abdel Moneim, A. Ashour Antidiabetic Effects of Hesperidin and Naringin in Type 2 Diabetic Rats. Diabetol. Croat. 2012, 41, 53–67. [Google Scholar]
- Singh, A.; Vr, D.; Keshari, A.; Rai, A.; Kumar, P.; Rawat, A.; Maity, B.; Kumar, D.; Prakash, A.; De, A.; et al. Isolated Mangiferin and Naringenin Exert Antidiabetic Effect via PPARγ/GLUT4 Dual Agonistic Action with Strong Metabolic Regulation. Chem.-Biol. Interact. 2018, 280, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Valdes, M.; Calzada, F.; Martínez-Solís, J.; Martínez-Rodríguez, J. Antihyperglycemic Effects of Annona Cherimola Miller and the Flavonoid Rutin in Combination with Oral Antidiabetic Drugs on Streptozocin-Induced Diabetic Mice. Pharmaceuticals 2023, 16, 112. [Google Scholar] [CrossRef]
- Prince, P.; Kamalakkannan, N. Rutin Improves Glucose Homeostasis in Streptozotocin Diabetic Tissues by Altering Glycolytic and Gluconeogenic Enzymes. J. Biochem. Mol. Toxicol. 2006, 20, 96–102. [Google Scholar] [CrossRef]
- Yi, H.; Peng, H.; Wu, X.; Xu, X.; Kuang, T.; Zhang, J.; Du, L.; Fan, G. The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence. Oxidative Med. Cell. Longev. 2021, 2021, e6678662. [Google Scholar] [CrossRef]
- Gupta, R.; Kesari, A.; Murthy, P.; Chandra, R.; Tandon, V.; Watal, G. Hypoglycemic and Antidiabetic Effect of Ethanolic Extract of Leaves of Annona Squamosa L. in Experimetal Animals. J. Ethnopharmacol. 2005, 99, 75–81. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A. Antidiabetic and Antioxidative Effects of Annona Squamosa Leaves Are Possibly Mediated through Quercetin-3-O-Glucoside. BioFactors 2007, 31, 201–210. [Google Scholar] [CrossRef]
- Kahssay, S.; Hailu, G.; Taye Desta, K. Design, Synthesis, Characterization and in Vivo Antidiabetic Activity Evaluation of Some Chalcone Derivatives. Drug Des. Dev. Ther. 2021, 15, 3119–3129. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, R.; Kaur, M. Bio-Medical Potential of Chalcone Derivatives and Their Metal Complexes as Antidiabetic Agents: A Review. J. Coord. Chem. 2021, 74, 725–742. [Google Scholar] [CrossRef]
- Parmar, H.S.; Jain, P.; Chauhan, D.; Bhinchar, M.; Munjal, V.; Yusuf, M.; Choube, K.; Tawani, A.; Tiwari, V.; Kumar, A. DPP-IV Inhibitory Potential of Naringin: An in Silico, in Vitro and in Vivo Study. Diabetes Res. Clin. Pract. 2012, 97, 105–111. [Google Scholar] [CrossRef]
- Srinivasan, S.; Vinothkumar, V.; Murali, R. Antidiabetic Efficacy of Citrus Fruits With Special Allusion to Flavone Glycosides. In Bioactive Food as Dietary Interventions for Diabetes; Academic Press: Cambridge, MA, USA, 2019; pp. 335–346. ISBN 978-0-12-813822-9. [Google Scholar]
- Sahiner, M.; Sahiner, N.; Sagbas, S.; Fullerton, M.; Blake, D. Fabrication of Biodegradable Poly(Naringin) Particles with Antioxidant Activity and Low Toxicity. ACS Omega 2018, 3, 17359–17367. [Google Scholar] [CrossRef]

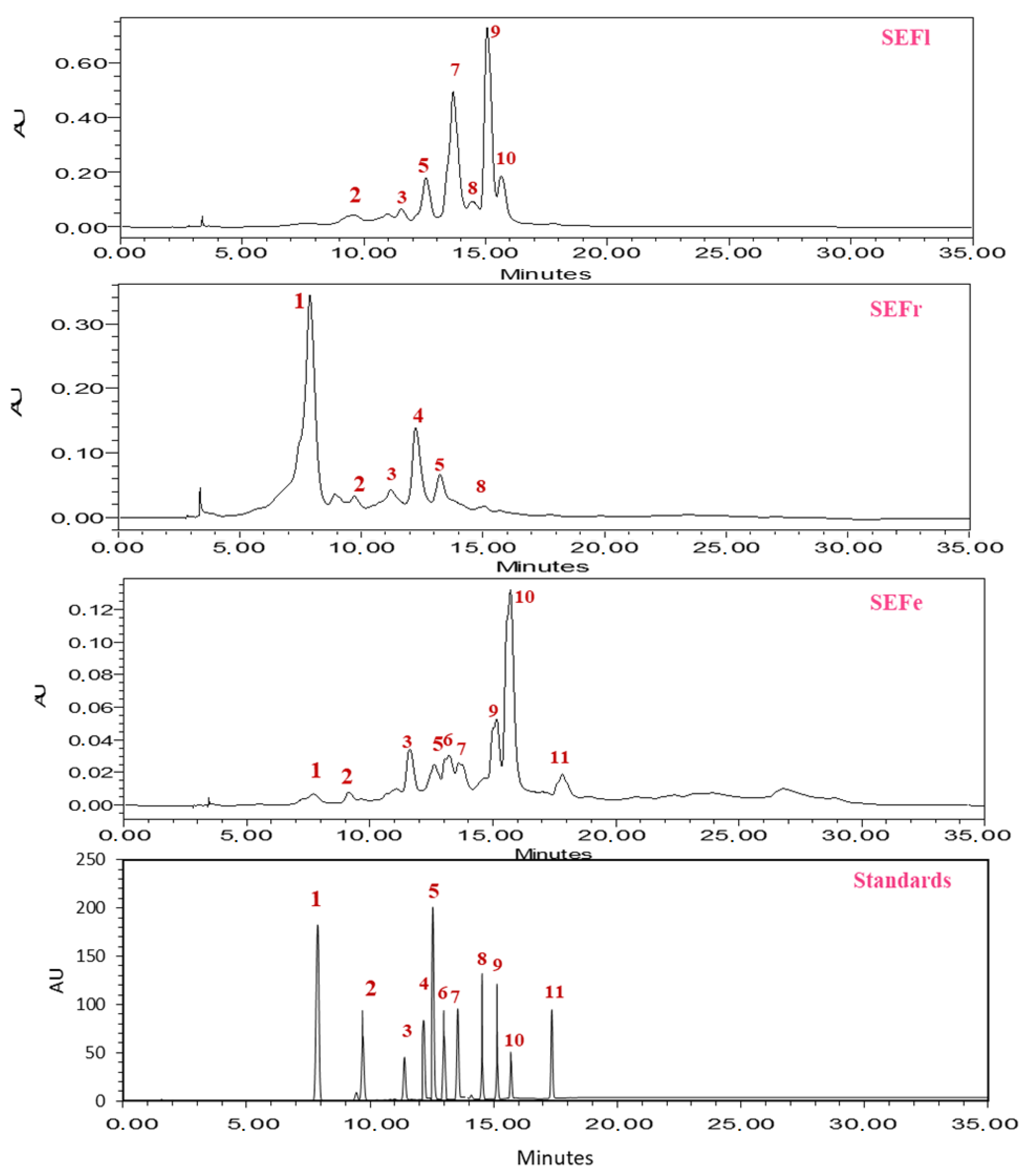

| Peak | Standards | Formula | Rt (min) | % Area | ||
|---|---|---|---|---|---|---|
| SEFr | SEFl | SEFe | ||||
| 1 | Salicylic acid | C7H6O3 | 7.88 | 68.39 | nd | 2.46 |
| 2 | Ferulic acid | C10H10O4 | 9.60 | 2.29 | 2.57 | nd |
| 3 | Sinapic acid | C11H12O5 | 11.30 | 3.81 | 1.94 | 8.35 |
| 4 | Cinnamic acid | C9H2O2 | 12.40 | 14.73 | nd | nd |
| 5 | Chlorogenic acid | C16H18O9 | 12.55 | 14.73 | 9.42 | 6.70 |
| 6 | Naringin | C15H12O5 | 12.95 | nd | nd | 2.85 |
| 7 | Quercetin | C10H10O4 | 13.50 | nd | 33.32 | 6.52 |
| 8 | Rutin | C27H30O16 | 14.53 | 4.52 | 5.19 | nd |
| 9 | Quercetin-3-O-beta-glucoside | C21H20O12 | 15.14 | nd | 36.99 | 13.33 |
| 10 | Kaempferol | C15H10O6 | 15.70 | nd | 9.51 | 36.19 |
| 11 | Chalcone | C15H12O | 17.39 | nd | nd | 4.13 |
| Samples | DPPH-IC50 (µg/mL) | TAC (μg AAE/mg) |
|---|---|---|
| SEFr | 71.21 ± 3.87 | 900.06 ± 4.01 |
| SEFe | 43.19 ± 1.46 | 792.10 ± 6.72 |
| SEFl | 132.13 ± 5.59 | 681.10 ± 3.02 |
| BHT | 67.17 ± 2.04 | 800.07 ± 3.11 |
| Samples | IC50 (μg/mL) | |
|---|---|---|
| α-Amylase | α-Glycosidase | |
| Acarbose | 44.65 ± 0.01 | 52.56 ± 2.67 |
| SEFl | 99.16 ± 1.17 | 41.14 ± 1.55 |
| SEFe | 79.16 ± 2.35 | 20.05 ± 0.12 |
| SEFr | 40.31 ± 2.04 | 20.53 ± 0.37 |
| 2RIP | |||
|---|---|---|---|
| Glide Gscore(Kcal/mol) | Glide Emodel(Kcal/mol) | Glide Energy(Kcal/mol) | |
| Rutin | −8.10 | −103.65 | −74.52 |
| Quercetin-3-O-beta-glucoside | −6.23 | −68.42 | −52.83 |
| Chalcone | −5.73 | −36.06 | −28.21 |
| Naringin | −5.37 | −68.48 | −53.20 |
| Chlorogenic acid | −5.12 | −55.94 | −42.80 |
| Kaempferol | −5.07 | −47.51 | −34.63 |
| Salicylic acid | −4.22 | −23.90 | −17.91 |
| Sinapic acid | −2.99 | −27.59 | −22.42 |
| Ferulic acid | −2.96 | −26.30 | −21.03 |
| Cinnamic acid | −2.63 | −17.22 | −14.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouslamti, M.; Loukili, E.H.; Elrherabi, A.; El Moussaoui, A.; Chebaibi, M.; Bencheikh, N.; Nafidi, H.-A.; Bin Jardan, Y.A.; Bourhia, M.; Bnouham, M.; et al. Phenolic Profile, Inhibition of α-Amylase and α-Glucosidase Enzymes, and Antioxidant Properties of Solanum elaeagnifolium Cav. (Solanaceae): In Vitro and In Silico Investigations. Processes 2023, 11, 1384. https://doi.org/10.3390/pr11051384
Bouslamti M, Loukili EH, Elrherabi A, El Moussaoui A, Chebaibi M, Bencheikh N, Nafidi H-A, Bin Jardan YA, Bourhia M, Bnouham M, et al. Phenolic Profile, Inhibition of α-Amylase and α-Glucosidase Enzymes, and Antioxidant Properties of Solanum elaeagnifolium Cav. (Solanaceae): In Vitro and In Silico Investigations. Processes. 2023; 11(5):1384. https://doi.org/10.3390/pr11051384
Chicago/Turabian StyleBouslamti, Mohammed, El Hassania Loukili, Amal Elrherabi, Abdelfattah El Moussaoui, Mohamed Chebaibi, Noureddine Bencheikh, Hiba-Allah Nafidi, Yousef A. Bin Jardan, Mohammed Bourhia, Mohamed Bnouham, and et al. 2023. "Phenolic Profile, Inhibition of α-Amylase and α-Glucosidase Enzymes, and Antioxidant Properties of Solanum elaeagnifolium Cav. (Solanaceae): In Vitro and In Silico Investigations" Processes 11, no. 5: 1384. https://doi.org/10.3390/pr11051384







