Dietary Fiber from Plant-Based Food Wastes: A Comprehensive Approach to Cereal, Fruit, and Vegetable Waste Valorization
Abstract
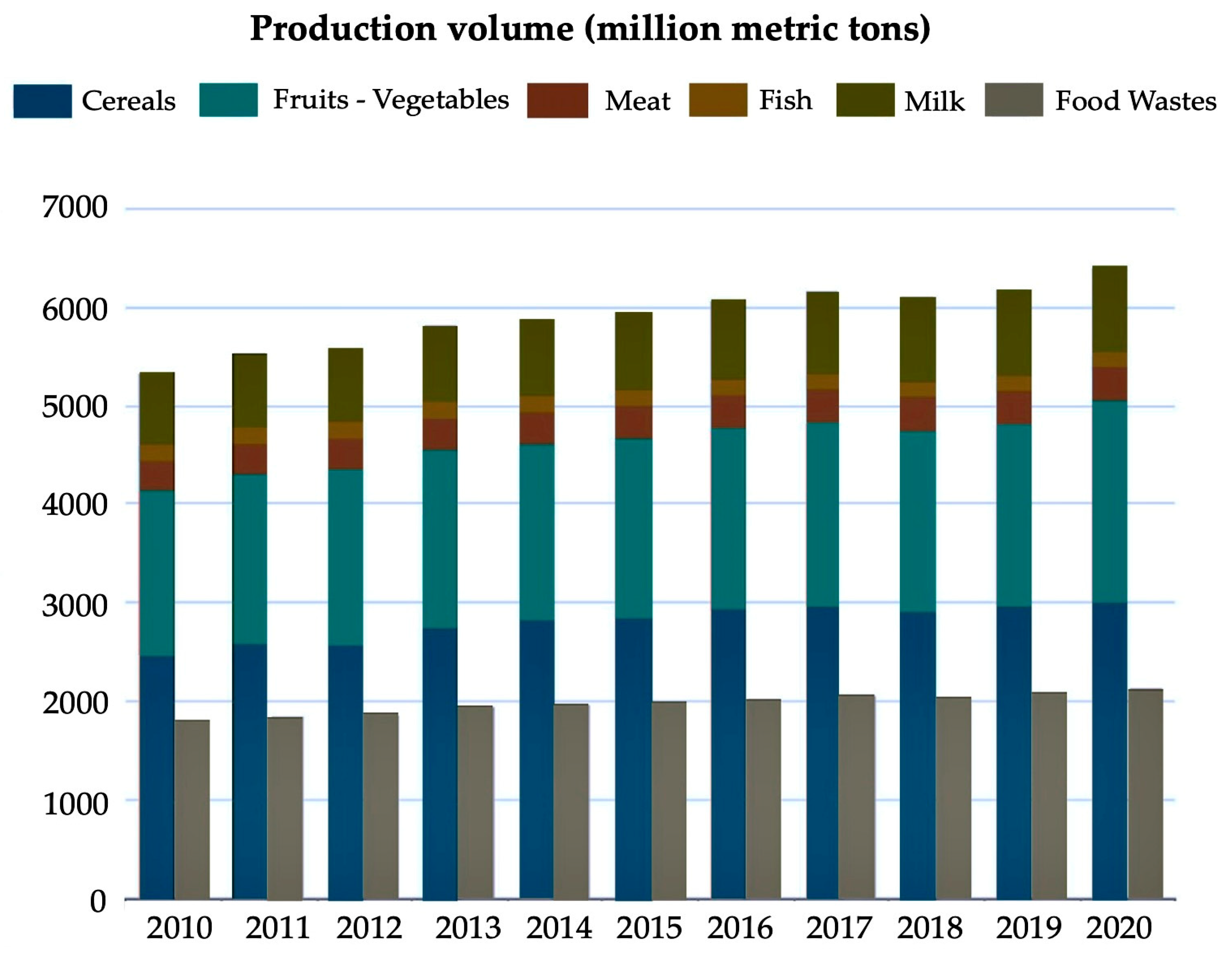
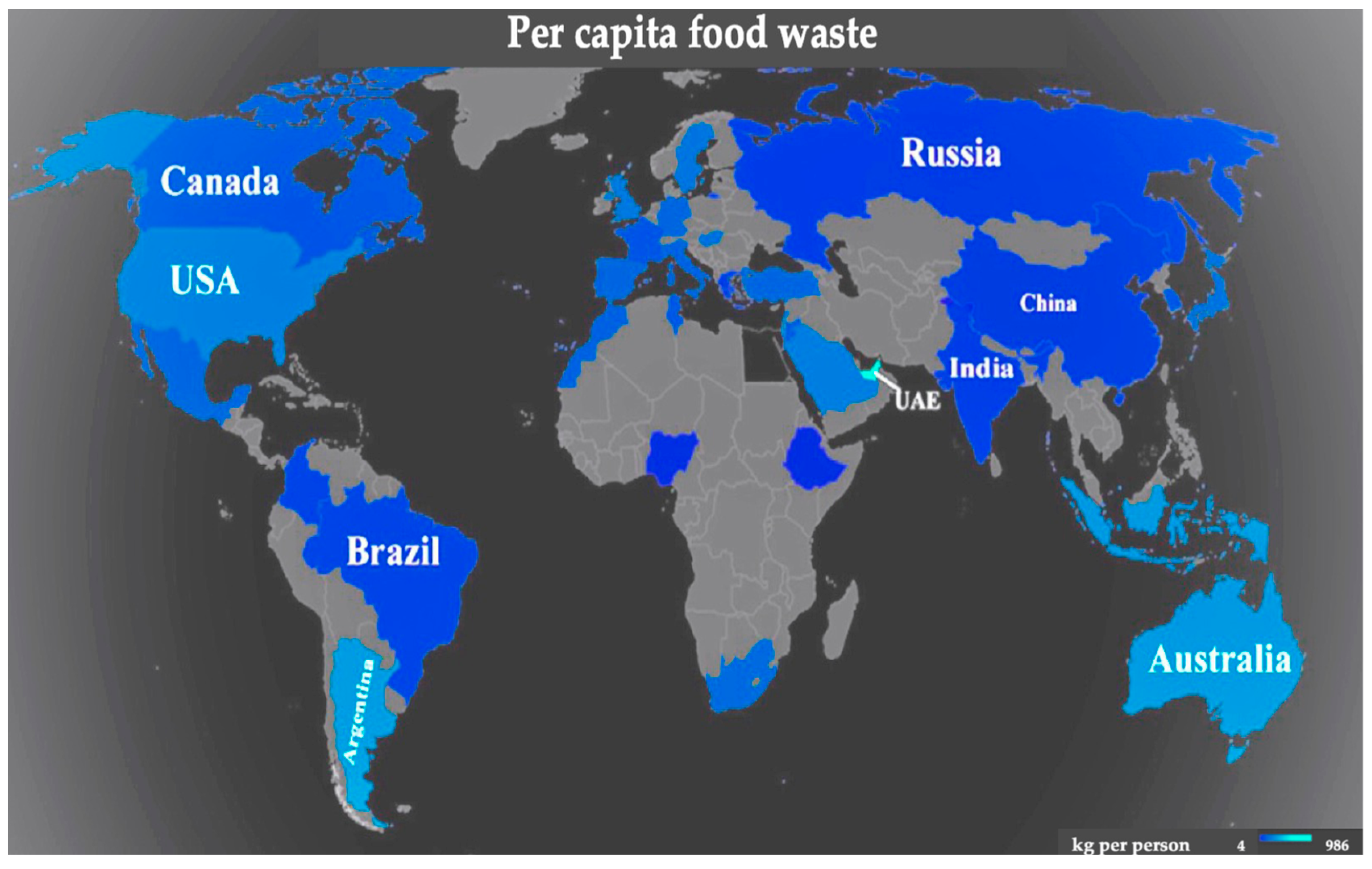
:1. Introduction
2. Valorization of Plant-Based Food Wastes
3. Utilization of Plant-Based Food Wastes and Byproducts on Circular Economy
4. Dietary Fiber from Plant-Based Food Wastes
4.1. Cereals
4.1.1. Wheat
4.1.2. Maize
4.1.3. Rice
4.2. Fruits
4.2.1. Apple
4.2.2. Citrus Fruits
4.2.3. Peach
4.2.4. Grape
4.3. Vegetables
4.3.1. Tomato
4.3.2. Onion
4.3.3. Potato
5. Application of Dietary Fibers in Food Industry
6. Application of Dietary Fibers in Nonfood Industry: The Case of Textiles
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). Technical Platform on the Measurement and Reduction of Food Loss and Waste. 2023. Available online: http://www.fao.org/save-food/resources/keyfindings/en/data (accessed on 1 April 2023).
- Parfitt, J.; Barthel, M.; MacNaughton, S. Food waste within food supply chains: Quantification and potential for change to 2050. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3065–3081. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Global Food Losses and Waste: Extent, Causes and Prevention; A Study Conducted for International Congress “Save Food” at Inter; FAO: Dusseldorf, Germany; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; Available online: http://www.fao.org/3/a-i2697e.pdf (accessed on 1 April 2023).
- Imbert, E. Food waste valorization options: Opportunities from the Bioeconomy. Open Agric. 2017, 2, 195–204. [Google Scholar] [CrossRef]
- Berkenkamp, J.; Nennich, T. Beyond Beauty: The Opportunities and Challenges of Cosmetically Imperfect Produce. Report 1: Survey Results from Minnesota Produce Growers. 2015. Available online: http://misadocuments.info/Beyond_Beauty_Grower_Survey_Results_052615.pdf (accessed on 1 April 2023).
- ReFED Collaborative. A Roadmap to Reduce U.S. Food Waste. 2016. Available online: https://refed.org/downloads/ReFED_Report_2016.pdf (accessed on 31 March 2023).
- Kumar, S.; Kushwaha, R.; Verma, M.L. Recovery and utilization of bioactives from food processing waste. In Biotechnological Production of Bioactive Compounds; Verma, M.L., Chandel, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 37–68. [Google Scholar]
- Gunders, D. Wasted: How America Is Losing Up to 40 Percent of Its Food from Farm to Fork to Landfill. NRDC Wasted Food Report, USA. 2012. Available online: https://www.nrdc.org/sites/default/files/wasted-food-IP.pdf (accessed on 31 March 2023).
- Papargyropoulou, E.; Lozano, R.; Steinberger, K.; Wright, N.; Ujang, Z.B. The food waste hierarchy as a framework for the management of food surplus and food waste. J. Clean. Prod. 2014, 76, 106–115. [Google Scholar] [CrossRef]
- Liu, Z.; de Souza, T.S.P.; Holland, B.; Dunshea, F.; Barrow, C.; Suleria, H.A.R. Valorization of food waste to produce value-added products based on its bioactive compounds. Processes 2023, 11, 840. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Definitional Framework of Food Losses and Waste; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; Available online: http://www.fao.org/3/a-at144e.pdf (accessed on 31 March 2023).
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary fiber from underutilized plant resources—A positive approach for valorization of fruit and vegetable wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Venkat, K. The climate change and economic impacts of food waste in the United States. Int. J. Food Syst. Dyn. 2011, 2, 431–446. [Google Scholar]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of fruit industrial by-products—A case study on circular economy approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef]
- Kibler, K.M.; Reinhart, D.; Hawkins, C.; Motlagh, A.M.; Wright, J. Food waste and the food-energy-water nexus: A review of food waste management alternatives. Waste Manag. 2018, 74, 52–62. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Q.; Qian, D.; Gong, L.; Zhang, W. The utilization of acid-tolerant bacteria on ethanol production from kitchen garbage. Renew. Energy 2009, 34, 1466–1470. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Yahia, E.M. The contribution of fruit and vegetable consumption to human health. In Fruit and Vegetable Phytochemicals- Chemistry and Human Health, 2nd ed.; De La Rosa, L.A., Alvarez-Parrilla, E., González Aguilar, G.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 3–51. [Google Scholar]
- Maphosa, Y.; Jideani, V.A. Dietary fiber extraction for human nutrition—A review. Food Rev. Int. 2016, 32, 98–115. [Google Scholar] [CrossRef]
- Takshak, S. Bioactive compounds in medicinal plants: A condensed review. SEJ Pharm. Nat. Med. 2018, 1, 13–35. [Google Scholar]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of green food processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Terra, L.D.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA-J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M.; Rodríguez-Gutiérrez, G.; Jaramillo-Carmona, S.; Espejo-Calvo, J.; Rodríguez-Arcos, R.; Fernández-Bolaños, J.; Guillén-Bejarano, R.; Jiménez-Araujo, A. Effect of extraction method on chemical composition and functional characteristics of high dietary fibre powders obtained from asparagus by-products. Food Chem. 2009, 113, 665–671. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008, 108, 977–985. [Google Scholar] [CrossRef]
- Vieira, G.S.; Cavalcanti, R.N.; Meireles, M.A.A.; Hubinger, M.D. Chemical and economic evaluation of natural antioxidant extracts obtained by ultrasound-assisted and agitated bed extraction from jussara pulp (Euterpe edulis). J. Food Eng. 2013, 119, 196–204. [Google Scholar] [CrossRef]
- Wang, C.; Tallian, C.; Su, J.; Vielnascher, R.; Silva, C.; Cavaco-Paulo, A.; Guebitz, G.M.; Fu, J. Ultrasound-assisted extraction of hemicellulose and phenolic compounds from bamboo bast fiber powder. PLoS ONE 2018, 13, e0197537. [Google Scholar] [CrossRef] [PubMed]
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Liguori, R.; Faraco, V. Biological processes for advancing lignocellulosic waste biorefinery by advocating circular economy. Bioresour. Technol. 2016, 215, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Elia, V.; Gnoni, M.G.; Tornese, F. Measuring circular economy strategies through index methods: A critical analysis. J. Clean. Prod. 2017, 142, 2741–2751. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Umesh, M.; Selvaraj, M.; Al-Shehri, B.M.; Chakraborty, P.; Duhan, L.; Sharma, S.; Pasrija, R.; Awasthi, M.K.; et al. Emerging challenges for the agro-industrial food waste utilization: A review on food waste biorefinery. Bioresour Technol. 2022, 362, 127790. [Google Scholar] [CrossRef]
- Mak, T.M.W.; Xiong, X.; Tsang, D.C.W.; Yu, I.K.M.; Poon, C.S. Sustainable food waste management towards circular bioeconomy: Policy review, limitations and opportunities. Bioresour. Technol. 2020, 297, 122497. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Varjani, S.; Kim, S.H.; Wong, J.W.C. Sustainable processing of food waste for production of bio-based products for circular bioeconomy. Bioresour. Technol. 2021, 325, 124684. [Google Scholar] [CrossRef]
- Kumar Awasthi, M.; Paul, A.; Kumar, V.; Sar, T.; Kumar, D.; Sarsaiya, S.; Liu, H.; Zhang, Z.; Binod, P.; Sindhu, R.; et al. Recent trends and developments on integrated biochemical conversion process for valorization of dairy waste to value added bioproducts: A review. Bioresour. Technol. 2022, 344, 126193. [Google Scholar] [CrossRef]
- FAOSTAT. Statistics Division; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 April 2023).
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 57, 59–81. [Google Scholar] [CrossRef]
- Gartaula, G.; Dhital, S.; Netzel, G.; Flanagan, B.M.; Yakubov, G.E.; Beahan, C.T.; Collins, H.M.; Burton, R.A.; Bacic, A.; Gidley, M.J. Quantitative structural organisation model for wheat endosperm cell walls: Cellulose as an important constituent. Carbohydr. Polym. 2018, 196, 199–208. [Google Scholar] [CrossRef]
- Evers, A.D.; Blakeney, A.B.; O’Brien, L. Cereal structure and composition. Aust. J. Agric. Res. 1999, 50, 629–650. [Google Scholar] [CrossRef]
- Ciudad-Mulero, M.; Fernández-Ruiz, V.; Matallana-González, M.C.; Morales, P. Dietary fiber sources and human benefits: The case study of cereal and pseudocereals. Adv. Food Nutr. Res. 2019, 90, 83–134. [Google Scholar] [PubMed]
- Sun, R.; Tomkinson, J.; Wang, S.; Zhu, W. Characterization of lignins from wheat straw by alkaline peroxide treatment. Polym. Degrad. Stabil. 2000, 67, 101–109. [Google Scholar] [CrossRef]
- Sun, R.C.; Tomkinson, J. Characterization of hemicelluloses obtained by classical and ultrasonically assisted extractions from wheat straw. Carbohydr. Polym. 2002, 50, 263–271. [Google Scholar] [CrossRef]
- Rashid, S.; Rakha, A.; Anjum, F.M.; Ahmed, W.; Sohail, M. Effects of extrusion cooking on the dietary fibre content and Water Solubility Index of wheat bran extrudates. Int. J. Food Sci. Technol. 2015, 50, 1533–1537. [Google Scholar] [CrossRef]
- Tejada-Ortigoza, V.; Garcia-Amezquita, L.E.; Serna-Saldıvar, S.O.; Welti-Chanes, J. Advances in the functional characterization and extraction processes of dietary fiber. Food Eng. Rev. 2016, 8, 251–271. [Google Scholar] [CrossRef]
- Shiferaw, B.; Prasanna, B.; Hellin, J.; Banziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Arendt, E.K.; Zannini, E. Cereal Grains for the Food and Beverage Industries; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar]
- De Santis, M.A.; Kosik, O.; Passmore, D.; Flagella, Z.; Shewry, P.R.; Lovegrove, A. Comparison of the dietary fibre composition of old and modern durum wheat (Triticum turgidum spp. durum) genotypes. Food Chem. 2018, 244, 304–310. [Google Scholar] [CrossRef]
- Saulnier, L.; Vigouroux, J.; Thibault, J.F. Isolation and partial characterization of feruloylated oligosaccharides from maize bran. Carbohydr. Res. 1995, 272, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, H.M.; Xie, A.J.; Zhu, C.Y.; Qin, G.Y. Dietary fiber extraction from defatted corn hull by hot-compressed water. Pol. J. Food Nutr. Sci. 2018, 68, 133–140. [Google Scholar] [CrossRef]
- Li, H.; Xu, L.; Liu, W.; Fang, M.; Wang, N. Assessment of the nutritive value of whole corn stover and its morphological fractions. Asian-Australas. J. Anim. Sci. 2014, 27, 194. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.S.S.; Hymavathi, A.; Babu, V.R.; Longvah, T. Nutritional composition in relation to glycemic potential of popular Indian rice varieties. Food Chem. 2018, 238, 29–34. [Google Scholar] [CrossRef]
- Ji, C.M.; Shin, J.A.; Cho, J.W.; Lee, K.T. Nutritional evaluation of immature grains in two Korean rice cultivars during maturation. Food Sci. Biotechnol. 2013, 22, 903–908. [Google Scholar] [CrossRef]
- Fernando, B. Rice as a Source of Fibre. Rice Res. Open Access 2013, 1, e101. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredient: A systematic review and meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple pomace as a functional and healthy ingredient in food products: A review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Sudha, M.; Baskaran, V.; Leelavathi, K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Yan, H.; Kerr, W.L. Total phenolics content, anthocyanins, and dietary fiber content of apple pomace powders produced by vacuum-belt drying. J. Sci. Food Agric. 2013, 93, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, X.; Lv, Y.; He, Q. Extraction and functional properties of water-soluble dietary fiber from apple pomace. J. Food Process. Eng. 2014, 37, 293–298. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Szymanska-Chargot, M.; Chylinska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Rubenthaler, G.; Leung, H.; Baranowski, J. Chemical, physical, and baking properties of apple fiber compared with wheat and oat bran. Cereal Chem. 1988, 65, 244–247. [Google Scholar]
- John, I.; Yaragarla, P.; Muthaiah, P.; Ponnusamy, K.; Appusamy, A. Statistical optimization of acid catalyzed steam pretreatment of citrus peel waste for bioethanol production. Resour. Technol. 2017, 3, 429–433. [Google Scholar] [CrossRef]
- Grigelmo-Miguel, N.; Martin-Belloso, O. Dietary fiber as a by-product of orange fruit extraction. In Book of Abstracts, Institute of Food Technologists Annual Meeting; Institute of Food Technologists: Orlando, FL, USA, 1997; p. 39. [Google Scholar]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern Extraction and purification techniques for obtaining high purity food-Grade bioactive compounds and value-added co-products from citrus wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef]
- Steger, E. Physiochemical properties of citrus fiber and potential use. In Book of Abstracts, Institute of Food Technologists Annual Meeting; Institute of Food Technologists: Chicago, CA, USA, 1991; p. 214. [Google Scholar]
- Lundberg, B.; Pan, X.; White, A.; Chau, H.; Hotchkiss, A. Rheology and composition of citrus fiber. J. Food Eng. 2014, 125, 97–104. [Google Scholar] [CrossRef]
- Rafiq, S.; Kaul, R.; Sofi, S.; Bashir, N.; Nazir, F.; Nayik, G.A. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agri. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef]
- Dervisoglu, M.; Yazici, F. Note. The effect of citrus fibre on the physical, chemical and sensory properties of ice cream. Food Sci. Technol. Int. 2006, 12, 159–164. [Google Scholar] [CrossRef]
- Gorinstein, S.; Martín-Belloso, O.; Park, Y.S.; Haruenkit, R.; Lojek, A.; Cíž, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- Chang, S.; Tan, C.; Frankel, E.N.; Barrett, D.M. Low-density lipoprotein antioxidant activity of phenolic compounds and polyphenol oxidase activity in selected clingstone peach cultivars. J. Agric. Food Chem. 2000, 48, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Grigelmo-Miguel, N.; Gorinstein, S.; Martín-Belloso, O. Characterisation of peach dietary fibre concentrate as a food ingredient. Food Chem. 1999, 65, 175–181. [Google Scholar] [CrossRef]
- Kurz, C.; Carle, R.; Schieber, A. Characterisation of cell wall polysaccharide profiles of apricots (Prunus armeniaca L.), peaches (Prunus persica L.), and pumpkins (Cucurbita sp.) for the evaluation of fruit product authenticity. Food Chem. 2008, 106, 421–430. [Google Scholar] [CrossRef]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Matharu, A.S.; de Melo, E.M.; Houghton, J.A. Opportunity for high value-added chemicals from food supply chain wastes. Bioresour. Technol. 2016, 215, 123–130. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Schieber, A.; Carle, R. A novel process for the recovery of polyphenols from grape (Vitis vinifera L.) pomace. J. Food Sci. 2005, 70, 157–163. [Google Scholar] [CrossRef]
- Valiente, C.; Arrigoni, E.; Esteban, R.; Amado, R. Grape pomace as a potential food fiber. J. Food Sci. 1995, 60, 818–820. [Google Scholar] [CrossRef]
- Llobera, A.; Cañellas, J. Antioxidant activity and dietary fibre of Prensal Blanc white grape (Vitis vinifera) by-products. Int. J. Food Sci. Technol. 2008, 43, 1953–1959. [Google Scholar] [CrossRef]
- Bender, A.B.B.; Speroni, C.S.; Moro, K.I.B.; Morisso, F.D.P.; Dos Santos, D.R.; Da Silva, L.P.; Penna, N.G. Effects of micronization on dietary fiber composition, physicochemical properties, phenolic compounds, and antioxidant capacity of grape pomace and its dietary fiber concentrate. LWT-Food Sci. Techol. 2020, 117, 108652. [Google Scholar] [CrossRef]
- Del Valle, M.; Cámara, M.; Torija, M.E. Chemical characterization of tomato pomace. J. Sci. Food Agric. 2006, 86, 1232–1236. [Google Scholar] [CrossRef]
- Herrera, P.G.; Sánchez-Mata, M.; Cámara, M. Nutritional characterization of tomato fiber as a useful ingredient for food industry. Innov. Food Sci. Emerg. Technol. 2010, 11, 707–711. [Google Scholar] [CrossRef]
- Méndez-Llorente, F.; Aguilera-Soto, J.I.; López-Carlos, M.A.; Ramírez, R.G.; Carrillo-Muro, O.; Escareño-Sánchez, L.M.; Medina-Flores, C.A. Preservation of fresh tomato waste by silage. Interciencia 2014, 39, 432–434. [Google Scholar]
- Tadeu Pontes, M.; Carvalheiro, F.; Roseiro, J.; Amaral Collaco, M. Evaluation of product composition profile during an extrusion based process of tomato pomace transformation. Agro Food Ind. Hi Tech 1996, 7, 39–40. [Google Scholar]
- Bakshi, M.; Kaur, J.; Wadhwa, M. Nutritional evaluation of sun dried tomato pomace as livestock feed. Indian J. Anim. Nutr. 2012, 29, 6–19. [Google Scholar]
- EUROSTAT. Where Do We Grow Our Fruit and Vegetables? Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/DDN-20191003-1?fbclid=IwAR2kI_mKqpIvax2YS08dw1Ky6-fm1T2-54mDghBUPXKcGnFIXrj3yjEeXXI (accessed on 2 April 2023).
- EU-Onions. Conversion of Environmentally-Unfriendly Onion Waste into Food Ingredients|EU Onions Project|FP4|Cordis|European Commission. 1997. Available online: https://cordis.europa.eu/project/id/FAIR961184 (accessed on 6 April 2023).
- Chandrasekaran, M. Valorization of Food Processing By-Products, 1st ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Sharma, K.; Mahato, N.; Nile, S.H.; Lee, E.T.; Lee, Y.R. Economical and environmentally-friendly approaches for usage of onion (Allium cepa L.) waste. Food Funct. 2016, 7, 3354–3369. [Google Scholar] [CrossRef]
- Jaime, L.; Mollá, E.; Fernández, A.; Martín-Cabrejas, M.A.; López-Andréu, F.J.; Esteban, R.M. Structural carbohydrate differences and potential source of dietary fiber of onion (Allium cepa L.) tissues. J. Agric. Food Chem. 2002, 50, 122–128. [Google Scholar] [CrossRef]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Terry, L.A.; Esteban, R.M. The impact of pasteurisation and sterilisation on bioactive compounds of onion by-products. Food Bioprocess Technol. 2013, 6, 1979–1989. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Chaves Morillo, D.; Bolanos Patino, V.; Bucheli Jurado, M.; Osorio Mora, O. Microwave-assisted extraction of antioxidants com- pounds from potato peel (Solanum tuberosum). Vitae 2016, 23, S635–S639. [Google Scholar]
- Camire, M.E.; Violette, D.; Dougherty, M.P.; McLaughlin, M.A. Potato peel dietary fiber composition: Effects of peeling and extrusion cooking processes. J. Agric. Food Chem. 1997, 45, 1404–1408. [Google Scholar] [CrossRef]
- Camire, M.E.; Flint, S.I. Thermal processing effects on dietary fiber composition and hydration capacity in corn meal, oatmeal and potato peels. Cereal Chem. 1991, 68, 645–647. [Google Scholar]
- Arora, A.; Zhao, J.; Camire, M.E. Extruded potato peel functional properties affected by extrusion conditions. J. Food Sci. 1993, 58, 335–337. [Google Scholar] [CrossRef]
- Javed, A.; Ahmad, A.; Tahir, A.; Shabbir, U.; Nouman, M.; Hameed, A. Potato peel waste—Its nutraceutical, industrial and biotechnological applacations. AIMS Agric. Food 2019, 4, 807. [Google Scholar] [CrossRef]
- Ncobela, C.; Kanengoni, A.; Hlatini, V.; Thomas, R.; Chimonyo, M. A review of the utility of potato by-products as a feed resource for smallholder pig production. Anim. Feed Sci. Technol. 2017, 227, 107–117. [Google Scholar] [CrossRef]
- Sharoba, A.M.; Farrag, M.; Abd El-Salam, A. Utilization of some fruits and vegetables waste as a source of dietary fiber and its effect on the cake making and its quality attributes. J. Agroaliment. Proc. Technol. 2013, 19, 429–444. [Google Scholar]
- Afifi, M. Enhancement of lactic acid production by utilizing liquid potato wastes. Int. J. Biol. Chem. 2011, 5, 91–102. [Google Scholar] [CrossRef]
- Byg, I.; Diaz, J.; Øgendal, L.H.; Harholt, J.; Jørgensen, B.; Rolin, C.; Svava, R.; Ulvskov, P. Large-scale extraction of rhamnogalacturonan I from industrial potato waste. Food Chem. 2012, 131, 1207–1216. [Google Scholar] [CrossRef]
- Rainakari, A.-I.; Rita, H.; Putkonen, T.; Pastell, H. New dietary fibre content results for cereals in the Nordic countries using AOAC 2011.25 method. J. Food Compos. Anal. 2016, 51, 1–8. [Google Scholar] [CrossRef]
- Dodevska, M.S.; Djordjevic, B.I.; Sobajic, S.S.; Miletic, I.D.; Djordjevic, P.B.; Dimitrijevic-Sreckovic, V.S. Characterisation of dietary fibre components in cereals and legumes used in Serbian diet. Food Chem. 2013, 141, 1624–1629. [Google Scholar] [CrossRef]
- Prosky, L.; Asp, N.-G.; Scheweizer, T.F.; DeVries, J.W.; Furda, I. Determination of insoluble, soluble, and total dietary fiber in foods and food products: Interlaboratory study. J. Assoc. Off. Anal. Chem. 1988, 71, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Prasanthi, P.S.; Naveena, N.; Vishnuvardhana Rao, M.; Bhaskarachary, K. Compositional variability of nutrients and phytochemicals in corn after processing. J. Food Sci. Technol. 2017, 54, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Aniola, J.; Gawecki, J.; Czarnocinska, J.; Galinski, G. Corncobs as a source of dietary fiber. Pol. J. Food Nutr. Sci. 2009, 59, 247–249. [Google Scholar]
- Amalraj, A.; Pius, A. Influence of oxalate, phytate, tannin, dietary fiber, and cooking on calcium bioavailability of commonly consumed cereals and millets in India. Cereal Chem. 2015, 92, 389–394. [Google Scholar] [CrossRef]
- Renard, C.; Thibault, J. Composition and physico-chemical properties of apple fibres from fresh fruits and industrial products. Lebenson. Wiss. Technol. 1991, 24, 523–527. [Google Scholar]
- Bengtsson, H.; Tornberg, E. Physicochemical characterization of fruit and vegetable fiber suspensions. I: Effect of homogenization. J. Texture Stud. 2011, 42, 268–280. [Google Scholar] [CrossRef]
- Toushik, S.H.; Lee, K.T.; Lee, J.S.; Kim, K.S. Functional applications of lignocellulolytic enzymes in the fruit and vegetable processing industries. J. Food Sci. 2017, 82, 585–593. [Google Scholar] [CrossRef]
- Dranca, F.; Vargas, M.; Oroian, M. Physicochemical properties of pectin from Malus domestica ‘Fălticeni’ apple pomace as affected by non-conventional extraction techniques. Food Hydrocoll. 2020, 100, 105383. [Google Scholar] [CrossRef]
- Pagan, J.; Ibarz, A. Extraction and rheological properties of pectin from fresh peach pomace. J. Food Eng. 1999, 39, 193–201. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Femenia, A.; Garau, M.; Meza-Velázquez, J.; Simal, S.; Rosselló, C. Ultrasound-assisted extraction of pectins from grape pomace using citric acid: A response surface methodology approach. Carbohydr. Polym. 2014, 106, 179–189. [Google Scholar] [CrossRef]
- Alvarado, A.; Pacheco-Delahaye, E.; Hevia, P. Value of a tomato byproduct as a source of dietary fiber in rats. Plant. Foods Hum. Nutr. 2001, 56, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.P.; Rhim, J.W. Extraction and characterization of cellulose microfibers from agricultural wastes of onion and garlic. J. Nat. Fibers 2018, 15, 465–473. [Google Scholar] [CrossRef]
- Liu, Q.; Tarn, R.; Lynch, D.; Skjodt, N.M. Physicochemical properties of dry matter and starch from potatoes grown in Canada. Food Chem. 2007, 105, 897–907. [Google Scholar] [CrossRef]
- Jeddou, K.B.; Bouaziz, F.; Zouari-Ellouzi, S.; Chaari, F.; Ellouz-Chaabouni, S.; Ellouz-Ghorbel, R.; Nouri-Ellouz, O. Improvement of texture and sensory properties of cakes by addition of potato peel powder with high level of dietary fiber and protein. Food Chem. 2017, 217, 668–677. [Google Scholar] [CrossRef]
- Sharma, S.K.; Bansal, S.; Mangal, M.; Dixit, A.K.; Gupta, R.K.; Mangal, A.K. Utilization of food processing by-products as dietary, functional, and novel fiber: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1647–1661. [Google Scholar] [CrossRef]
- Yangilar, F. The application of dietary fibre in food industry: Structural features, effects on health and definition, obtaining and analysis of dietary fibre: A review. J. Food Nutr. Res. 2013, 1, 13–23. [Google Scholar]
- Tungland, B.C.; Meyer, D. Non digestible oligo-and polysaccharides (Dietary Fiber): Their physiology and role in human health and food. Compr. Rev. Food Sci. Food Saf. 2002, 1, 90–109. [Google Scholar] [CrossRef]
- Alexander, R.J. Moving toward low-calorie dairy products. Food Prod. Des. 1997, 7, 74–98. [Google Scholar]
- Sanz, T.; Salvador, A.; Jimenez, A.; Fiszman, S.M. Yogurt enrichment with functional asparagus fibre. Effect of fibre extraction method on rheological properties, colour, and sensory acceptance. Eur. Food Res. Technol. 2008, 227, 1515–1521. [Google Scholar] [CrossRef]
- Sendra, E.; Fayos, P.; Lario, Y.; Fernandez-Lopez, J.A.; Sayas-Barbera, E.; Perez-Alvarez, J.A. Incorporation of citrus fibres in fermented milk containing probiotic bacteria. Food Microbiol. 2008, 25, 13–21. [Google Scholar] [CrossRef]
- Staffolo, M.D.; Bertola, N.; Martino, M.; Bevilacqua, Y.A. Influence of dietary fibre addition on sensory and rheological properties of yogurt. Int. Dairy J. 2004, 14, 263–268. [Google Scholar] [CrossRef]
- Hashim, I.B.; Khalil, A.H.; Afifi, H.S. Quality characteristics and consumer acceptance of yogurt fortified with date fibre. J. Dairy Sci. 2009, 92, 5403–5407. [Google Scholar] [CrossRef] [PubMed]
- Tudoric, C.M.; Kuri, V.; Brennan, C.S. Nutritional and physicochemical characteristics of dietary fibre enriched pasta. J. Agric. Food Chem. 2002, 50, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Kruk, M. Asian Noodle Technology; Technical Bulletin 1998, XX(12); American Institute of Baking: Manhattan, KS, USA, 1998. [Google Scholar]
- Chen, J.S.; Fei, M.J.; Shi, C.L.; Tian, J.C.; Sun, C.L.; Zhang, H.; Ma, Z.; Dong, H.X. Effect of particle size and addition level of wheat bran on quality of dry white Chinese noodles. J. Cereal Sci. 2011, 53, 217–224. [Google Scholar] [CrossRef]
- Toma, R.B.; Orr, P.H.; Appolonia, B.D.; Dintzis, F.R.; Tabekhia, M.M. Physical and chemical properties of potato peel as a source of dietary fibre in bread. J. Food Sci. 1979, 44, 1403–1407. [Google Scholar] [CrossRef]
- Nassar, A.G.; AbdEl-Hamied, A.A.; El-Naggar, E.A. Effect of citrus by-products flour incorporation on chemical, rheological and organoleptic characteristics of biscuits. World J. Agric. Sci. 2008, 4, 612–616. [Google Scholar]
- Antonic, B.; Dordevic, D.; Jancikova, S.; Holeckova, D.; Tremlova, B.; Kulawik, P. Effect of Grape Seed Flour on the Antioxidant Profile, Textural and Sensory Properties of Waffles. Processes 2021, 9, 131. [Google Scholar] [CrossRef]
- Sharif, M.K.; Masood, S.B.; Faqir, M.A.; Nawaz, H. Preparation of fibre and mineral enriched defatted rice bran supplemented cookies. Pak. J. Nutr. 2009, 8, 571–577. [Google Scholar] [CrossRef]
- Talukder, S. Effect of dietary fiber on properties and acceptance of meat products: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1005–1011. [Google Scholar] [CrossRef]
- Chevance, F.F.V.; Farmer, L.J.; Desmond, E.M.; Novelli, E.; Troy, D.J.; Chizzolini, R. Effect of some fat replacers on the release of volatile aroma compound from low-fat meat products. J. Agric. Food Chem. 2000, 48, 3476–3484. [Google Scholar] [CrossRef]
- Verma, A.K.; Banerjee, R. Dietary fibre as functional ingredient in meat products: A novel approach for healthy living—A review. J. Food Sci. Technol. 2010, 47, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Sharma, B.D.; Banerjee, R. Quality characteristics and storage stability of low fat functional chicken nuggets with salt substitute blend and high fibre ingredients. Fleischwirtsch Int. 2009, 24, 54–57. [Google Scholar]
- Choi, Y.S.; Park, K.S.; Choi, J.H.; Kim, H.W.; Song, D.H.; Kim, J.M.; Chung, H.J.; Kim, C.J. Physico-chemical properties of chicken meat emulsion systems with dietary fiber extracted from makgeolli lees. Food Sci. Anim. Res. 2010, 30, 910–917. [Google Scholar] [CrossRef]
- Agar, B.; Gençcelep, H.; Saricaoglu, F.T.; Turhan, S. Effect of sugar beet fiber concentrations on rheological properties of meat emulsions and their correlation with texture profile analysis. Food Bioprod. Process. 2016, 100, 118–131. [Google Scholar] [CrossRef]
- European Topic Centre Circular Economy and Resource Use (ETC/CE). Textiles and the Environment—The Role of Design in Europe’s Circular Economy, Eionet Report No ETC/CE 2022/2A. 2022. Available online: https://www.eionet.europa.eu/etcs/etc-ce/products/etc-ce-products/etc-ce-report-2-2022-textiles-and-the-environment-the-role-of-design-in-europes-circular-economy) (accessed on 14 May 2023).
- Narasimhan, S.; Srikanth, B.S.; Poltronieri, P. Plants by-products and fibers’ industrial exploitation. In Biotransformation of Agricultural Waste and By-Products: The Food, Feed, Fibre, Fuel (4F) Economy; Poltronieri, P., D’Urso, O.F., Eds.; Elsevier: Amsterdam, Netherlands, 2016; pp. 49–67. [Google Scholar]
- Izwan, S.M.; Sapuan, S.M.; Zuhri, M.Y.M.; Mohamed, A.R.; Ilyas, R.A. A comprehensive review of natural fiber reinforced polymer bio- composites and their applications. In Design for Sustainability: Green Materials and Processes; Sapuan, S.M., Mansor, M.R., Eds.; Elsevier: Amsterdam, Netherlands, 2021; pp. 287–305. [Google Scholar]
- Reddy, N.; Yang, Y. Structure and properties of high quality natural cellulose fibers from cornstalks. Polymer 2005, 46, 5494–5500. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Natural cellulose fibers from soybean straw. Bioresour. Technol. 2009, 100, 3593–3598. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Stergiou, M.; Panagiotatos, G.; Kiskira, K.; Priniotakis, G. Regenerated Cellulosic Fibers from Agricultural Waste. AIP Conf. Proc. 2022, 2430, 080006. [Google Scholar]
- Lapsa, V.; Betkers, T.; Shulga, G. Composite materials from pulp and papermaking wastes. In Cellulosic Pulps, Fibres and Materials; Kennedy, J.F., Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Limited: Sawston, UK, 2000; pp. 297–303. [Google Scholar]
- Seçkin, M.; Üçgül, I. Investigation of lignin as a textile fiber. Celal Bayar Univ. J. Sci 2020, 16, 183–190. [Google Scholar]
- Campos, N.F.; Guedes, G.A.J.C.; Oliveira, L.P.S.; Gama, B.M.V.; Sales, D.C.S.; Rodríguez-Díaz, J.M.; Barbosa, C.M.B.M.; Duarte, M.M.M.B. Competitive adsorption between Cu2+ and Ni2+ on corn cob activated carbon and the difference of thermal effects on mono and bicomponent systems. J. Environ. Chem. Eng. 2020, 8, 104232. [Google Scholar] [CrossRef]
- Laudes Foundation. Spinning Future Threads-the Potential of Agricultural Residues as Textile Fibre Feedstock. 2021. Available online: https://www.laudesfoundation.org/learning/research/2021-07-01-spinning-future-threads (accessed on 12 April 2023).
- Ditzel, F.I.; Prestes, E.; Carvalho, B.M.; Demiate, I.M.; Pinheiro, L.A. Nanocrystalline cellulose extracted from pine wood and corncob. Carbohydr. Polym. 2017, 157, 1577–1585. [Google Scholar] [CrossRef]
- Longaresi, R.H.; de Menezes, A.J.; Pereira-da-Silva, M.A.; Baron, D.; Mathias, S.L. The maize stem as a potential source of cel- lulose nanocrystal: Cellulose characterization from its phenological growth stage dependence. Ind. Crops Prod. 2019, 133, 232–240. [Google Scholar] [CrossRef]
- Araújo, D.; Castro, M.C.R.; Figueiredo, A.; Vilarinho, M.; Machado, A. Green synthesis of cellulose acetate from corncob: Physicochemical properties and assessment of environmental impacts. J. Clean Prod. 2020, 260, 120865. [Google Scholar] [CrossRef]
- Bilal, M.; Wang, Z.; Cui, J.; Ferreira, L.F.R.; Bharagava, R.N.; Iqbal, H.M.N. Environmental impact of lignocellulosic wastes and their effective exploitation as smart carriers—A drive towards greener and eco-friendlier biocatalytic systems. Sci. Total Environ. 2020, 722, 137903. [Google Scholar] [CrossRef] [PubMed]
- Coiado, R.D.S.; Lazo, G.D.; Oliveira, R.R.; Rodrigues, R.C.L.B.; Moura, E.A.B. Polymer blend based on recycled polyethylene and ethylene vinyl acetate copolymers reinforced with natural fibers from agricultural wastes. In Minerals, Metals and Materials Series: Vol. Part F7; Springer: Cham, Switzerland, 2017; pp. 689–697. [Google Scholar]
- Orlandelli, R.C.; Santos, M.S.; Polonio, J.C.; de Azevedo, J.L.; Pamphile, J.A. Use of agro-industrial wastes as substrates for α-amylase production by endophytic fungi isolated from Piper hispidum Sw.|Uso de resíduos agroindustriais para a produção de α-amilase por fungos endofíticos isolados de Piper hispidum Sw. Acta Sci. Technol. 2017, 39, 255–261. [Google Scholar] [CrossRef]
- Gautério, G.V.; da Silva, L.G.G.; Hübner, T.; da Rosa Ribeiro, T.; Kalil, S.J. Maximization of xylanase production by Aureobasid- ium pullulans using a by-product of rice grain milling as xylan source. Biocatal. Agric. Biotechnol. 2020, 23, 101511. [Google Scholar] [CrossRef]
- Hafemann, E.; Battisti, R.; Bresolin, D.; Marangoni, C.; Machado, R.A.F. Enhancing chlorine-free purification routes of rice husk biomass waste to obtain cellulose nanocrystals. Waste Biomass Valoriz. 2020, 11, 6595–6611. [Google Scholar] [CrossRef]
- Ribeiro, G.A.C.; Silva, D.S.A.; Dos Santos, C.C.; Vieira, A.P.; Bezerra, C.W.B.; Tanaka, A.A.; Santana, S.A.A. Removal of Remazol brilliant violet textile dye by adsorption using rice hulls. Polimeros 2017, 27, 16–26. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; De Freitas Rosa, M.; Cioffi, M.O.H.; De Carvalho Benini, K.C.C.; Milanese, A.C.; Voorwald, H.J.C.; Mulinari, D.R. Vegetal fibers in polymeric composites: A review. Polimeros 2015, 25, 9–22. [Google Scholar] [CrossRef]
- Camassola, M.; Dillon, A.J.P. Production of cellulases and hemi-cellulases by Penicillium echinulatum grown on pretreated sugar cane bagasse and wheat bran in solid-state fermentation. J. Appl. Microbiol. 2007, 103, 2196–2204. [Google Scholar] [CrossRef]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. A green approach based on reactive extrusion to produce nanofibrillated cellulose from oat hull. Waste Biomass Valoriz. 2021, 12, 1051–1060. [Google Scholar] [CrossRef]
- Miranda, K.W.E.; Mattoso, L.H.C.; Bresolin, J.D.; Hubinger, S.Z.; Medeiros, E.S.; de Oliveira, J.E. Polystyrene bioactive nanofibers using orange oil as an ecofriendly solvent. J. Appl. Polym. Sci. 2019, 136, 47337. [Google Scholar] [CrossRef]
- Mariño, M.A.; Rezende, C.A.; Tasic, L. A multistep mild process for preparation of nanocellulose from orange bagasse. Cellulose 2018, 25, 5739–5750. [Google Scholar] [CrossRef]
- Nascimento, G.E.D.; Duarte, M.M.M.B.; Campos, N.F.; Rocha, O.R.S.D.; Silva, V.L.D. Adsorption of azo dyes using peanut hull and orange peel: A comparative study. Environ. Technol. 2014, 35, 1436–1453. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, J.; Giraldi Fisch, A.; Zimnoch Dos Santos, J.H.; Gutterres, M. Hybrid sol–gel silica adsorbent material based on grape stalk applied to cationic dye removal. Environ. Progress Sustain. Energy 2020, 39, e13398. [Google Scholar] [CrossRef]
- Oliveira, A.P.D.; Módenes, A.N.; Bragião, M.E.; Hinterholz, C.L.; Trigueros, D.E.G.; Bezerra, I.G.D.O. Use of grape pomace as a biosorbent for the removal of the Brown KROM KGT dye. Bioresour. Technol. Rep. 2018, 2, 92–99. [Google Scholar] [CrossRef]
- Okino-Delgado, C.H.; Prado, D.Z.; Fleuri, L.F. Brazilian fruit processing, wastes as a source of lipase and other biotechnological products: A review. Anais Acad. Bras. Cienc. 2018, 90, 2927–2943. [Google Scholar] [CrossRef]
- Wei, W.; Luo, Q.; Liu, Y.; Qu, R.; Sun, D.; Gao, F.; Li, B.; Wu, M. Feasibility of preparing nanofiber reinforcer of gelatin hydrogel from waste peach branches. Biomass Conv. Bioref. 2023, 13, 5831–5841. [Google Scholar] [CrossRef]
- Silveira, M.B.; Pavan, F.A.; Gelos, N.F.; Lima, E.C.; Dias, S.L.P. Punica granatum shell preparation, characterization, and use for crystal violet removal from aqueous solution. Clean 2014, 42, 939–946. [Google Scholar]
- Bazzo, A.; Adebayo, M.A.; Dias, S.L.P.; Lima, E.C.; Vaghetti, J.C.P.; de Oliveira, E.R.; Leite, A.J.B.; Pavan, F.A. Avocado seed powder: Characterization and its application for crystal violet dye removal from aqueous solutions. Desalin. Water Treat. 2016, 57, 15873–15888. [Google Scholar] [CrossRef]
- Bonetto, L.R.; Crespo, J.S.; Guégan, R.; Esteves, V.I.; Giovanela, M. Removal of methylene blue from aqueous solutions using a solid residue of the apple juice industry: Full factorial design, equilibrium, thermodynamics and kinetics aspects. J. Mol. Struct. 2021, 1224, 129296. [Google Scholar] [CrossRef]
- Pereira, C.R.; Resende, J.T.V.; Guerra, E.P.; Lima, V.A.; Martins, M.D.; Knob, A. Enzymatic conversion of sweet potato granular starch into fermentable sugars: Feasibility of sweet potato peel as alternative substrate for α-amylase production. Biocatal. Agric. Biotechnol. 2017, 11, 231–238. [Google Scholar] [CrossRef]
- Pang, X.; Sellaoui, L.; Franco, D.; Dotto, G.L.; Georgin, J.; Bajahzar, A.; Belmabrouk, H.; Ben Lamine, A.; Bonilla-Petriciolet, A.; Li, Z. Adsorption of crystal violet on biomasses from pecan nutshell, para chestnut husk, araucaria bark and palm cactus: Experimental study and theoretical modeling via monolayer and double layer statistical physics models. Chem. Eng. J. 2019, 378, 122101. [Google Scholar] [CrossRef]

| Product | Waste Type | Total Dietary Fiber (TDF) | Insoluble Dietary Fiber (IDF) | Soluble Dietary Fiber (SDF) | Cellulose | Hemicellulose | Lignin | Pectin | References |
|---|---|---|---|---|---|---|---|---|---|
| Cereals | |||||||||
| Wheat | Seeds | 11.6–17.0 | 10.2–14.7 | 1.4–2.3 | - | - | - | - | [48] |
| Wheat | Seeds | 10.2–15.7 | 7.2–11.4 | 1.9–2.9 | - | - | - | - | [104] |
| Wheat | Seeds | 9.2 | - | - | - | - | - | - | [105] |
| Wheat | Bran | 44.46 | 41.59 | 2.87 | - | - | - | - | [106] |
| Wheat | Bran | 44.0 | 41.1 | 2.9 | - | - | - | - | [67] |
| Maize | Seeds | 3.7–8.6 | 3.1–6.1 | 0.5–2.5 | - | - | - | - | [107] |
| Maize | Seeds | 13.1–19.6 | 11.6–16.0 | 1.5–3.6 | - | - | - | - | [48] |
| Maize | Bran | 87.87 | 87.47 | 0.40 | - | - | - | - | [106] |
| Maize | Corncobs | 90.0–93.0 | - | 0.8–2.0 | 35.0–39.0 | 43.0–46.0 | 3.0–6.0 | - | [108] |
| Rice | Seeds | 9.9 | 5.4 | 4.4 | - | - | - | - | [109] |
| Rice | Seeds | 2.7–4.9 | 1.9–4.2 | 0.6–1.1 | - | - | - | - | [55] |
| Fruits | |||||||||
| Apple | Whole | 86.0 | 63.0 | 22.0 | - | - | - | 6.0–8.0 | [110,111] |
| Apple | Pomace | 78.20–89.8 | - | - | 40.0–43.6 | 19.0–24.4 | 15.0–20.4 | 9.0–11.7 | [112] |
| Apple | Pomace | 89.0 | 70.0 | 19.0 | 44.0 | 24.0 | 20.0 | 7.0–23.0 | [110,113] |
| Orange | Pulp | 35.40–36.9 | - | - | 25.32 | 5.35 | 2.2–3.0 | 15.7–16.3 | [112] |
| Peach | Pomace | 31.0–36.0 | - | - | 28.7–30.0 | 18.6–20.0 | 5.35–6.0 | 20.5–23.8 | [112] |
| Peach | Pomace/pit | 54.0 | 36.0 | 19.0 | 31.0 | 22.0 | 27.0 | - | [111,114] |
| Grape | Pomace | 66.50–77.89 | - | - | 6.0–17.75 | 18.0–31.0 | 59.0–64.0 | 0.25–4.0 | [112] |
| Grape | Pomace, stalk | 74.0 | 64.0 | 11.0 | 38.0 | 14.0 | 33.0 | 32.0 | [115] |
| Vegetables | |||||||||
| Tomato | Pomace | 44.90–59.03 | - | - | 19.02 | 12.0 | 36.0 | 7.55 | [112] |
| Tomato | Pomace | 59.0 | - | - | 9.0 | 5.0 | 3.0 | - | [64,83] |
| Tomato | Whole | 49.5 | 40.5 | 8.9 | - | - | - | 6.4–6.9 | [111,116] |
| Onion | Skin/leaves | 68.3 | - | - | 41.0 | 16.0 | 39.0 | - | [92,117] |
| Potato | Pulp | 84.3–91.3 | - | - | 17.0–21.7 | 14.0 | 2.6 | 2.2 | [112] |
| Potato | Pulp/whole | - | - | - | 4.0 | 14.0 | 0.4 | 10.0–12.0 | [111] |
| Potato | Peel | 73.0 | 20.0–53.0 | 10.0–20.0 | [118,119] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plakantonaki, S.; Roussis, I.; Bilalis, D.; Priniotakis, G. Dietary Fiber from Plant-Based Food Wastes: A Comprehensive Approach to Cereal, Fruit, and Vegetable Waste Valorization. Processes 2023, 11, 1580. https://doi.org/10.3390/pr11051580
Plakantonaki S, Roussis I, Bilalis D, Priniotakis G. Dietary Fiber from Plant-Based Food Wastes: A Comprehensive Approach to Cereal, Fruit, and Vegetable Waste Valorization. Processes. 2023; 11(5):1580. https://doi.org/10.3390/pr11051580
Chicago/Turabian StylePlakantonaki, Sofia, Ioannis Roussis, Dimitrios Bilalis, and Georgios Priniotakis. 2023. "Dietary Fiber from Plant-Based Food Wastes: A Comprehensive Approach to Cereal, Fruit, and Vegetable Waste Valorization" Processes 11, no. 5: 1580. https://doi.org/10.3390/pr11051580









