The Use of Response Surface Methodology to Optimize Assisted Extraction of Bioactive Compounds from Cucurbita maxima Fruit By-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Sampling
2.3. Extraction Protocols
2.3.1. Conventional High-Energy Ultrasonic-Assisted Extraction
2.3.2. Low-Energy Extraction Modeling and Optimization Using RSM
2.3.3. Screening Test of Factors and Level Range for Phenolic and Antiradical Compound Extraction
2.3.4. Experimental Design
2.4. Determination of Total Phenolic Compound Content
2.5. Phenolic Compounds by HPLC
2.6. DPPH Radical Scavenging Activity Assay
2.7. Antimicrobial Activity
2.8. Statistical Analyses
3. Results and Discussion
3.1. Modeling and Numerical Optimization of the Extraction Process
3.1.1. Response Surface Analysis of DPPH Radical Scavenging Activity
3.1.2. Response Surface Analysis of Total Phenolic Compound Content
3.1.3. Optimization of Extraction Parameters
3.2. RSM Extraction and Comparison to Conventional High-Energy Extraction
3.3. Phenolic Compounds’ Identification
3.4. Antioxidant and Phenolic Composition Statistical Interactions
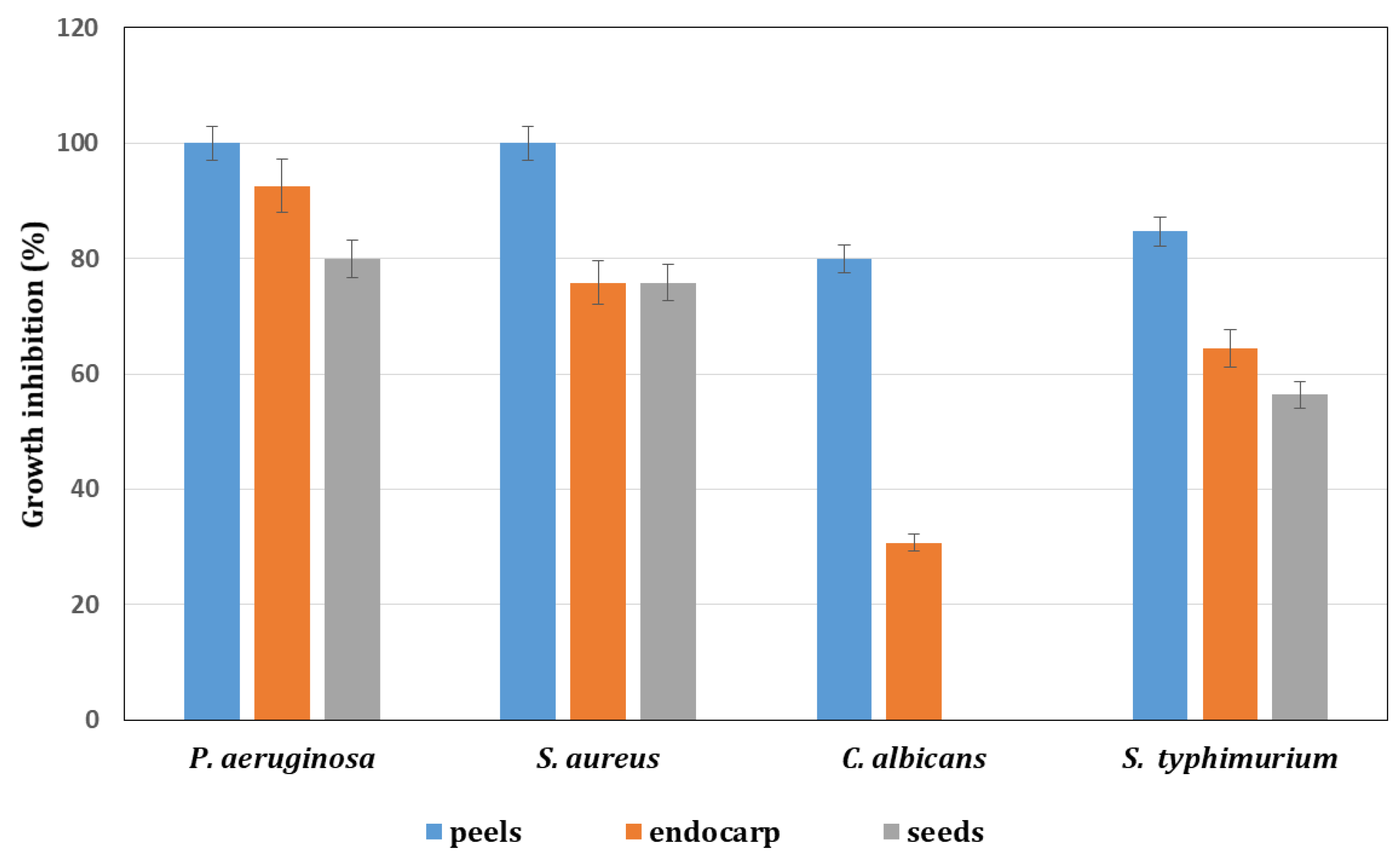
3.5. Antimicrobial and Antifungal Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bisht, B.; Gururani, P.; Aman, J.; Vlaskin, M.S.; Kurbatova, A.I.; Adarchenko, I.A.; Joshi, S.; Kumar, S.; Kumar, V. A review on holistic approaches for fruits and vegetables biowastes valorization. Mater. Today Proc. 2022, 73, 54–63. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Marin, J.; Rivas, J.; Sanhueza, L.; Soto, Y.; Vera, N.; Puente-Díaz, L. Pumpkin seeds (Cucurbita maxima). A review of functional attributes and by-products. Rev. Chil. Nutr. 2019, 46, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Kaźmińska, K.; Sobieszek, K.; Targońska-Karasek, M.; Korzeniewska, A.; Niemirowicz-Szczytt, K.; Bartoszewski, G. Genetic diversity assessment of a winter squash and pumpkin (Cucurbita maxima Duchesne) germplasm collection based on genomic Cucurbita-conserved SSR markers. Sci. Hortic. 2017, 219, 37–44. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Mita, G. Supercritical carbon dioxide extraction of carotenoids from pumpkin (Cucurbita spp.): A review. Int. J. Mol. Sci. 2014, 15, 6725–6740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzortzakis, N.; Chrysargyris, A.; Petropoulos, S.A. Phytochemicals content and health effects of cultivated and underutilized species of the cucurbitaceae Family. In Phytochemical in Vegetables: A Valuable Source of Bioactive Compounds; Petropoulos, S.A., Ferreira, I.C.F.R., Barros, L., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2018; pp. 99–165. ISBN 9781681087399. [Google Scholar]
- Leichtweis, M.G.; Molina, A.K.; Pires, T.C.S.; Dias, M.I.; Calhelha, R.; Bachari, K.; Ziani, B.E.C.; Oliveira, M.B.P.P.; Pereira, C.; Barros, L. Biological activity of pumpkin byproducts: Antimicrobial and antioxidant properties. Molecules 2022, 27, 8366. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Calhelha, R.C.; Rouphael, Y.; Petrović, J.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. Antimicrobial properties, cytotoxic effects, and fatty acids composition of vegetable oils from purslane, linseed, luffa, and pumpkin seeds. Appl. Sci. 2021, 11, 5738. [Google Scholar] [CrossRef]
- Chouaibi, M.; Daoued, K.B.; Riguane, K.; Rouissi, T.; Ferrari, G. Production of bioethanol from pumpkin peel wastes: Comparison between response surface methodology (RSM) and artificial neural networks (ANN). Ind. Crops Prod. 2020, 155, 112822. [Google Scholar] [CrossRef]
- de Escalada Pla, M.; Rojas, A.M.; Gerschenson, L.N. Effect of butternut (Cucurbita moschata Duchesne ex Poiret) fibres on bread making, quality and staling. Food Bioprocess Technol. 2013, 6, 828–838. [Google Scholar] [CrossRef]
- Genevois, C.; Flores, S.; de Escalada Pla, M. Byproduct from pumpkin (Cucurbita moschata Duchesne ex poiret) as a substrate and vegetable matrix to contain Lactobacillus casei. J. Funct. Foods 2016, 23, 210–219. [Google Scholar] [CrossRef]
- Pająk, P.; Przetaczek-Rożnowska, I.; Juszczak, L. Development and physicochemical, thermal and mechanical properties of edible films based on pumpkin, lentil and quinoa starches. Int. J. Biol. Macromol. 2019, 138, 441–449. [Google Scholar] [CrossRef]
- Stevenson, D.G.; Eller, F.J.; Wang, L.; Jane, J.L.; Wang, T.; Inglett, G.E. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J. Agric. Food Chem. 2007, 55, 4005–4013. [Google Scholar] [CrossRef] [Green Version]
- Achu, M.B.; Fokou, E.; Tchiégang, C.; Fotso, M.; Tchouanguep, F.M. Nutritive value of some Cucurbitaceae oilseeds from different regions in Cameroon. Afr. J. Biotechnol. 2005, 4, 1329–1334. [Google Scholar]
- Díaz Obregón, D.; Lloja Lozano, L.; Carbajal Zúñiga, V. Preclinical studies of Cucurbita maxima (pumpkin seeds) a traditional intestinal antiparasitic in rural urban areas. Rev. Gastroenterol. Peru 2004, 24, 323–327. [Google Scholar]
- Daiuto, E.R.; Vieites, R.L.; Pigoli, D.R.; de Carvalho, L.D. Alterações nutricionais em casca e polpa de abóbora decorrentes de diferentes métodos de cozimento. Rev. Iberoam. Tecnol. Postcosecha 2012, 13, 196–203. [Google Scholar]
- Kim, M.Y.; Kim, E.J.; Kim, Y.N.; Choi, C.; Lee, B.H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012, 6, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Kulczyński, B.; Gramza-Michałowska, A.; Królczyk, J.B. Optimization of extraction conditions for the antioxidant potential of different pumpkin varieties (Cucurbita maxima). Sustainability 2020, 12, 1305. [Google Scholar] [CrossRef] [Green Version]
- Staichok, C.A.B.; Mendonça, K.B.R.; dos Santos, A.P.G.; Garcia, L.G.C.; Damiani, C. Pumpkin Peel Flour (Cucurbita máxima L.)-Characterization and Technological Applicability. J. Food Nutr. Res. 2016, 4, 327–333. [Google Scholar]
- Bochnak, J.; Świeca, M. Potentially bioaccessible phenolics, antioxidant capacities and the colour of carrot, pumpkin and apple powders—Effect of drying temperature and sample structure. Int. J. Food Sci. Technol. 2020, 55, 136–145. [Google Scholar] [CrossRef]
- Asif, M.; Naqvi, S.A.R.; Sherazi, T.A.; Ahmad, M.; Zahoor, A.F.; Shahzad, S.A.; Hussain, Z.; Mahmood, H.; Mahmood, N. Antioxidant, antibacterial and antiproliferative activities of pumpkin (cucurbit) peel and puree extracts—An in vitro study. Pak. J. Pharm. Sci. 2017, 30, 1327–1334. [Google Scholar]
- Que, F.; Mao, L.; Fang, X.; Wu, T. Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int. J. Food Sci. Technol. 2008, 43, 1195–1201. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Antioxidants in hypertension and cardiovascular disease. Mol. Interv. 2010, 10, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Sendi, N.; Mkadmini-Hammi, K.; Mansour, R.B.; Selmi, S.; Trabelsi, N.; Isoda, H.; Ksouri, R.; Megdiche-Ksouri, W. Simultaneous optimization of ultrasound-assisted extraction of flavonoid compounds and antiradical activity from Artemisia herba-Alba using response surface methodology. Prep. Biochem. Biotechnol. 2020, 50, 943–953. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Bessa, L.C.B.A.; Ferreira, M.C.; Rodrigues, C.E.C.; Batista, E.A.C.; Meirelles, A.J.A. Simulation and process design of continuous countercurrent ethanolic extraction of rice bran oil. J. Food Eng. 2017, 202, 99–113. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Mass transfer process during extraction of phenolic compounds from milled berries. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Hammi, K.M.; Jdey, A.; Abdelly, C.; Majdoub, H.; Ksouri, R. Optimization of ultrasound-assisted extraction of antioxidant compounds from Tunisian Zizyphus lotus fruits using response surface methodology. Food Chem. 2015, 184, 80–89. [Google Scholar] [CrossRef]
- BenJemaa, M.; Rahali, F.Z.; Falleh, H.; Beji-Serairi, R.; Megdiche-Ksouri, W.; Hamrouni, I.; Hammami, M.; Nehme, R.; Pereira, R.B.; Andrés, S.; et al. Essential oil stabilisation by response surface methodology (RSM): Nanoemulsion formulation, physicochemical, microbiological, and sensory investigations. Molecules 2022, 27, 7330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, H.; Zhang, Z.; Hou, T. Optimization of ultrasound-assisted extraction of polysaccharides from perilla seed meal by response surface methodology: Characterization and in vitro antioxidant activities. J. Food Sci. 2021, 86, 306–318. [Google Scholar] [CrossRef]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef]
- Dahmoune, F.; Remini, H.; Dairi, S.; Aoun, O.; Moussi, K.; Bouaoudia-Madi, N.; Adjeroud, N.; Kadri, N.; Lefsih, K.; Boughani, L.; et al. Ultrasound assisted extraction of phenolic compounds from P. lentiscus L. leaves: Comparative study of artificial neural network (ANN) versus degree of experiment for prediction ability of phenolic compounds recovery. Ind. Crops Prod. 2015, 77, 251–261. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ghitescu, R.E.; Volf, I.; Carausu, C.; Bühlmann, A.M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Yue, P.X. Optimization of Ultrasound-Assisted Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef]
- Mansour, R.B.; Wided, M.K.; Cluzet, S.; Krisa, S.; Richard, T.; Ksouri, R. LC-MS identification and preparative HPLC isolation of Frankenia pulverulenta phenolics with antioxidant and neuroprotective capacities in PC12 cell line. Pharm. Biol. 2017, 55, 880–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulaaba, M.; Medini, F.; Hajlaoui, H.; Mkadmini, K.; Falleh, H.; Ksouri, R.; Isoda, H.; Smaoui, A.; Abdelly, C. Biological activities and phytochemical analysis of phenolic extracts from Salsola kali L. Role of endogenous factors in the selection of the best plant extracts. S. Afr. J. Bot. 2019, 123, 193–199. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef]
- Morelli, L.L.L.; Prado, M.A. Extraction optimization for antioxidant phenolic compounds in red grape jam using ultrasound with a response surface methodology. Ultrason. Sonochem. 2012, 19, 1144–1149. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Xue, J.; Fan, E. Plant phenolics extraction from flos chrysanthemi: Response surface methodology based optimization and the correlation between extracts and free radical scavenging activity. J. Food Sci. 2017, 82, 2726–2733. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.H.; Hsiao, F.W.; Chen, J.H.; Hsieh, C.W.; Liu, Y.C.; Shieh, C.J. Kinetic aspects of ultrasound-accelerated lipase catalyzed acetylation and optimal synthesis of 4′-acetoxyresveratrol. Ultrason. Sonochem. 2013, 20, 546–552. [Google Scholar] [CrossRef]
- Singh, J.; Singh, V.; Shukla, S.; Rai, A.K. Phenolic content and antioxidant capacity of selected cucurbit fruits extracted with different solvents. J. Nutr. Food Sci. 2016, 6, 6. [Google Scholar] [CrossRef]
- Valenzuela, G.M.; Soro, A.S.; Tauguinas, A.L.; Gruszycki, M.R.; Cravzov, A.L.; Giménez, M.C.; Wirth, A. Evaluation polyphenol content and antioxidant activity in extracts of Cucurbita spp. Open Access Libr. J. 2014, 1, 1–6. [Google Scholar] [CrossRef]
- Kiat, V.V.; Siang, W.K.; Madhavan, P.; Chin, J.H.; Ahmad, M.; Akowuah, G.A. FT-IR profile and antiradical activity of dehulled kernels of apricot, almond and pumpkin. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 112–120. [Google Scholar]
- Martínez, C.; Valenzuela, J.L.; Jamilena, M. Genetic and pre-and postharvest factors influencing the content of antioxidants in cucurbit crops. Antioxidants 2021, 10, 894. [Google Scholar] [CrossRef]
- Busuioc, A.C.; Botezatu, A.V.D.; Furdui, B.; Vinatoru, C.; Maggi, F.; Caprioli, G.; Dinica, R.M. Comparative study of the chemical compositions and antioxidant activities of fresh juices from romanian Cucurbitaceae varieties. Molecules 2020, 25, 5468. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A. Extraction kinetic modelling of total polyphenols and total anthocyanins from saffron floral bio-residues: Comparison of extraction methods. Food Chem. 2018, 258, 137–143. [Google Scholar] [CrossRef]
- Dahmoune, F.; Spigno, G.; Moussi, K.; Remini, H.; Cherbal, A.; Madani, K. Pistacia lentiscus leaves as a source of phenolic compounds: Microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crops Prod. 2014, 61, 31–40. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Saeed, R.; Ahmed, D.; Mushtaq, M. Ultrasound-aided enzyme-assisted efficient extraction of bioactive compounds from Gymnema sylvestre and optimization as per response surface methodology. Sustain. Chem. Pharm. 2022, 29, 100818. [Google Scholar] [CrossRef]
- Bashi, D.S.; Dowom, S.A.; Bazzaz, B.S.F.; Khanzadeh, F.; Soheili, V.; Mohammadpour, A. Evaluation, prediction and optimization the ultrasoundassisted extraction method using response surface methodology: Antioxidant and biological properties of Stachys parviflora L. Iran. J. Basic Med. Sci. 2016, 19, 829–841. [Google Scholar]
- Oloyede, F.M.; Obisesan, I.O.; Agbaje, G.O.; Obuotor, E.M. Effect of NPK fertilizer on chemical composition of pumpkin (Cucurbita pepo Linn.) seeds. Sci. World J. 2012, 2012, 808196. [Google Scholar] [CrossRef] [Green Version]
- Mokhtar, M.; Bouamar, S.; Di Lorenzo, A.; Temporini, C.; Daglia, M.; Riazi, A. The influence of ripeness on the phenolic content, antioxidant and antimicrobial activities of pumpkins (Cucurbita moschata Duchesne). Molecules 2021, 26, 3623. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, L.; Qi, Y.; Xie, C.; Zhao, W.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Effect of processing on polyphenols in butternut pumpkin (Cucurbita moschata). Food Biosci. 2022, 49, 101925. [Google Scholar] [CrossRef]
- Iswaldi, I.; Gómez-Caravaca, A.M.; Lozano-Sánchez, J.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of phenolic and other polar compounds in zucchini (Cucurbita pepo L.) by reverse-phase high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Res. Int. 2013, 50, 77–84. [Google Scholar] [CrossRef]
- Hussain, A.; Kausar, T.; Sehar, S.; Sarwar, A.; Ashraf, A.H.; Jamil, M.A.; Noreen, S.; Rafique, A.; Iftikhar, K.; Quddoos, M.Y.; et al. A Comprehensive review of functional ingredients, especially bioactive compounds present in pumpkin peel, flesh and seeds, and their health benefits. Food Chem. Adv. 2022, 1, 100067. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Maciel, L.; Nunes, D.S. Chemical perspective and criticism on selected analytical methods used to estimate the total content of phenolic compounds in food matrices. TrAC Trends Anal. Chem. 2016, 80, 266–279. [Google Scholar] [CrossRef]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Biasoto, A.C.; Schwember, A.R.; Granato, D.; Rasera, G.B.; Franchin, M.; Rosalen, P.L.; Alencar, S.M.; Shahidi, F. Should we ban total phenolics and antioxidant screening methods? The link between antioxidant potential and activation of NF-κB using phenolic compounds from grape by-products. Food Chem. 2019, 290, 229–238. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. Antimicrobial activity of anthocyanins and catechins against foodborne pathogens Escherichia coli and Salmonella. Food Control 2019, 106, 106712. [Google Scholar] [CrossRef]
- Caili, F.; Huan, S.; Quanhong, L. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum. Nutr. 2006, 61, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.; Boyer, R.R.; Neilson, A.P.; O’Keefe, S.F.; Chu, H.S.S.; Williams, R.C.; Dorenkott, M.R.; Goodrich, K.M. Evaluation of peanut skin and grape seed extracts to inhibit growth of foodborne pathogens. Food Sci. Nutr. 2017, 5, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.G.; Chen, Y.J.; Tong, J.W.; Huang, J.A.; Li, J.; Gong, Y.S.; Liu, Z.H. Tea polyphenol epigallocatechin gallate inhibits Escherichia coli by increasing endogenous oxidative stress. Food Chem. 2017, 217, 196–204. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D.; Tsai, H.L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Hsu, H.; Sheth, C.C.; Veses, V. Herbal extracts with antifungal activity against Candida albicans: A systematic review. Mini-Rev. Med. Chem. 2021, 21, 90–117. [Google Scholar] [CrossRef] [PubMed]
- Kabbashi, A.; Koko, W.; Mohammed, S.; Musa, N.; Osman, E.; Dahab, M.; Allah, E.; Mohammed, A. In vitro amoebicidal and antioxidant activities of the plants Adansonia digitata and Cucurbit maxima. Adv. Med. Plant Res. 2014, 2, 50–57. [Google Scholar]


| Experiments a | Seeds | Peels | Endocarp | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent Variables | Responses | Independent Variables | Responses | Independent Variables | Responses | ||||||||||
| Time (min) X1 | % Alcohol X2 | Temp (°C) X3 | YIP50 b (%I) | YTPC c (mg GAE/g E) | Time (min) X1 | % Alcohol X2 | Temp (°C) X3 | YIP50 (%I) | YTPC (mg GAE/g E) | Time (min) X1 | % Alcohol X2 | Temp (°C) X3 | YIP50 (%I) | YTPC (mg GAE/g E) | |
| 1 | 22.50 | 15.00 | 55.00 | 49.48 | 10.52 | 5.00 | 50.00 | 10.00 | 65.39 | 14.23 | 10.00 | 25.00 | 30.00 | 54.26 | 11.51 |
| 2 | 22.50 | 25.00 | 55.00 | 44.85 | 12.35 | 15.00 | 50.00 | 10.00 | 66.73 | 15.24 | 10.00 | 35.00 | 30.00 | 45.29 | 11.58 |
| 3 | 22.50 | 15.00 | 65.00 | 31.70 | 11.59 | 5.00 | 60.00 | 10.00 | 65.61 | 16.03 | 10.00 | 25.00 | 40.00 | 60.45 | 14.97 |
| 4 | 22.50 | 25.00 | 65.00 | 45.67 | 12.43 | 15.00 | 60.00 | 10.00 | 64.68 | 15.23 | 10.00 | 35.00 | 40.00 | 43.71 | 11.63 |
| 5 | 15.00 | 15.00 | 60.00 | 41.36 | 11.70 | 5.00 | 55.00 | 5.00 | 65.06 | 14.92 | 5.00 | 25.00 | 35.00 | 49.82 | 11.82 |
| 6 | 15.00 | 25.00 | 60.00 | 38.89 | 12.02 | 15.00 | 55.00 | 5.00 | 66.84 | 16.42 | 5.00 | 35.00 | 35.00 | 45.47 | 11.81 |
| 7 | 30.00 | 15.00 | 60.00 | 27.57 | 10.05 | 5.00 | 55.00 | 15.00 | 66.16 | 16.25 | 15.00 | 25.00 | 35.00 | 55.49 | 14.08 |
| 8 | 30.00 | 25.00 | 60.00 | 39.38 | 13.60 | 15.00 | 55.00 | 15.00 | 68.01 | 15.63 | 15.00 | 35.00 | 35.00 | 38.58 | 9.98 |
| 9 | 15.00 | 20.00 | 55.00 | 54.09 | 13.21 | 10.00 | 50.00 | 5.00 | 66.64 | 16.23 | 5.00 | 30.00 | 30.00 | 52.18 | 13.44 |
| 10 | 15.00 | 20.00 | 65.00 | 32.56 | 11.93 | 10.00 | 60.00 | 5.00 | 68.83 | 15.82 | 5.00 | 30.00 | 40.00 | 43.98 | 12.28 |
| 11 | 30.00 | 20.00 | 55.00 | 37.38 | 12.45 | 10.00 | 50.00 | 15.00 | 68.43 | 15.83 | 15.00 | 30.00 | 30.00 | 43.54 | 12.72 |
| 12 | 30.00 | 20.00 | 65.00 | 42.29 | 12.99 | 10.00 | 60.00 | 15.00 | 69.70 | 16.82 | 15.00 | 30.00 | 40.00 | 51.76 | 11.92 |
| 13 | 22.50 | 20.00 | 60.00 | 53.68 | 14.46 | 10.00 | 55.00 | 10.00 | 70.94 | 17.72 | 10.00 | 30.00 | 35.00 | 66.19 | 15.88 |
| 14 | 22.50 | 20.00 | 60.00 | 53.23 | 14.41 | 10.00 | 55.00 | 10.00 | 72.80 | 17.92 | 10.00 | 30.00 | 35.00 | 67.11 | 15.86 |
| 15 | 22.50 | 20.00 | 60.00 | 55.90 | 14.67 | 10.00 | 55.00 | 10.00 | 72.69 | 17.82 | 10.00 | 30.00 | 35.00 | 66.90 | 15.95 |
| 16 | 22.50 | 20.00 | 60.00 | 52.02 | 14.98 | 10.00 | 55.00 | 10.00 | 73.78 | 18.10 | 10.00 | 30.00 | 35.00 | 66.29 | 15.00 |
| 17 | 22.50 | 20.00 | 60.00 | 54.69 | 14.69 | 10.00 | 55.00 | 10.00 | 73.66 | 17.82 | 10.00 | 30.00 | 35.00 | 67.48 | 15.16 |
| DPPH | TPC | |||
|---|---|---|---|---|
| Coefficient | Significance % | Coefficient | Significance % | |
| Peels | ||||
| b 0 | 75.374 | *** | 17.876 | *** |
| Linear effect | ||||
| b 1 | 0.925 | * | 0.136 | 11.3% |
| b 2 | 0.734 | * | 0.296 | ** |
| b 3 | 0.416 | 18.6% | 0.142 | 10.0% |
| Quadratic effect | ||||
| b 11 | −7.027 | *** | −1.532 | *** |
| b 22 | −4.745 | *** | −1.162 | *** |
| b 33 | −2.230 | *** | −0.539 | ** |
| Interaction effect | ||||
| b 12 | −1.067 | * | −0.452 | ** |
| b 13 | −0.582 | 19.0% | −0.530 | ** |
| b 23 | −0.230 | 59.1% | 0.350 | * |
| Endocarp | ||||
| b 0 | 66.794 | *** | 15.570 | *** |
| Linear effect | ||||
| b 1 | −5.871 | *** | −0.922 | * |
| b 2 | 0.579 | 12.6% | 0.194 | 54.0% |
| b 3 | −0.260 | 46.9% | −0.081 | 78.7% |
| Quadratic effect | ||||
| b 11 | −8.196 | *** | −1.907 | ** |
| b 22 | −7.671 | *** | −1.240 | * |
| b 33 | −11.258 | *** | −1.740 | ** |
| Interaction effect | ||||
| b 12 | −1.943 | ** | −0.853 | 8.0% |
| b 13 | −3.140 | *** | −1.022 | * |
| b 23 | 4.105 | *** | 0.090 | 83% |
| Seeds | ||||
| b 0 | 53.9.03 | *** | 14.64 | *** |
| Linear effect | ||||
| b 1 | 2.333 | ** | 0.817 | *** |
| b 2 | −4.199 | *** | 0.050 | 69.9% |
| b 3 | −2.334 | ** | 0.030 | 81.0% |
| Quadratic effect | ||||
| b 11 | −7.879 | *** | −1.858 | *** |
| b 22 | −3.099 | ** | −1.061 | *** |
| b 33 | −9.223 | *** | −0.936 | ** |
| Interaction effect | ||||
| b 12 | 4.651 | *** | −0.249 | 19.3% |
| b 13 | 3.570 | ** | 0.807 | ** |
| b 23 | 6.611 | *** | 0.454 | * |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Square | FOBS | Significance |
|---|---|---|---|---|---|
| Peels (response YIP) | |||||
| Regression | 372.2282 | 9 | 41.3587 | 63.6624 | *** |
| Residuals | 4.5476 | 7 | 0.6497 | ||
| Validity | 3.6833 | 3 | 1.2278 | 5.6820 | 6.5% |
| Pure error | 0.8643 | 4 | 0.2161 | ||
| Total | 376.7758 | 16 | |||
| FOBS (63.6624) ˃ Ftabulated (3.68) | |||||
| Peels (response YTPC) | |||||
| Regression | 21.8515 | 9 | 2.4279 | 52.7332 | *** |
| Residuals | 0.3223 | 7 | 0.0460 | ||
| Validity | 0.2396 | 3 | 0.0799 | 3.8616 | 11.3% |
| Pure error | 0.0827 | 4 | 0.0207 | ||
| Total | 22.1738 | 16 | |||
| FOBS (52.7332) ˃ Ftabulated (3.68) | |||||
| Endocarp (response YIP) | |||||
| Regression | 1.5850 | 9 | 1.7612 | 194.8989 | *** |
| Residuals | 6.3255 | 7 | 9.0364 | ||
| Validity | 5.1250 | 3 | 1.7083 | 5.6920 | 6.5% |
| Pure error | 1.20052 | 4 | 3.0013 | ||
| Total | 1.59141 | 16 | |||
| FOBS (194.8989) ˃ Ftabulated (3.68) | |||||
| Endocarp (response YTPC) | |||||
| Regression | 52.7183 | 9 | 5.8576 | 8.3155 | ** |
| Residuals | 4.9309 | 7 | 0.7044 | ||
| Validity | 4.1133 | 3 | 1.3711 | 6.7080 | 5.0% |
| Pure error | 0.8176 | 4 | 0.2044 | ||
| Total | 57.6492 | 16 | |||
| FOBS (8.3155) ˃ Ftabulated (3.68) | |||||
| Seeds (response YIP) a | |||||
| Regression | 34.37 | 9 | 1.412 | 72.3968 | *** |
| Residuals | 1.365 | 7 | 1.951 | ||
| Validity | 5.0117 | 3 | 1.676 | 0.7729 | 56.8% |
| Pure error | 8.645 | 4 | 2.161 | ||
| Total | 1.289 | 16 | |||
| FOBS (72.39) ˃ Ftabulated (3.68) | |||||
| Seeds (response YTPC) b | |||||
| Regression | 34.37 | 9 | 3.01 | 31.5245 | *** |
| Residuals | 0.848 | 7 | 0.121 | ||
| Validity | 0.645 | 3 | 0.2152 | 4.2553 | 9.9% |
| Pure error | 0.202 | 4 | 0.0506 | ||
| Total | 35.22 | 16 | |||
| FOBS (31.52) ˃ Ftabulated (3.68) | |||||
| Process Variables | Experimental Value a | Predicted Value | |||||
|---|---|---|---|---|---|---|---|
| Run | Time (min) X1 | % Alcohol X2 | Temp (°C) X3 | DPPH Scavenging | TPC (mg/g) | DPPH Scavenging | TPC (mg/g) |
| Peels | 11.23 | 12.17 | 55.15 | 75 ± 0.31 | 17.5 ± 0.27 | 75.37 | 17.88 |
| Seeds | 22.5 | 20.00 | 60.00 | 53.5 ± 0.22 | 14.5 ± 0.38 | 53.90 | 14.64 |
| Endocarp | 10.54 | 28.45 | 36.88 | 65.9 ± 0.44 | 16 ± 0.32 | 66.79 | 15.57 |
| By-Products | Peel | Seeds | Endocarp | |||
|---|---|---|---|---|---|---|
| MUS | RSM | MUS | RSM | MUS | RSM | |
| TPC | 76 ± 1.5 a | 17.5 ± 0.3 | 121.2 ± 3.5 | 14.5 ± 0.4 | 57.6 ± 0.2 | 16 ± 0.3 |
| DPPH Test | 650 ± 10.7 | 75 ± 2.3 | 340 ± 7.2 | 53.5 ± 0.2 | 730 ± 0.8 | 65.9 ± 0.4 |
| Compounds | RT (min) | Content (mg g−1) | R2 | Standard Curve (Regression Equation) | Recovery (%) | LOD (µg/mL) | LOQ (µg/mL) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Seeds | Peels | Endocarp | ||||||||
| 1 | Vanillic acid | 5.12 ± 0.07 | 0.177 ± 0.02 b | 0.152 ± 0.09 b | 0.801 ± 0.01 a | 1 | y = 22.285x + 1.68 | 98.7 ± 2.13 | 1.23 | 4.33 |
| 2 | Gallic acid | 6.1 ± 0.03 | 0.035 ± 0.01 a | - | - | 1 | y = 22.28x + 1.68 | 100.2 ± 3.13 | 1.50 | 5.1 |
| 3 | Catechin gallate | 7.3 ± 0.10 | 2.18 ± 0.14 a | 1.69 ± 0.03 b | 2.003 ± 0.03 a | 1 | y = 3.63x + 1.8 | 100 ± 3.04 | 0.27 | 0.87 |
| 4 | Hydroxytyrosol | 9.15 ± 0.21 | - | 2.71 ± 0.22 a | 1.7 ± 0.31 b | 0.95 | y = 9.878x − 4.308 | 100 ± 1.84 | 0.97 | 3.88 |
| 5 | Epigallocatechin | 10.6 ± 0.71 | 1.129 ± 0.12 c | 2.92 ± 0.11 b | 22.42 ± 1.12 a | 1 | y = 3.63x + 1.8 | 97.7 ± 1.20 | 0.21 | 1.13 |
| 6 | Resorcinol | 11.5 ± 0.1 | - | 0.198 ± 0.07 a | - | 0.99 | y = 9.878x − 4.308 | 99.6 ± 5.12 | 0.07 | 0.84 |
| 7 | Chlorogenic acid | 11.6 ± 0.09 | 0.657 ± 0.03 a | - | 0.576 ± 0.02 a | 0.99 | y = 9.02x − 1.55 | 97.2 ± 0.83 | 0.15 | 1.09 |
| 8 | Epicatechin | 13.8 ± 0.12 | 2.296 ± 0.15 c | 4.243 ± 0.51 b | 9.78 ± 0.05 a | 1 | y = 3.63x + 1.8 | 97.1 ± 1.51 | 1.08 | 4.67 |
| 9 | Rutin | 16.44 ± 0.5 | 0.480 ± 0.04 a | 0.240 ± 0.03 b | 0.511 ± 0.0 a | 1 | y = 9.58x − 7.41 | 95.9 ± 2.74 | 1.49 | 4.51 |
| 10 | Ferulic acid | 18.87 ± 0.23 | 0.198 ± 0.03 a | - | - | 1 | y = 20.50x − 8.72 | 100.6 ± 4.08 | 0.42 | 1.26 |
| 12 | Myrecitin | 22.50 ± 0.07 | - | 0.050 ± 0.02 a | - | 0.99 | y = 67.91x − 35.35 | 99.8 ± 1.01 | 0.09 | 0.77 |
| 13 | Resveratrol | 24.5 ± 0.47 | 0.538 ± 0.03 b | 0.908 ± 0.05 a | - | 0.99 | y = 9.87x − 4.308 | 99.2 ± 0.99 | 0.4 | 1.30 |
| Total | 7.69 c | 13.10 b | 37.79 a | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, R.B.; Falleh, H.; Hammami, M.; Barros, L.; Petropoulos, S.A.; Tarchoun, N.; Ksouri, R. The Use of Response Surface Methodology to Optimize Assisted Extraction of Bioactive Compounds from Cucurbita maxima Fruit By-Products. Processes 2023, 11, 1726. https://doi.org/10.3390/pr11061726
Mansour RB, Falleh H, Hammami M, Barros L, Petropoulos SA, Tarchoun N, Ksouri R. The Use of Response Surface Methodology to Optimize Assisted Extraction of Bioactive Compounds from Cucurbita maxima Fruit By-Products. Processes. 2023; 11(6):1726. https://doi.org/10.3390/pr11061726
Chicago/Turabian StyleMansour, Rim Ben, Hanen Falleh, Majdi Hammami, Lillian Barros, Spyridon A. Petropoulos, Neji Tarchoun, and Riadh Ksouri. 2023. "The Use of Response Surface Methodology to Optimize Assisted Extraction of Bioactive Compounds from Cucurbita maxima Fruit By-Products" Processes 11, no. 6: 1726. https://doi.org/10.3390/pr11061726





