Experimental Investigation on Pressure-Control Characteristics of Liquid Hydrogen Tank Based on Active and Passive Thermodynamic Venting System Technology
Abstract
1. Introduction
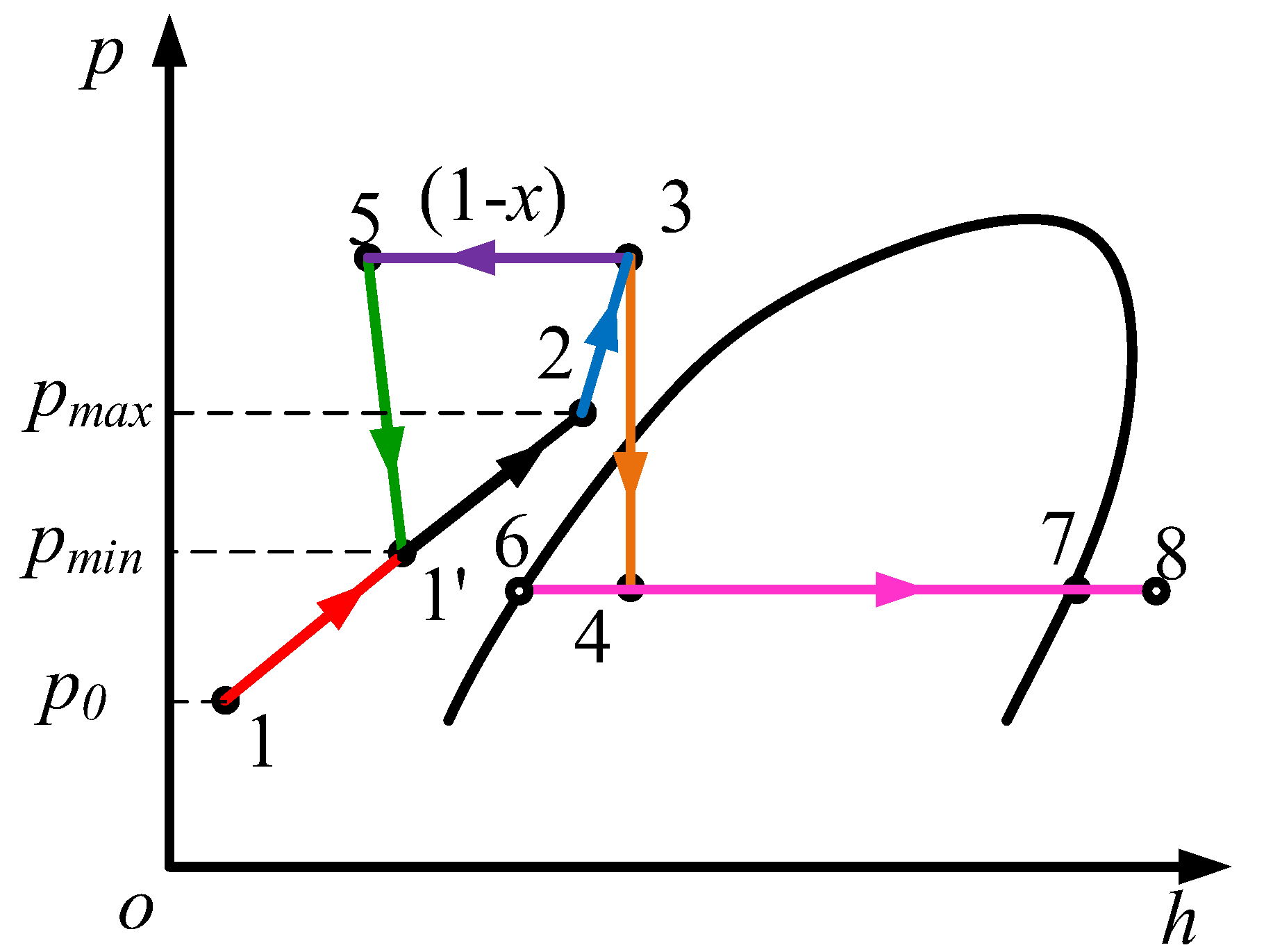
2. Pressure-Control Strategy
3. Mathematical Theoretical Model
3.1. Thermal Model Analysis of Storage Tanks
3.2. Throttling Refrigeration Analysis
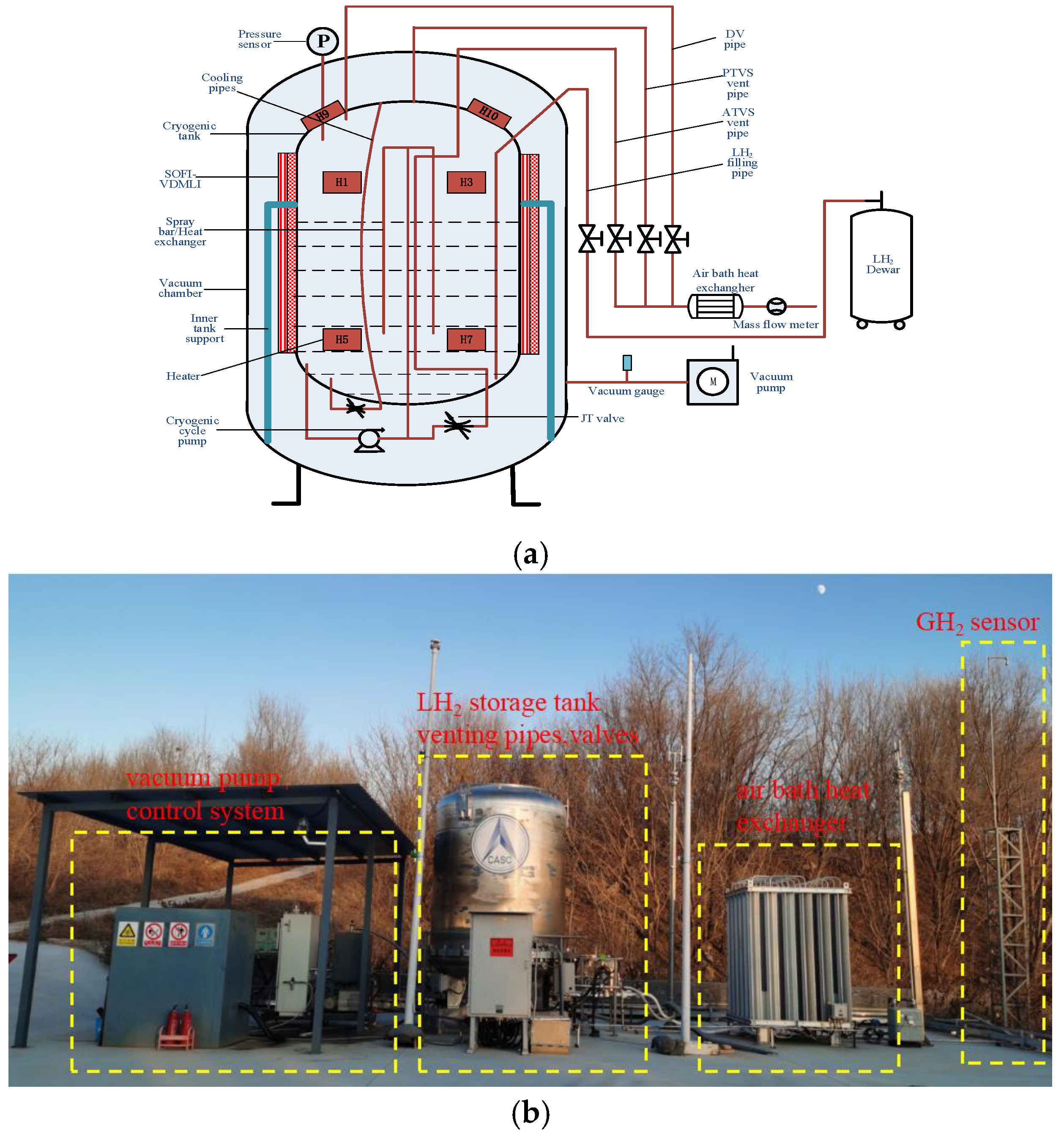
4. Experimental Apparatus and Measurement Uncertainties
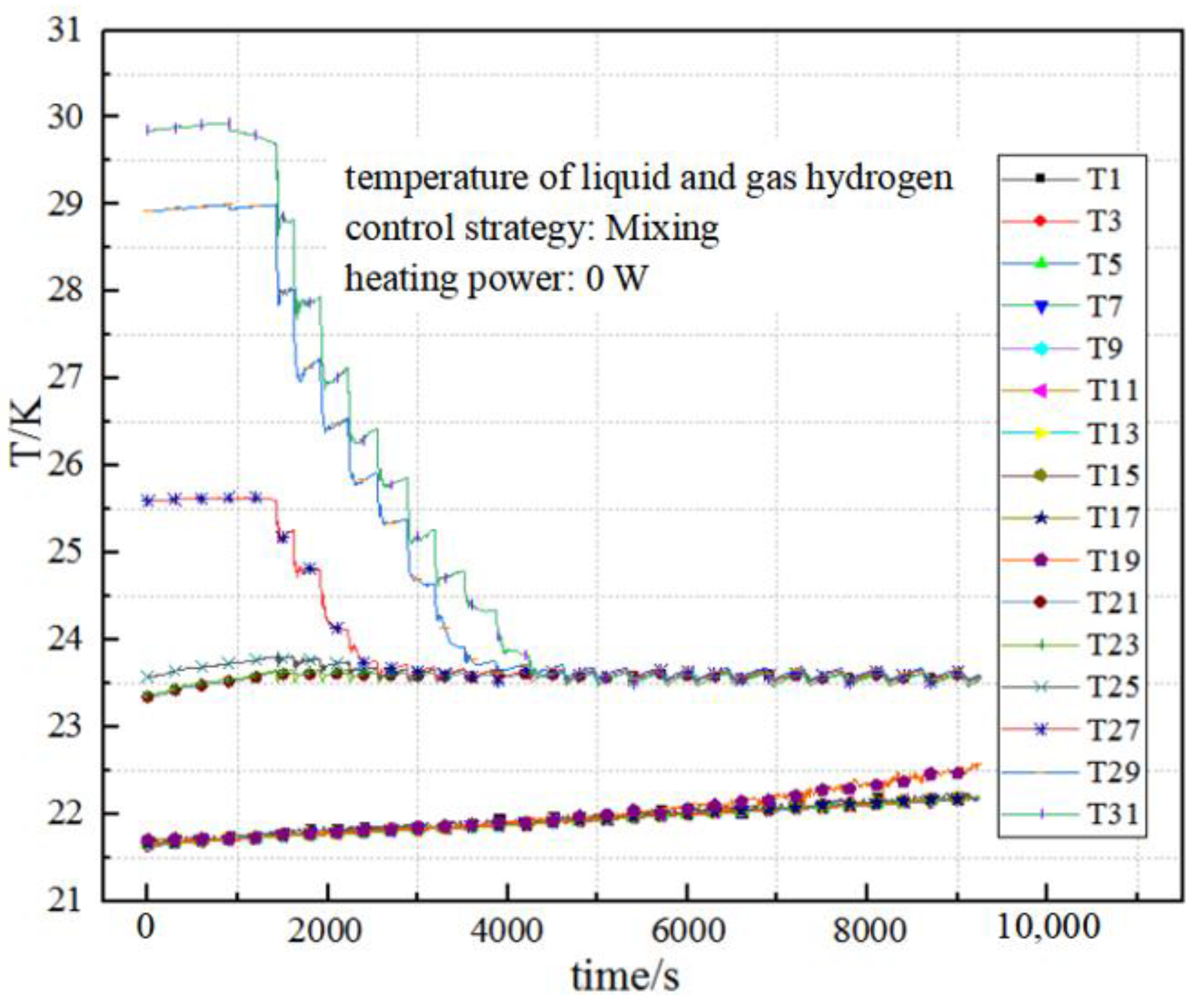
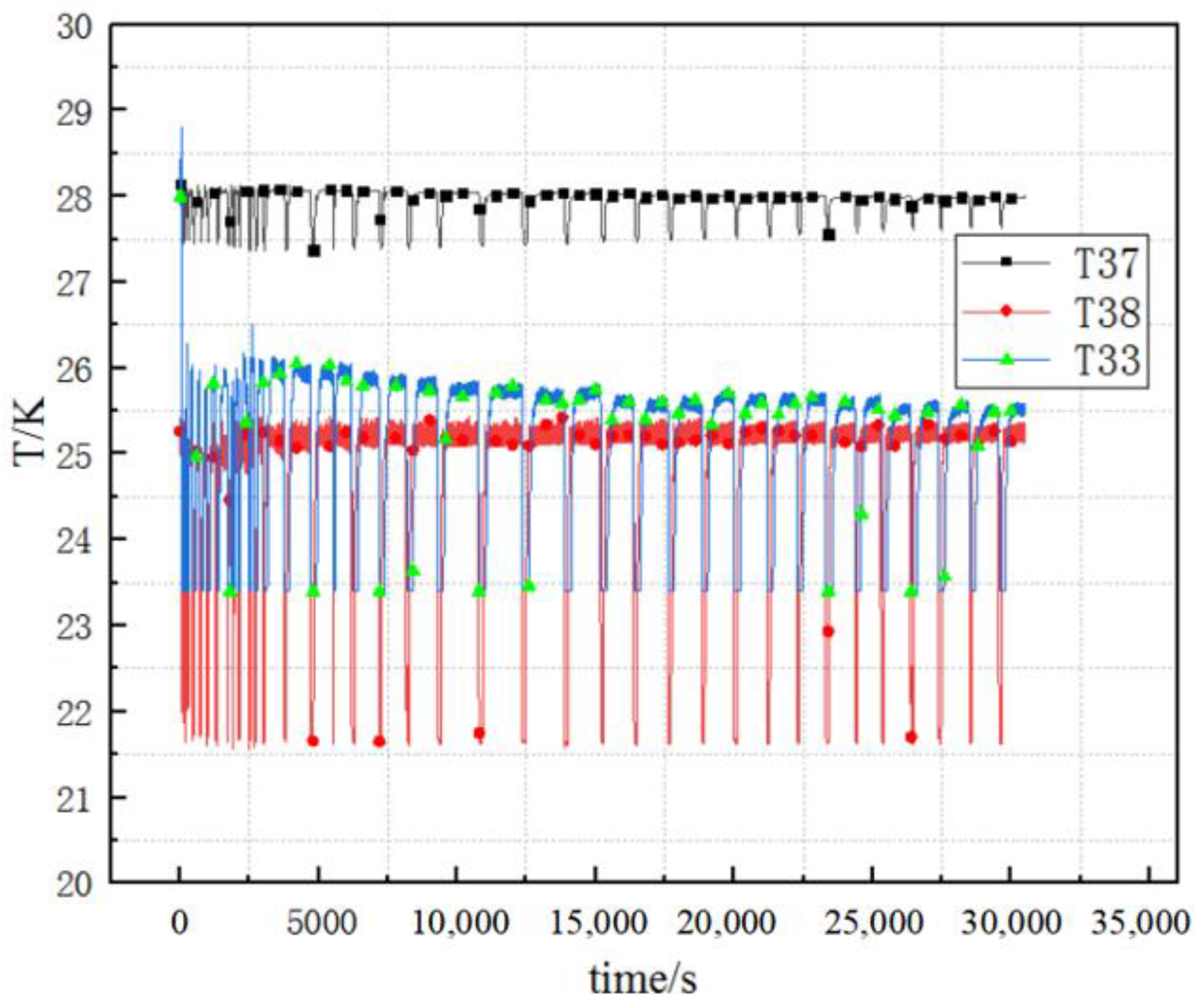
5. Results and Discussion
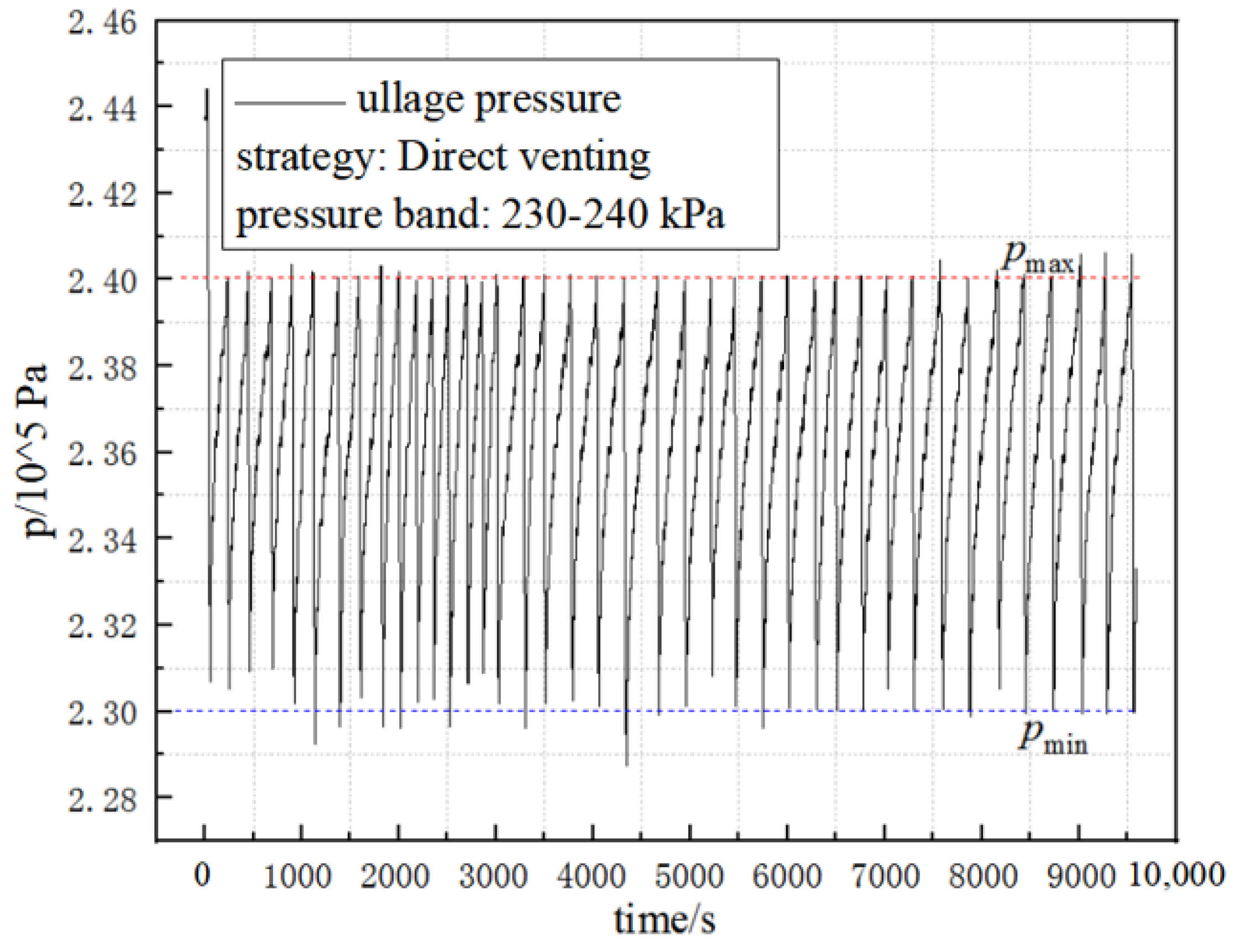
5.1. Effect of Different Pressure-Control Strategies on the Duty Cycle
5.2. Effect of Heating Power on Characteristics of Pressure Control
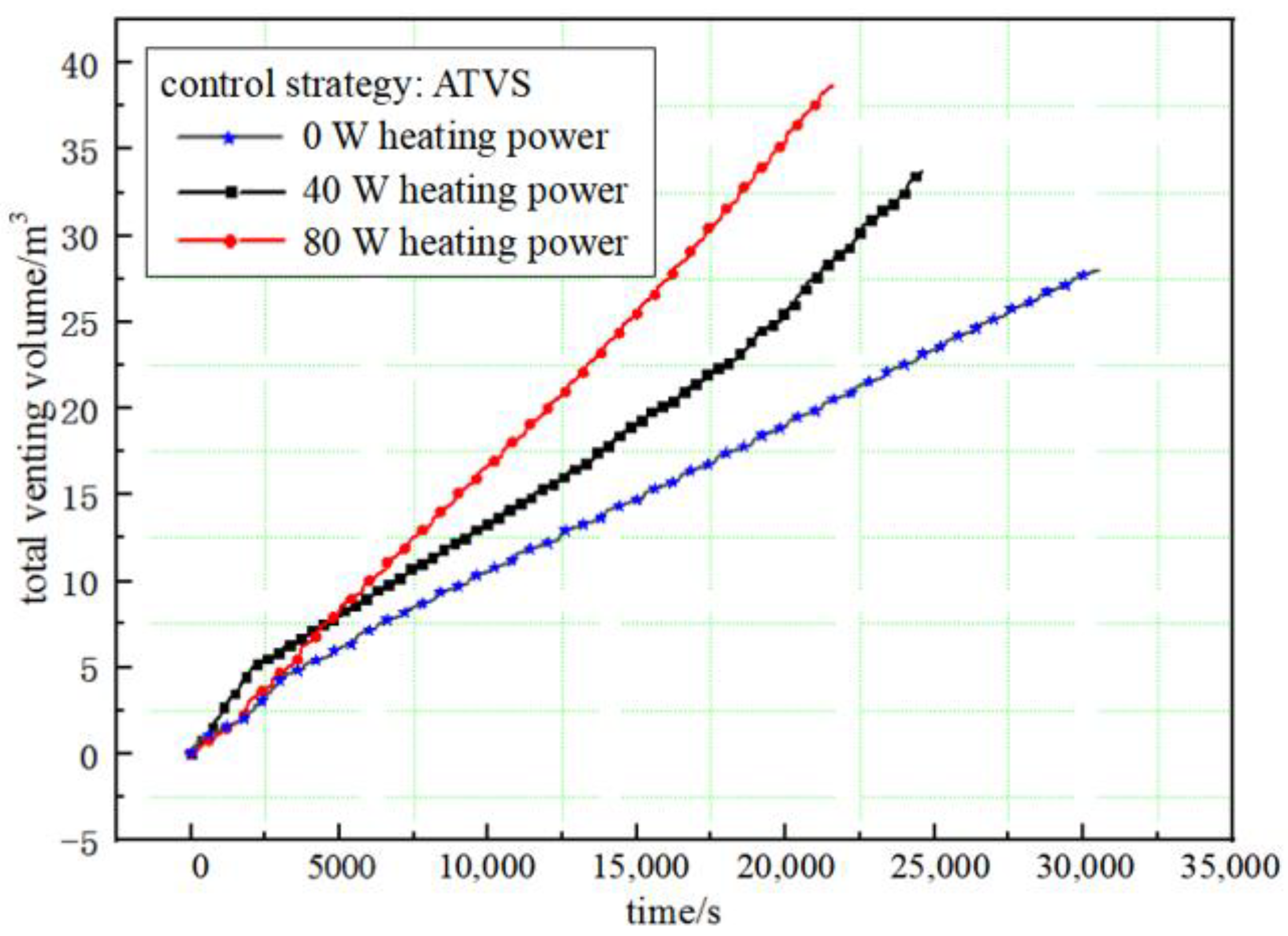
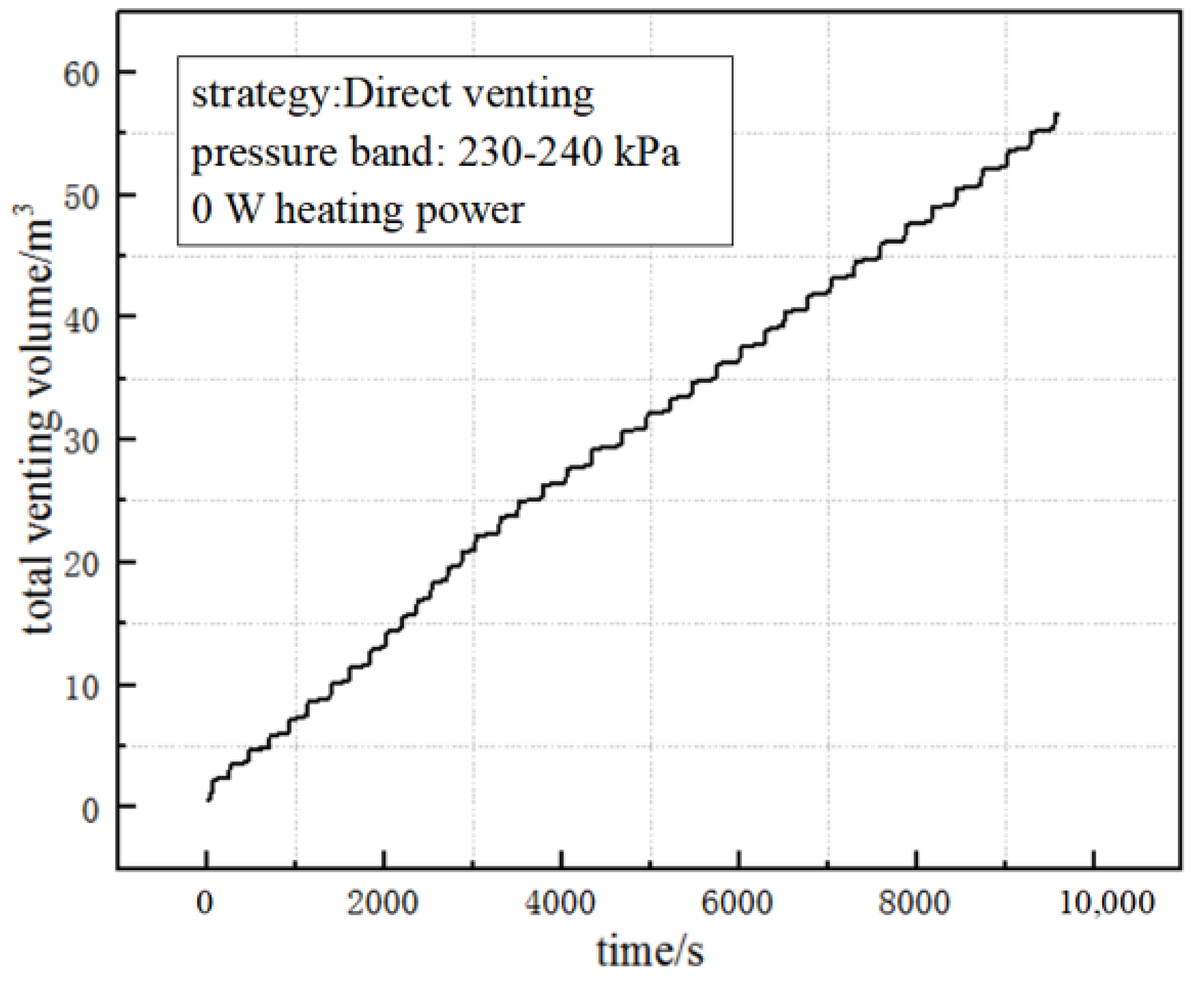
5.3. Effect of Pressure-Control Strategies on Volume of Vent GH2
6. Conclusions
- (1)
- The two-phase hydrogen with a lower temperature generated in the J-T throttle increases the temperature difference in the heat transfer and reduces the time of the heat transfer under the same thermal conditions, leading to the lowest single cycle time of the ATVS strategy in different tests;
- (2)
- There is a gradual increase in the single cycle time when the heating power is constant in the tests using the mixing strategy, while in the ATVS and PTVS tests, the single cycle time increases gradually only when there is no heating power input. The pressurization time becomes shorter when the heating power increases, β reflects the variation degree in the single cycle time, and β increases as the heating power increases;
- (3)
- The volume of the venting gas increases with the increases in the external heating power whether in the PTVS or ATVS tests. The venting volume in the unit time of ATVS is much smaller than that of the PTVS. The venting volume in unit time by direct venting is close to that of the PTVS test with a heating power of 40 W. In addition, the venting volume in the unit time with the ATVS strategy decreased by 87.3% compared to the direct-venting test.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| SOFI | spray-on foam insulation |
| VDMLI | variable density multilayer insulation |
| TVS | thermodynamic venting system |
| PTVS | passive thermodynamic venting system |
| ATVS | active thermodynamic venting system |
| LH2 | liquid hydrogen |
| GH2 | gas hydrogen |
| LO2 | liquid oxygen |
| MHTB | Marshall Space Flight Center |
| J-T | Joule-Thomson |
| SCT | single cycle time |
| DC | duty cycle |
| pmax | pressure upper limit (Pa) |
| pmin | pressure lower limit (Pa) |
| integral throttling coefficient | |
| Qc-JT | unit throttling cooling capacity |
| cp | specific heat |
| β | increase amplitude of the single cycle time |
| χ | view factor |
| σ | Stefan-Boltzman constant |
References
- Kutter, B.; Zegler, F.; Lucas, S.; Hines, L.; Ragab, M.; Spradley, I.; Hopkins, J. Atlas Centaur Extensibility to Long-Duration In-Space Applications; Lockheed Martin Space Systems Company: Denver, CO, USA, 2005. [Google Scholar]
- Motil, S.M. Technology Demonstration Mission (TMD) Cryogenic Propellant Storage & Transfer (CPST). CPST Project Overview and Cryogenic Activities; NASA Glenn Research Center: Cleveland, OH, USA, 2013. [Google Scholar]
- The White House. National Strategy for Critical and Emerging Technology. Available online: https://new.qq.com/rain/a/20220210A041VW00.html (accessed on 8 February 2022).
- Johnson, W.L. Recent ground hold and rapid depressurization testing of multilayer system. In Proceedings of the 50th AIAA/ASME/SAE/ASEE Joint Propulsion Conference, Cleveland, OH, USA, 28–30 July 2014. [Google Scholar]
- Lin, C.S.; Van Dresar, N.T.; Hasan, M.M. Pressure control analysis of cryogenic storage system. J. Propuls. Power 2004, 20, 480–485. [Google Scholar] [CrossRef]
- Nguyen, H. Zero Thermodynamic Venting System (TVS) Performance Prediction Program; National Aeronautics and Space Administration: Washington, DC, USA, 1994; pp. 1–136. [Google Scholar]
- Candy, E.C. Design of thermodynamic vent/screen baffle cryogenic storage system. Eng. Notes 1975, 12, 501–502. [Google Scholar]
- Hastings, L.J.; Flachbart, R.H.; Lak, T.; Nguyen, H.; Bailey, J.W. Spray Bar Zero-Gravity Vent System for On-Orbit Liquid Hydrogen Storage; NASA/TM-2003-212926; NASA Center for AeroSpace Information: Hanover, MD, USA, 2023. [Google Scholar]
- Thibault, J.P.; Corre, C.; Demeure, L.; Mer, S. Thermodynamic control system for cryogenic propellant storage during long missions. In Proceeding of the ASME 2014 4th Joint US-European Fluids Engineering Division Meeting, Chicago, IL, USA, 12 August 2014. [Google Scholar]
- Mer, S.; Fernandez, D.; Thibault, J.-P.; Corre, C. Optimal design of a thermodynamic vent system for cryogenic propellant storage. Cryogenics 2016, 80, 127–137. [Google Scholar] [CrossRef]
- Bae, J.; Yoo, J.; Jin, L.; Jeong, S. Experimental investigation of passive thermodynamic vent system (TVS) with liquid nitrogen. Cryogenics 2018, 89, 147–156. [Google Scholar] [CrossRef]
- Zhou, Z.; Lei, G.; Wang, T.; Xuan, Z. Control method of pressurization and venting flow rate in cryogenic tank. J. Aerosp. Power 2018, 33, 1263–1269. [Google Scholar]
- Zhou, Z.; Liu, X. Analysis of on-orbit pressure control method and effectiveness comparison of cryogenic propellants. Cryogenics 2019, 2, 41–46. [Google Scholar]
- Zhou, Z.; Liu, X.; Zhang, S.; Wang, S. Analysis for the effects of TVS on the pressure control characteristics of the liquid nitrogen storage tank. J. Astronaut. 2020, 41, 599–607. [Google Scholar]
- Liu, Z.; Li, Y.; Xia, S.; Tan, H. Ground experimental investigation of thermodynamic vent system with HCFC123. Int. J. Therm. Sci. 2017, 122, 218–230. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Zhang, S.; Zhang, X.; Liu, X. Experimental study on thermodynamic vent system with different influence factors. Int. J. Energy Res. 2018, 42, 1040–1055. [Google Scholar] [CrossRef]
- Ren, J.; Xie, F.; Wang, L.; Li, Y. Analysis on throttling effect and cooling capacity utilization in thermodynamic vent system. J. Astronaut. 2020, 41, 490–498. [Google Scholar]
- Wang, B.; Huang, Y.; Chen, Z.; Wu, J.; Wang, T.; Lei, G. Performance of thermodynamic vent system for cryogenic propellant storage using different control strategies. Appl. Therm. Eng. 2017, 126, 100–107. [Google Scholar] [CrossRef]
- Delhaye, J.M. Jump conditions and entropy sources in two-phase systems. Local instant formulation. Int. J. Multiph. Flow 1974, 1, 395–409. [Google Scholar] [CrossRef]
- Meserole, J.S.; Jones Brennan, S.M.; Fortini, A. Mixing-Induced Ullage Condensation and Fluid Destratifcation. In Proceedings of the AIAA-87-2018, 23rd Joint Propulsion Conference, San Diego, CA, USA, 29 June–2 July 1987. [Google Scholar] [CrossRef]
- Xin, L.; Xiaoyu, Z. Experimental Pressure Control Investigation of Thermodynamic Vent System. J. Deep. Space Explor. 2018, 5, 292–298. [Google Scholar]








| Measuring Parameter | Units | Description | Sensors | Uncertainty |
|---|---|---|---|---|
| T | K | Temperature | platinum-resistor PT-1000 | ±0.1 K |
| p | Pa | Pressure | Endevco 8530B-500 piezoresistive | 0.2%FS |
| m3/h | Flow rate | WG40 flowmeter | ±1% | |
| L | m | Liquid level | HY1151/3351GP | 0.1%FS |
| Cases | Control Bank (kPa) | Pressure-Control Strategy | Heating Power (W) |
|---|---|---|---|
| C1 | 230–240 | PTVS | 0 |
| C2 | 230–240 | PTVS | 40 |
| C3 | 230–240 | PTVS | 80 |
| C4 | 230–240 | Mixing | 0 |
| C5 | 230–240 | Mixing | 40 |
| C6 | 230–240 | Mixing | 80 |
| C7 | 230–240 | ATVS | 0 |
| C8 | 230–240 | ATVS | 40 |
| C9 | 230–240 | ATVS | 80 |
| C10 | 230–240 | DV | 0 |
| Control Strategy | Heating Power/W | Average Cycle Time in the First 10% Cycles t1/s | Average Cycle Time in the Last 10% Cycles t2/s | β |
|---|---|---|---|---|
| PTVS | 0 | 1316 | 2026 | 54% |
| 40 | 1069 | 945 | −11.6% | |
| 80 | 928 | 442 | −52.4% | |
| Mixing | 0 | 450 | 563 | 25.1% |
| 40 | 362 | 525 | 45.0% | |
| 80 | 153 | 259 | 69.3% | |
| ATVS | 0 | 487 | 956 | 96.3% |
| 40 | 576 | 530 | −8.0% | |
| 80 | 788 | 550 | −30.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Wu, J.; Zhang, S.; Gong, M.; Liu, X. Experimental Investigation on Pressure-Control Characteristics of Liquid Hydrogen Tank Based on Active and Passive Thermodynamic Venting System Technology. Processes 2023, 11, 1831. https://doi.org/10.3390/pr11061831
Zhou Z, Wu J, Zhang S, Gong M, Liu X. Experimental Investigation on Pressure-Control Characteristics of Liquid Hydrogen Tank Based on Active and Passive Thermodynamic Venting System Technology. Processes. 2023; 11(6):1831. https://doi.org/10.3390/pr11061831
Chicago/Turabian StyleZhou, Zhenjun, Jun Wu, Shaohua Zhang, Mengmeng Gong, and Xin Liu. 2023. "Experimental Investigation on Pressure-Control Characteristics of Liquid Hydrogen Tank Based on Active and Passive Thermodynamic Venting System Technology" Processes 11, no. 6: 1831. https://doi.org/10.3390/pr11061831
APA StyleZhou, Z., Wu, J., Zhang, S., Gong, M., & Liu, X. (2023). Experimental Investigation on Pressure-Control Characteristics of Liquid Hydrogen Tank Based on Active and Passive Thermodynamic Venting System Technology. Processes, 11(6), 1831. https://doi.org/10.3390/pr11061831






