Immobilization of Pseudomonas fluorescens Lipase on Hollow Poly(o-phenylenediamine) Microspheres and Its Application in the Preparation of Citronellyl Acetate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Support
2.3. Immobilization of Lipase by Adsorption
2.4. Immobilization of Lipase by Covalent Binding
2.5. Determination of Enzyme Activity
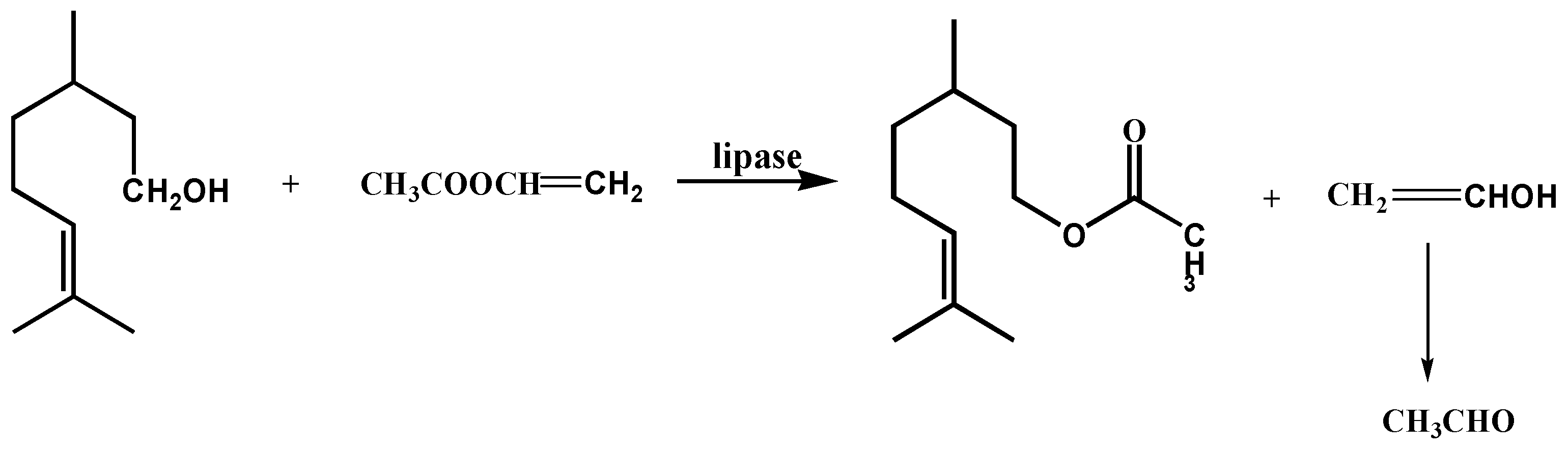
2.6. Synthesis of Citronellyl Acetate in the Reaction of Transesterification Using Immobilized PFL
2.7. Analysis Method of Citronellyl Acetate
2.8. Determination of Enzyme Reaction Stability
2.9. Determination of Enzyme Storage Stability
3. Results and Discussion
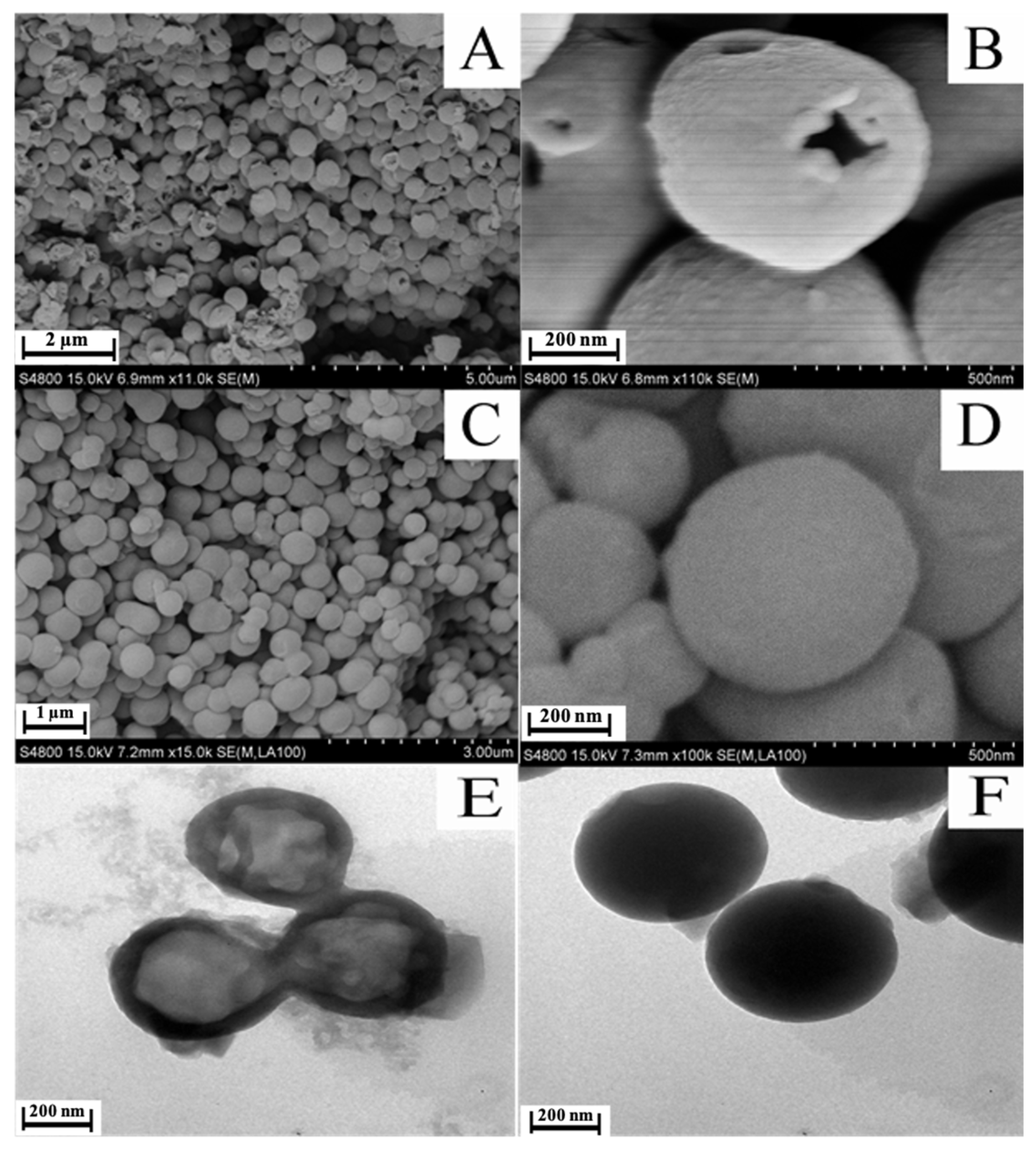
3.1. Characterization of Support Morphology
3.2. Effect of Different Supports on Lipase Immobilization by Adsorption
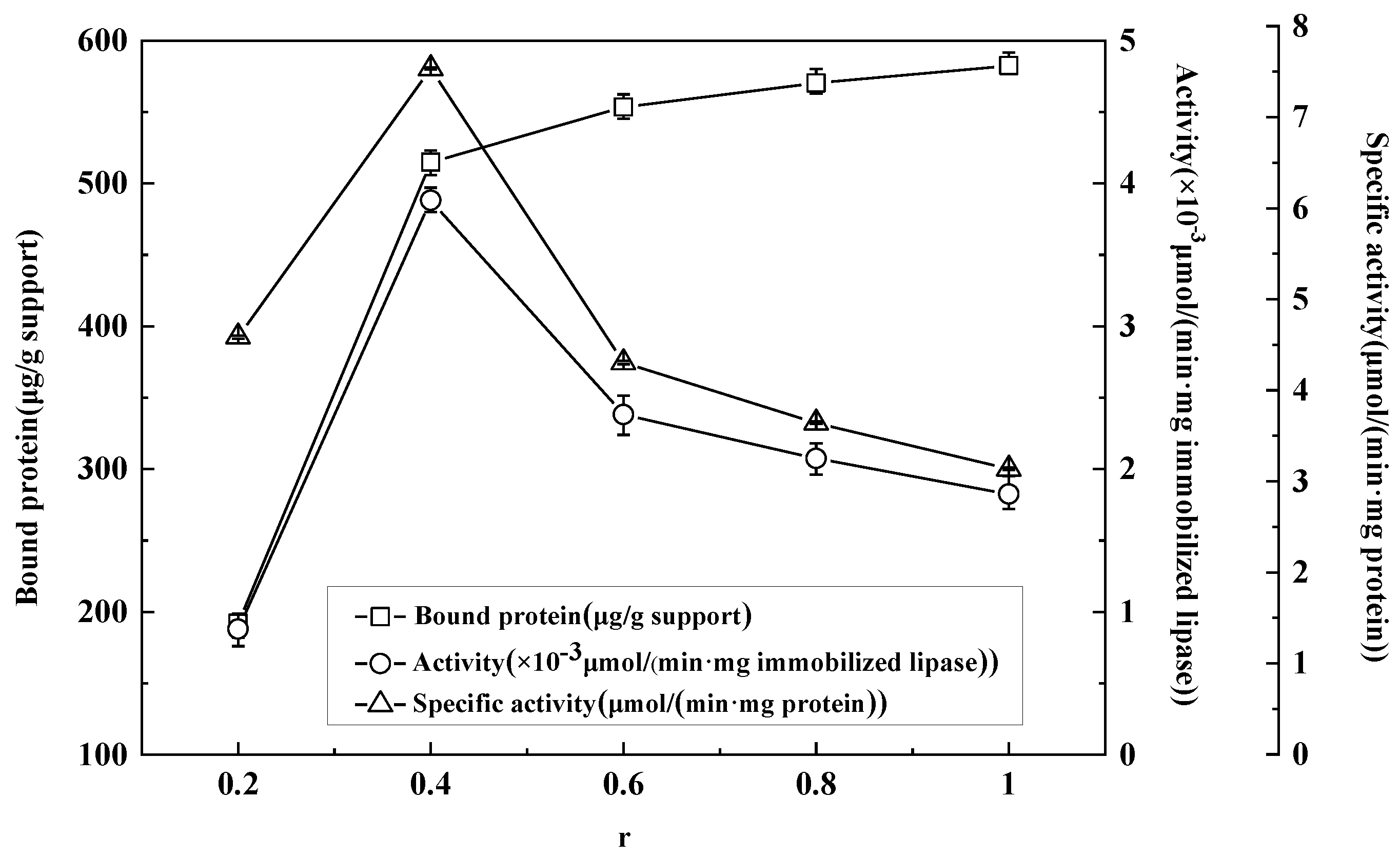
3.3. Effect of Different Enzyme/Support Ratios on Adsorption Immobilization
3.4. Effect of Glutaraldehyde Concentration on Lipase Immobilized by Covalent Binding
3.5. Determination of Covalent Binding Time
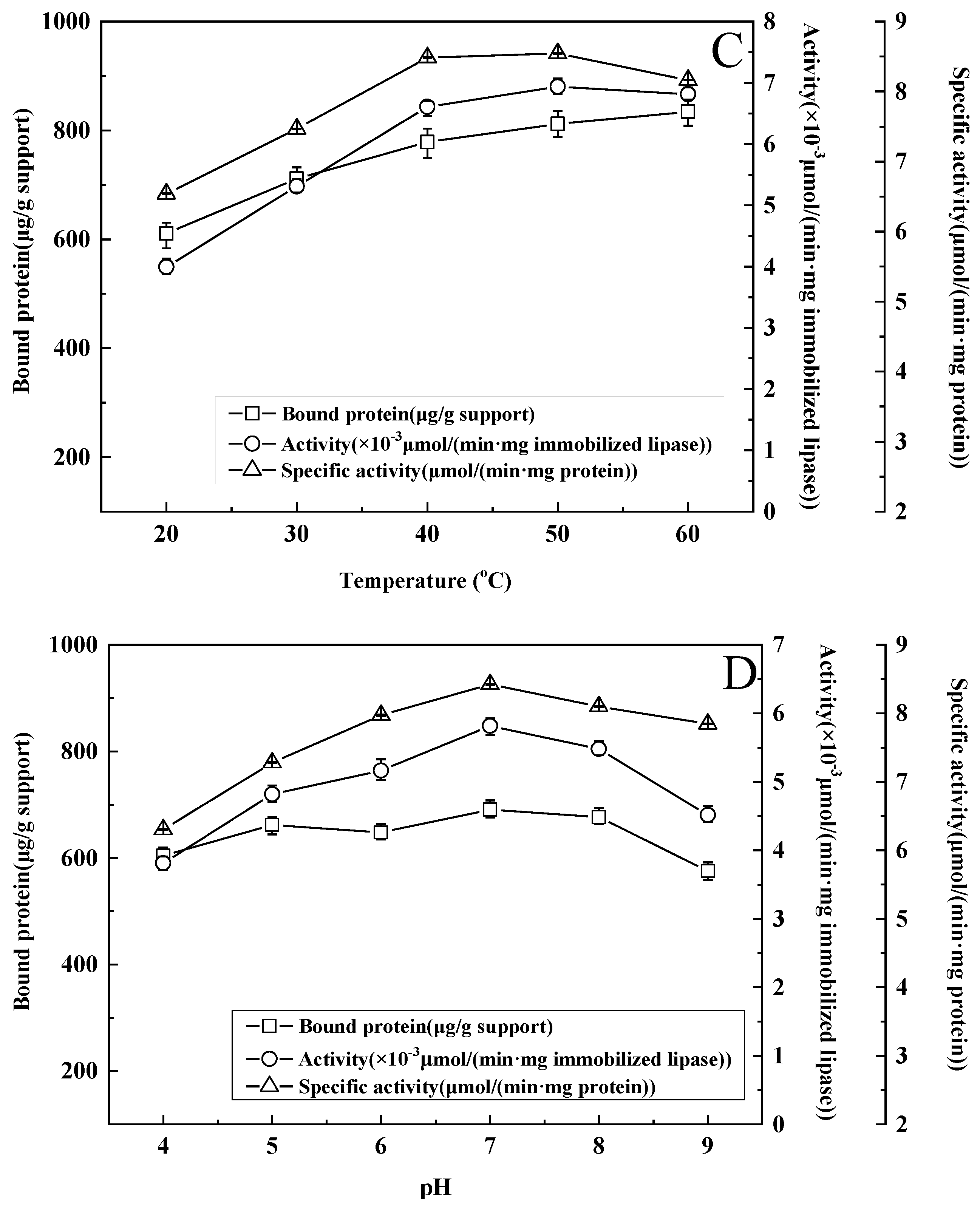
3.6. Effect of Temperature on Immobilization
3.7. Effect of Buffer pH on Immobilization
3.8. Comparison of Some Immobilized Lipases
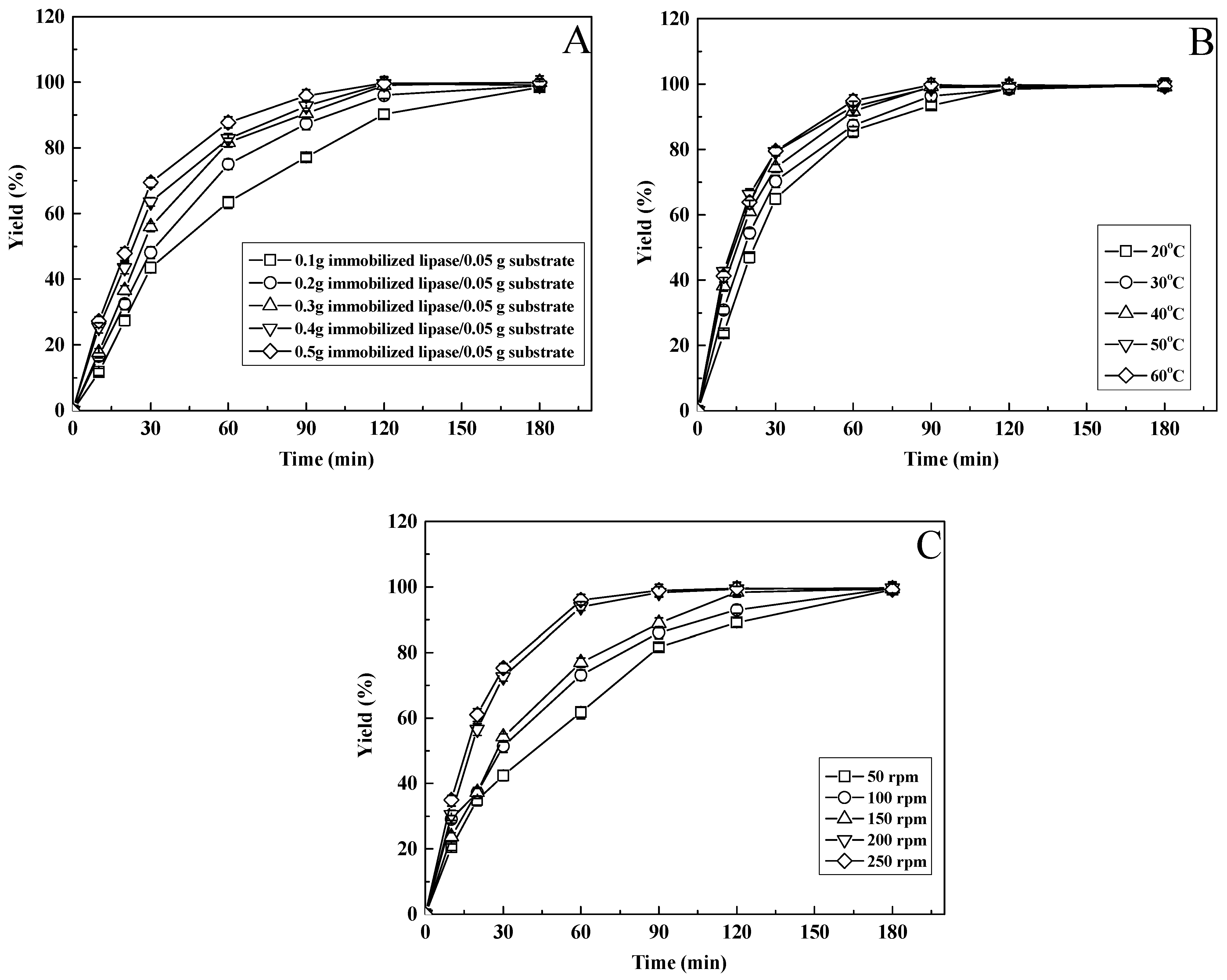
3.9. Effect of the Amount of Immobilized Enzyme on the Preparation of Citronellyl Acetate
3.10. Effect of Reaction Temperature on the Preparation of Citronellyl Acetate
3.11. Effect of Shaking Intensity on the Preparation of Citronellyl Acetate
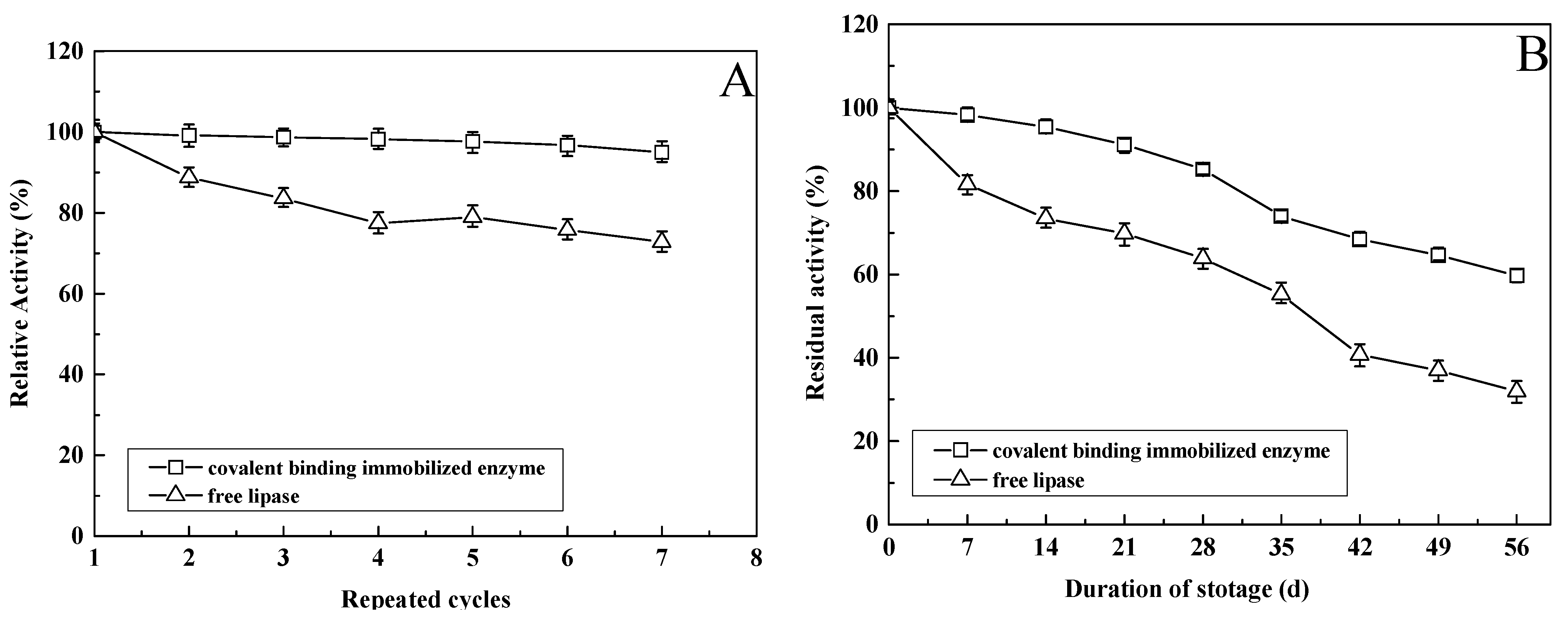
3.12. Operational Stability of Immobilized Enzyme
3.13. Storage Stability of Immobilized Enzyme
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, S.; Khan, S.A.; Hamayun, M.; Lee, I.J. The recent advances in the utility of microbial lipases: A review. Microorganisms 2023, 11, 510. [Google Scholar] [CrossRef]
- Akram, F.; Mir, A.S.; ul Haq, I.; Roohi, A. An appraisal on prominent industrial and biotechnological applications of bacterial lipases. Mol. Biotechnol. 2022, 65, 521–543. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Chang, P.S. Kinetic modeling of lipase-catalysed hydrolysis of triacylglycerol in a reverse micelle system for the determination of integral stereoselectivity. Catal. Sci. Technol. 2022, 12, 2819–2828. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.H.; Cong, F.D.; Xu, Y.L.; Kang, J.; Zhang, Y.; Zhou, M.J.; Xing, K.Z.; Zhang, G.R.; Pan, H. Synthesis of cinnamyl acetate catalysed by highly reusable cotton-immobilized Pseudomonas fluorescens lipase. Biocatal. Biotransform. 2018, 36, 332–339. [Google Scholar] [CrossRef]
- Martelli, G.; Cirillo, M.; Giraldi, V.; Giacomini, D. Chemoenzymatic enantioselective route to get (+) and (−) 4-acetoxy-aze-tidin-2-one by lipase-catalysed kinetic resolution and their applications. Bioorg. Chem. 2022, 120, 105580. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Ortiz, P.A.; Gama, R.S.; Gomez, O.C.; Luiz, J.H.H.; Fernandez-Lafuente, R.; Cren, E.C.; Mendes, A.A. Sustainable Enzymatic Synthesis of a Solketal Ester-Process Optimization and Evaluation of Its Antimicrobial Activity. Catalysts 2020, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Rios, N.S.; Pinheiro, B.B.; Pinheiro, M.P.; Bezerra, R.M.; dos Santos, J.C.S.; Goncalves, L.R.B. Biotechnological potential of lipases from Pseudomonas: Sources, properties and applications. Process Biochem. 2018, 75, 99–120. [Google Scholar] [CrossRef]
- Cao, Y.P.; Zhi, G.Y.; Han, L.; Chen, Q.; Zhang, D.H. Biosynthesis of benzyl cinnamate using an efficient immobilized lipase entrapped in nano-molecular cages. Food Chem. 2021, 364, 130428. [Google Scholar] [CrossRef]
- Remonatto, D.; Miotti, R.H.; Monti, R.; Bassan, J.C.; de Paula, A.V. Applications of immobilized lipases in enzymatic reactors: A review. Process Biochem. 2022, 114, 1–20. [Google Scholar] [CrossRef]
- de Souza, W.F.C.; Almeida, F.L.C.; de Melo, A.M.; Soares, A.S.P.; Forte, M.B.S.; de Castro, R.J.S.; Sato, H.H. Immobilization techniques on bioprocesses: Current applications regarding enzymes, microorganisms, and essential oils. Food Bioprocess Technol. 2022, 15, 1449–1476. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Cavalcante, A.L.G.; de Sousa, I.G.; Neto, F.S.; dos Santos, J.C.S. Current status and future perspectives of supports and protocols for enzyme immobilization. Catalysts 2021, 11, 1222. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R. Immobilization of lipases—A review. Part II: Carrier materials. ChemBioEng Rev. 2019, 6, 167–194. [Google Scholar] [CrossRef]
- Samanta, S.; Roy, P.; Kar, P. Influence of pH of the reaction medium on the structure and property of conducting poly (o-Phenylenediamine). Mater. Today Proc. 2015, 2, 1301–1308. [Google Scholar] [CrossRef]
- Samanta, S.; Roy, P.; Kar, P. Influence of structure of poly (o-phenylenediamine) on the doping ability and conducting property. Ionics 2017, 23, 937–947. [Google Scholar] [CrossRef]
- Vu, C.M.; Nguyen, V.H.; Bach, Q.V. Influences of electric field strength on rheological properties of electrorheological fluid based on hollow poly (o-phenylenediamine-co-o-toluidine) dispersed on silicone oil. J. Mol. Liq. 2020, 314, 113762. [Google Scholar] [CrossRef]
- Kannapiran, N.; Muthusamy, A.; Renganathan, B.; Ganesan, A.R.; Meena, S.S. Magnetic, electrical and gas sensing properties of poly (o-phenylenediamine)/MnCoFe2O4 nanocomposites. Appl. Phys. A 2020, 126, 959. [Google Scholar] [CrossRef]
- Wang, X.Y.; Sun, D.P.; Tong, Y.L.; Zhong, Y.S.; Chen, Z.G. A voltammetric aptamer-based thrombin biosensor exploiting signal amplification via synergetic catalysis by DNAzyme and enzyme decorated AuPd nanoparticles on a poly(o-phenylenediamine) support. Microchim. Acta 2017, 184, 1791–1799. [Google Scholar] [CrossRef]
- Feng, L.J.; Zhang, X.H.; Zhao, D.M.; Wang, S.F. Electrochemical studies of bovine serum albumin immobilization onto the poly-o-phenylenediamine and carbon-coated nickel composite film and its interaction with papaverine. Sens. Actuators B 2011, 152, 88–93. [Google Scholar] [CrossRef]
- Moonla, C.; Chenkhuruthum, S.; Ouiram, T.; Preechaworapun, A.; Tapala, W.; Ngamchuea, K.; Tangkuaram, T. A Novel label-free chronoamperometric immunosensor based on a biocomposite material for rapid detection of carcinoembryonic antigen. Electroanalysis 2022, 34, 1289–1298. [Google Scholar] [CrossRef]
- Bu, L.J.; Guo, L.; Xie, J.W. An in situ assay of nerve agents enabled by a self-assembled bienzymatic electrochemical biosensor. New J. Chem. 2020, 44, 7460–7466. [Google Scholar] [CrossRef]
- Mousa, H.M.; Aggas, J.R.; Guiseppi, E. An Electropolymerization of aniline and (N-phenyl-o-phenylenediamine) for glucose biosensor application. Mater. Lett. 2018, 238, 267–270. [Google Scholar] [CrossRef]
- Ganesana, M.; Trikantzopoulos, E.; Maniar, Y.; Lee, S.T.; Venton, B.J. Development of a novel micro biosensor for in vivo monitoring of glutamate release in the brain. Biosens. Bioelectron. 2019, 130, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kuralay, F.; Bayramli, Y. Electrochemical determination of mitomycin C and its interaction with double-stranded DNA using a poly(o-phenylenediamine)-multi-walled carbon nanotube modified pencil graphite electrode. Anal. Lett. 2021, 54, 1295–1308. [Google Scholar] [CrossRef]
- Xiao, Z.; Luo, J.; Niu, Y.; Wang, P.; Wang, R.; Sun, X. Olfactory impact of esters on rose essential oil floral alcohol aroma expression in model solution. Food Res. Inter. 2019, 116, 211–222. [Google Scholar] [CrossRef]
- Oda, S.; Nakanishi, M.; Ishikawa, A.; Baba, T. Modified liquid-liquid interface cultivation system with floating microspheres and binder micro-pieces for slow-growing or unicellular microorganisms: Application to interfacial bioconversions with an actinomycete and yeasts. Process Biochem. 2019, 80, 1–8. [Google Scholar] [CrossRef]
- Buettner, A.; Elsharif, S. Influence of the chemical structure on the odor characters of beta-citronellol and its oxygenated derivatives. Food Chem. 2017, 232, 704–711. [Google Scholar]
- Maja, H.; Saga, S.; Muzafera, P. Lipase-catalyzed esterification of citronellol with lauric acid in supercritical carbon dioxide/co-solvent media. J. Supercrit. Fluids 2007, 43, 199–203. [Google Scholar]
- Yuan, M.; Cong, F.D.; Zhai, Y.L.; Li, P.; Yang, W.; Zhang, S.L.; Su, Y.P.; Li, T.; Wang, Y.C.; Luo, W.; et al. Rice straw enhancing catalysis of Pseudomonas fuorescens lipase for synthesis of citronellyl acetate. Bioprocess Biosyst. Eng. 2022, 45, 453–464. [Google Scholar] [CrossRef]
- Yadav, G.D.; Borkar, I.V. Kinetic and mechanistic investigation of microwave-assisted lipase catalyzed synthesis of citronellyl acetate. Ind. Eng. Chem. Res. 2009, 48, 7915–7922. [Google Scholar] [CrossRef]
- Macedo, G.A.; Lozano, M.M.S.; Pastore, G.M. Enzymatic synthesis of short chain citronellyl esters by a new lipase from Rhizopus sp. Electron. Electron. J. Biotechnol. 2004, 7, 72–75. [Google Scholar]
- Han, J.; Liu, Y.; Guo, R. A novel templateless method to nanofibers of polyaniline derivatives with size control. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 740–746. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Winkler, U.K.; Stuckmann, M. Glycogen, hyaluronate and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Bacteriol. 1979, 138, 663–670. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Xu, Y.; Ma, H.; Zhang, Q.; Evans, D.G.; Duan, X. Effect of surface hydrophobicity/hydrophilicity of mesoporous supports on the activity of immobilized lipase. J. Colloid Interface Sci. 2006, 298, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Chaudhury, N.K. Entrapment of biomolecules in sol-gel matrix for applications in biosensors: Problems and future prospects. Biosens. Bioelectron. 2007, 22, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, G.O.; Faba, E.M.S.; Vaschetto, E.G.; Eimer, G.A. Heterogeneous enzymatic catalysts: Comparing their efficiency in the production of biodiesel from alternative oils. ChemistrySelect 2023, 8, e202203962. [Google Scholar] [CrossRef]
- Wang, J.H.; Li, K.; He, Y.J.; Wang, Y.; Han, X.T.; Yan, Y.J. Enhanced performance of lipase immobilized onto Co2+-chelated magnetic nanoparticles and its application in biodiesel production. Fuel 2019, 255, 115794. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.Y.; Bai, W.J.; Liang, Y.X. P(GMA-HEMA)/SiO2 nanofilm constructed macroporous monolith for immobilization of Pseudomonas fluorescens lipase. ChemistrySelect 2020, 5, 4885–4892. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, H.; Zhang, Z.Y.; Liang, Y.X. Preparation of mesoporous nanofilm constructed millimeter-sized macroporous SiO2 for lipase immobilization. Microporous Mesoporous Mater. 2021, 323, 111227. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Scaramuzzo, F.A.; Fioravanti, R.; di Nitto, A.; Cerra, S.; Palocci, C.; Fratoddi, I. Noble metal nanoparticle-based networks as a new platform for lipase immobilization. Int. J. Biol. Macromol. 2020, 146, 790–797. [Google Scholar] [CrossRef]
- De Castro, H.F.; de Oliveira, P.C.; Pereira, E.B. Evaluation of different approaches for lipase catalysed synthesis of citronellyl acetate. Biotech. Lett. 1997, 19, 229–232. [Google Scholar] [CrossRef]
- Weber, H.K.; Stecher, H.; Faber, K. Sensitivity of microbial lipases to acetaldehyde formed by acyl-transfer reactions from vinyl esters. Biotechnol. Lett. 1995, 17, 803–808. [Google Scholar] [CrossRef]
- Dwivedee, B.P.; Soni, S.; Bhimpuria, R.; Laha, J.K.; Banerjee, U.C. Tailoring a robust and recyclable nanobiocatalyst by immobilization of Pseudomonas fluorescens lipase on carbon nanofiber and its application in synthesis of enantiopure carboetomidate analogue. Int. J. Biol. Macromol. 2019, 133, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Pitzalis, F.; Carucci, C.; Naseri, M.; Fotouhi, L.; Magner, E.; Salis, A. Lipase encapsulation onto ZIF-8. a comparison between biocatalysts obtained at low and high Zinc:2-methylimidazole molar ratio in aqueous medium. ChemCatChem 2018, 10, 1578–1585. [Google Scholar] [CrossRef]

| Support | Bound Protein (μg/g Support) | Activity (μmol/(min·mg Lipase)) | Specific Activity (μmol/(min·mg Protein)) |
|---|---|---|---|
| Solid support | 313.59 ± 6.07 | 1.06 ± 0.08 × 10−3 | 3.39 ± 0.013 |
| Hollow support | 387.24 ± 3.94 | 2.48 ± 0.02 × 10−3 | 6.41 ± 0.005 |
| Free lipase | — | 5.40 ± 0.02 × 10−3 | 1.56 ± 0.008 |
| Free Lipase | Support | Immobilized Lipase | Activity Recovery (%) | Substrate | Reference | ||
|---|---|---|---|---|---|---|---|
| Activity | Specific Activity | Activity | Specific Activity | ||||
| 0.005 | 1.56 | PoPD | 0.003 | 8.26 | 530 | p-NPP | This study. |
| 0.02 | 0.47 | SBA-15 | 0.05 | 0.92 | 196 | sunflower oil/ethanol | [36] |
| 0.02 | 0.47 | Na/SBA-15 | 0.06 | 1.24 | 264 | [36] | |
| 0.02 | 0.47 | Ca/SBA-15 | 0.06 | 1.24 | 264 | [36] | |
| - | - | AGMNP-Co2+ | - | - | 2125 | dodecanoic acid/1-dodecanol | [37] |
| - | 445.6 | P(GMA-HEMA)/ SiO2 | 3773 | 1033.70 | 232 | 1-dodecanol/dodecanoic acid | [38] |
| - | 445.4 | MNCMMS | 4897 | 1136.19 | 255 | p-NPL | [39] |
| - | 5.56 | AuNPs-BI | - | 5.03 | 90 | tributyrin | [40] |
| - | 5.56 | AgNPs-BI | - | 5.92 | 106 | tributyrin | [40] |
| - | 5.56 | AuNPs-Pt-DEBP | - | 2.87 | 52 | tributyrin | [40] |
| - | 5.56 | AgNPs-Pt-DEBP | - | 2.78 | 50 | tributyrin | [40] |
| - | 5.56 | poly-Pt-DEBP | - | 4.79 | 86 | tributyrin | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, J.; Wang, Q.; Xu, H.; Sun, W. Immobilization of Pseudomonas fluorescens Lipase on Hollow Poly(o-phenylenediamine) Microspheres and Its Application in the Preparation of Citronellyl Acetate. Processes 2023, 11, 1842. https://doi.org/10.3390/pr11061842
Xiong J, Wang Q, Xu H, Sun W. Immobilization of Pseudomonas fluorescens Lipase on Hollow Poly(o-phenylenediamine) Microspheres and Its Application in the Preparation of Citronellyl Acetate. Processes. 2023; 11(6):1842. https://doi.org/10.3390/pr11061842
Chicago/Turabian StyleXiong, Jian, Qi Wang, Hanghang Xu, and Wenyuan Sun. 2023. "Immobilization of Pseudomonas fluorescens Lipase on Hollow Poly(o-phenylenediamine) Microspheres and Its Application in the Preparation of Citronellyl Acetate" Processes 11, no. 6: 1842. https://doi.org/10.3390/pr11061842
APA StyleXiong, J., Wang, Q., Xu, H., & Sun, W. (2023). Immobilization of Pseudomonas fluorescens Lipase on Hollow Poly(o-phenylenediamine) Microspheres and Its Application in the Preparation of Citronellyl Acetate. Processes, 11(6), 1842. https://doi.org/10.3390/pr11061842







