Enhancing Lipid Production of Chlorella sp. by Mixotrophic Cultivation Optimization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Samples, Establishment and Identification of Algal Strains
2.2. Serum Bottle Cultivation of Isolated Chlorella sp. G3H3-1-2
2.3. Microscopic Observation
2.4. Analytical Method
2.5. Total Fatty Acids Methyl Esters (FAMEs)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Nitrogen Sources Effect
3.2. Nitrogen Concentrations Effect
3.3. Carbon and Nitrogen Sources Concentration Effect
3.4. pH Effect
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Osman, A.I.; Chen, L.; Yang, M.; Msigwa, G.; Farghali, M.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Cost, Environmental Impact, and Resilience of Renewable Energy under a Changing Climate: A Review. Environ. Chem. Lett. 2023, 21, 741–764. [Google Scholar] [CrossRef]
- Wu, W.; Tan, L.; Chang, H.; Zhang, C.; Tan, X.; Liao, Q.; Zhong, N.; Zhang, X.; Zhang, Y.; Ho, S.-H. Advancements on Process Regulation for Microalgae-Based Carbon Neutrality and Biodiesel Production. Renew. Sustain. Energy Rev. 2023, 171, 112969. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High Lipid Induction in Microalgae for Biodiesel Production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef] [Green Version]
- Mofijur, M.; Rasul, M.G.; Hassan, N.M.S.; Nabi, M.N. Recent Development in the Production of Third Generation Biodiesel from Microalgae. Energy Procedia 2019, 156, 53–58. [Google Scholar] [CrossRef]
- Lin, T.-S.; Wu, J.-Y. Effect of Carbon Sources on Growth and Lipid Accumulation of Newly Isolated Microalgae Cultured under Mixotrophic Condition. Bioresour. Technol. 2015, 184, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, G.; Gargano, I.; Andreozzi, R.; Marotta, R.; Marzocchella, A.; Pinto, G.; Pollio, A. Effects of Photobioreactors Design and Operating Conditions on Stichococcus Bacillaris Biomass and Biodiesel Production. Biochem. Eng. J. 2013, 74, 8–14. [Google Scholar] [CrossRef]
- Yeh, K.-L.; Chen, C.-Y.; Chang, J.-S. PH-Stat Photoheterotrophic Cultivation of Indigenous Chlorella Vulgaris ESP-31 for Biomass and Lipid Production Using Acetic Acid as the Carbon Source. Biochem. Eng. J. 2012, 64, 1–7. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Lay, C.-H.; Chen, C.-C.; Wu, S.-Y. Lipid Accumulating Microalgae Cultivation in Textile Wastewater: Environmental Parameters Optimization. J. Taiwan Inst. Chem. Eng. 2017, 79, 1–6. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M.; Koniuszy, A. Utilisation of CO2 from Sodium Bicarbonate to Produce Chlorella Vulgaris Biomass in Tubular Photobioreactors for Biofuel Purposes. Sustainability 2021, 13, 9118. [Google Scholar] [CrossRef]
- Yu, H.; Kim, J.; Rhee, C.; Shin, J.; Shin, S.G.; Lee, C. Effects of Different PH Control Strategies on Microalgae Cultivation and Nutrient Removal from Anaerobic Digestion Effluent. Microorganisms 2022, 10, 357. [Google Scholar] [CrossRef]
- Skorupskaite, V.; Makareviciene, V.; Levisauskas, D. Optimization of Mixotrophic Cultivation of Microalgae Chlorella Sp. for Biofuel Production Using Response Surface Methodology. Algal Res. 2015, 7, 45–50. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, S.; Rong, J.; Li, X.; Chen, H.; He, C.; Wang, Q. Effective Biological DeNOx of Industrial Flue Gas by the Mixotrophic Cultivation of an Oil-Producing Green Alga Chlorella Sp. C2. Environ. Sci. Technol. 2016, 50, 1620–1627. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Chen, J.-J. Optimization of Simultaneous Biomass Production and Nutrient Removal by Mixotrophic Chlorella Sp. Using Response Surface Methodology. Water Sci. Technol. 2016, 73, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-H.; Cho, D.-H.; Heo, J.; Lee, Y.J.; Lee, B.; Chang, Y.K.; Kim, H.-S. Evaluation of the Potential of Chlorella Sp. HS2, an Algal Isolate from a Tidal Rock Pool, as an Industrial Algal Crop under a Wide Range of Abiotic Conditions. J. Appl. Phycol. 2019, 31, 2245–2258. [Google Scholar] [CrossRef]
- Zhan, J.; Rong, J.; Wang, Q. Mixotrophic Cultivation, a Preferable Microalgae Cultivation Mode for Biomass/Bioenergy Production, and Bioremediation, Advances and Prospect. Int. J. Hydrog. Energy 2017, 42, 8505–8517. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Lay, C.-H.; Chiong, M.-C.; Chew, K.W.; Chen, C.-C.; Wu, S.-Y.; Zhou, D.; Kumar, G.; Show, P.L. Immobilized Chlorella Species Mixotrophic Cultivation at Various Textile Wastewater Concentrations. J. Water Process Eng. 2020, 38, 101609. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, P.; Sun, H.; Chen, M.; Lu, S.; Li, P. Effects of Various Organic Carbon Sources on the Growth and Biochemical Composition of Chlorella Pyrenoidosa. Bioresour. Technol. 2014, 173, 52–58. [Google Scholar] [CrossRef]
- Wan, M.; Liu, P.; Xia, J.; Rosenberg, J.N.; Oyler, G.A.; Betenbaugh, M.J.; Nie, Z.; Qiu, G. The Effect of Mixotrophy on Microalgal Growth, Lipid Content, and Expression Levels of Three Pathway Genes in Chlorella Sorokiniana. Appl. Microbiol. Biotechnol. 2011, 91, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Alishah Aratboni, H.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and Lipid Induction Strategies in Microalgae for Biofuel Production and Other Applications. Microb. Cell Fact. 2019, 18, 178. [Google Scholar] [CrossRef] [Green Version]
- Kumar, L.R.; Yellapu, S.K.; Tyagi, R.D.; Drogui, P. Purified Crude Glycerol by Acid Treatment Allows to Improve Lipid Productivity by Yarrowia Lipolytica SKY7. Process Biochem. 2020, 96, 165–173. [Google Scholar] [CrossRef]
- Kumar, L.R.; Yellapu, S.K.; Yan, S.; Tyagi, R.; Drogui, P. Elucidating the Effect of Impurities Present in Different Crude Glycerol Sources on Lipid and Citric Acid Production by Yarrowia Lipolytica SKY7. J. Chem. Technol. Biotechnol. 2021, 96, 227–240. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chang, J.-S.; Lai, Y.-Y.; Chen, C.-N.N. Achieving High Lipid Productivity of a Thermotolerant Microalga Desmodesmus Sp. F2 by Optimizing Environmental Factors and Nutrient Conditions. Bioresour. Technol. 2014, 156, 108–116. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of Nitrogen Sources on Cell Growth and Lipid Accumulation of Green Alga Neochloris Oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Wu, W.-T. Cultivation of Microalgae for Oil Production with a Cultivation Strategy of Urea Limitation. Bioresour. Technol. 2009, 100, 3921–3926. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Chu, R.; Sun, D.; Deng, X.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. Mixotrophic Culture of Bait Microalgae for Biomass and Nutrients Accumulation and Their Synergistic Carbon Metabolism. Bioresour. Technol. 2023, 367, 128301. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Chinnasamy, S.; Singh, M.; Das, K.C. Renewable Biomass Production by Mixotrophic Algae in the Presence of Various Carbon Sources and Wastewaters. Appl. Energy 2011, 88, 3425–3431. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Torpee, S. Enhanced Growth and Lipid Production of Microalgae under Mixotrophic Culture Condition: Effect of Light Intensity, Glucose Concentration and Fed-Batch Cultivation. Bioresour. Technol. 2012, 110, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Saratale, R.; Ponnusamy, V.K.; Jeyakumar, R.B.; Sirohi, R.; Piechota, G.; Shobana, S.; Dharmaraja, J.; Lay, C.; Dattatraya Saratale, G.; Seung Shin, H.; et al. Microalgae Cultivation Strategies Using Cost–Effective Nutrient Sources: Recent Updates and Progress towards Biofuel Production. Bioresour. Technol. 2022, 361, 127691. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Potential of Using Organic Fertilizer to Cultivate Chlorella Vulgaris for Biodiesel Production. Appl. Energy 2012, 94, 303–308. [Google Scholar] [CrossRef]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J. CO2 Biofixation and Fatty Acid Composition of Scenedesmus Obliquus and Chlorella Pyrenoidosa in Response to Different CO2 Levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef]
- Bartley, M.L.; Boeing, W.J.; Dungan, B.N.; Holguin, F.O.; Schaub, T. PH Effects on Growth and Lipid Accumulation of the Biofuel Microalgae Nannochloropsis Salina and Invading Organisms. J. Appl. Phycol. 2014, 26, 1431–1437. [Google Scholar] [CrossRef]
- Zeng, X.; Danquah, M.K.; Chen, X.D.; Lu, Y. Microalgae Bioengineering: From CO2 Fixation to Biofuel Production. Renew. Sustain. Energy Rev. 2011, 15, 3252–3260. [Google Scholar] [CrossRef]
- Zhu, J.; Rong, J.; Zong, B. Factors in Mass Cultivation of Microalgae for Biodiesel. Chin. J. Catal. 2013, 34, 80–100. [Google Scholar] [CrossRef]
- Basu, S.; Roy, A.S.; Mohanty, K.; Ghoshal, A.K. Enhanced CO2 Sequestration by a Novel Microalga: Scenedesmus Obliquus SA1 Isolated from Bio-Diversity Hotspot Region of Assam, India. Bioresour. Technol. 2013, 143, 369–377. [Google Scholar] [CrossRef]
- Wu, L.F.; Chen, P.C.; Lee, C.M. The Effects of Nitrogen Sources and Temperature on Cell Growth and Lipid Accumulation of Microalgae. Int. Biodeterior. Biodegrad. 2013, 85, 506–510. [Google Scholar] [CrossRef]
- Wan, C.; Bai, F.-W.; Zhao, X.-Q. Effects of Nitrogen Concentration and Media Replacement on Cell Growth and Lipid Production of Oleaginous Marine Microalga Nannochloropsis Oceanica DUT01. Biochem. Eng. J. 2013, 78, 32–38. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Karthikeyan, O.P.; Verma, P. Influence of Carbon Sources on Biomass and Biomolecule Accumulation in Picochlorum Sp. Cultured under the Mixotrophic Condition. Int. J. Environ. Res. Public Health 2022, 19, 3674. [Google Scholar] [CrossRef]
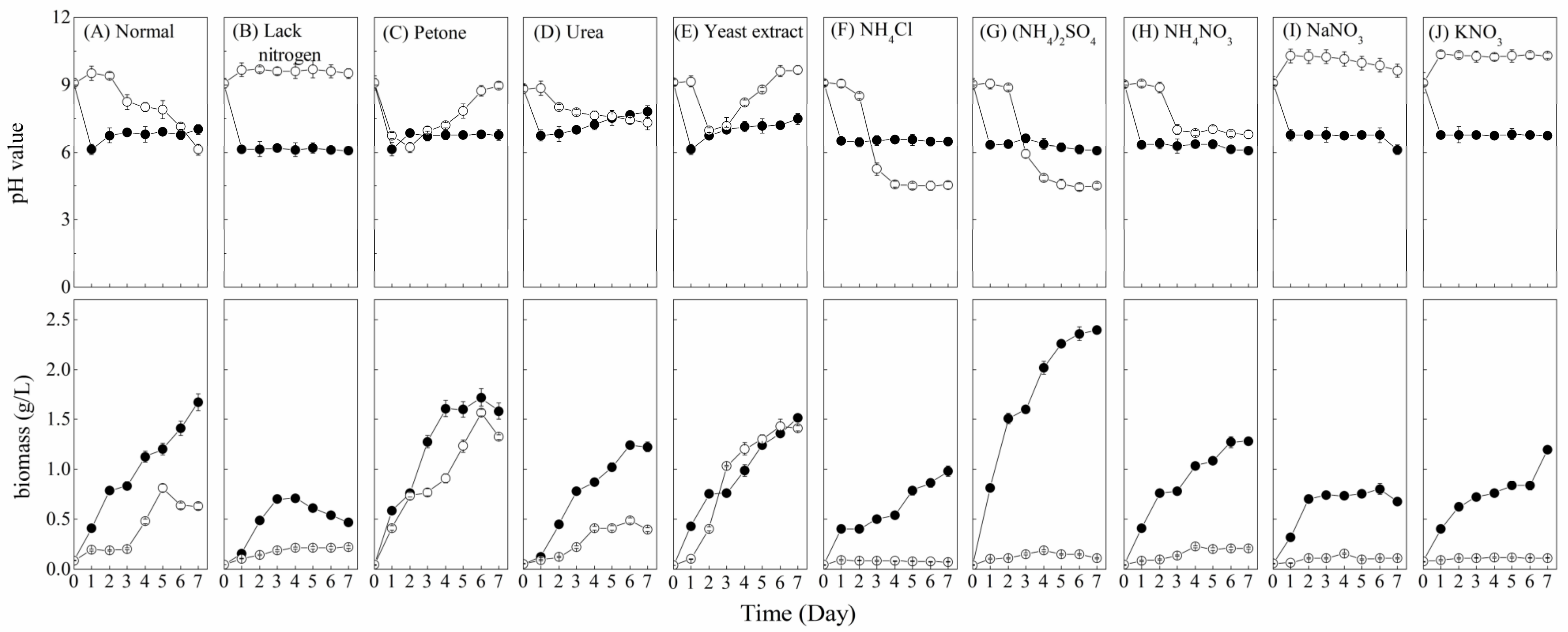
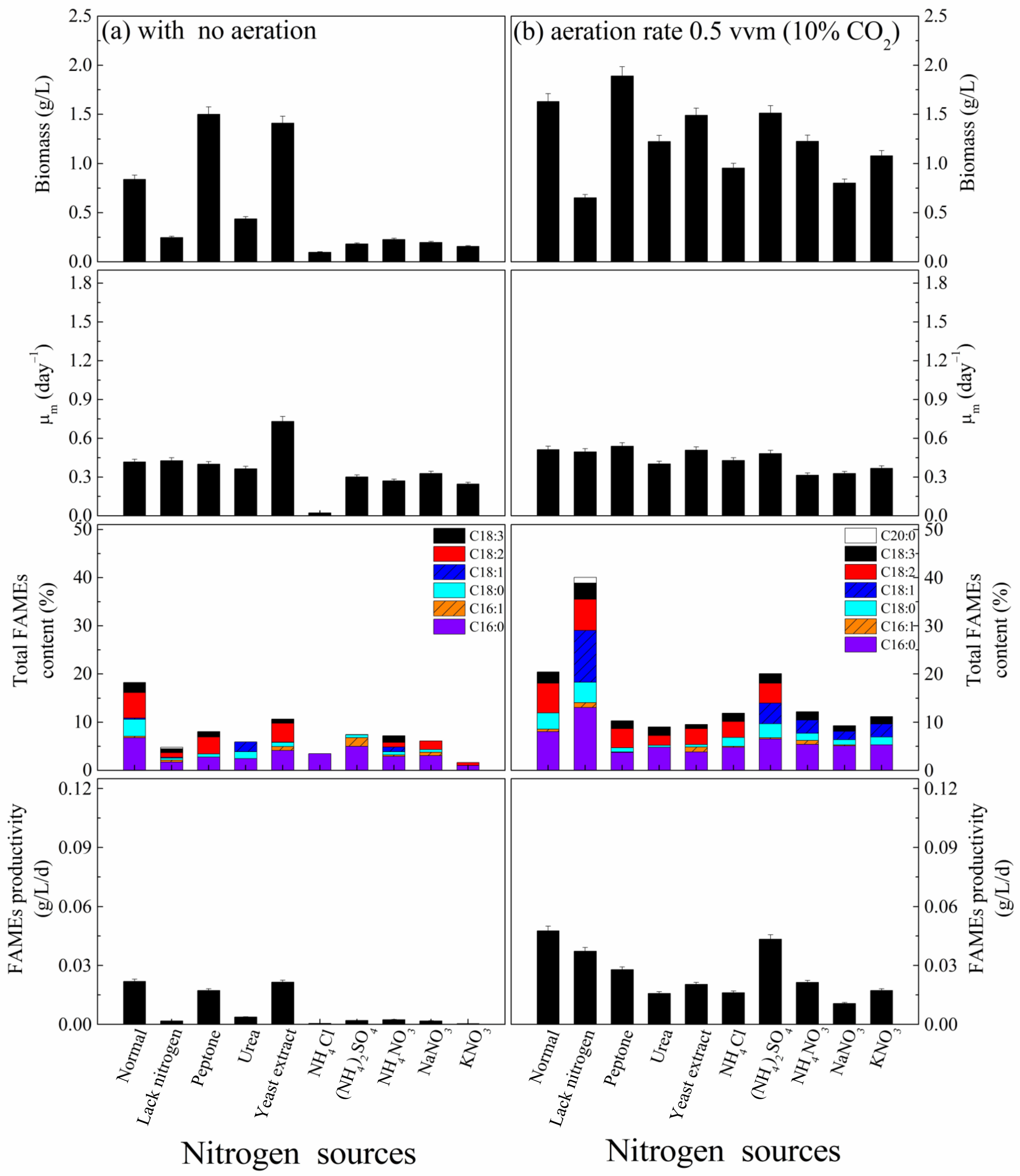

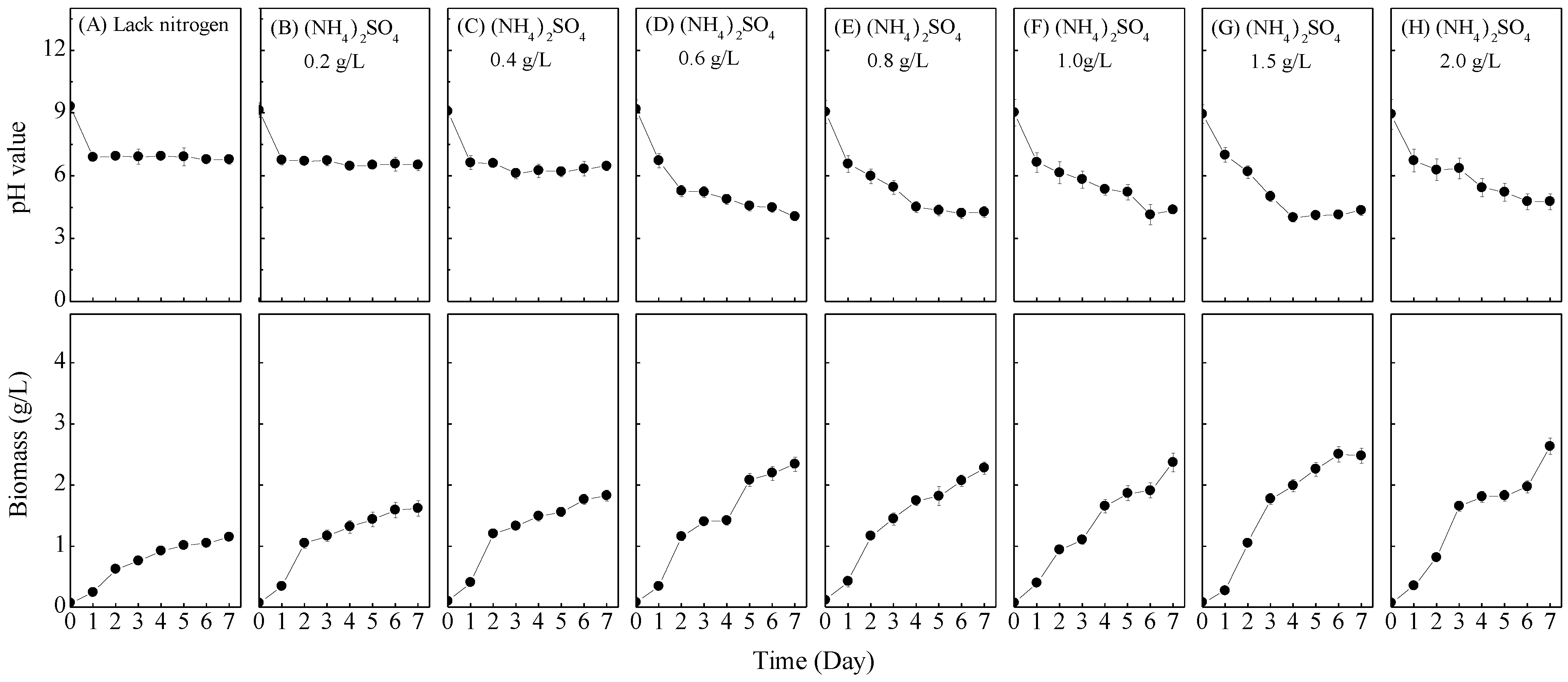
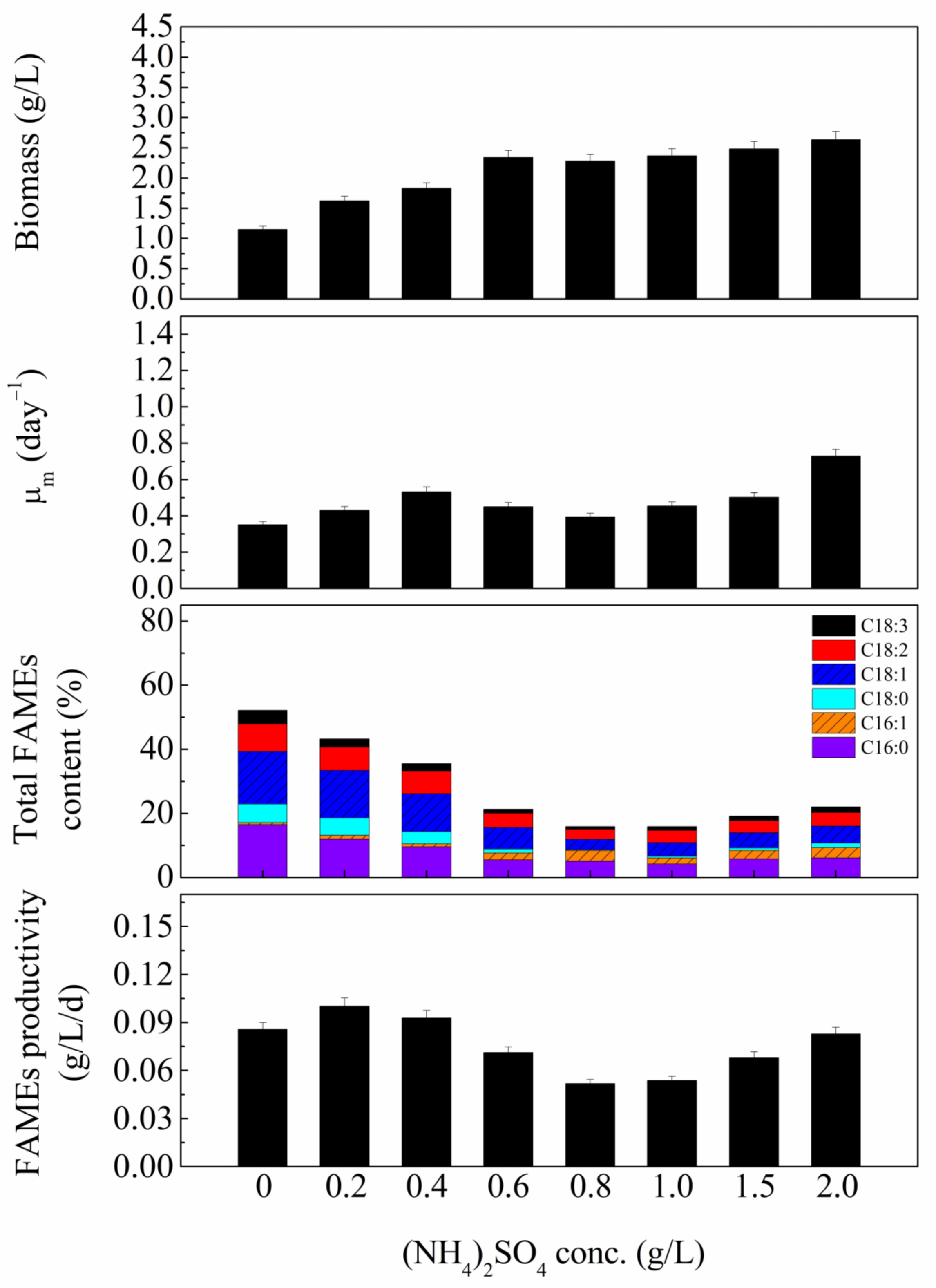
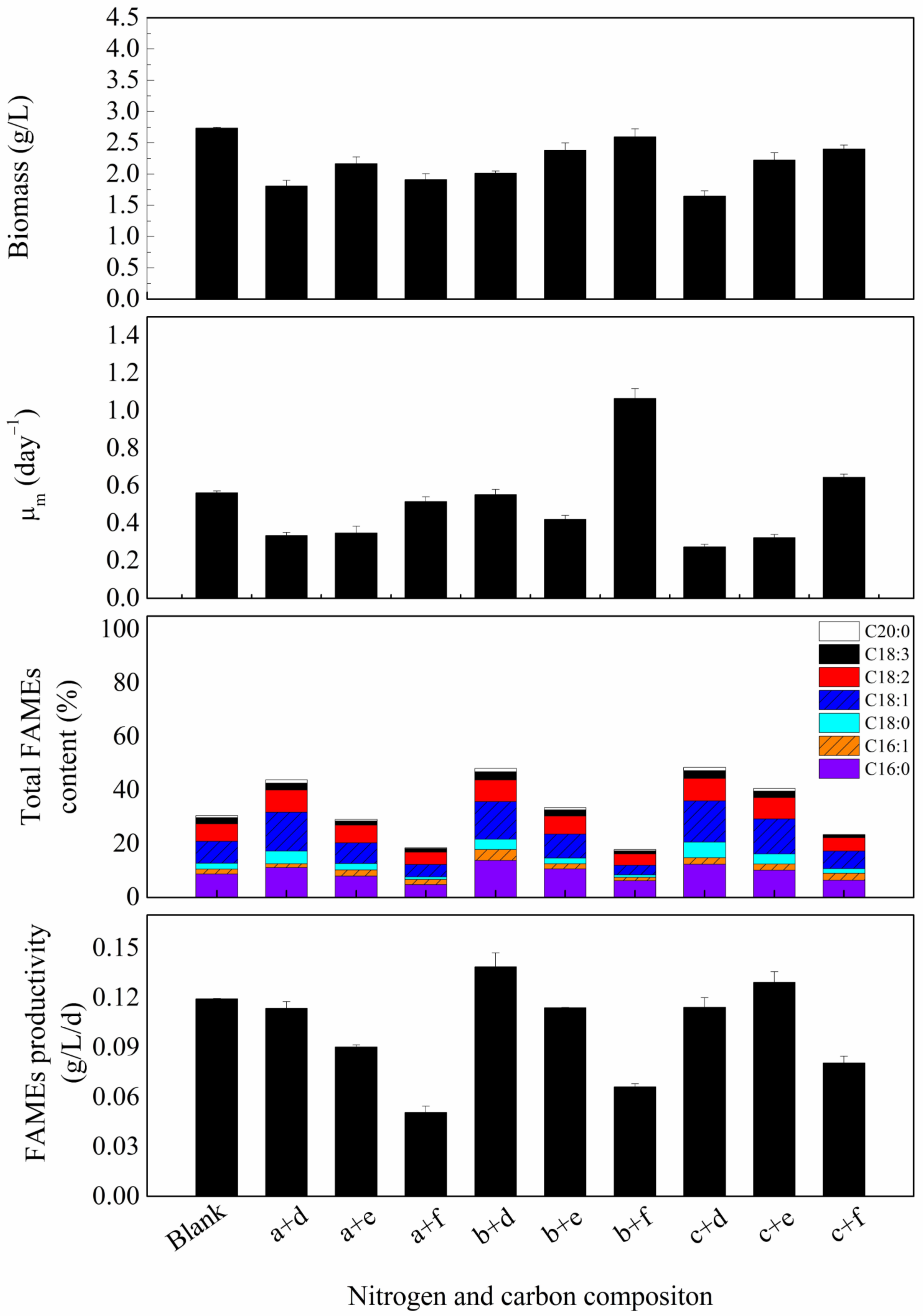

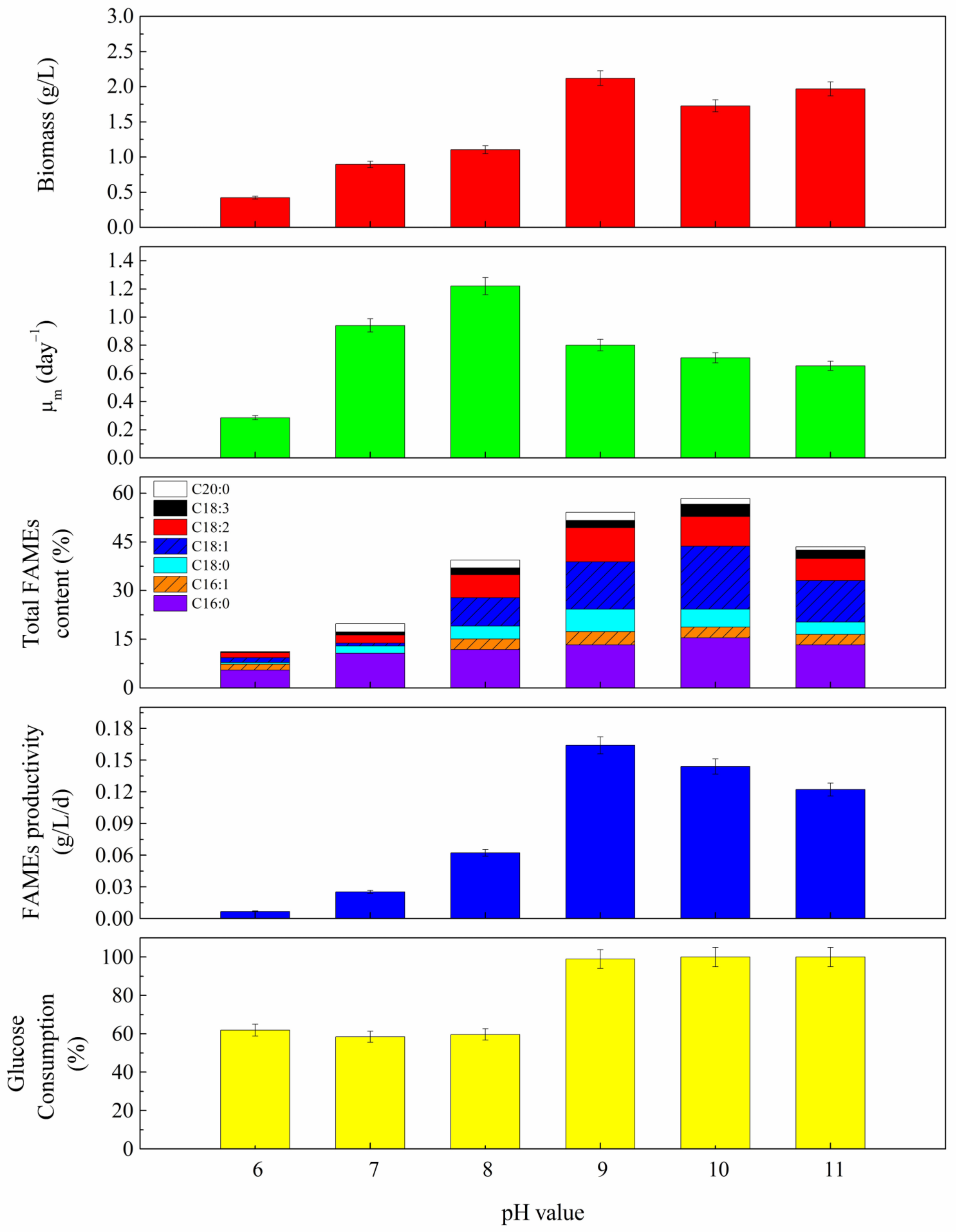
| Experiment | Nitrogen Sources | Carbon Sources | Initial pH |
|---|---|---|---|
| 1. Nitrogen sources effect | Peptone, urea, yeast extract, NH4Cl, (NH4)2SO4, NH4NO3, NaNO3 and KNO3 (1 g/L) | Sucrose 1.0 g/L | 9 |
| 2. Nitrogen sources concentrations effect | (NH4)2SO4 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5, 2.0 g/L | Sucrose 1.0 g/L | 9 |
| 3. Carbon and nitrogen sources combination effect | (NH4)2SO4 0.2, 0.4, 0.6 g/L | Glucose, glycerol, sucrose (1 g/L) | 9 |
| 4. Cultivation pH effect | (NH4)2SO4 0.2 g/L | Glucose 1 g/L | 6, 7, 8, 9, 10, 11 |
| Strains | Growth Type | Carbon Source | Nitrogen Source | pH | Temp (°C) | Biomass Concentration (g/L) | Lipid Content (w/w, %) | Lipid Concentration (g/L) | Lipid Productivity (mg/L/day) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Marine Chlorella sp. | Mixotrophy | Glucose | NaNO3 | 7.3 | 30 | 4.48 | 25.1 | 1.1237 | 112.4 | [27] |
| Nannochloropsis sp. | Mixotrophy | Glucose | NaNO3 | 7.3 | 30 | 5.87 | 25.3 | 1.4825 | 148.3 | [27] |
| Chlorella vulgaris ESP-31 | pH-stat photoheterotrophy | Acetic acid | NaNO3 | 7 | 25 | 2.13 | 49.7 | 0.986 | 70 | [7] |
| Scenedesmus obliquus SA1 | – | – | – | – | 25 | 4.975 | 33.04 | – | – | [34] |
| Monoraphidium sp. SB2 | – | – | KNO3 | 7.5 | 30 | 0.65 | 31.5 | – | 29.2 | [35] |
| Stichococcus bacillaris | Inclined bubble column | CO2 | NaNO3 | 7 | 23 | 4.27 | 32 | – | 81 | [6] |
| Nannochloropsis oceanica DUT01 | – | – | NaNO3 | 8 | 25 | 1.4 | 33.9 | 0.6 | 31 | [36] |
| Desmodesmus sp. F2 | Autotrophy | CO2 | NaNO3 | 7.6 | 35 | 3.32 | 64.13 | – | 263 | [22] |
| Chlorella sp. Y8-1 | Mixotrophy | Sucrose | urea | 9 | 30 | 0.45 | 35.5 | – | – | [5] |
| Picochlorum sp. BDUG100241 | Mixotrophy | Sodium acetate | – | 7 | 25 | 2.4 | 53.5 | – | – | [37] |
| Chlorella sp. | Mixotrophy | Acetic acid | KNO3 | 7 | 25 | 8.78 (protein content) | 37.1 | – | – | [25] |
| Thalassiosira pseudonana | Mixotrophy | Acetic acid | KNO3 | 7 | 25 | 10.71 (protein content) | 19.0 | – | – | [25] |
| Chlorella sp. G3H3-1-2 | Mixotrophy | Glucose | (NH4)2SO4 | 10 | 40 | 1.75 | 59 (FAMEs) | 49 (FAMEs) | 144 (FAMEs) | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-C.; Lay, C.-H.; Abdul, P.M.; Wu, J.-Y. Enhancing Lipid Production of Chlorella sp. by Mixotrophic Cultivation Optimization. Processes 2023, 11, 1892. https://doi.org/10.3390/pr11071892
Yu H-C, Lay C-H, Abdul PM, Wu J-Y. Enhancing Lipid Production of Chlorella sp. by Mixotrophic Cultivation Optimization. Processes. 2023; 11(7):1892. https://doi.org/10.3390/pr11071892
Chicago/Turabian StyleYu, Hao-Cheng, Chyi-How Lay, Peer Mohamed Abdul, and Jane-Yii Wu. 2023. "Enhancing Lipid Production of Chlorella sp. by Mixotrophic Cultivation Optimization" Processes 11, no. 7: 1892. https://doi.org/10.3390/pr11071892
APA StyleYu, H.-C., Lay, C.-H., Abdul, P. M., & Wu, J.-Y. (2023). Enhancing Lipid Production of Chlorella sp. by Mixotrophic Cultivation Optimization. Processes, 11(7), 1892. https://doi.org/10.3390/pr11071892










