Understanding the Impact of Sintering Temperature on the Properties of Ni–BCZY Composite Anode for Protonic Ceramic Fuel Cell Application
Abstract
Highlights
- NiO–BCZY powder prepared by citrate sol–gel method has homogenous particles;
- NiO–BCZY powder showed an average particle size of 51 ± 16 nm;
- NiO–BCZY powder calcined at 1100 °C showed a cubic phase without any impurities;
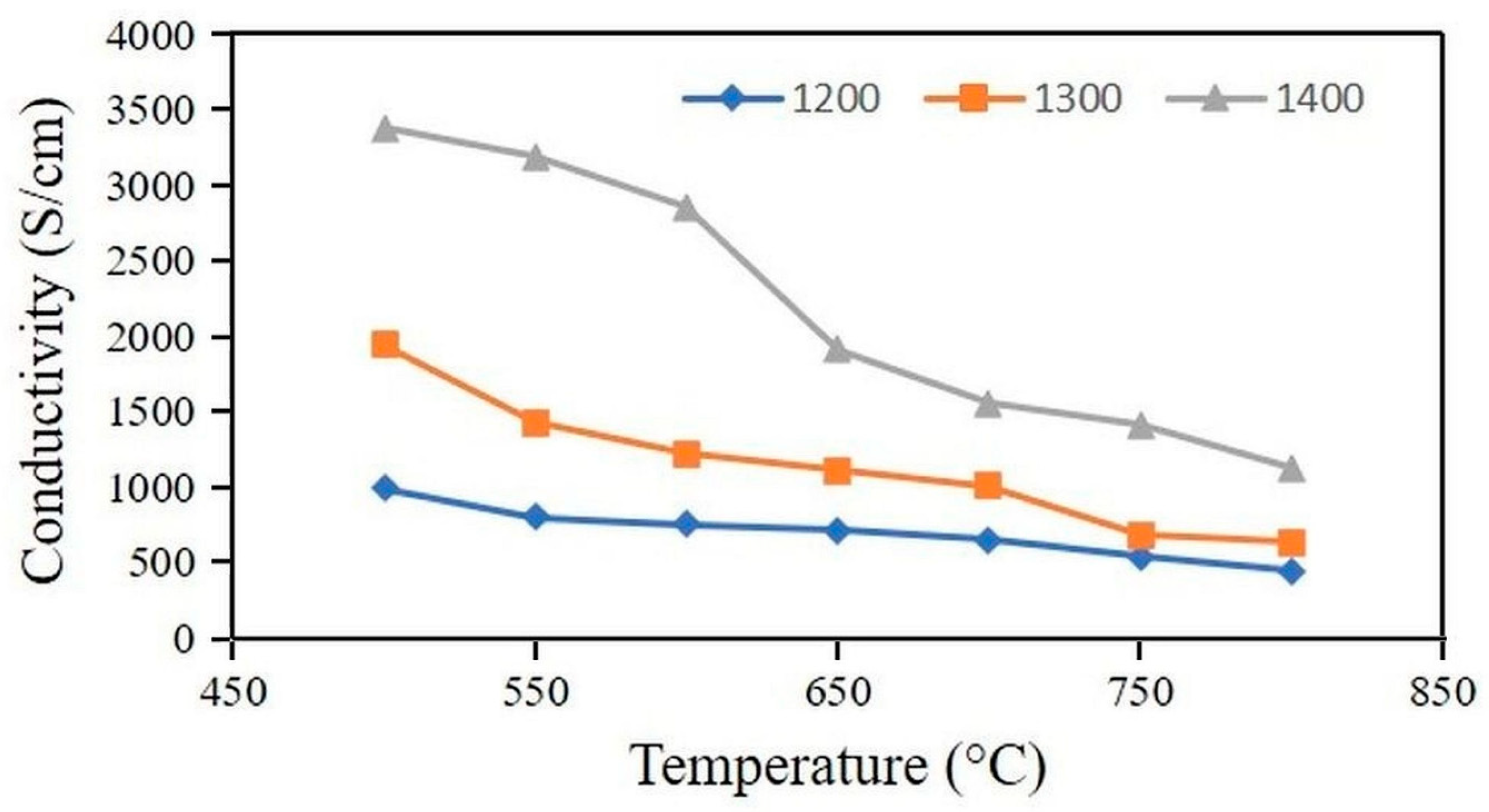
- The conductivity of anode sintered at 1200–1400 °C was 443–1124 S/cm at 800 °C;
- Anode sintered at 1400 °C exhibited the lowest ASR of 1.165 Ω cm2 at 800 °C.
Abstract
1. Introduction
2. Materials and Method
2.1. Powder Preparation
2.2. Physicochemical Characterisation of Synthesised Powder
2.3. Fabrication of Anode Screen–Printing Ink
2.4. Electrochemical Characterisation of Anode Symmetrical Cells
2.4.1. Electrical Conductivity Measurement
2.4.2. Electrochemical Performance Measurement
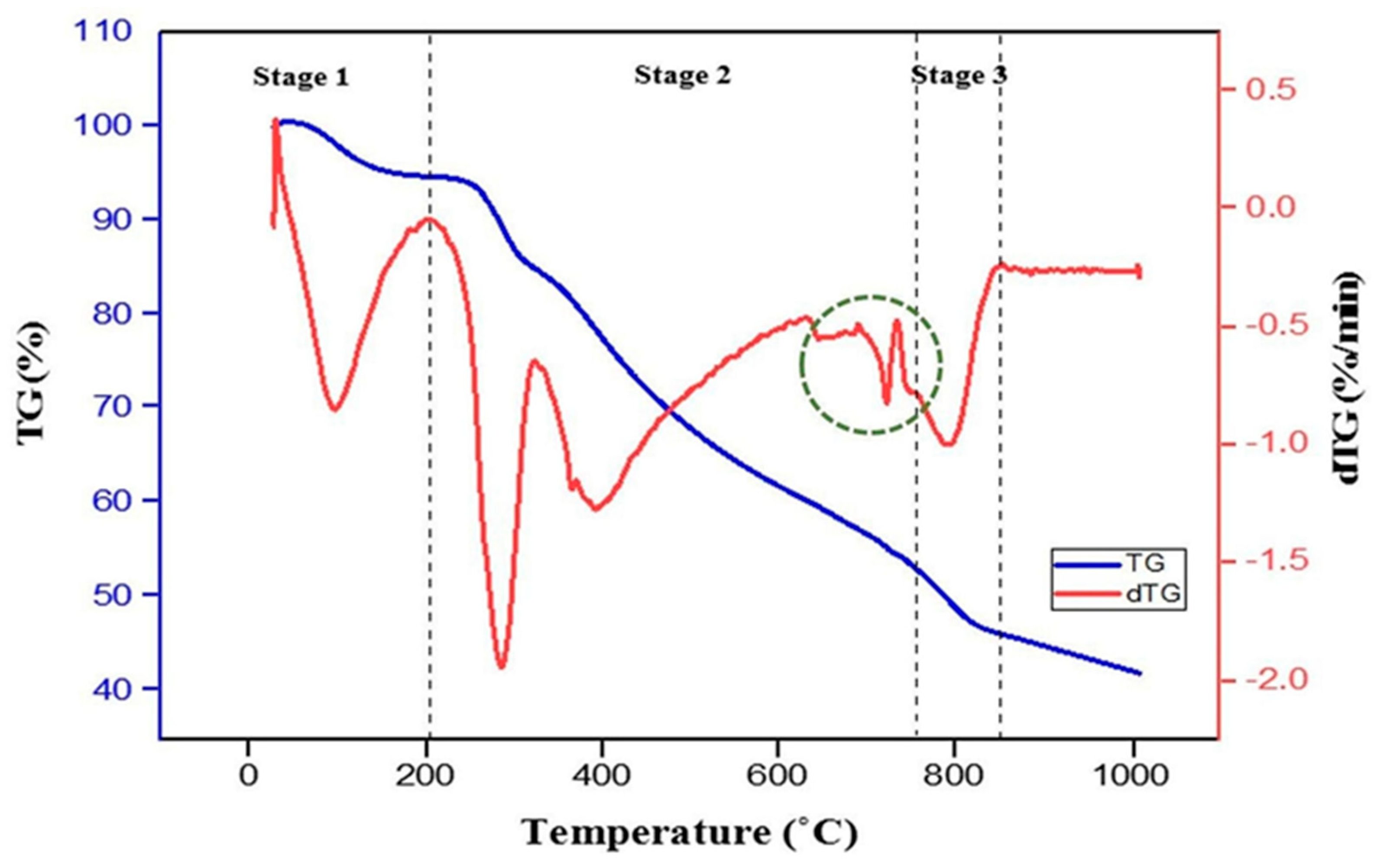
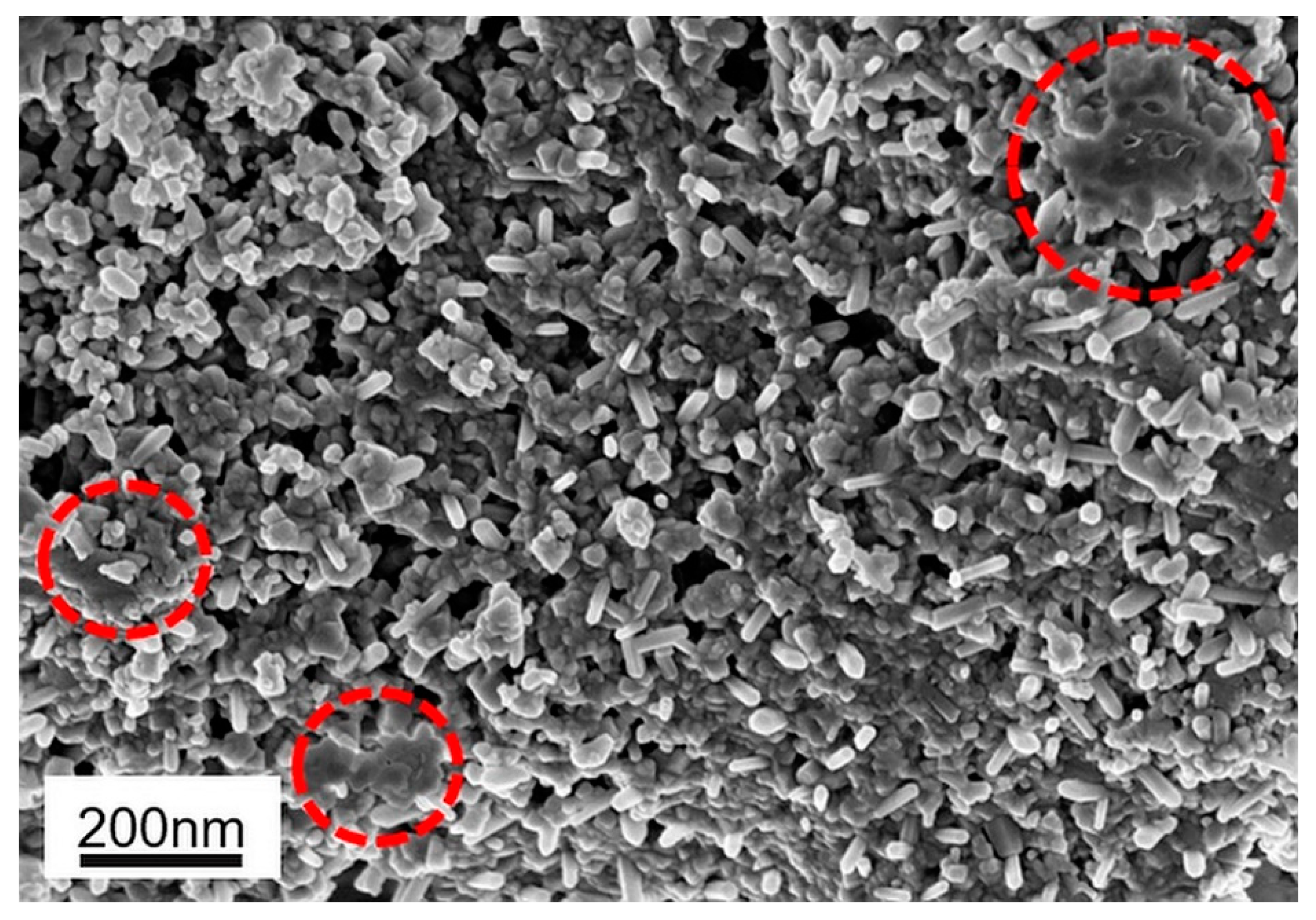
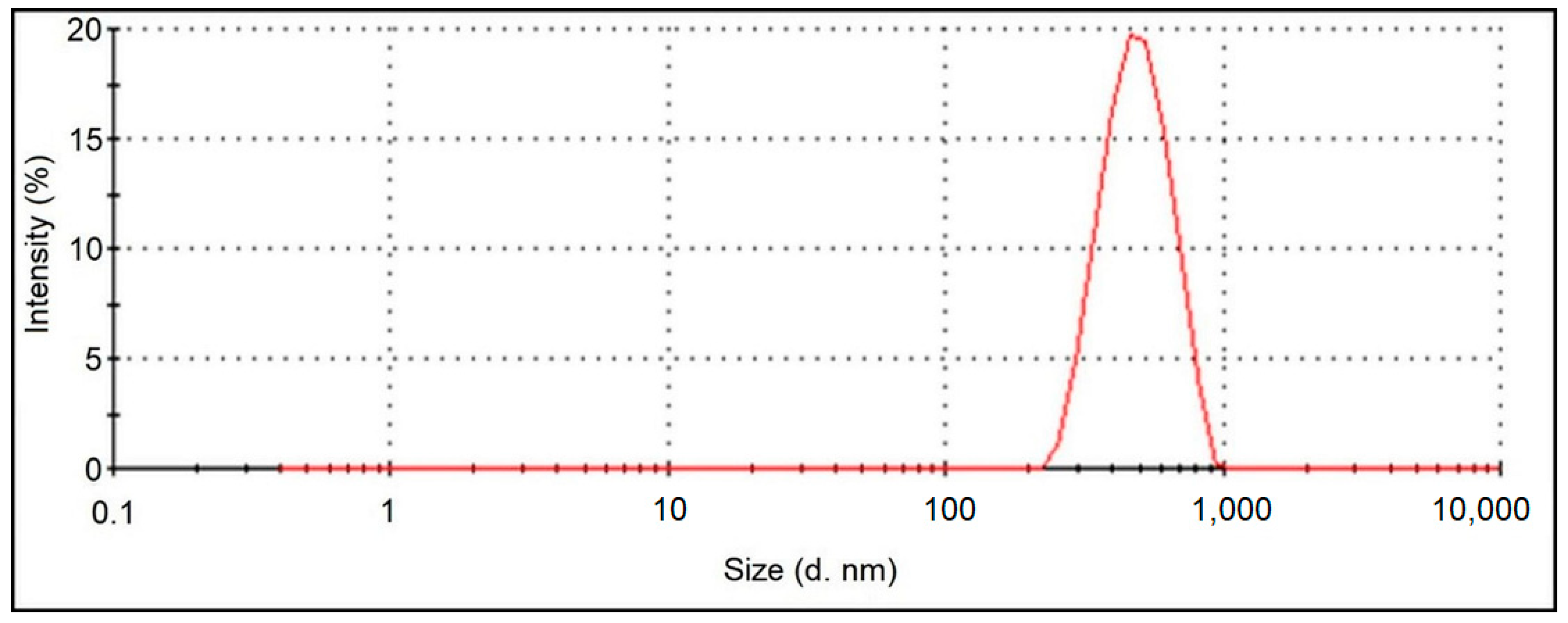
3. Results and Discussion
3.1. Physicochemical Characterisation
3.2. Characterisation of Anode Film
3.2.1. Electrical Conductivity of the Anode
3.2.2. Electrochemical Performance of Anode
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, D.; Li, K.; Yang, J.; Yan, D.; Jia, L.; Chi, B.; Pu, J.; Li, J. High-Performance La0.5(Ba0.75Ca0.25)0.5Co0.8Fe0.2O3-δ Cathode for Proton-Conducting Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2021, 46, 10007–10014. [Google Scholar] [CrossRef]
- Liu, B.; Jia, L.; Chi, B.; Pu, J.; Li, J. A Novel PrBaCo2O5+σ-BaZr0.1Ce0.7Y0.1Yb0.1O3 Composite Cathode for Proton Conducting Solid Oxide Fuel Cells. Compos. B Eng. 2020, 191, 107936. [Google Scholar] [CrossRef]
- Kei Hoa, N.; Rahman, H.A.; Rao Somalu, M. Influence of Silver Addition on the Morphological and Thermal Characteristics of Nickel Oxide-Samarium Doped Ceria Carbonate (NiO-SDCC) Composite Anode. Int. J. Integr. Eng. 2018, 10, 196–201. [Google Scholar] [CrossRef]
- Miyamoto, K.; Koga, H.; Izumi, M.; Mizui, M.; Nishiguchi, H. Study on Fabrication of Anodes for SOFCs with 3D Printing Technology. ECS Trans. 2020, 96, 219–226. [Google Scholar] [CrossRef]
- Su, H.; Hu, Y.H. Progress in Low-Temperature Solid Oxide Fuel Cells with Hydrocarbon Fuels. Chem. Eng. J. 2020, 402, 126235. [Google Scholar] [CrossRef]
- Batool, R.; Gill, R.; Altaf, F.; Ahmad, M.A.; Raza, R.; Khan, M.A.; Hussain, F.; ur Rehman, Z.; Abbas, G. Structural and Electrochemical Study of Ba0.15Cu0.15Ni0.10Zn0.60 Oxide Anode for Low Temperature Solid Oxide Fuel Cell. J. Alloys Compd. 2019, 780, 653–659. [Google Scholar] [CrossRef]
- Escudero, M.J.; Yeste, M.P.; Cauqui, M.Á.; Muñoz, M.Á. Performance of a Direct Methane Solid Oxide Fuel Cell Using Nickel-Ceria-Yttria Stabilized Zirconia as the Anode. Materials 2020, 13, 599. [Google Scholar] [CrossRef]
- Tarutin, A.; Kasyanova, A.; Vdovin, G.; Lyagaeva, J.; Medvedev, D. Nickel-Containing Perovskites, PrNi0.4Fe0.6O3–δ and PrNi0.4Co0.6O3–δ, as Potential Electrodes for Protonic Ceramic Electrochemical Cells. Materials 2022, 15, 2166. [Google Scholar] [CrossRef]
- Ricote, S.; Kee, R.J.; Coors, W.G. Slip Casting and Solid-State Reactive Sintering of BCZY(BaCex Zr0.9−x Y0.1 O3−d)-NiO/BCZY Half-Cells. Membranes 2022, 12, 242. [Google Scholar] [CrossRef]
- Antonova, E.P.; Osinkin, D.A.; Bogdanovich, N.M. On a Variation of the Kinetics of Hydrogen Oxidation on Ni–BaCe(Y,Gd)O3 Anode for Proton Ceramic Fuel Cells. Int. J. Hydrogen Energy 2021, 46, 22638–22645. [Google Scholar] [CrossRef]
- Lv, H.; Jin, Z.; Peng, R.; Liu, W.; Gong, Z. BaCoxFe0.7-xZr0.3O3-δ(0.2≤x≤0.5) as Cathode Materials for Proton-Based SOFCs. Ceram. Int. 2019, 45, 23948–23953. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, X.; Luo, Y.; He, Y.; Liu, H.; Geng, S.; Yu, K.; Dong, Y. Ionic Conduction Mechanism of a Nanostructured BCY Electrolyte for Low-Temperature SOFC. Int. J. Hydrogen Energy 2020, 45, 24108–24115. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.H. Progress in Proton-conducting Oxides as Electrolytes for Low-temperature Solid Oxide Fuel Cells: From Materials to Devices. Energy Sci. Eng. 2021, 9, 984–1011. [Google Scholar] [CrossRef]
- Ismail, A.M.; Menazea, A.A.; Kabary, H.A.; El-Sherbiny, A.E.; Samy, A. The Influence of Calcination Temperature on Structural and Antimicrobial Characteristics of Zinc Oxide Nanoparticles Synthesized by Sol–Gel Method. J. Mol. Struct. 2019, 1196, 332–337. [Google Scholar] [CrossRef]
- El Nahrawy, A.M.; Abou Hammad, A.B.; Bakr, A.M.; Shaheen, T.I.; Mansour, A.M. Sol–Gel Synthesis and Physical Characterization of High Impact Polystyrene Nanocomposites Based on Fe2O3 Doped with ZnO. Appl. Phys. A 2020, 126, 654. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Kalinina, M.V.; Simonenko, N.P.; Simonenko, E.P.; Glumov, O.v.; Mel’nikova, N.A.; Murin, I.v.; Shichalin, O.O.; Papynov, E.K.; Shilova, O.A.; et al. Synthesis of BaCe0.9-xZrxY0.1O3-δ Nanopowders and the Study of Proton Conductors Fabricated on Their Basis by Low-Temperature Spark Plasma Sintering. Int. J. Hydrogen Energy 2019, 44, 20345–20354. [Google Scholar] [CrossRef]
- Skalar, T.; Zupan, K.; Marinšek, M. Microstructure Tailoring of Combustion-Derived Ni-GDC and Ni-SDC Composites as Anode Materials for Intermediate Temperature Solid Oxide Fuel Cells. J. Aust. Ceram. Soc. 2019, 55, 123–133. [Google Scholar] [CrossRef]
- Shazana, M.S.; Nafisah, O.; Md Jani, A.M. Characterization of NiO-BCZY Composite Anode Prepared by One-Step Sol-Gel Method. Solid State Sci. Technol. 2017, 25, 135–141. [Google Scholar] [CrossRef]
- An, H.; Shin, D.; Ji, H.I. Effect of Nickel Addition on Sintering Behavior and Electrical Conductivity of BaCe0.35Zr0.5Y0.15O3-δ. J. Korean Ceram. Soc. 2019, 56, 91–97. [Google Scholar] [CrossRef]
- Pornprasertsuk, R.; Piyaworapaiboon, M.; Jinawath, S. Fabrication of Y-Doped Barium Cerium Zirconate Thin Films by Electrostatic Spray Deposition Technique for Protonic Ceramic Fuel Cell Application. Ceram. Int. 2014, 40, 9319–9326. [Google Scholar] [CrossRef]
- Mustapha, S.; Tijani, J.O.; Ndamitso, M.M.; Abdulkareem, A.S.; Shuaib, D.T.; Amigun, A.T.; Abubakar, H.L. Facile Synthesis and Characterization of TiO2 Nanoparticles: X-Ray Peak Profile Analysis Using Williamson–Hall and Debye–Scherrer Methods. Int. Nano Lett. 2021, 11, 241–261. [Google Scholar] [CrossRef]
- Radhika, D.; Samson, A. Preparation and Characterization of NiO Based Nano-Ceramic Composites as Alternative Anode Materials for Solid Oxide Fuel Cells (SOFCs). J. Nanostruct. 2020, 10, 463–485. [Google Scholar]
- Anwar, M.; Muhammed Ali, S.A.; Abdalla, A.M.; Somalu, M.R.; Muchtar, A. Effect of Sintering Temperature on the Microstructure and Ionic Conductivity of Ce0.8Sm0.1Ba0.1O2-δ Electrolyte. Process. Appl. Ceram. 2017, 11, 67–74. [Google Scholar] [CrossRef]
- Baharuddin, N.A.; Abdul Rahman, N.F.; Rahman, H.; Somalu, M.R.; Azmi, M.A.; Raharjo, J. Fabrication of High-Quality Electrode Films for Solid Oxide Fuel Cell by Screen Printing: A Review on Important Processing Parameters. Int. J. Energy Res. 2020, 44, 8296–8313. [Google Scholar] [CrossRef]
- Somalu, M.R.; Muchtar, A.; Daud, W.R.W.; Brandon, N.P. Screen-Printing Inks for the Fabrication of Solid Oxide Fuel Cell Films: A Review. Renew. Sustain. Energy Rev. 2017, 75, 426–439. [Google Scholar] [CrossRef]
- Aznam, I.; Mah, J.C.W.; Muchtar, A.; Somalu, M.R.; Ghazali, M.J. Electrophoretic Deposition of (Cu,Mn,Co)3O4 Spinel Coating on SUS430 Ferritic Stainless Steel: Process and Performance Evaluation for Solid Oxide Fuel Cell Interconnect Applications. J. Eur. Ceram. Soc. 2021, 41, 1360–1373. [Google Scholar] [CrossRef]
- Zhang, B.; Gong, J.; Wei, K.; Yue, S.; Jiang, C.; Ma, J. The Instructive Role of Mechanical Properties of Polyvinyl Alcohol Film in the Process of YSZ Tape Calendering. Ceram. Int. 2020, 46, 17010–17017. [Google Scholar] [CrossRef]
- Shi, R.; Chen, W.; Hu, W.; Liu, J.; Li, H.; Wang, H.; Sheng, L. Low Melting Point Glass Powder (Glass) Used as an Additive to Zr0.88Y0.08Eu0.04O2-α (ZYE) Electrolyte for Intermediate Temperature SOFCs. Int. J. Electrochem. Sci. 2018, 13, 10821–10828. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Hatakeyama, H. Introduction to Thermal Analysis; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2005; Volume 4, ISBN 1402023545. [Google Scholar]
- Yoo, Y.S.; Namgung, Y.; Bhardwaj, A.; Song, S.J. A Facile Combustion Synthesis Route for Performance Enhancement of La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCF6428) as a Robust Cathode Material for IT-SOFC. J. Korean Ceram. Soc. 2019, 56, 497–505. [Google Scholar] [CrossRef]
- Samat, A.A.; Senari, S.M.; Somalu, M.R.; Muchtar, A.; Hassan, O.H.; Osman, N. Heat Treatment Effect on the Phase and Morphology of NiO-BCZY Prepared by an Evaporation and Decomposition of Solution and Suspension Method. Sains Malays. 2018, 47, 589–594. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Oxygen Vacancy and Dopant Concentration Dependent Magnetic Properties of Mn Doped TiO2 Nanoparticle. Curr. Appl. Phys. 2013, 13, 1025–1031. [Google Scholar] [CrossRef]
- Plekhanov, M.S.; Lesnichyova, A.S.; Stroeva, A.Y.; Ananyev, M.V.; Farlenkov, A.S.; Bogdanovich, N.M.; Belyakov, S.A.; Kuzmin, A.v. Novel Ni Cermets for Anode-Supported Proton Ceramic Fuel Cells. J. Solid State Electrochem. 2019, 23, 1389–1398. [Google Scholar] [CrossRef]
- Mendez Torrecillas, C.; Halbert, G.W.; Lamprou, D.A. A Novel Methodology to Study Polymodal Particle Size Distributions Produced during Continuous Wet Granulation. Int. J. Pharm. 2017, 519, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Baharuddin, N.A.; Muchtar, A.; Somalu, M.R.; Anwar, M.; Muhammed Ali, S.A.; Mah, J. Effects of Sintering Temperature on the Structural and Electrochemical Properties of SrFe0.5Ti0.5O3-δ Perovskite Cathode. Int. J. Appl. Ceram. Technol. 2018, 15, 338–348. [Google Scholar] [CrossRef]
- Sawant, P.; Varma, S.; Gonal, M.R.; Wani, B.N.; Prakash, D.; Bharadwaj, S.R. Effect of Ni Concentration on Phase Stability, Microstructure and Electrical Properties of BaCe0.8Y0.2O3-δ—Ni Cermet SOFC Anode and Its Application in Proton Conducting ITSOFC. Electrochim. Acta 2014, 120, 80–85. [Google Scholar] [CrossRef]
- Jais, A.A.; Ali, S.A.M.; Anwar, M.; Somalu, M.R.; Muchtar, A.; Isahak, W.N.R.W.; Baharudin, N.A.; Lim, K.L.; Brandon, N.P. Performance of Ni/10Sc1CeSZ Anode Synthesized by Glycine Nitrate Process Assisted by Microwave Heating in a Solid Oxide Fuel Cell Fueled with Hydrogen or Methane. J. Solid State Electrochem. 2020, 24, 711–722. [Google Scholar] [CrossRef]
- Somalu, M.R.; Muchtar, A.; Brandon, N.P. Properties of Screen-Printed Nickel/Scandia-Stabilized-Zirconia Anodes Fabricated Using Rheologically Optimized Inks during Redox Cycles. J. Mater. Sci. 2017, 52, 7175–7185. [Google Scholar] [CrossRef]
- Somalu, M.R.; Yufit, V.; Cumming, D.; Lorente, E.; Brandon, N.P. Fabrication and Characterization of Ni/ScSZ Cermet Anodes for IT-SOFCs. Int. J. Hydrogen Energy 2011, 36, 5557–5566. [Google Scholar] [CrossRef]
- Rahman, H.A.; Ng, K.H.; Ahmad, S.; Taib, H.; Mahzan, S.; Salleh, S.M.; Ismail, A.; Muchtar, A. Influence of Microstructure on the Electrochemical Behaviour of LSCF-SDCC. IOP Conf. Ser. Mater. Sci. Eng. 2019, 494, 012062. [Google Scholar] [CrossRef]
- Samat, A.A.; Yusoff, W.N.A.W.; Baharuddin, N.A.; Somalu, M.R.; Muchtar, A.; Osman, N. Electrochemical Performance of Sol-Gel Derived La0.6Sr0.4CoO3-δ Cathode Material for Proton-Conducting Fuel Cell: A Comparison between Simple and Advanced Cell Fabrication Techniques. Process. Appl. Ceram. 2018, 12, 277–286. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, S.K.; Nam, J.T.; Park, J.S. Fabrication of an Electrolyte-Supported Protonic Ceramic Fuel Cell with Nano-Sized Powders of Ni-Composite Anode. Int. J. Hydrogen Energy 2021, 46, 1076–1084. [Google Scholar] [CrossRef]
- Osman, N.; Malek, N.I.A.; Ismail, I.; Habiballah, A.S.; Jani, A.M.M. Development of MCSF (M=La, Ba) Cathode Materials for Proton Conducting Fuel Cell Application. AIP Conf. Proc. 2020, 2221, 020001. [Google Scholar]
- Baral, A.K.; Choi, S.; Kim, B.K.; Lee, J.-H. Processing and Characterizations of a Novel Proton-Conducting BaCe0.35Zr0.50Y0.15O3-δ Electrolyte and Its Nickel-Based Anode Composite for Anode-Supported IT-SOFC. Mater Renew Sustain. Energy 2014, 3, 35. [Google Scholar] [CrossRef]
- Zhang, J.; Lenser, C.; Menzler, N.H.; Guillon, O. Comparison of Solid Oxide Fuel Cell (SOFC) Electrolyte Materials for Operation at 500 °C. Solid State Ion. 2020, 344, 115138. [Google Scholar] [CrossRef]
- Osman, N.; Mazlan, N.A.; Affandi, N.S.M.; Mazlan, N.W.; Md Jani, A.M. Optimization of Electrolyte Performance by Tailoring the Structure and Morphology of Ba(Ce,Zr)O3 Ceramics with Different Types of Surfactants. Ceram. Int. 2020, 46, 27401–27409. [Google Scholar] [CrossRef]
- Bach, M.; Schemmel, T.; Hubálková, J.; Bühringer, M.; Jansen, H.; Aneziris, C.G. Effect of Thermal Treatment Conditions on the Solid-State Synthesis of Barium Zirconate from Barium Carbonate and Monoclinic Zirconia. Ceram. Int. 2021, 47, 25839–25845. [Google Scholar] [CrossRef]
- Pers, P.; Mao, V.; Taillades, M.; Taillades, G. Electrochemical Behavior and Performances of Ni-BaZr0.1Ce0.7Y0.1Yb0.1O3−δ Cermet Anodes for Protonic Ceramic Fuel Cell. Int. J. Hydrogen Energy 2018, 43, 2402–2409. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C. BaFe0.6Co0.3Ce0.1O3-δ as Cathode Materials for Proton-Conducting Solid Oxide Fuel Cells. Ceram. Int. 2016, 42, 10511–10515. [Google Scholar] [CrossRef]
- He, F.; Liang, M.; Wang, W.; Ran, R.; Yang, G.; Zhou, W.; Shao, Z. High-Performance Proton-Conducting Fuel Cell with B-Site-Deficient Perovskites for All Cell Components. Energy Fuels 2020, 34, 11464–11471. [Google Scholar] [CrossRef]
- Shimada, H.; Yamaguchi, Y.; Sumi, H.; Mizutani, Y. Enhanced La0.6Sr0.4Co0.2Fe0.8O3–δ-Based Cathode Performance by Modification of BaZr0.1Ce0.7Y0.1Yb0.1O3–δ Electrolyte Surface in Protonic Ceramic Fuel Cells. Ceram. Int. 2021, 47, 16358–16362. [Google Scholar] [CrossRef]
- Hsu, K.T.; Song, S.M.; Tsai, P.H.; Jang, J.S.C.; Lin, J.C.; Lee, S.W.; Tseng, C.J.; Hung, I.M.; Hsi, C.S. Effect of the Reactive Surface Area of Proton-Conducting Ni–Ba0.8Sr0.2Ce0.6Zr0.2Y0.2O3-δ Anodes on Cell Performance. Ceram. Int. 2019, 45, 14524–14532. [Google Scholar] [CrossRef]





| Ingredients | Name of Ingredients | Weight (wt%) |
| Solid | NiO–BCZY (prepared by sol–gel) | 70.0 |
| Solvent | Terpineol | 27.2 |
| Binder | Ethyl cellulose N7 grade | 1.4 |
| Dispersant | Hypermer KD9 | 1.4 |
| Material | a (Å) | ρ (g cm−3) |
|---|---|---|
| NiO (this work) | 4.1768 | 6.63 |
| NiO (JCPDS card 010780643) | 4.1760 | 6.81 |
| BCZY (this work) | 4.3315 | 6.12 |
| Ba(Ce,Zr)O3 (JCPDS card 010892485) | 4.3436 | 6.30 |
| Element | Mass Percentage from EDX (wt%) | Calculated Mass Percentage (wt%) | Nominal Mole Ratio | Calculated Mole Ratio |
|---|---|---|---|---|
| Ni | 21.40 | 18.73 | 1.00 | 1.00 |
| Ba | 35.60 | 40.11 | 1.00 | 1.00 |
| Ce | 19.10 | 22.10 | 0.54 | 0.60 |
| Zr | 6.20 | 9.59 | 0.36 | 0.30 |
| Y | 2.10 | 2.60 | 0.10 | 0.10 |
| Sintering Temperature (°C) | Film Thickness (µm) | Porosity (%) |
|---|---|---|
| 1200 | 28.95 ± 2.34 | 33.98 ± 0.52 |
| 1300 | 27.29 ± 2.53 | 29.47 ± 0.62 |
| 1400 | 26.18 ± 1.20 | 26.93 ± 0.65 |
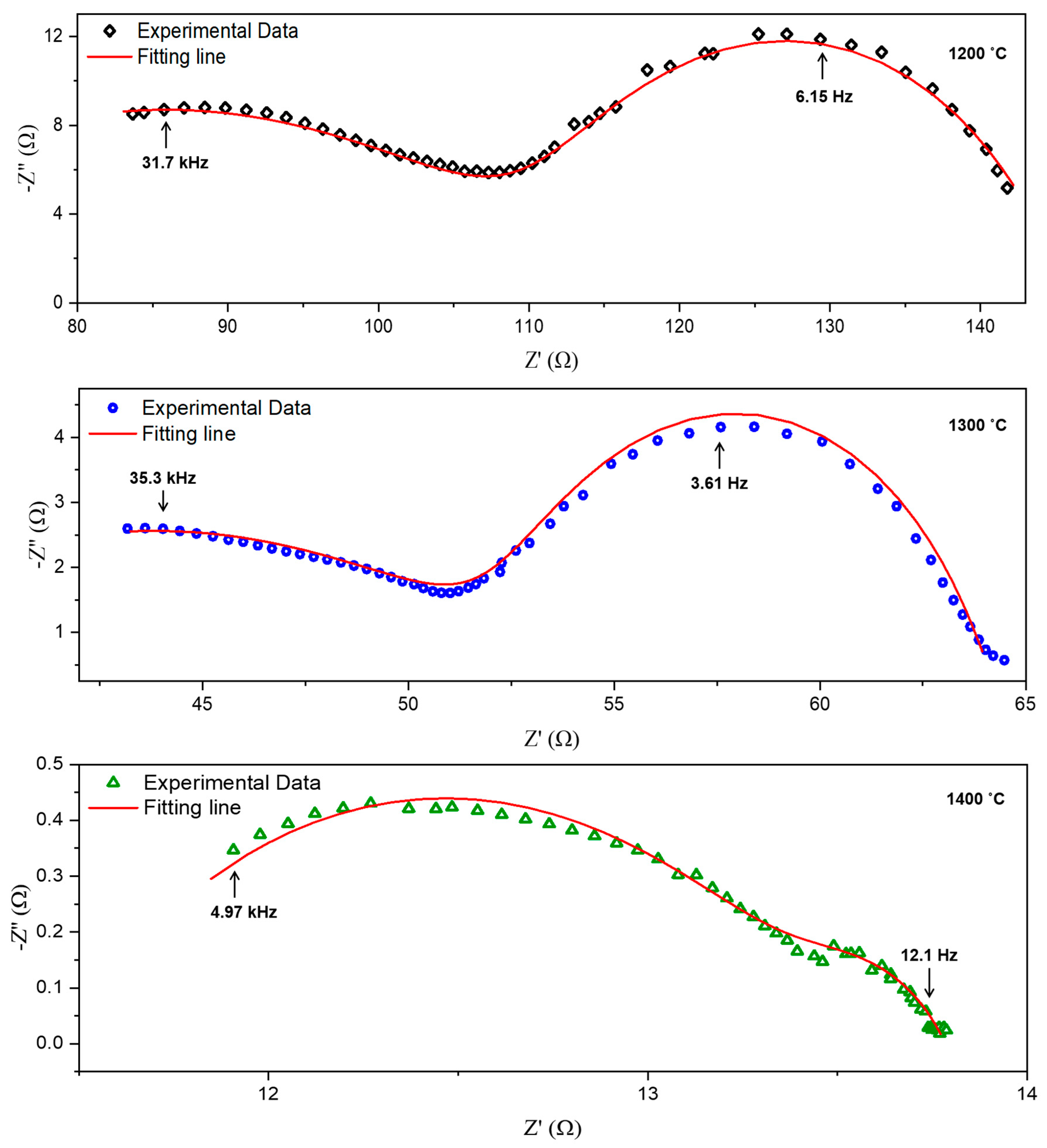
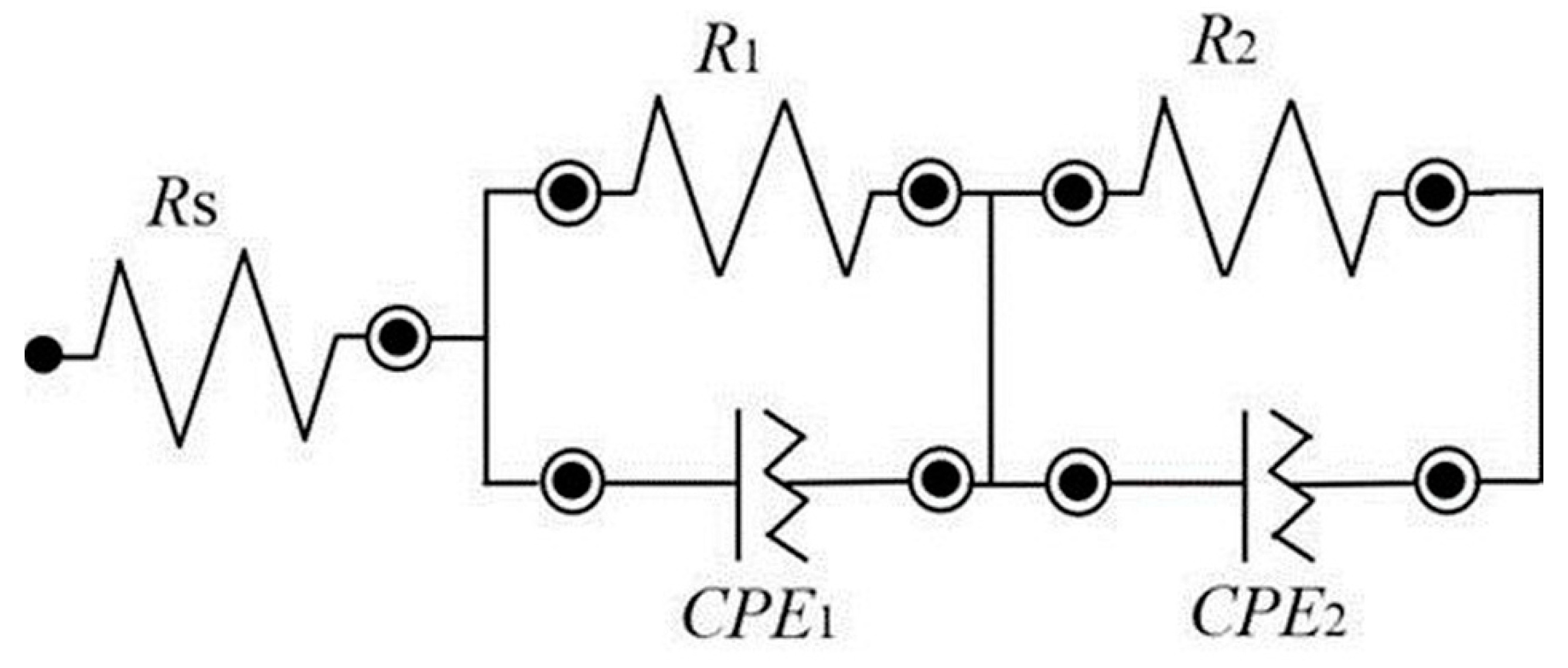
| Sintering Temperature (°C) | Resistance 1, R1 (Ω cm2) | Resistance 2, R2 (Ω cm2) | Area Specific Resistance ASR, (Ω cm2) | Capacitance, C (F) | Frequency, fmax (Hz) | ||
|---|---|---|---|---|---|---|---|
| CPE1 | CPE2 | fCPE1 | fCPE2 | ||||
| 1200 | 55.827 | 32.668 | 16.334 | 9.00 × 10−8 | 7.92 × 10−4 | 3.17 × 104 | 6.15 |
| 1300 | 19.978 | 10.692 | 5.346 | 2.25 × 10−7 | 4.12 × 10−3 | 3.53 × 104 | 3.61 |
| 1400 | 1.982 | 0.348 | 1.165 | 1.62 × 10−5 | 3.78 × 10−2 | 4.97 × 103 | 12.1 |
| Anode | Powder Preparation Method | Anode Preparation Technique | Electrolyte | Sintering Condition | Reducing Condition | Activation Energy (eV) | ASR (Ω cm2) | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 Ni–BCZY | Sol–gel | Screen–printing | 2 BCY | 1400 (3 h) | Humidified (3%H2O) 10%H2–90%N2, 800 °C (2 h) | _ | 1.165 (800 °C, 10%H2–90%N2, 3%H2O) | In this work |
| 3 Ni–BCY | Ball milling | Brush–Painting | 4 BCY | 1200 (2 h) | Wet H2, 900 °C (1 h) | 0.26 | 1.06–0.39 (600–900 °C, wet H2) | [10] |
| 5 Ni–BCGC | Ball milling | Brush–Painting | 6 BCGC | 1200 (2 h) | Wet H2, 900 °C (1 h) | 0.46 | 0.7–0.15 (600–900 °C, wet H2) | [10] |
| 7 Ni–BZCYYb | Ball milling | Dry pressing | 8 BZCYYb-ZnO | 1300 (4 h) | 5%H2 in N2, 700 (2 h) | 0.52 | 0.0245 (600 °C, pure H2) | [48] |
| 9 Ni–BZCYYb | Ball milling | Dry pressing | 10 BZCYYb | 1450 (5 h) | Humidified H2 (~3% H2O) | 0.82 | 0.31 (550 °C), 0.23 (600 °C), 0.19 (650 °C) (~3% H2O) | [49] |
| 11 Ni–BZCY | Sol–gel | Dry pressing | 12 BZCY | 1400 (5 h) | _ | _ | _ | [11] |
| 13 Ni–BZCYYb | Ball milling | Dry pressing | 14 BZCYYb | 1450 (10 h) | 10% H2–90%N2 | 0.85 | 0.15 (600 °C) | [50] |
| 15 Ni–BZCYYb | Ball milling | Extrusion | 16 BZCYYb | 900 (1 h) 1350 (3 h) 950 (1 h) | Humidified H2 (3%H2O) | 1.18 | _ | [51] |
| 17 Ni–BSCZY | Ball milling | Tape casting | 18 BSCIY | 1450 (4 h) | 95%Ar–5%H2, 800 °C (4 h) | 0.74 | _ | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadi, N.H.; Somalu, M.R.; Samat, A.A.; Yusoff, W.N.A.W.; Muchtar, A.; Baharuddin, N.A.; Abdul, M.A.S.; Raharjo, J.; Khaerudini, D.S.; Abdalla, A.M.; et al. Understanding the Impact of Sintering Temperature on the Properties of Ni–BCZY Composite Anode for Protonic Ceramic Fuel Cell Application. Processes 2023, 11, 1902. https://doi.org/10.3390/pr11071902
Hadi NH, Somalu MR, Samat AA, Yusoff WNAW, Muchtar A, Baharuddin NA, Abdul MAS, Raharjo J, Khaerudini DS, Abdalla AM, et al. Understanding the Impact of Sintering Temperature on the Properties of Ni–BCZY Composite Anode for Protonic Ceramic Fuel Cell Application. Processes. 2023; 11(7):1902. https://doi.org/10.3390/pr11071902
Chicago/Turabian StyleHadi, Nur Hanisah, Mahendra Rao Somalu, Abdullah Abdul Samat, Wan Nor Anasuhah Wan Yusoff, Andanastuti Muchtar, Nurul Akidah Baharuddin, Muhammed Ali Shaikh Abdul, Jarot Raharjo, Deni Shidqi Khaerudini, Abdalla M. Abdalla, and et al. 2023. "Understanding the Impact of Sintering Temperature on the Properties of Ni–BCZY Composite Anode for Protonic Ceramic Fuel Cell Application" Processes 11, no. 7: 1902. https://doi.org/10.3390/pr11071902
APA StyleHadi, N. H., Somalu, M. R., Samat, A. A., Yusoff, W. N. A. W., Muchtar, A., Baharuddin, N. A., Abdul, M. A. S., Raharjo, J., Khaerudini, D. S., Abdalla, A. M., & Azad, A. K. (2023). Understanding the Impact of Sintering Temperature on the Properties of Ni–BCZY Composite Anode for Protonic Ceramic Fuel Cell Application. Processes, 11(7), 1902. https://doi.org/10.3390/pr11071902













