Minimising Leachate Wastewater Generated from NaOH-Catalysed Biodiesel Synthesis from Methanol
Abstract
:1. Introduction
2. Literature Review
2.1. Triglyceride Source Selection
2.2. Basic Reagent Selection
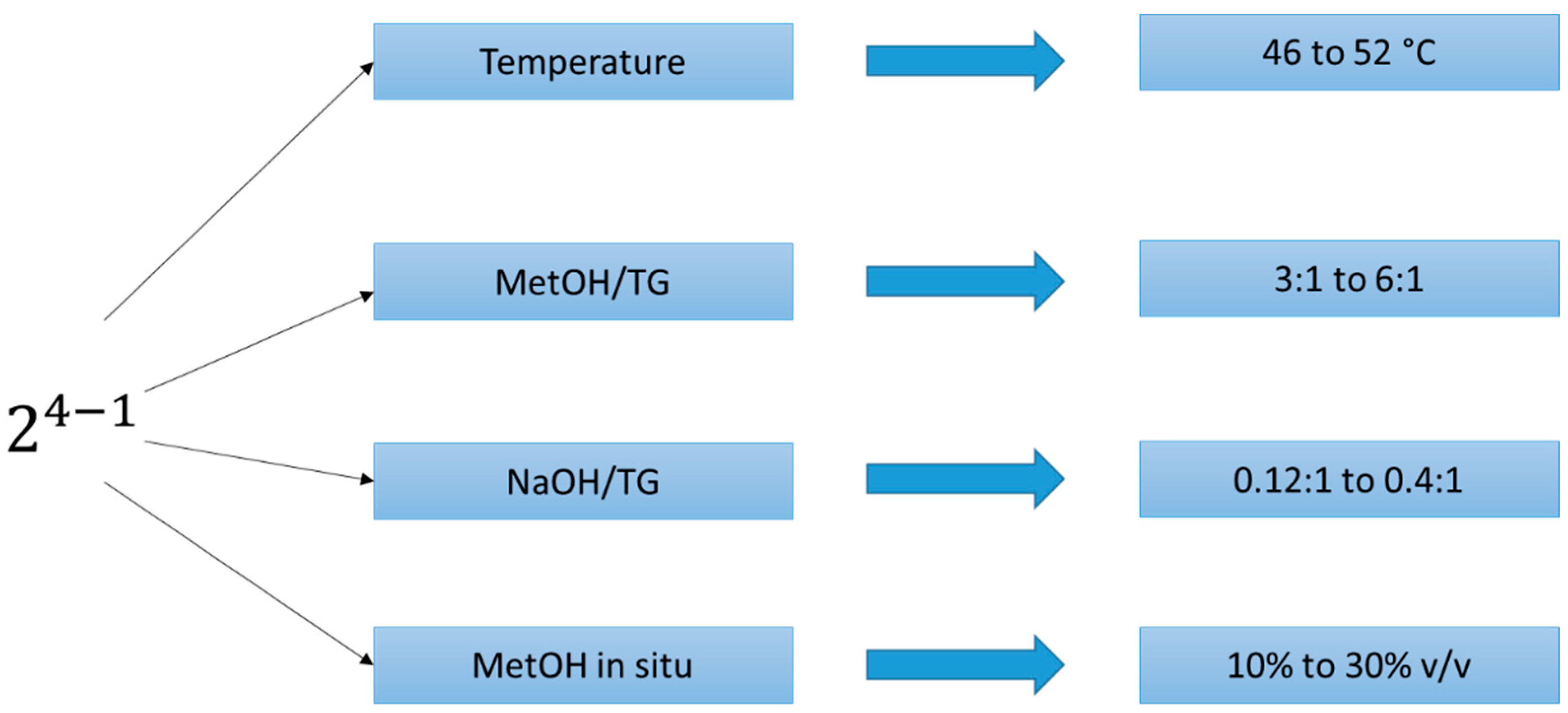
2.3. Experimental Designs
- ✓
- As part of the alkoxide blend (sodium methoxide), called the MetOH/TG ratio.
- ✓
- Combined into the triglyceride system, called MetOH in situ. This addition is bounded by the values 10 vol.% and 30 vol%, because a higher amount of MetOH in situ could cause an increase in the leaching water amount [34].
3. Materials and Methods
3.1. Design for Virgin Oil Experiments
3.2. Design of Experiments for Model Waste Cooking Oil Preparation
4. Results
4.1. Virgin Soybean Oil
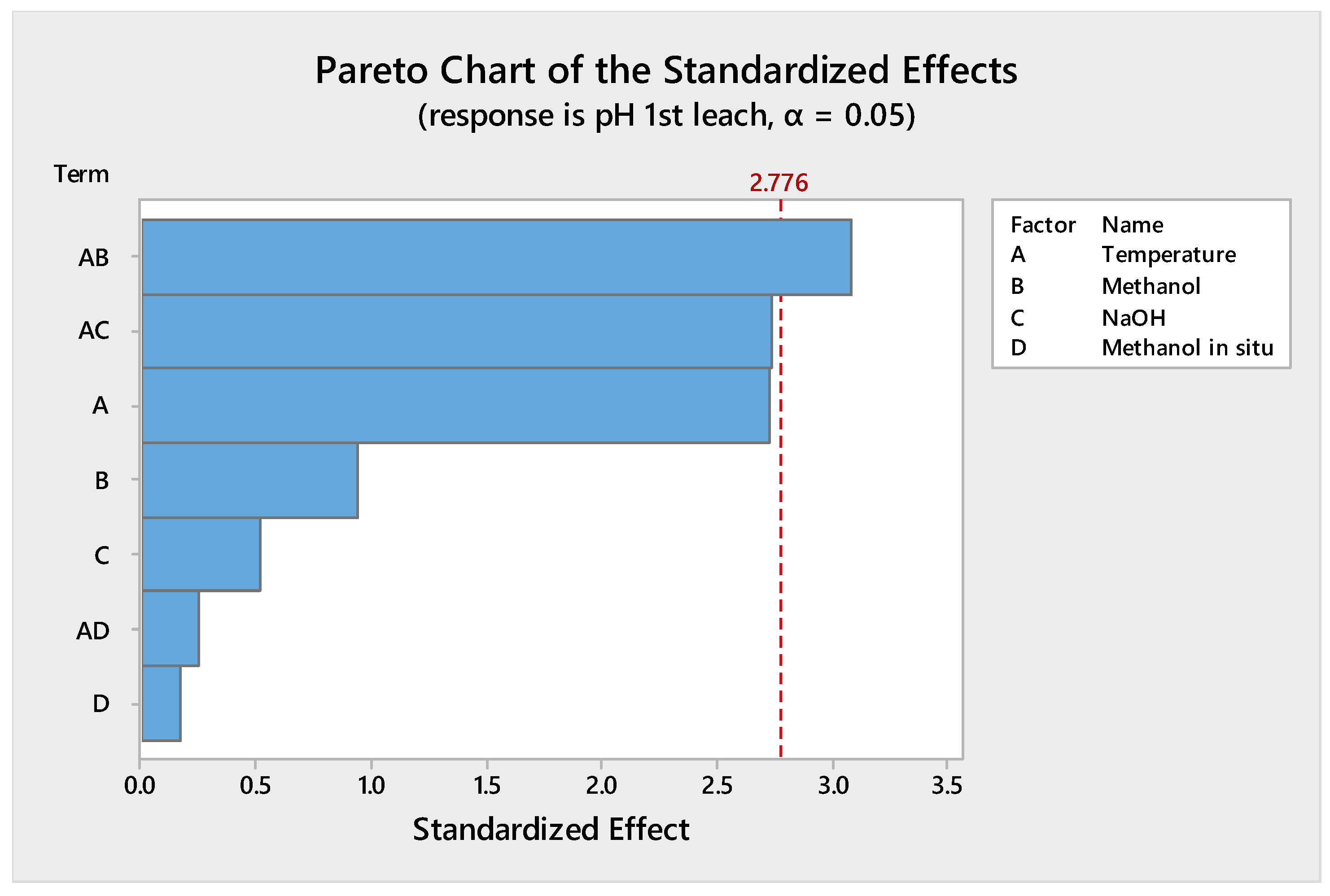
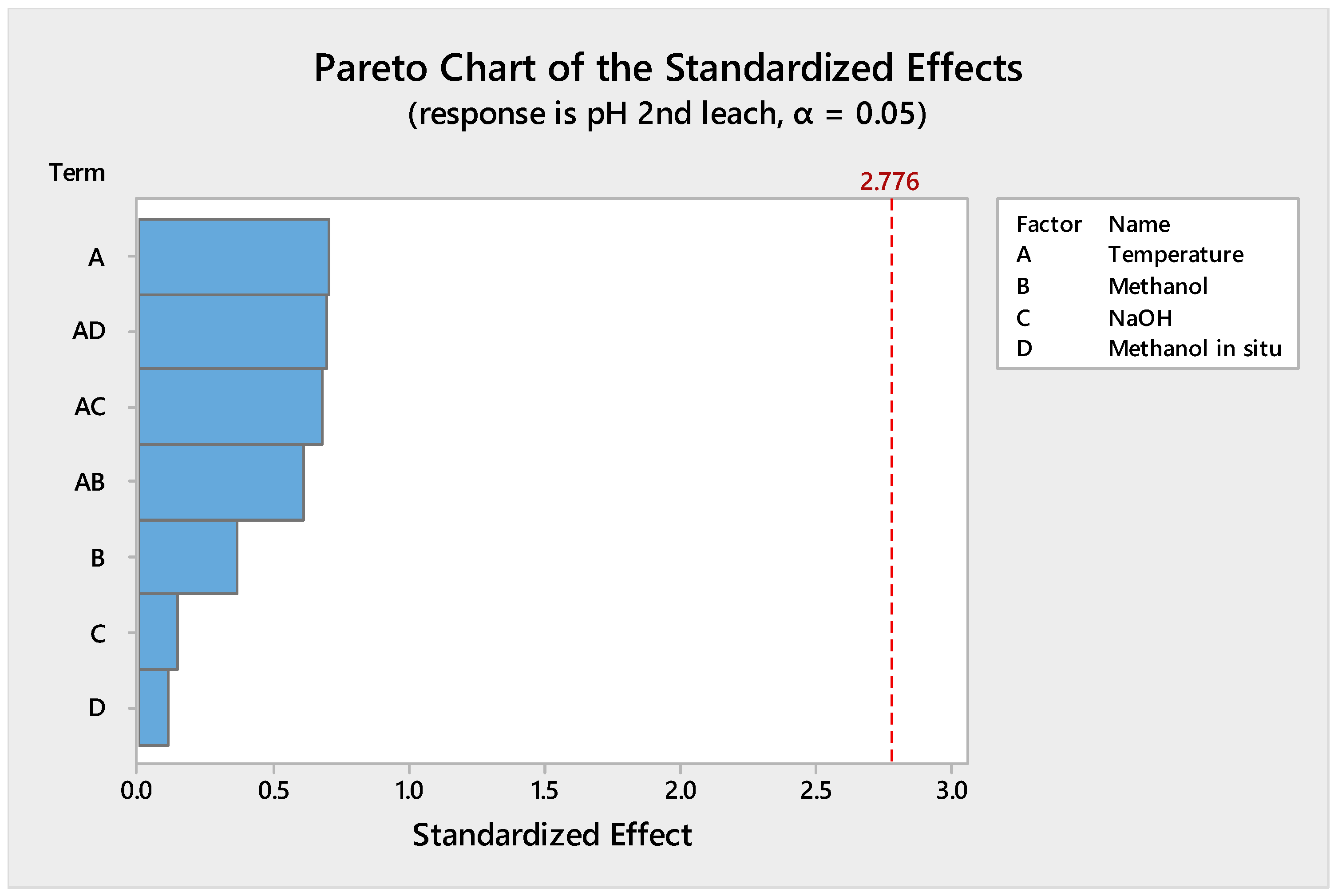
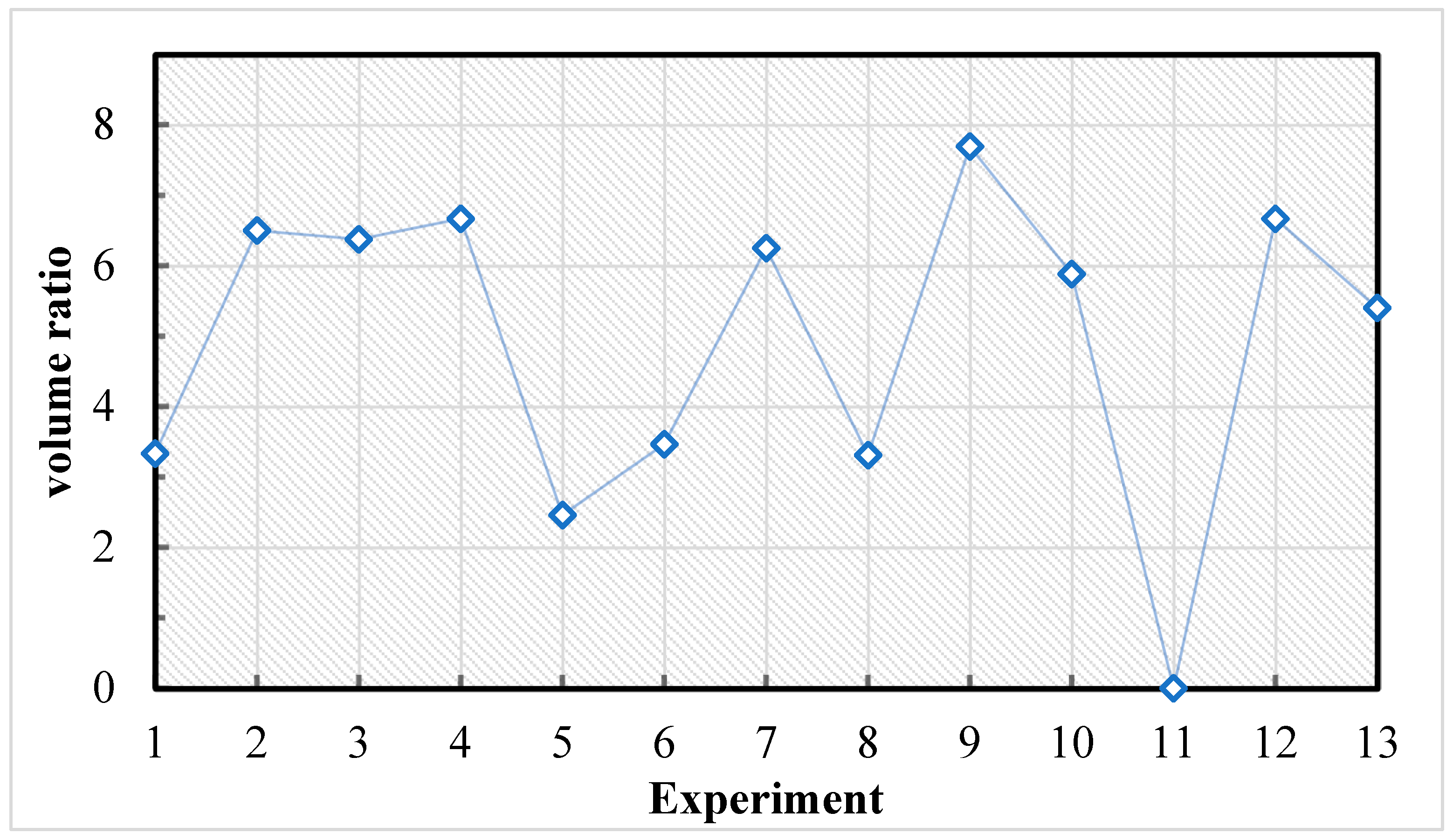
4.1.1. Reduction of the Design of Experiments
4.1.2. Leaching Stage
4.2. Model Waste Cooking Oil
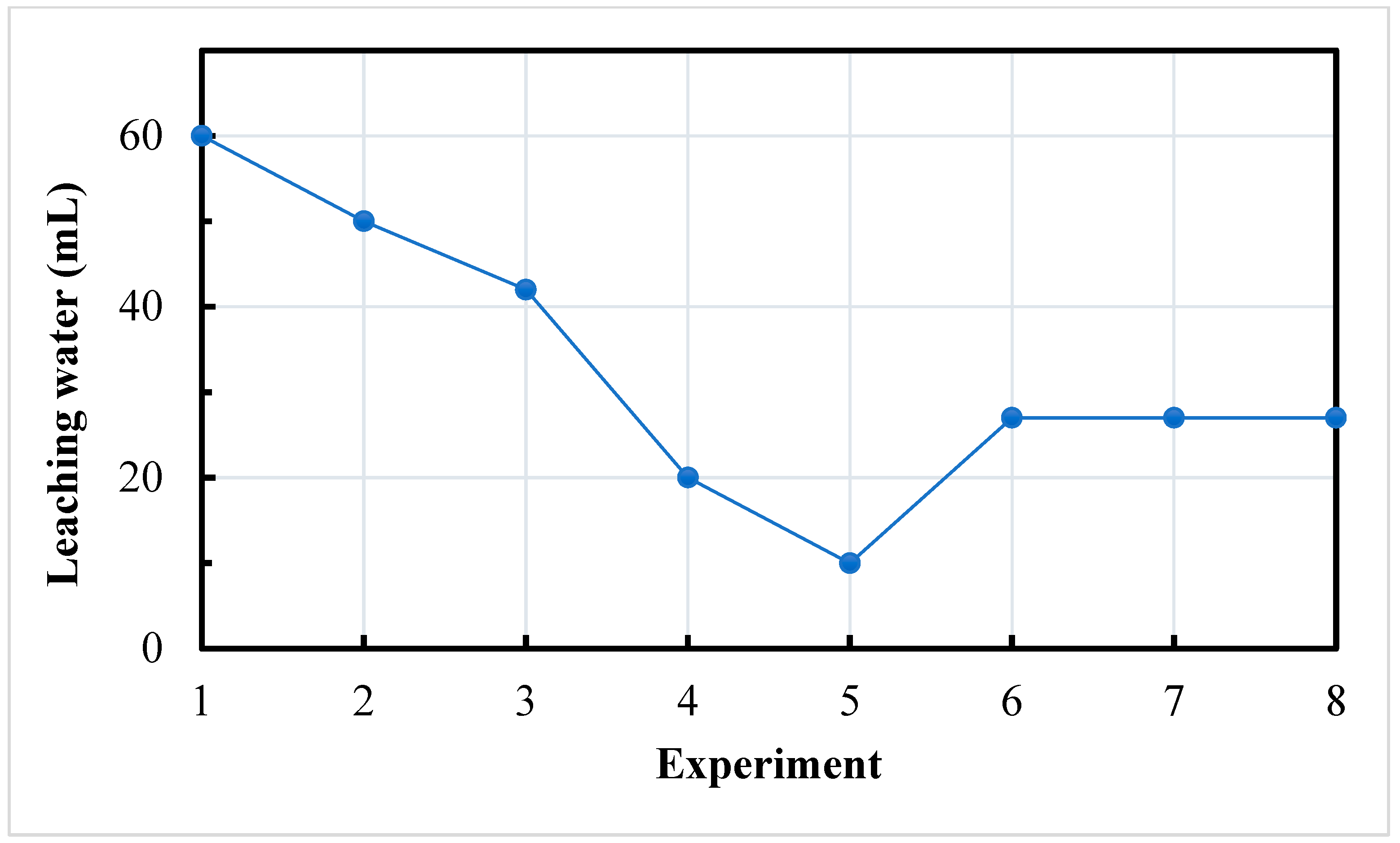
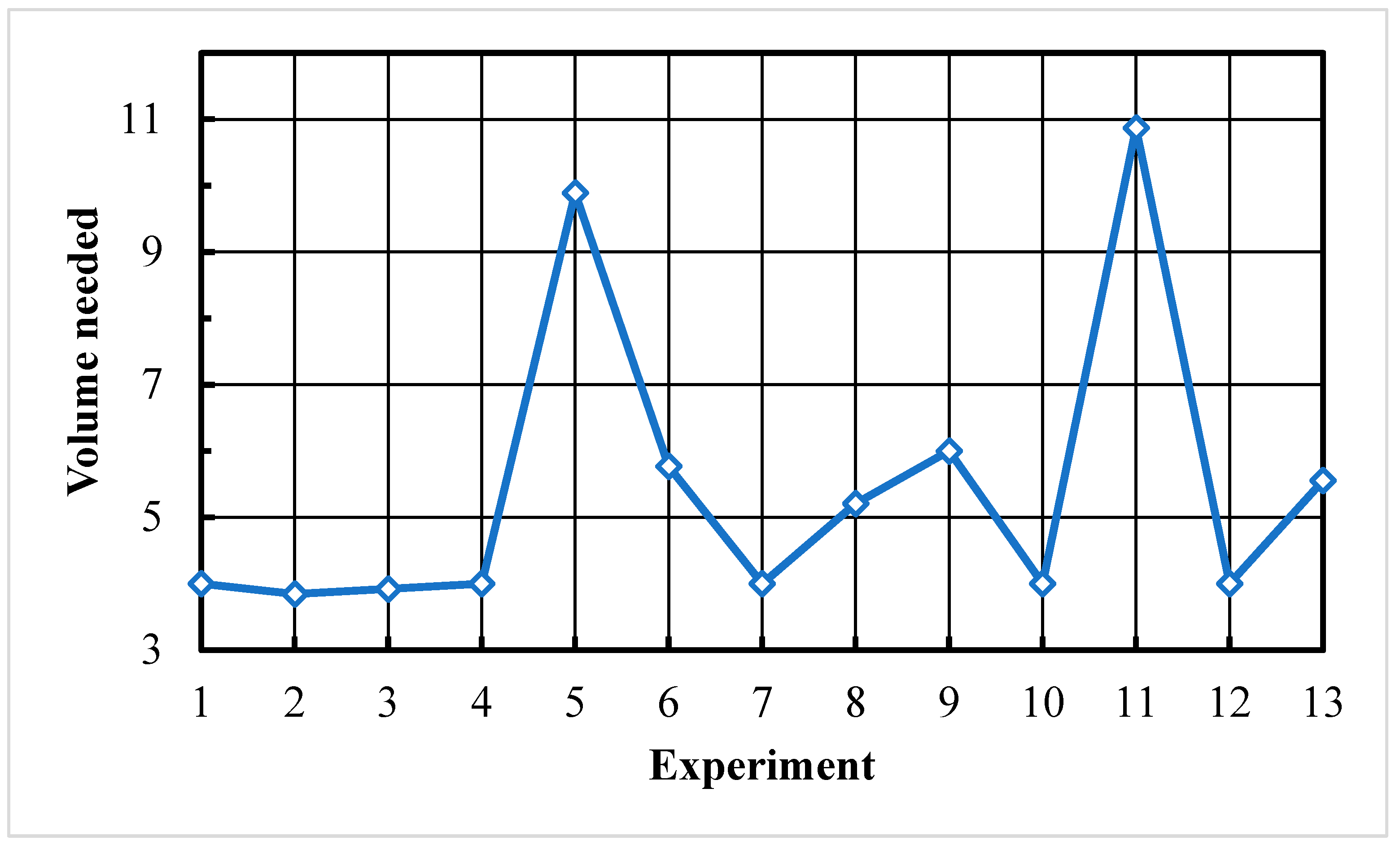
4.2.1. Leaching Water
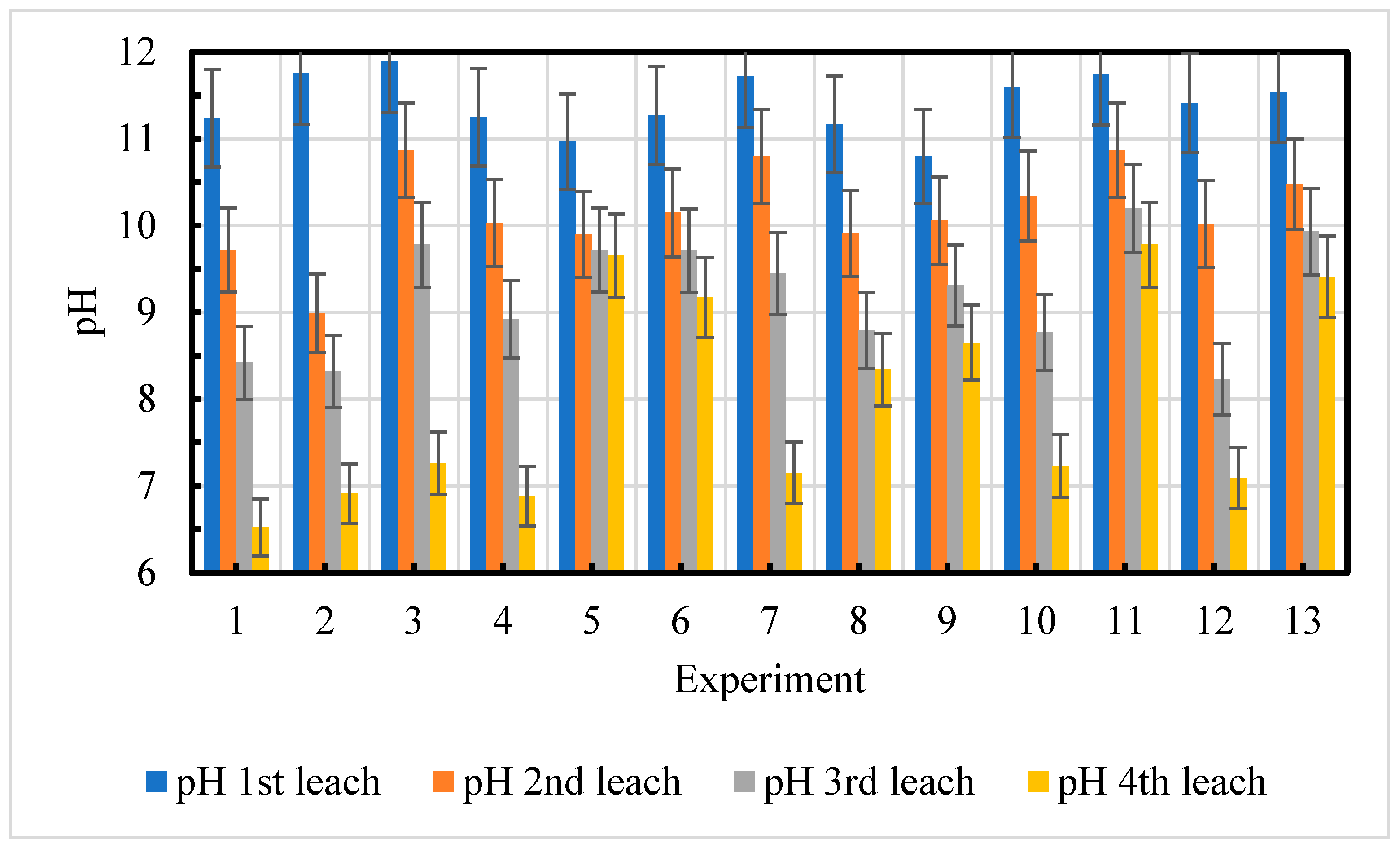
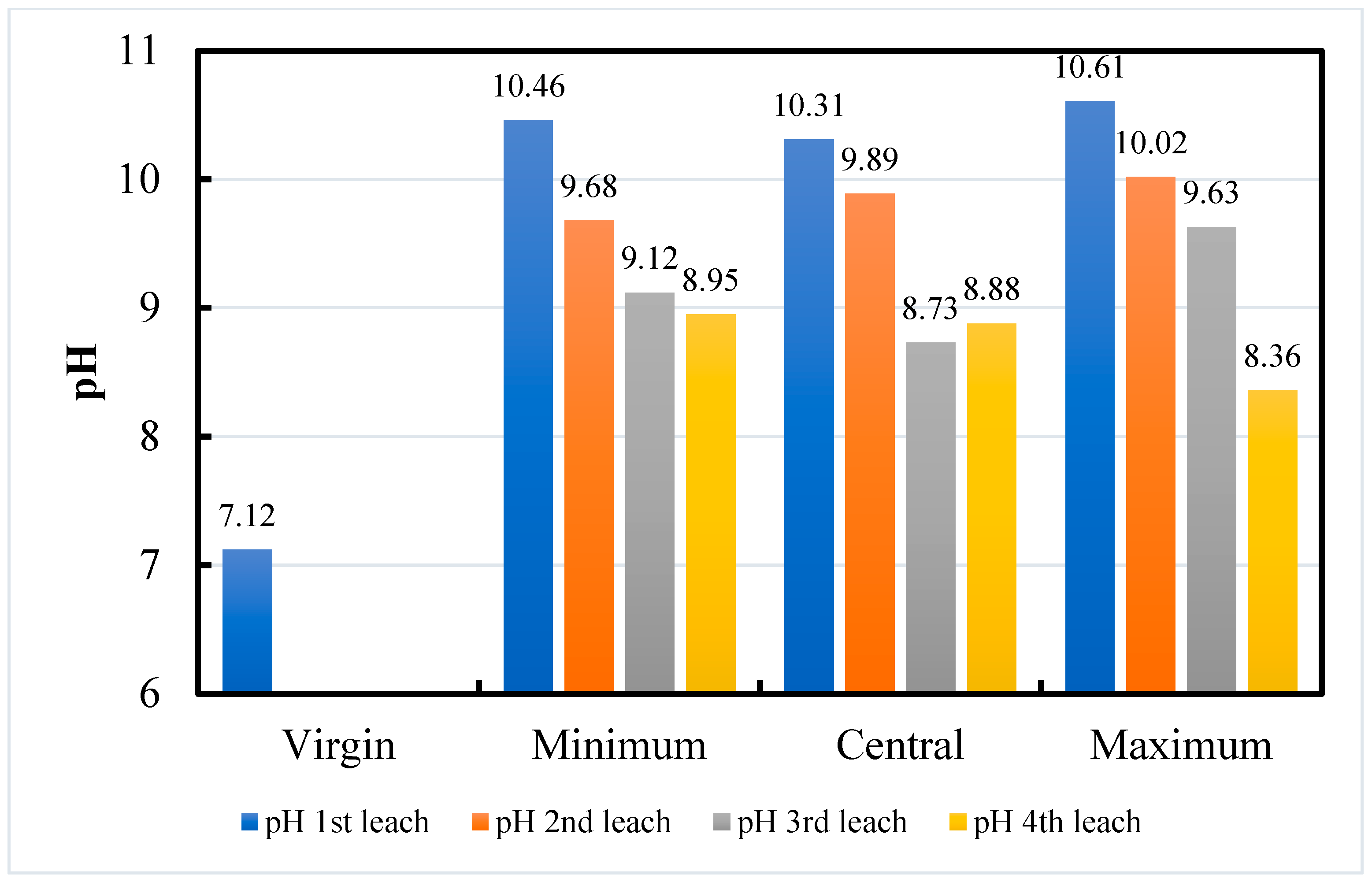
4.2.2. Changes of pH of Leaching Water
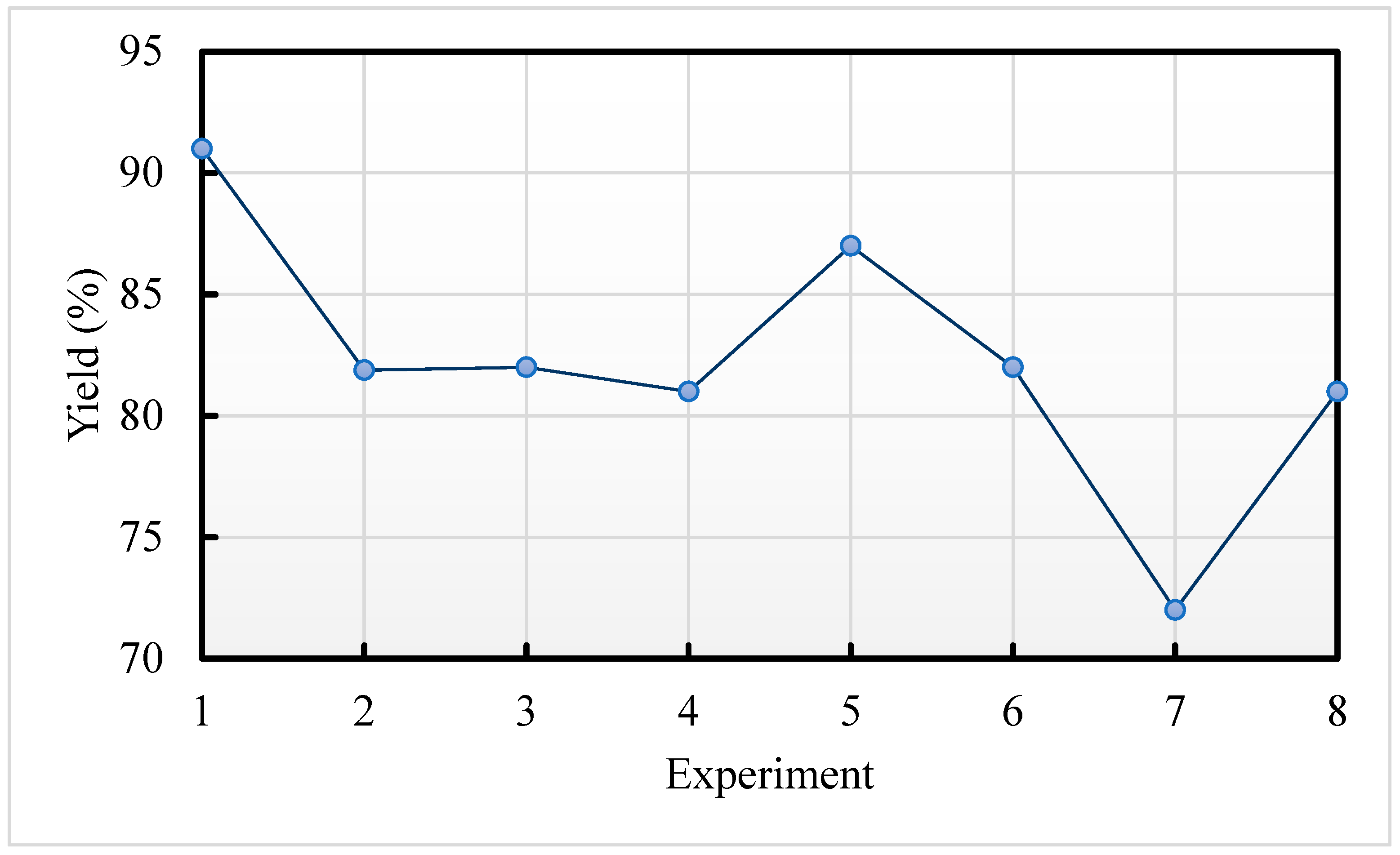
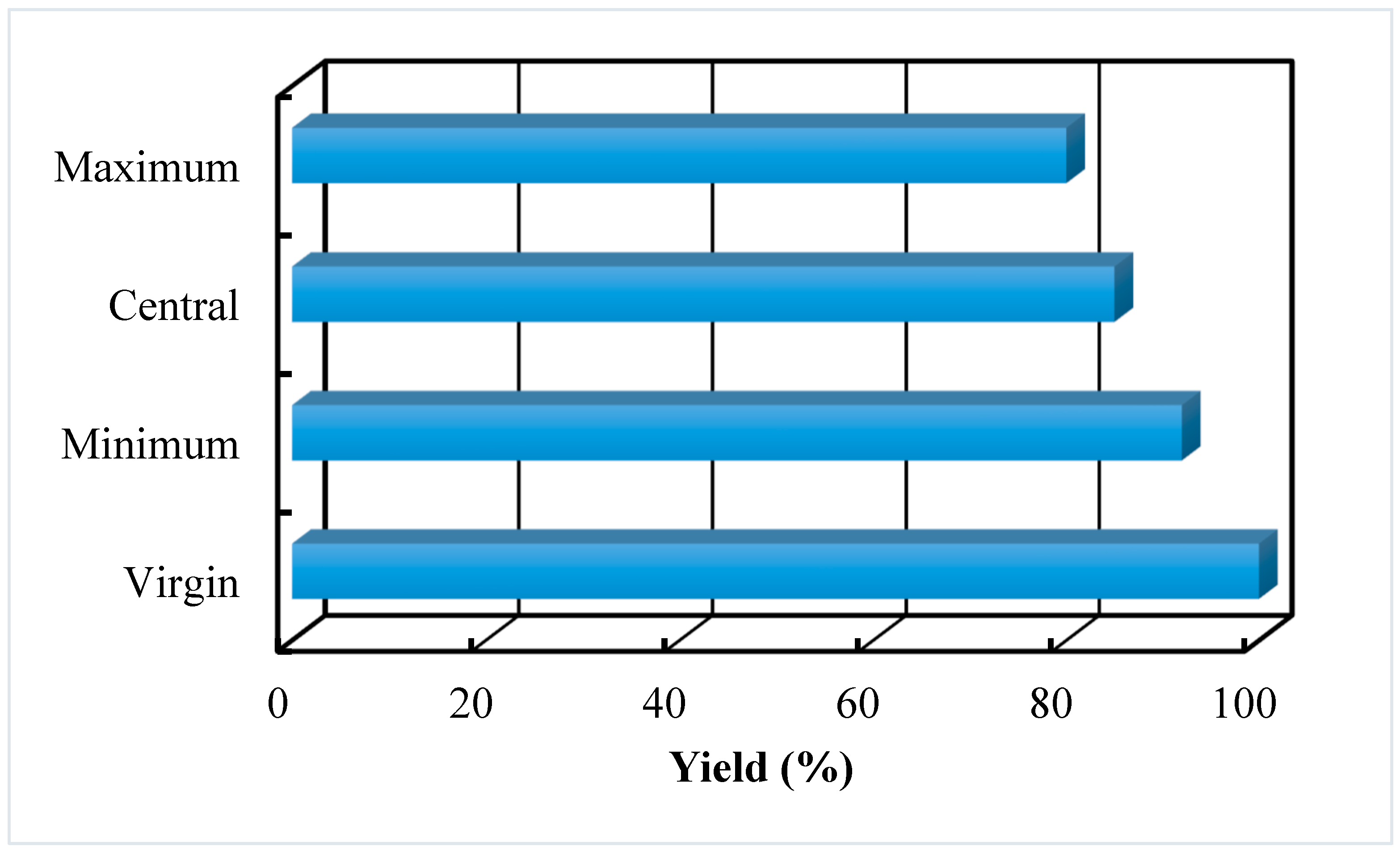
4.2.3. Yield to Biodiesel
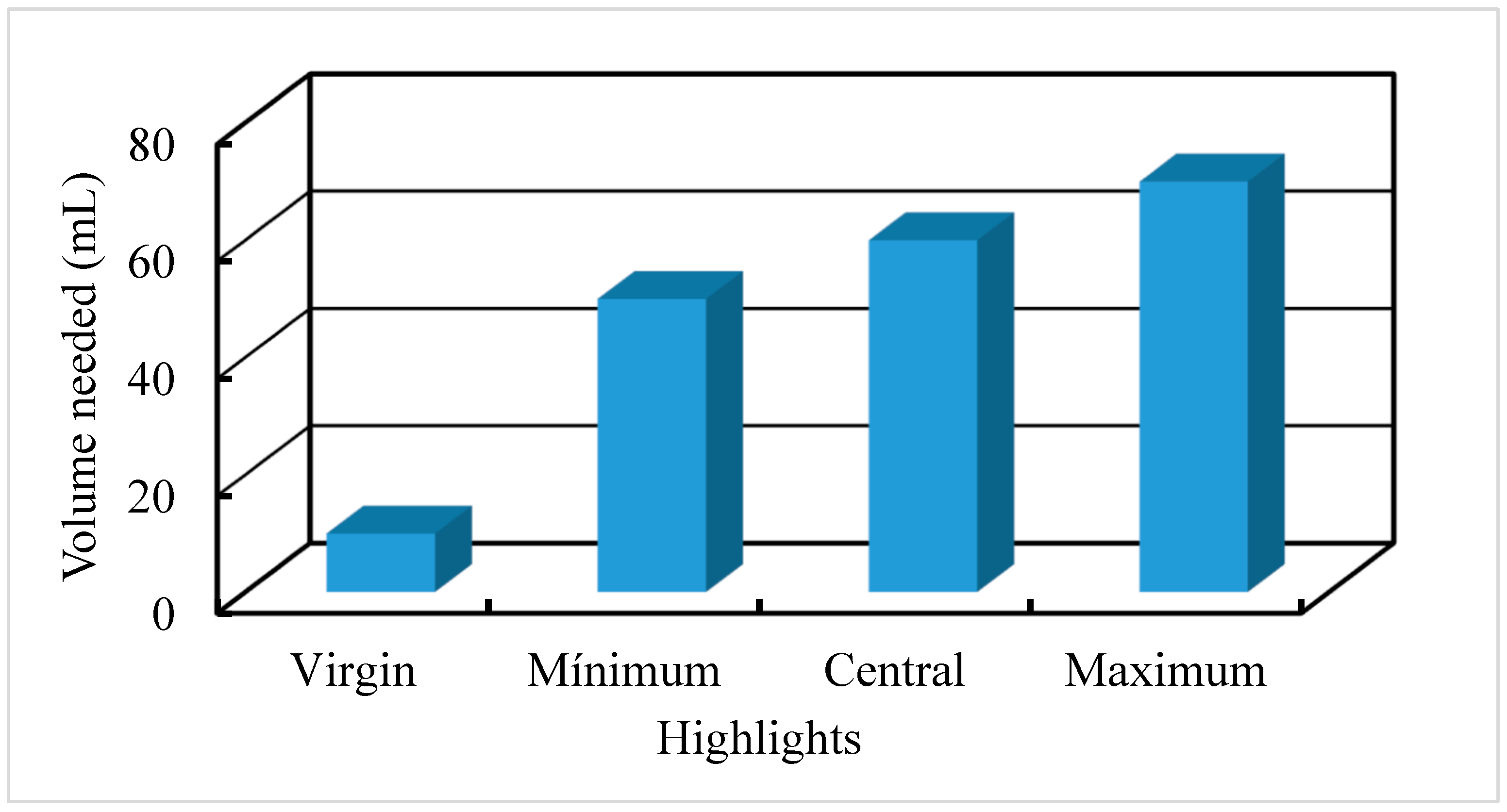
4.2.4. Comparative Leaching Water/Biodiesel
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, Y.V.; Perry, S.; Kleměs, J.J.; Lee, C.T. A review on air emissions assessment: Transportation. J. Clean. Prod. 2018, 194, 673–684. [Google Scholar] [CrossRef]
- De Mello, M.; Young, A.; Villardi, H.; Pessoa, F.; Salgado, A. Biodiesel production by the methylic-alkaline and ethylic-enzymatic routes: Discussion of some environmental aspects. J. Clean. Prod. 2017, 144, 347–357. [Google Scholar] [CrossRef]
- Mikulcic, H.; Baleta, J.; Klemes, J.J. Sustainability through combined development of energy, water and environment systems. J. Clean. Prod. 2020, 251, 119727. [Google Scholar] [CrossRef]
- Young, A.F.; Pessoa, F.L.P.; Queiroz, E.M. Comparison Between Biodiesel Production from Soybean Oil and Palm Oil with Ethanol: Design and Economic Evaluation. Chem. Eng. Trans. 2015, 43, 325–330. [Google Scholar]
- Dickinson, S.; Mientus, M.; Frey, D.; Hajibashi, A.; Ozturki, S.; Shaikh, F.; Sengupta, D.; El-Halwagi, M. A review of biodiesel production from microalgae. Clean Technol. Environ. Policy 2017, 19, 637–668. [Google Scholar] [CrossRef]
- Monari, C.; Righi, S.; Olsen, S.I. Greenhouse gas emissions and energy balance of biodiesel production from microalgae cultivated in photobioreactors in Denmark: A life-cycle modeling. J. Clean. Prod. 2016, 112, 4084–4092. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, S.; Kagawa, S.; Okamoto, S. Environmental and economic performance of a biodiesel plant using waste cooking oil. J. Clean. Prod. 2015, 101, 245–250. [Google Scholar] [CrossRef]
- Jamil, F.; Al-Muhatseb, A.H.; Myint, M.T.Z.; Al-Hinai, M.; Al-Haj, L.; Baawain, M.; AlAbri, M.; Kumar, G.; Atabani, A.E. Biodiesel production by valorizing waste Phoenix dactylifera L. Kernel oil in the presence of synthesized heterogeneous metallic oxide catalyst (Mn@MgO-ZrO2). Energy Convers. Manag. 2018, 155, 128–137. [Google Scholar] [CrossRef]
- Han, H.; Weiliang, C.; Jingchang, Z. Preparation of biodiesel from soybean oil using supercritical methanol and CO2 as co-solvent. Process Biochem. 2005, 40, 3148–3151. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Miguel, V.U.; Errazu, A.F. Techno-economic study of different alternatives for biodiesel production. Fuel Process. Technol. 2008, 89, 740–748. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Kumari, D. Chemical compositions, properties, and standards for different generation biodiesels: A review. Fuel 2019, 253, 60–71. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2009, 14, 200–216. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Liu, L.; Mu, B.; Li, W.; Xu, H.; Yang, J.; Yang, Y. Clean cotton dyeing in circulated dyebath of waste cooking oil: A feasible industrialization strategy for pollution minimization. J. Clean. Prod. 2021, 278, 123799. [Google Scholar] [CrossRef]
- Rodrigues Machado, E.; Marmesat, S.; Abrantes, S.; Dobarganes, C. Uncontrolled variables in frying studies: Differences in repeatability between thermoxidation and frying experiments. Grasas Aceites 2007, 58, 283–288. [Google Scholar]
- Ruiz-Méndez, M.V.; Marmesat, S.; Liotta, A.; Dobarganes, M.C. Analysis of used frying fats for the production of biodiesel. Grasas Aceites 2008, 59, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Sharma, D.; Sonu, S.L.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of biodiesel production from waste cooking oil and its use as fuel in compression ignition engines: 3rd generation cleaner feedstock. J. Clean. Prod. 2021, 307, 127299. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Dalai, A.K. Waste Cooking OilAn Economical Source for Biodiesel: A Review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Sander, A.; Antonije Koscak, M.; Kosir, D.; Milosavljevic, N.; Parlov Vukovic, J.; Magic, L. The influence of animal fat type and purification conditions on biodiesel quality. Renew. Energy 2018, 118, 752–760. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P. Review of process parameters for biodiesel production from different feedstocks. Renew. Sustain. Energy Rev. 2016, 62, 1063–1071. [Google Scholar] [CrossRef]
- Leong, W.-H.; Lim, J.-W.; Lam, M.-K.; Uemura, Y.; Ho, Y.-C. Third generation biofuels: A nutritional perspective in enhancing microbial lipid production. Renew. Sustain. Energy Rev. 2018, 91, 950–961. [Google Scholar] [CrossRef]
- Mofijur, M.; Arafat Siddiki, S.Y.; Ahmed, M.B.; Djavanroodi, F.; Fattah, I.M.R.; Ong, H.C.; Chowdhury, M.A.; Mahlia, T.M.I. “Effect of nanocatalysts on the transesterification reaction of first, second and third generation biodiesel sources—A mini-review”. Chemosphere 2020, 270, 128642. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.J.; Zaini, T.; Mahlia, T.M.I.; Azad, A.K. Elemental, morphological and thermal analysis of mixed microalgae species from drain water. Renew. Energy 2019, 131, 617–624. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; Sulaiman, N.M.N. “The effects of catalysts in biodiesel production: A review”. J. Ind. Eng. Chem. 2013, 19, 14–26. [Google Scholar] [CrossRef]
- Mat Yasin, M.H.; Mamat, R.; Najafi, G.; Ali, O.M.; Yusop, A.F.; Ali, M.H. Potentials of palm oil as new feedstock oil for a global alternative fuel: A review. Renew. Sustain. Energy Rev. 2017, 79, 1034–1049. [Google Scholar] [CrossRef] [Green Version]
- Adepoju, F.T.; Betiku, E. Methanolysis optimization of sesame (Sesamum indicum) oil to biodiesel and fuel quality characterization. Int. J. Energy Environ. Eng. 2013, 4, 9. [Google Scholar]
- Loizides, M.I.; Loizidou, X.L.; Orthodoxou, D.L.; Petsa, D. Circular Bioeconomy in Action: Collection and Recycling of Domestic Used Cooking Oil through a Social, Reverse Logistics System. Recycling 2019, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, J.; Miguel, V.; Errazu, A. Possible methods for biodiesel production. Renew. Sustain. Energy Rev. 2007, 11, 1300–1311. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Leung, Y.D.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Dermibas, A. Comparison of transesterification methods for production of biodiesel from vegetable oils and fats. Energy Conserv. Manag. 2008, 49, 125–130. [Google Scholar]
- Vicente, G.; Martínez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dube, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour. Technol. 2003, 90, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Arcos, J.; Sandoval-Salas, F.; Del Ángel-Ramos, G. Evaluación del efecto de cantidad de catalizador y relación metanol/aceite para la producción de biodiesel a partir del Cocos nucifera L. Rev. Energía Química Física 2016, 3, 46–55. [Google Scholar]
- Leung, D.Y.C.; Koo, B.C.P.; Guo, Y. Degradation of biodiesel under different storage conditions. Bioresour. Technol. 2006, 97, 250–256. [Google Scholar] [CrossRef]
- Eevera, T.; Rajendran, K.; Saradha, S. Biodiesel production process optimization and characterization to assess the suitability of the product for varied environmental conditions. Renew. Energy 2009, 34, 762–765. [Google Scholar] [CrossRef]
- Pérez-Méndez, M.A.; Jiménez-García, G.; Huirache-Acuña, R.; Maya-Yescas, R. Estimation of Reaction Rates of Transesterification Pathways. Front. Chem. Eng. 2021, 3, 673970. [Google Scholar] [CrossRef]
- Safari, A.; Salamat, R.; Baik, O.D. A review on heat and mass transfer coefficients during deep-fat frying: Determination methods and influencing factors. J. Food Eng. 2018, 230, 114–123. [Google Scholar] [CrossRef]
- Cao, G.; Ruan, D.; Chen, Z.; Hong, Y.; Cai, Z. Recent developments and applications of mass spectrometry for the quality and safety assessment of cooking oil. TrAC Trends Anal. Chem. 2017, 96, 201–211. [Google Scholar] [CrossRef]
- Lam, M.; Lee, K.; Mohamed, A. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef]

| Vegetable Oil | Free Fatty Acid Composition, wt.% | Acid Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 16:1 | 18:0 | 20:0 | 22:0 | 24:0 | 18:1 | 22:1 | 18:2 | 18:3 | ||
| Corn | 11.67 | 1.85 | 0.24 | 0.00 | 0.00 | 25.16 | 0.00 | 60.60 | 0.48 | 0.11 |
| Cotton | 28.33 | 0.89 | 0.00 | 0.00 | 0.00 | 13.27 | 0.00 | 57.51 | 0.00 | 0.07 |
| Crambe | 20.7 | 0.70 | 2.09 | 0.80 | 1.12 | 18.86 | 58.50 | 9.00 | 6.85 | 0.36 |
| Peanut | 11.38 | 2.39 | 1.32 | 2.52 | 1.23 | 48.28 | 0.00 | 31.95 | 0.93 | 0.20 |
| Rapeseed | 3.49 | 0.85 | 0.00 | 0.00 | 0.00 | 64.4 | 0.00 | 22.30 | 8.23 | 1.14 |
| Soybean | 11.75 | 3.15 | 0.00 | 0.00 | 0.00 | 23.26 | 0.00 | 55.53 | 6.31 | 0.20 |
| Sunflower | 6.08 | 3.26 | 0.00 | 0.00 | 0.00 | 16.93 | 0.00 | 73.73 | 0.00 | 0.15 |
| Compound | Temperature °C | Alcohol (Molar Ratio Alcohol/Oil) | Added Amount wt.% | Reaction Time H | Yield to BD mol% | Observations | References |
|---|---|---|---|---|---|---|---|
| NaOH | 60 | Methanol (7:1) | 1.1 | 0.33 | 88.80% | Soap production, cheap | [30] |
| KOH | 87 | Methanol (9:1) | 6.0 | 2 | 87% | expensive | [31] |
| Monitored Parameter | Virgin Oil | BEP | WCO * |
|---|---|---|---|
| Leaching water volume | 4:1 | 1:1 | 5:1 |
| pH after 4th leaching | 7.12 | 7.10 * | 8.36 |
| Yield to biodiesel production | 98% | 87% | 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Méndez, M.A.; Fraga-Cruz, G.S.; Jiménez-García, G.; Maya-Yescas, R.; Nápoles-Rivera, F. Minimising Leachate Wastewater Generated from NaOH-Catalysed Biodiesel Synthesis from Methanol. Processes 2023, 11, 1946. https://doi.org/10.3390/pr11071946
Pérez-Méndez MA, Fraga-Cruz GS, Jiménez-García G, Maya-Yescas R, Nápoles-Rivera F. Minimising Leachate Wastewater Generated from NaOH-Catalysed Biodiesel Synthesis from Methanol. Processes. 2023; 11(7):1946. https://doi.org/10.3390/pr11071946
Chicago/Turabian StylePérez-Méndez, Mario Alberto, Guadalupe Selene Fraga-Cruz, Gladys Jiménez-García, Rafael Maya-Yescas, and Fabricio Nápoles-Rivera. 2023. "Minimising Leachate Wastewater Generated from NaOH-Catalysed Biodiesel Synthesis from Methanol" Processes 11, no. 7: 1946. https://doi.org/10.3390/pr11071946








