Laboratory Test of Fluid Physical Property Parameters of Well Fluid Containing CO2
Abstract
:1. Introduction
2. Experimental Apparatus and Method for Physical Property Parameters of Well Fluid
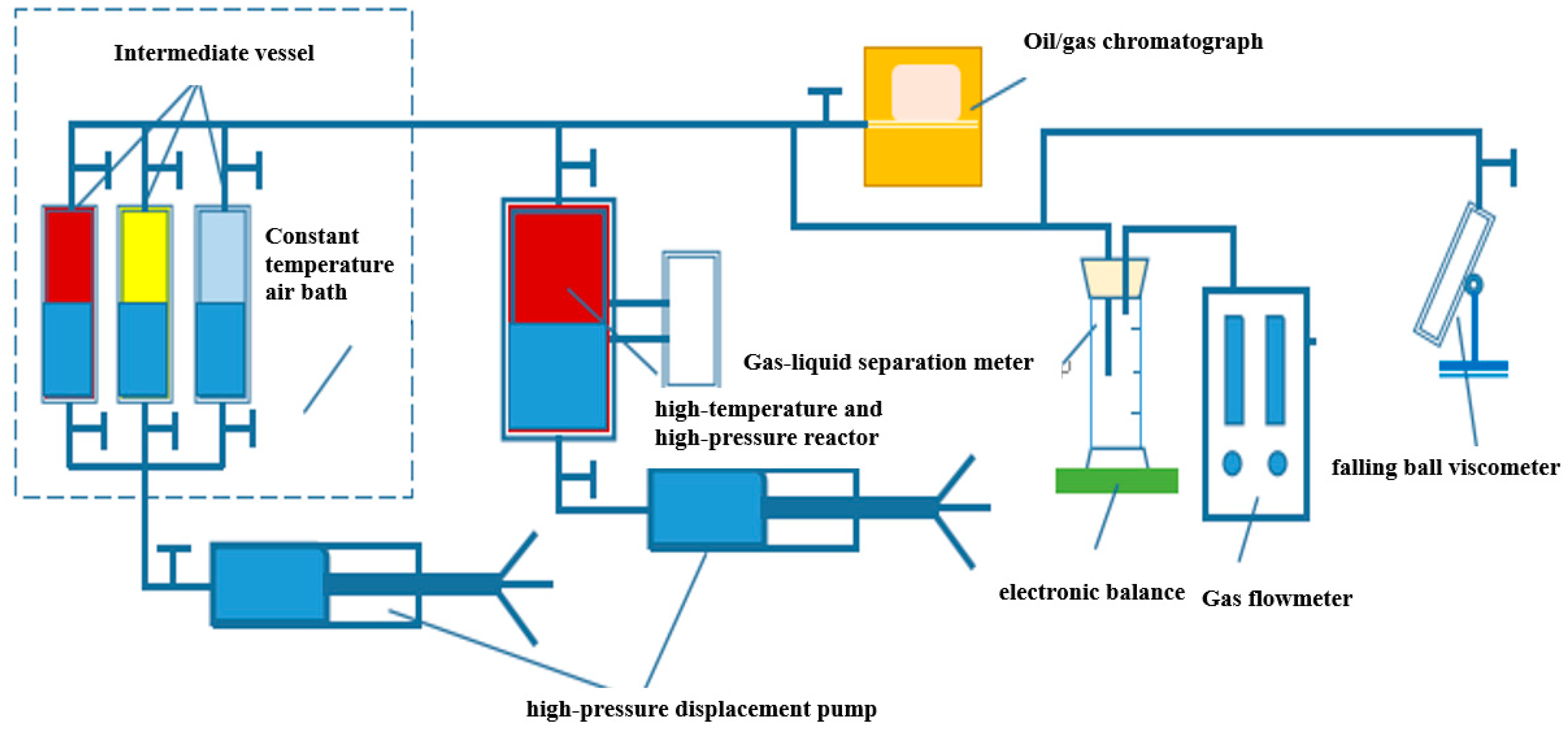
2.1. Experimental Equipment
2.2. Experimental Materials
2.3. Experimental Method
- (1)
- Natural gas preparation
- (2)
- Oil preparation
- (3)
- Parameter test
- Pour 300 mL crude oil into the reactor and adjust the temperature to the design temperature of the experiment. Inject the formation water sample into the dead oil in a certain proportion according to the water content requirement (20%). According to the molar content of CO2 in natural gas, inject various gases into the intermediate vessel and pressurize to 30 MPa to form standard gas. To prepare live oil, inject the standard gas into the reactor at a constant pressure of 30 MPa to fully dissolve it into crude oil. Discharge the free gas, then connect the capillary to the reactor and the gas–liquid separation meter (test tube and gas flowmeter). Open the valve of the reactor to let the crude oil enter the test tube, and the gas enters the gas flowmeter. Record the values before and after the pressure pump, and test the volume factor. Remove the connection device after the test. Using a capillary connection reactor, use the densitometer and kinematic viscosity tester as follows: Open the valve of the reactor, and the fluid will enter the kinematic viscosity tester for testing the viscosity. Stop the test when the viscosity value shows stability, and measure density with the weighing method. Lower the pressure to the next pressure point, and after it is constant for 1 h repeat the above steps. When measuring the parameters below the bubble point pressure, it is necessary to open the release valve of the reactor to exhaust and reduce the pressure, and the stability time should be extended from 4 h to 5 h.
- Pour 300 mL crude oil into the reactor and adjust the temperature to the design temperature of the experiment. According to the gas–oil ratio, inject various gases into the intermediate vessel and pressurize to 30 MPa to form standard gas. To prepare live oil, inject the standard gas into the reactor at a constant pressure of 30 MPa to fully dissolve it into crude oil. Inject excess CO2 (recorded amount of CO2 added) into the reactor and allow it to dissolve fully, leaving undissolved CO2 above the reservoir. Connect the capillary to the reactor and the gas flowmeter and discharge the free gas. Collect the gas sample in the gas collection bag and test it using the gas chromatograph (Agilent 7890). Record the total amount of gas discharged until the oil is produced. Lower the pressure to the next pressure point, and after it is constant for 1 h repeat the above steps. Calculate CO2 solubility from the volume of free gas and oil.
3. Experimental Results and Analysis
3.1. Experiment on Volume Factor of Watered oil Containing CO2
3.2. Experiment on Viscosity of Watered Oil Containing CO2
3.3. Experiment on Density of Watered Oil Containing CO2
3.4. Experiment on Solubility of CO2 in Watered Oil
4. Conclusions
- (1)
- The solubility of CO2 in watered oil increases with increasing pressure and decreases with increasing temperature. Therefore, in the recovery process, as the pressure decreases, the solubility will gradually decrease, and a large amount of CO2 will be precipitated.
- (2)
- Under different temperature conditions, with the increase in pressure, the volume factor first increases and then decreases, mainly because the increase in pressure improves the compression degree of crude oil, while the increase in temperature causes volume expansion, resulting in an increase in CO2 gas that can be dissolved, resulting in an increase in volume factor. However, above the bubble point pressure, CO2 is already saturated, the fluid is compressed under the influence of pressure, and the volume factor decreases.
- (3)
- The viscosity curve decreases first and then increases with increasing pressure. The main reason is that live crude oil is very sensitive to pressure. When the pressure is lower than the saturation pressure, the gas will dissolve into the oil as the pressure rises, improving the composition of the crude oil and making the viscosity of the crude oil drop sharply. When the pressure is higher than the saturated pressure, the oil will be squeezed with rising pressure, resulting in an increase in the density and viscosity of the oil.
- (4)
- With increasing temperature, the viscosity decreases sharply. When the temperature is above 20 °C, the viscosity changes greatly because the increase in temperature near the freezing point has an obvious effect on the viscosity reduction of crude oil, and the increase in temperature above the freezing point weakens the viscosity reduction effect.
- (5)
- Under certain CO2 content and temperature conditions, the density showed a trend of decreasing first and then increasing. When the pressure is less than the saturation pressure, the dissolved gas increases with increasing pressure, so the density of crude oil decreases. When the pressure is higher than the saturation pressure, the gas has been completely dissolved, and the oil is compressed with increasing pressure, so the density of the oil increases.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.; Zhou, D.; Fan, K.; Lian, L.; Zhang, L. Eor technology practice of natural gas flooding: A case study on donghe i carboniferous reservoir with the characteristics of ultra-deep, very thick and 3 highs, International Petroleum Technology Conference 2020, IPTC 2020. In Proceedings of the International Petroleum Technology Conference (IPTC), Dhahran, Saudi Arabia, 13–15 January 2020. [Google Scholar]
- Xiong, X.; Liao, T.; Xing, X.; Zhang, Z.; Dong, Z.; Bi, F. Study Progress on Characteristics and Separation of Produced Fluid of CO2 Flooding. XINJANG OIL GAS 2022, 18, 33–39. [Google Scholar]
- Cao, L.; Qian, W.; Gong, P.; Guan, S.; Duan, Y.; He, Y. Application Practices and Evaluation of Gas Injection Technology for CO2 Flooding in Subei Oilfield. XINJANG OIL GAS 2022, 18, 46–50. [Google Scholar]
- Li, Z.; Su, Y.; Li, L.; Hao, Y.; Wang, W.; Meng, Y.; Zhao, A. Evaluation of CO2 storage of water alter nating gas flooding using experimental and numerical simulation methods. Fuel 2022, 311, 122489. [Google Scholar] [CrossRef]
- Klewiah, I.; Berawala, D.S.; Walker, H.C.A.; Andersen, P.Ø.; Nadeau, P.H. Review of experi mental sorption studies of CO2 and CH4 in shales. J. Nat. Gas Sci. Eng. 2020, 73, 103045. [Google Scholar] [CrossRef]
- Berawala, D.S.; Andersen, P.Ø. Evaluation of multicomponent adsorption kinetics for carbon dioxide enhanced gas re covery from tight shales. SPE Reserv. Eval. Eng. 2020, 23, 1060–1076. [Google Scholar] [CrossRef]
- Hossein, D.M.; Hassan, D. Quantification of convective and diffusive transport during CO2 dissolution in oil: A numerical and analytical study. Phys. Fluids 2020, 32, 085110. [Google Scholar]
- Amarasinghe, W.; Fjelde, I.; Guo, Y. CO2 dissolution and convection in oil at realistic reservoir conditions: A visualization study. J. Nat. Gas Sci. Eng. 2021, 95, 104113. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Su, Y.; Fan, L.; Hao, Y.; Huang, L.; Ren, S.; Sun, Y.; Liu, R. Influences of diffusion and advection on dynamic oil-CO2 mixing during CO2 EOR and storage process: Experimental study and numerical modeling at pore-scales. Energy 2023, 267, 126567. [Google Scholar] [CrossRef]
- Kang, S.; Gao, C.; Zhang, S. Scientific Research and Field Application of CO2 immiscible flooding in heavy oil recovery. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2–4 July 2013. [Google Scholar]
- Saner, W.B.; Patton, J.T. CO2 recovery of heavy oil: Wilmington field test. J. Pet. Technol. 1986, 38, 769–776. [Google Scholar] [CrossRef]
- Zhou, X.; Yuan, Q.; Peng, X.; Zeng, F.; Zhang, L. A critical review of the CO2 huff ‘n’puff process for enhanced heavy oil recovery. Fuel 2018, 215, 813–824. [Google Scholar] [CrossRef]
- Talib, M.Q.A.; Al-Jawad, M.S. Assessment of the Common PVT Correlations in Iraqi Oil Fields. J. Pet. Res. Stud. 2022, 34, 68–87. [Google Scholar]
- Almashan, M.; Narusue, Y.; Morikawa, H. Estimating PVT Properties of Crude Oil Systems Based on a Boosted Decision Tree Regression Modelling Scheme with K-Means Clustering. In Proceedings of the SPE Latin America and Caribbean Petroleum Engineering Conference, Bali, Indonesia, 29–31 October 2019. [Google Scholar]
- Wang, Z.; Sun, B.; Yan, L. Improved Density Correlation for Supercritical CO2. Chem. Eng. Technol. 2015, 38, 75–84. [Google Scholar] [CrossRef]
- Ghasemi, M.; Whitson, C.H. Pvt modeling of complex heavy oil mixtures. J. Pet. Sci. Eng. 2021, 205, 108510. [Google Scholar] [CrossRef]
- Izurieta, A.J.; Iza, A. The PVT Properties of the Ecuadorian Crude Oils. In Proceedings of the SPE Latin America and Caribbean Petroleum Engineering Conference, Buenos Aires, Argentina, 17–19 May 2017. [Google Scholar]
- Middleton, R.S.; Carey, J.W.; Currier, R.P.; Hyman, J.D.; Kang, Q.; Karra, S.; Jiménez-Martínez, J.; Porter, M.L.; Viswanathan, H.S. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Sanai, K.; Kentaro, N.; Takayuki, S.; Takeo, K.; Kenji, H. Design factors in gas-lift advanced dissolution (GLAD) system for CO2 sequestration into the ocean. Chem. Eng. Sci. 2001, 56, 6205–6210. [Google Scholar]
- Shafaei, M.J.; Abedi, J.; Hassanzadeh, H.; Chen, Z. Reverse gas-lift technology for CO2 storage into deep saline aquifers. Energy 2012, 45, 840–849. [Google Scholar] [CrossRef]
- Li, Y.G.; Park, C.B.; Li, H.B.; Wang, J. Measurement of the PVT property of PP/CO2 solution. Fluid Phase Equilibria 2008, 270, 15–22. [Google Scholar] [CrossRef]
- Hao, Y.; Wu, Z.; Ju, B.; Chen, Y.; Luo, X.; Xinjiang, P. Laboratory investigation of CO2 flooding. In Proceedings of the Nigeria Annual International Conference and Exhibition, Abuja, Nigeria, 2–4 August 2004. [Google Scholar]
- Nguyen, T.A.; Ali, S.F. Effect of nitrogen on the solubility and diffusivity of carbon dioxide into oil and oil recovery by the immiscible WAG process. J. Can. Pet. Technol. 1998, 37, PETSOC-98-02-02. [Google Scholar] [CrossRef]
- Gao, J.; Dan, S.; Yang, T.; Zhang, X.; Yue, H.; Yu, Q. Study on CO2 solubility in heavy oil in Well Ji7, Changji oilfield, Junggar Basin. China Pet. Explor. 2018, 23, 65. [Google Scholar]










| Component | Live Oil | |
|---|---|---|
| mol/% | wt% | |
| CO2 | 3.64 | 1.23 |
| N2 | 0.2 | 0.04 |
| C1 | 35.27 | 4.36 |
| C2 | 6.29 | 1.46 |
| C3 | 3.5 | 1.19 |
| iC4 | 0.35 | 0.16 |
| nC4 | 1.38 | 0.62 |
| iC5 | 0.5 | 0.28 |
| nC5 | 1.04 | 0.58 |
| C6 | 1.91 | 1.23 |
| C7 | 2.83 | 2.09 |
| C8 | 4.15 | 3.42 |
| C9 | 3.39 | 3.16 |
| C10 | 2.92 | 3.01 |
| C11 | 2.41 | 2.73 |
| C12 | 2.2 | 2.73 |
| C13 | 2.21 | 2.98 |
| C14 | 2.05 | 3 |
| C15 | 2.12 | 3.37 |
| C16 | 1.6 | 2.73 |
| C17 | 1.64 | 2.98 |
| C18 | 1.41 | 2.73 |
| C19 | 1.29 | 2.61 |
| C20 | 1.22 | 2.58 |
| C21 | 1.08 | 2.43 |
| C22 | 0.97 | 2.28 |
| C23 | 0.96 | 2.35 |
| C24 | 0.86 | 2.2 |
| C25 | 0.83 | 2.22 |
| C26 | 0.76 | 2.09 |
| C27 | 0.78 | 2.25 |
| C28 | 0.8 | 2.39 |
| C29 | 0.81 | 2.5 |
| C30 | 0.78 | 2.5 |
| C31 | 0.58 | 1.93 |
| C32 | 0.57 | 1.93 |
| C33 | 0.43 | 1.5 |
| C34 | 0.38 | 1.37 |
| C35 | 0.33 | 1.23 |
| C36+ | 3.56 | 17.56 |
| Total | 100 | 100 |
| Experimental Project | Parameter | Parameter Range |
|---|---|---|
| Viscosity, density, volume factor test | Temperature/°C | 20, 70, 120 |
| Pressure/MPa | 5–30 | |
| CO2 concentration/mol% | 10, 40, 90 | |
| Watered/% | 20 | |
| Solubility experiment | Temperature/°C | 20, 70, 120 |
| Pressure/MPa | 5, 8, 10, 12, 15, 18, 20, 30 | |
| Watered/% | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, M.; Yu, J.; Chen, H.; Li, M.; Wu, G.; Shi, H.; Bian, H.; Liao, X.; Huang, L. Laboratory Test of Fluid Physical Property Parameters of Well Fluid Containing CO2. Processes 2023, 11, 1954. https://doi.org/10.3390/pr11071954
Zou M, Yu J, Chen H, Li M, Wu G, Shi H, Bian H, Liao X, Huang L. Laboratory Test of Fluid Physical Property Parameters of Well Fluid Containing CO2. Processes. 2023; 11(7):1954. https://doi.org/10.3390/pr11071954
Chicago/Turabian StyleZou, Minghua, Jifei Yu, Huan Chen, Menglong Li, Guang‘ai Wu, Haowen Shi, Hanqing Bian, Xiaobo Liao, and Lijuan Huang. 2023. "Laboratory Test of Fluid Physical Property Parameters of Well Fluid Containing CO2" Processes 11, no. 7: 1954. https://doi.org/10.3390/pr11071954
APA StyleZou, M., Yu, J., Chen, H., Li, M., Wu, G., Shi, H., Bian, H., Liao, X., & Huang, L. (2023). Laboratory Test of Fluid Physical Property Parameters of Well Fluid Containing CO2. Processes, 11(7), 1954. https://doi.org/10.3390/pr11071954






