Reaction–Diffusion Process for Hydrogels with a Tailored Layer Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
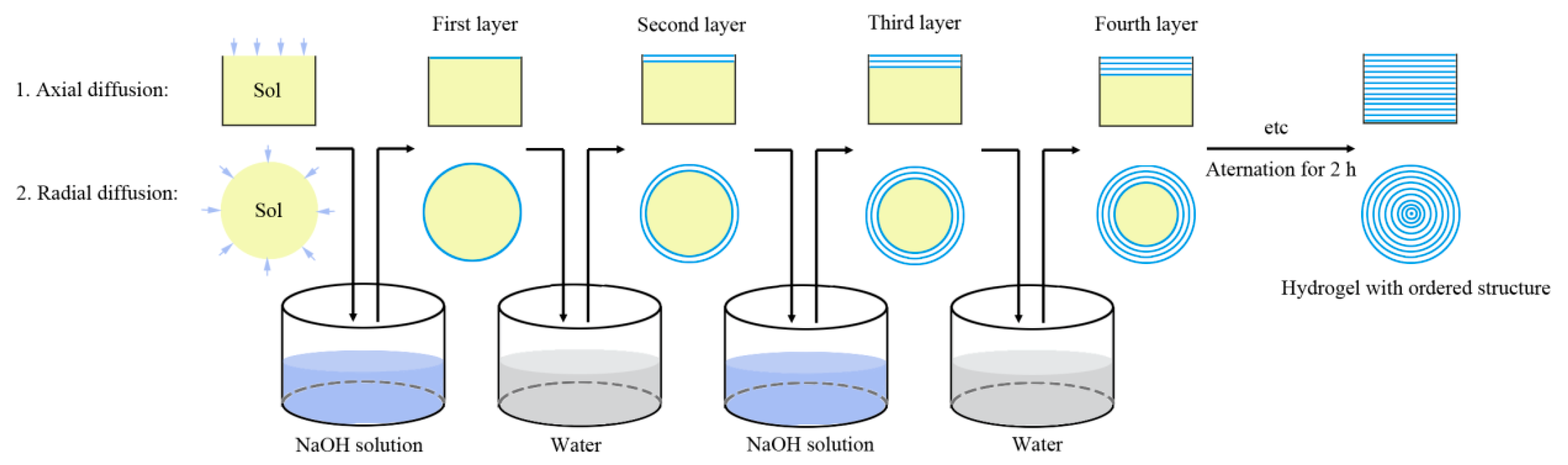
2.2. Synthesis of Layered Magnetic Chitosan Hydrogels (L-MCH)
2.3. Morphologies of Layered Hydrogels
2.4. Determination of Mechanical Properties of Hydrogels
3. Results and Discussion
3.1. Morphologies of Layered Chitosan Hydrogels
3.2. Effect of Interval Time on the Layer Structure of Hydrogels
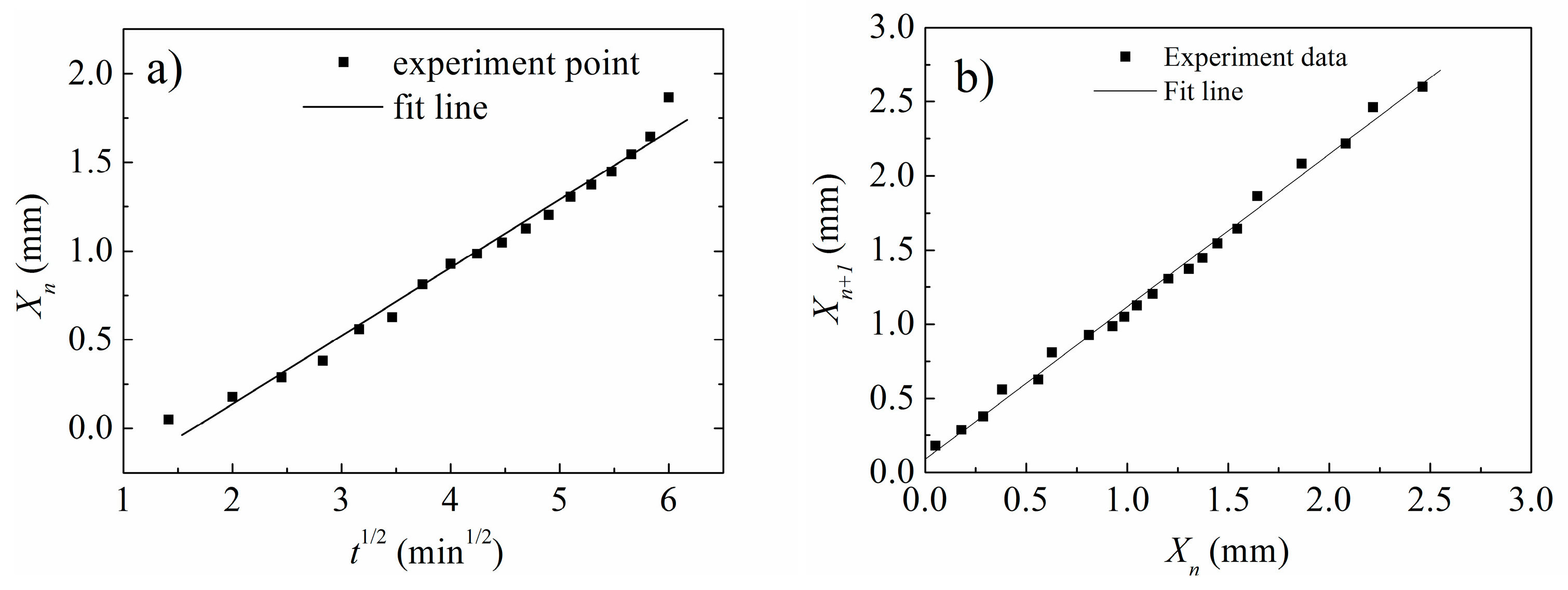
3.3. Verification of Time Law and Spacing Law of L-MCHs
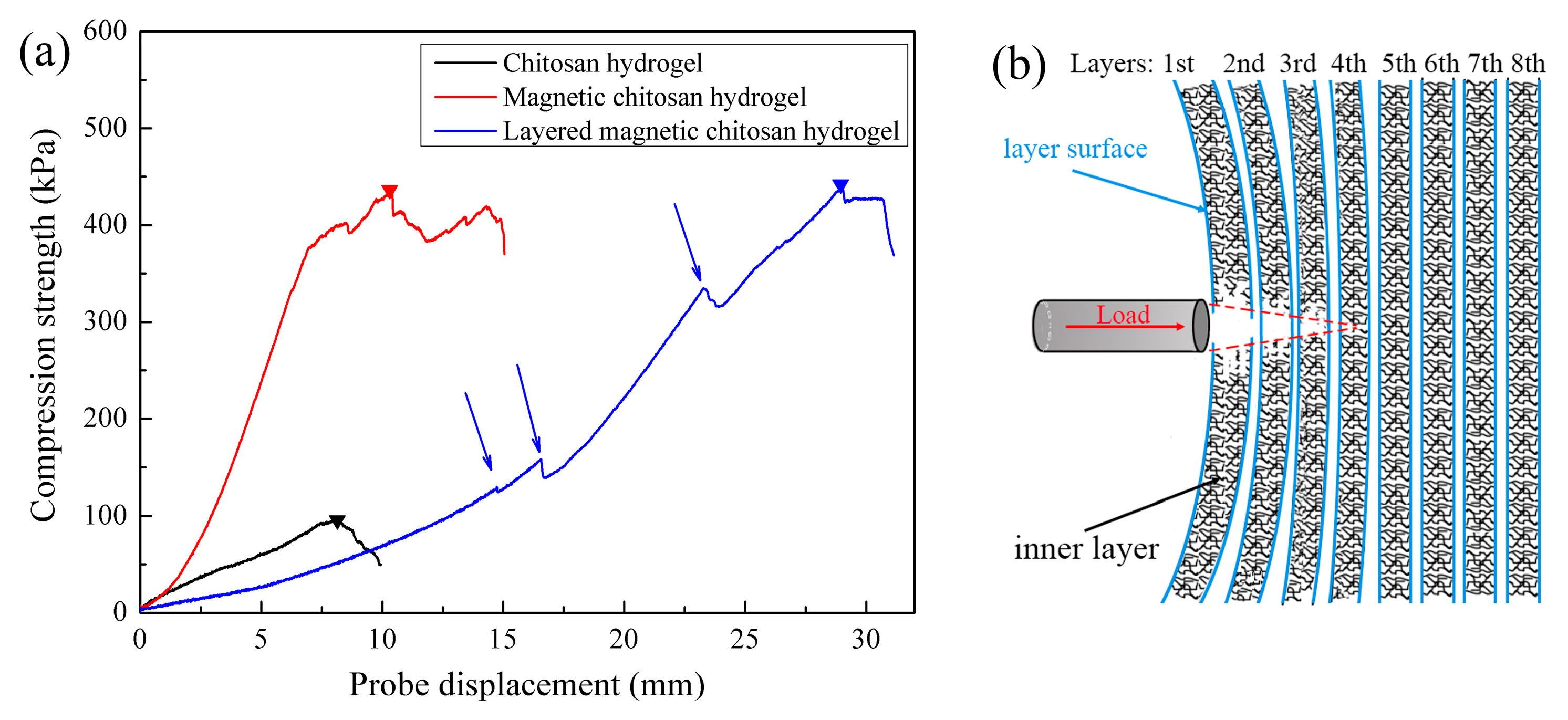
3.4. Mechanical Properties of Layered Hydrogels
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ribeiro, E.F.; Polachini, T.C.; Locali-Pereira, A.R.; Janzantti, N.S.; Quiles, A.; Hernando, I.; Nicoletti, V.R. Storage stability of spray- and freeze-dried chitosan-based pickeringemulsions containing roasted coffee oil: Color evaluation, lipid oxidation, and volatile compounds. Processes 2023, 11, 1048. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-based nanoparticles as effective drug delivery systems—A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Urbanska, A.M.; Gholizadeh, S.S.; Goodarzi, V.; Saeb, M.R.; Mozafari, M. A facile route to the synthesis of anilinic electroactive colloidal hydrogels for neural tissue engineering applications. J. Colloid Interface Sci. 2018, 516, 57. [Google Scholar] [CrossRef] [PubMed]
- Khubiev, O.M.; Esakova, V.E.; Egorov, A.R.; Bely, A.E.; Golubev, R.A.; Tachaev, M.V.; Kirichuk, A.A.; Lobanov, N.N.; Tskhovrebov, A.G.; Kritchenkov, A.S. Novel non-toxic highly antibacterial chitosan/Fe(III)-based nanoparticles that contain a deferoxamine—Trojan horse ligands: Combined synthetic and biological studies. Processes 2023, 11, 870. [Google Scholar] [CrossRef]
- Meira, D.I.; Proença, M.; Rebelo, R.; Barbosa, A.I.; Rodrigues, M.S.; Borges, J.; Vaz, F.; Reis, R.L.; Correlo, V.M. Chitosan micro-membranes with integrated gold nanoparticles as an LSPR-based sensing platform. Biosensors 2022, 12, 951. [Google Scholar] [CrossRef]
- Song, W.; Huang, T.; Zuo, H.; Deng, D.; Tang, C. Application of microbial immobilization on chitosan composite membrane for manganese removal in water treatment. Polymer 2022, 243, 124531. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.M.; Liu, Y.F.; Ding, G.W.; Pei, Y.; Li, J.; Hua, G.F.; Huang, J. Preparation and characterization of magnetic targeted drug controlled-release hydrogel microspheres. Macromol. Symp. 2005, 225, 71–80. [Google Scholar] [CrossRef]
- Sun, H.; Kabb, C.P.; Sims, M.B.; Sumerlin, B.S. Architecture-transformable polymers: Reshaping the future of stimuli-responsive polymers. Prog. Polym. Sci. 2019, 89, 61–75. [Google Scholar] [CrossRef]
- Liu, M.J.; Ishida, Y.; Ebina, Y.; Sasaki, T.; Hikima, T.; Hikima, T.; Takata, M.; Aida, T. An anisotropic hydrogel with electrostatic repulsion between cofacially aligned nanosheets. Nature 2014, 517, 68–72. [Google Scholar] [CrossRef]
- Sakr, O.S.; Jordan, O.; Borchard, G. Sustained protein release from hydrogel microparticles using layer-by-layer (LbL) technology. Drug Deliv. 2015, 23, 2747–2755. [Google Scholar] [CrossRef]
- Dedroog, L.M.; Deschaume, O.; Abrego, C.J.G.; Koos, E.; de Coene, Y.; Vananroye, A.; Thielemans, W.; Bartic, C.; Lettinga, M.P. Stress-controlled shear flow alignment of collagen type I hydrogel systems. Acta Biomater. 2022, 150, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Roehm, K.D.; Madihally, S.V. Bioprinted chitosan-gelatin thermosensitive hydrogels using an inexpensive 3D printer. Biofabrication 2018, 10, 015002. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.A.; Jin, Y.; Bouyer, J.; DeOre, B.J.; Suprewicz, L.; Figel, A.; Walens, H.; Fischer, I.; Galie, P.A. Magnetic alignment of injectable hydrogel scaffolds for spinal cord injury repair. Biomater. Sci. 2022, 10, 2237–2247. [Google Scholar] [CrossRef]
- Grzybowski, B.A.; Bishop, K.J. Micro- and nanoprinting into solids using reaction-diffusion etching and hydrogel stamps. Small 2009, 5, 22–27. [Google Scholar] [CrossRef]
- Erb, R.M.; Sander, J.S.; Grisch, R.; Studart, A.R. Self-shaping composites with programmable bioinspired microstructures. Nat. Commun. 2013, 4, 1712. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- Kumar, S.; Jiwari, R.; Mittal, C. Numerical simulation for computational modelling of reaction–diffusion Brusselator model arising in chemical processes. J. Math. Chem. 2019, 57, 149–179. [Google Scholar] [CrossRef]
- Jonášová, E.P.; Stokke, B.T.; Prot, V. Interrelation between swelling, mechanical constraints and reaction–diffusion processes in molecular responsive hydrogels. Soft Matter 2022, 18, 1510–1524. [Google Scholar] [CrossRef]
- Walliser, R.M.; Boudoire, F.; Orosz, E.; Tóth, R.; Braun, A.; Constable, E.C.; Rácz, Z.; Lagzi, I. Growth of nanoparticles and microparticles by controlled reaction-diffusion processes. Langmuir 2015, 31, 1828–1834. [Google Scholar] [CrossRef]
- Campbell, C.J.; Baker, E.; Fialkowski, M.; Grzybowski, B.A. Arrays of microlenses of complex shapes prepared by reaction-diffusion in thin films of ionically doped gels. Appl. Phys. Lett. 2004, 85, 1871–1873. [Google Scholar] [CrossRef]
- Klajn, R.; Fialkowski, M.; Bensemann, I.T.; Bitner, A.; Campbell, C.J.; Bishop, K.; Smoukov, S.; Grzybowski, B.A. Multicolour micropatterning of thin films of dry gels. Nat. Mater. 2004, 3, 729–735. [Google Scholar] [CrossRef]
- Lagzi, I.; Kowalczyk, B.; Grzybowski, B.A. Liesegang Rings Engineered from Charged Nanoparticles. J. Am. Chem. Soc. 2010, 132, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, Y.; Feng, Y.; Ma, B.; Zhu, R.; Zhou, Y. Formation of concentric multilayers in a chitosan hydrogel inspired by liesegang ring phenomena. J. Biomater. Sci. Polym. Ed. 2011, 22, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Zhou, Y.; Han, Z.; Feng, Y.; Wei, D. A facile concentric-layered magnetic chitosan hydrogel with magnetic field remote stimulated drug release. J. Control. Release 2013, 172, e90. [Google Scholar] [CrossRef]
- Dai, H.; Li, X.; Long, Y.; Wu, J.; Liang, S.; Zhang, X.; Zhao, N.; Xu, J. Multi-membrane hydrogel fabricated by facile dynamic self-assembly. Soft Matter 2009, 5, 1987–1989. [Google Scholar] [CrossRef]
- Rajurkar, N.; Ambekar, B. Studies on Liesegang rings of cobalt hydroxide in 1% agar gel medium. J. Mol. Liq. 2015, 204, 205–209. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Xu, F.; Han, Z.; Wei, D.; Jia, D.; Zhou, Y. Tough magnetic chitosan hydrogel nanocomposites for remotely stimulated drug release. Biomacromolecules 2018, 19, 3351–3360. [Google Scholar] [CrossRef]



| T (min) | L-CHs | L-MCHs | ||
|---|---|---|---|---|
| Layer Number | Width of Layer (μm) | Layer Number | Width of Layer (μm) | |
| 2 | 46 ± 3 | 80 ± 4.2 | 48 ± 3 | 50 ± 5.8 |
| 5 | 22 ± 2 | 140 ± 7.3 | 24 ± 2 | 90 ± 6.4 |
| 10 | 14 ± 1 | 648 ± 10.3 | 10 ± 1 | 487 ± 9.8 |
| Samples | Yield Strength (kPa) | Probe Displacement (mm) |
|---|---|---|
| Chitosan hydrogel | 95.1 ± 7.6 | 8.2 ± 0.8 |
| Magnetic chitosan hydrogel | 401.7 ± 12.1 | 10.16 ± 1.3 |
| Layered magnetic chitosan hydrogel | 441.0 ± 15.1 | 28.93 ± 2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, Y.; Wang, Y.; Li, B.; Wang, C.; Han, Z.; Weng, L. Reaction–Diffusion Process for Hydrogels with a Tailored Layer Structure. Processes 2023, 11, 1975. https://doi.org/10.3390/pr11071975
Wang Y, Xu Y, Wang Y, Li B, Wang C, Han Z, Weng L. Reaction–Diffusion Process for Hydrogels with a Tailored Layer Structure. Processes. 2023; 11(7):1975. https://doi.org/10.3390/pr11071975
Chicago/Turabian StyleWang, Yongliang, Yaxin Xu, Yunfei Wang, Baoqiang Li, Chunfeng Wang, Zhidong Han, and Ling Weng. 2023. "Reaction–Diffusion Process for Hydrogels with a Tailored Layer Structure" Processes 11, no. 7: 1975. https://doi.org/10.3390/pr11071975
APA StyleWang, Y., Xu, Y., Wang, Y., Li, B., Wang, C., Han, Z., & Weng, L. (2023). Reaction–Diffusion Process for Hydrogels with a Tailored Layer Structure. Processes, 11(7), 1975. https://doi.org/10.3390/pr11071975








