Application of the Hybrid Chemical-Biocatalytic Approach for Conversion of Nitrocellulose-Containing Sewage Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Biocatalyst
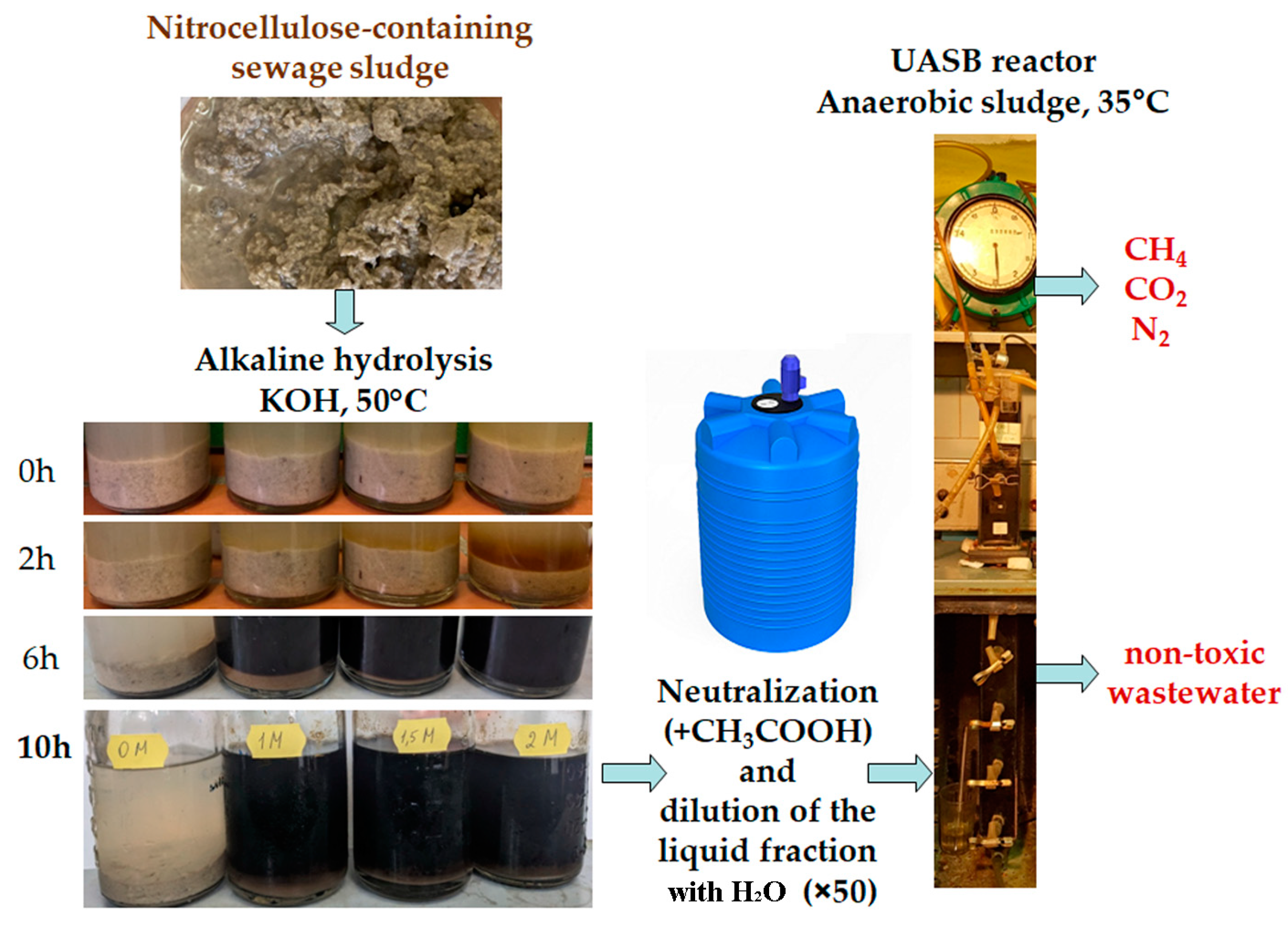
2.2. Alkaline Hydrolysis of NCS
2.3. Anaerobic Fermentation in UASB Reactor
2.4. Analytical Methods
2.5. Accumulation of Biogas and Determination of Its Content
2.6. Characterisation
3. Results
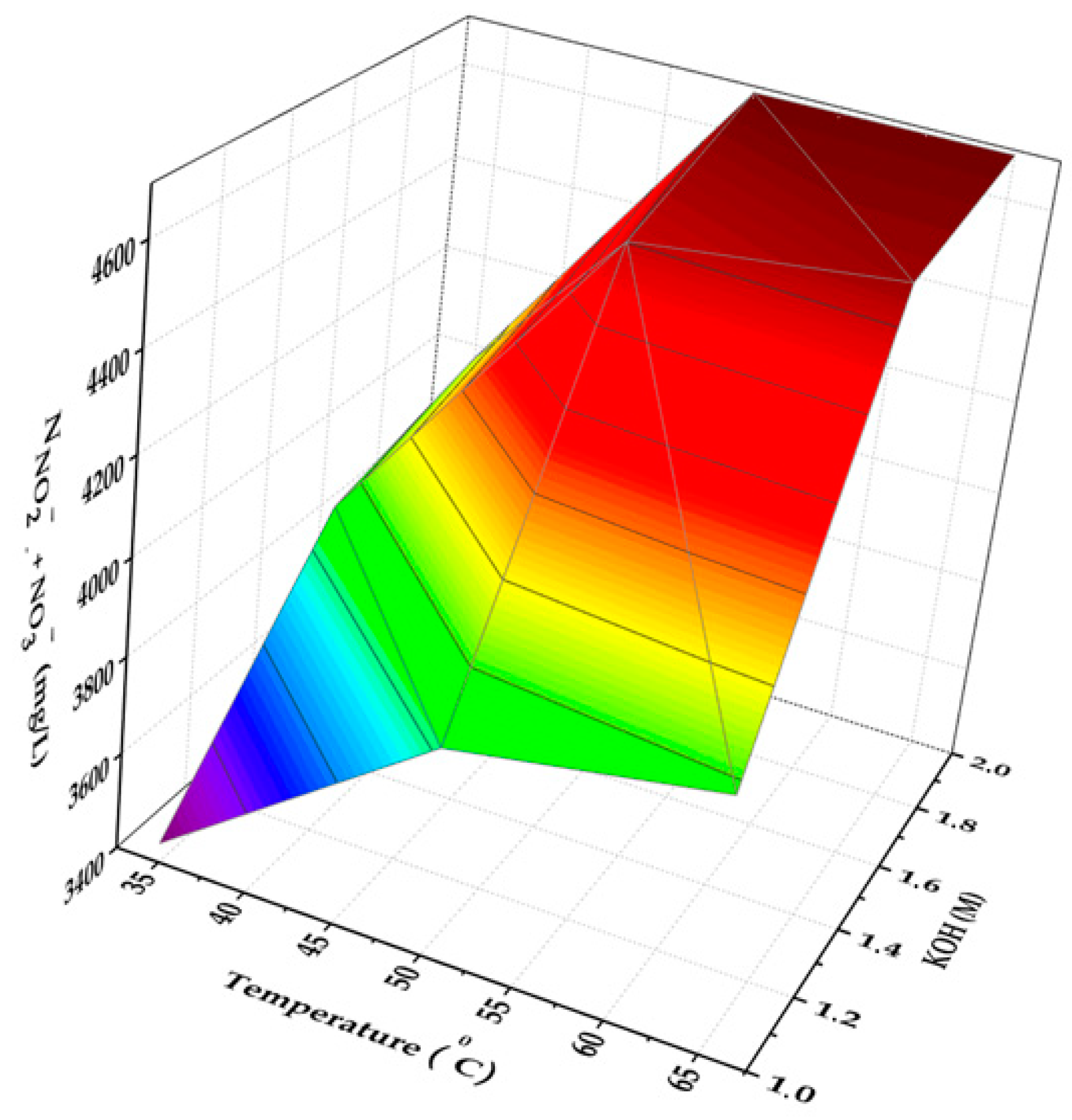
3.1. Optimizing the Conditions for Performing Alkaline Hydrolysis of NCS
3.2. Biocatalytic Conversion of the Liquid Fraction Produced via the Alkaline Treatment of NCS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattar, H.; Baz, Z.; Saleh, A.; Shalaby, A.S.; Azzazy, A.E.; Salah, H.; Ismail, I. Nitrocellulose: Structure, synthesis, characterization, and applications. Water Energy Food Environ. J. 2020, 1, 1–15. [Google Scholar]
- Pouretedal, H.R.; Shamsi, M.; Arabiyan, D. Statistical optimization of nitrocellulose removal from industrial wastewater by electrocoagulation using response surface method. Desalination Water Treat. 2021, 212, 212–219. [Google Scholar] [CrossRef]
- Gladchenko, M.A.; Rogozin, A.D.; Cherenkov, P.G.; Murygina, V.P.; Gaidamaka, S.N.; Lifshits, A.B. Regulation of the physicochemical and biotechnological process parameters of the liquid-phase aerobic degradation of nitrocellulose-containing wastewater sludge. Russ. J. Phys. Chem. B 2016, 10, 504–510. [Google Scholar] [CrossRef]
- Maslova, O.; Senko, O.; Akopyan, A.; Lysenko, S.; Anisimov, A.; Efremenko, E. Nanocatalysts for oxidative desulfurization of liquid fuel: Modern solutions and the perspectives of application in hybrid chemical-biocatalytic processes. Catalysts 2021, 11, 1131. [Google Scholar] [CrossRef]
- Bhanot, P.; Celin, S.M.; Sreekrishnan, T.R.; Kalsi, A.; Sahai, S.K.; Sharma, P. Application of integrated treatment strategies for explosive industry wastewater—A critical review. J. Water Process. Eng. 2020, 35, 101232. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Maslova, O.; Lyagin, I.; Aslanli, A.; Stepanov, N. Destruction of mycotoxins in poultry waste under anaerobic conditions within methanogenesis catalyzed by artificial microbial consortia. Toxins 2023, 15, 205. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Stepanov, N.; Senko, O.; Maslova, O.; Lyagin, I.; Aslanli, A. Progressive Biocatalysts for the Treatment of Aqueous Systems Containing Pharmaceutical Pollutants. Life 2023, 13, 841. [Google Scholar] [CrossRef]
- Maslova, O.; Senko, O.; Gladchenko, M.A.; Gaydamaka, S.N.; Efremenko, E. Prospects for Combined Applications of Nanostructured Catalysts and Biocatalysts for Elimination of Hydrocarbon Pollutants. Appl. Sci. 2023, 13, 5815. [Google Scholar] [CrossRef]
- Hassan, G.K.; Mahmoud, W.H.; Al-sayed, A.; Ismail, S.H.; El-Sherif, A.A.; Abd El Wahab, S.M. Multi-functional of TiO2@Ag core–shell nanostructure to prevent hydrogen sulfide formation during anaerobic digestion of sewage sludge with boosting of bio-CH4 production. Fuel 2023, 333, 126608. [Google Scholar] [CrossRef]
- Hassan, G.K.; Abdel-Karim, A.; Al-Shemy, M.T.; Rojas, P.; Sanz, J.L.; Ismail, S.H.; Mohamed, G.G.; El-gohary, F.A.; Al-sayed, A. Harnessing Cu@Fe3O4 core shell nanostructure for biogas production from sewage sludge: Experimental study and microbial community shift. Renew. Energy 2022, 188, 1059–1071. [Google Scholar] [CrossRef]
- Alabresm, A.; Chen, Y.P.; Decho, A.W.; Lead, J. A novel method for the synergistic remediation of oil-water mixtures using nanoparticles and oil-degrading bacteria. Sci. Total Environ. 2018, 630, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Li, S.; Ding, Y.; Xiao, Z. The preparation and properties of low-nitrogen nitrocellulose by alkaline denitration. Cent. Eur. J. Energetic Mater. 2020, 17, 535–551. [Google Scholar] [CrossRef]
- Alinat, E.; Delaunay, N.; Archer, X.; Mallet, J.M.; Gareil, P. A new method for the determination of the nitrogen content of nitrocellulose based on the molar ratio of nitrite-to-nitrate ions released after alkaline hydrolysis. J. Hazard. Mater. 2015, 286, 92–99. [Google Scholar] [CrossRef]
- Li, J.; Yin, X.; Liu, Z.; Gu, Z.; Niu, J. Reaction yield model of nitrocellulose alkaline hydrolysis. J. Hazard. Mater. 2019, 371, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liang, X.; Li, J.; Liu, Z. Heuristic-based alkaline hydrolysis mechanism of nitrate ester (Nitrocellulose monomer) and nitroamine (Hexogen) compounds: Electrostatic attraction effect of the nitro group. J. Phys. Chem. A 2023, 127, 1609–1618. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Liang, X.; Liu, Z. Automated reaction mechanisms and kinetics with the nudged elastic band method-based AMK_Mountain and its description of the preliminary alkaline hydrolysis of nitrocellulose monomer. J. Comput. Chem. 2022, 43, 1513–1523. [Google Scholar] [CrossRef]
- Christodoulatos, C.; Su, T.L.; Koutsospyros, A. Kinetics of the alkaline hydrolysis of nitrocellulose. Water Environ. Res. 2001, 73, 185–191. [Google Scholar] [CrossRef]
- Maslova, O.; Senko, O.; Stepanov, N.; Gladchenko, M.; Gaydamaka, S.; Akopyan, A.; Eseva, E.; Anisimov, A.; Efremenko, E. Sulfur containing mixed wastes in anaerobic processing by new immobilized synthetic consortia. Bioresour. Technol. 2022, 362, 127794. [Google Scholar] [CrossRef]
- Senko, O.; Gladchenko, M.; Maslova, O.; Efremenko, E. Long-term storage and use of artificially immobilized anaerobic sludge as a powerful biocatalyst for conversion of various wastes including those containing xenobiotics to biogas. Catalysts 2019, 9, 326. [Google Scholar] [CrossRef]
- Trukhina, A.I.; Gladchenko, M.A.; Kalyuzhnyi, S.V. Optimizations of sulphide and organic modifications of the deamox process. Appl. Biochem. Microbiol. 2011, 47, 841–845. [Google Scholar] [CrossRef]
- Gladchenko, M.A.; Kovalev, D.A.; Kovalev, A.A.; Litti, Y.V.; Nozhevnikova, A.N. Methane production by anaerobic digestion of organic waste from vegetable processing facilities. Appl. Biochem. Microbiol. 2017, 53, 242–249. [Google Scholar] [CrossRef]
- Dubber, D.; Gray, N. Replacement of chemical oxygen demand (COD) with total organic carbon (TOC) for monitoring wastewater treatment performance to minimize disposal of toxic analytical waste. J. Environ. Sci. Health A 2010, 45, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Ilma, Q.; Dinoto, A.; Setianingrum, N.; Mulyadi, M.; Agustyani, D.; Radiastuti, N.; Julistiono, H. Isolation and identification of bacteria removing nitrite, nitrate and ammonium from bioballs filter. Indones. Aquac. J. 2022, 17, 13–22. [Google Scholar] [CrossRef]
- Oakley, S.M.; Gold, A.J.; Oczkowski, A.J. Nitrogen control through decentralized wastewater treatment: Process performance and alternative management strategies. Ecol. Eng. 2010, 36, 1520–1531. [Google Scholar] [CrossRef]
- Shi, J.; Huang, W.; Han, H.; Xu, C. Pollution control of wastewater from the coal chemical industry in China: Environmental management policy and technical standards. Renew. Sustain. Energy Rev. 2021, 143, 110883. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, K.; Xia, Q.; Yu, S.; Zhang, M.; An, Y.; Zhao, X.; Zhou, Z. Up-concentration of nitrogen from domestic wastewater: A sustainable strategy from removal to recovery. Chem. Eng. J. 2023, 451, 138789. [Google Scholar] [CrossRef]
- Liu, X.W.; Gu, Y.M.; Sun, T.J.; Guo, Y.; Wei, X.L.; Zhao, S.S.; Wang, S.D. Water resistant and flexible MOF materials for highly efficient separation of methane from nitrogen. Ind. Eng. Chem. Res. 2019, 58, 20392–20400. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, S.Y.; Yoon, T.U.; Kim, M.B.; Park, W.; Han, H.H.; Kong, C.I.; Park, C.Y.; Kim, J.H.; Bae, Y.S. Improved methane/nitrogen separation properties of zirconium-based metal–organic framework by incorporating highly polarizable bromine atoms. Chem. Eng. J. 2020, 399, 125717. [Google Scholar] [CrossRef]
- Wu, Y.; Weckhuysen, B.M. Separation and purification of hydrocarbons with porous materials. Angew. Chem. Int. Ed. 2021, 60, 18930–18949. [Google Scholar] [CrossRef]
- Sharma, A.; Shankhdhar, D.; Shankhdhar, S.C. Potassium-solubilizing microorganisms: Mechanism and their role in potassium solubilization and uptake. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V., Maurya, B., Verma, J., Meena, R., Eds.; Springer: New Delhi, India, 2016; pp. 203–219. [Google Scholar] [CrossRef]
- Renaudie, M.; Dumas, C.; Vuilleumier, S.; Ernst, B. New way of valorization of raw coffee silverskin: Biohydrogen and acetate production by dark fermentation without exogenous inoculum. Bioresour. Technol. Rep. 2022, 17, 100918. [Google Scholar] [CrossRef]
- Deng, G.; Shi, X. The effects of carbon source and COD/N ratio on simultaneous denitrification and methanogenesis in an upflow anaerobic sludge blanket reactor. Renew. Energy 2020, 157, 867–873. [Google Scholar] [CrossRef]
- Piotrowski, W.; Kubica, R. Integration of the Process for Production of Ethyl Acetate by an Enhanced Extraction Process. Processes 2021, 9, 1425. [Google Scholar] [CrossRef]
- Tariq, M.; Wang, J.; Bhatti, Z.A.; Bilal, M.; Malik, A.J.; Akhter, M.S.; Mahmood, Q.; Hussain, S.; Ghfar, A.; Al-Anazy, M.M.; et al. Bioenergy Potential of Albumin, Acetic Acid, Sucrose, and Blood in Microbial Fuel Cells Treating Synthetic Wastewater. Processes 2021, 9, 1289. [Google Scholar] [CrossRef]
- Kiefer, D.; Merkel, M.; Lilge, L.; Henkel, M.; Hausmann, R. From acetate to bio-based products: Underexploited potential for industrial biotechnology. Trends Biotechnol. 2021, 39, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, M.; Najafpour, G.D. Alkaline hydrolysis of waste nitrocellulose for recovery of pure cellulose. Iran. J. Energy Environ. 2011, 2, 221–228. [Google Scholar] [CrossRef]
- Mainardis, M.; Buttazzoni, M.; Goi, D. Up-Flow Anaerobic Sludge Blanket (UASB) Technology for Energy Recovery: A Review on State-of-the-Art and Recent Technological Advances. Bioengineering 2020, 7, 43. [Google Scholar] [CrossRef]
- Devi, M.K.; Manikandan, S.; Oviyapriya, M.; Selvaraj, M.; Assiri, M.A.; Vickram, S.; Subbaiya, R.; Karmegam, N.; Ravindran, B.; Chang, S.W.; et al. Recent advances in biogas production using Agro-Industrial Waste: A comprehensive review outlook of Techno-Economic analysis. Bioresour. Technol. 2022, 363, 127871. [Google Scholar] [CrossRef]
- Chen, H.; Luo, Z.; Wei, Y.; Deng, Z.; Yang, E.; Wang, H.; Chen, J.; Sanjaya, E.H.; Liu, Z.; Wu, S. Complex inhibitions on anaerobic degradation of monosodium glutamate in wastewater under low COD/sulfate ratios. Int. Biodeterior. Biodegrad. 2023, 177, 105526. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Wu, J.; Chen, X.; Liu, R.; Han, Y.; Xiao, B.; Yu, Z.; Peng, Y. Improving two-stage thermophilic-mesophilic anaerobic co-digestion of swine manure and rice straw by digestate recirculation. Chemosphere 2021, 274, 129787. [Google Scholar] [CrossRef]
- Chen, H.; Huang, R.; Wu, J.; Zhang, W.; Han, Y.; Xiao, B.; Wang, D.; Zhou, Y.; Liu, B.; Yu, G. Biohythane production and microbial characteristics of two alternating mesophilic and thermophilic two-stage anaerobic co-digesters fed with rice straw and pig manure. Bioresour. Technol. 2021, 320, 124303. [Google Scholar] [CrossRef]



| Parameter | Value | Parameter | Value |
|---|---|---|---|
| pH | 6.5 ± 0.05 | C (mg/kg on a dry matter basis) | 722 ± 17 |
| Moisture content (%) | 86 ± 0.2 | N-NH4 (mg/kg on a dry matter basis) | 69 ± 1 |
| NC (g/kg on a dry matter basis) | 617 ± 9 | C:N ratio | 25:1 |
| OM (g/kg on a dry matter basis) | 82 ± 10 | MM (g/kg on a dry matter basis) | 301 ± 19 |
| Operation Mode | I | II |
|---|---|---|
| Duration (day) | 20 | 22 |
| Reactor parameters | ||
| HRT (day) | 2 | 1 |
| NLR (mg N( + )/L/day) | 45.7 | 91.4 |
| OLR (mg COD/L/day) | 334 | 668 |
| Influent parameters | ||
| pH | 7.5 | 7.5 |
| N( + ) (mg/L) | 91.3 | 91.3 |
| COD (mg/L) | 668 | 668 |
| Effluent parameters ** | ||
| pH | 7.8 | 7.8 |
| N( + ) (mg/L) | 1.7 | 1.2 |
| COD (mg/L) | 13.4 | 13.4 |
| Biogas (mL/L) | 360 | 360 |
| CH4 in biogas (%) | 72 | 74 |
| N2 in biogas (%) | 10.0 | 10.0 |
| Calculated parameters ** | ||
| N( + )removal (%) | 98 | 99 |
| CODremoval (%) | 98 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaydamaka, S.; Gladchenko, M.; Maslova, O.; Senko, O.; Kornilova, A.; Kornilov, I. Application of the Hybrid Chemical-Biocatalytic Approach for Conversion of Nitrocellulose-Containing Sewage Sludge. Processes 2023, 11, 2017. https://doi.org/10.3390/pr11072017
Gaydamaka S, Gladchenko M, Maslova O, Senko O, Kornilova A, Kornilov I. Application of the Hybrid Chemical-Biocatalytic Approach for Conversion of Nitrocellulose-Containing Sewage Sludge. Processes. 2023; 11(7):2017. https://doi.org/10.3390/pr11072017
Chicago/Turabian StyleGaydamaka, Sergey, Marina Gladchenko, Olga Maslova, Olga Senko, Alla Kornilova, and Igor’ Kornilov. 2023. "Application of the Hybrid Chemical-Biocatalytic Approach for Conversion of Nitrocellulose-Containing Sewage Sludge" Processes 11, no. 7: 2017. https://doi.org/10.3390/pr11072017
APA StyleGaydamaka, S., Gladchenko, M., Maslova, O., Senko, O., Kornilova, A., & Kornilov, I. (2023). Application of the Hybrid Chemical-Biocatalytic Approach for Conversion of Nitrocellulose-Containing Sewage Sludge. Processes, 11(7), 2017. https://doi.org/10.3390/pr11072017






