Ultrasound and Eco-Detergents for Sustainable Cleaning
Abstract
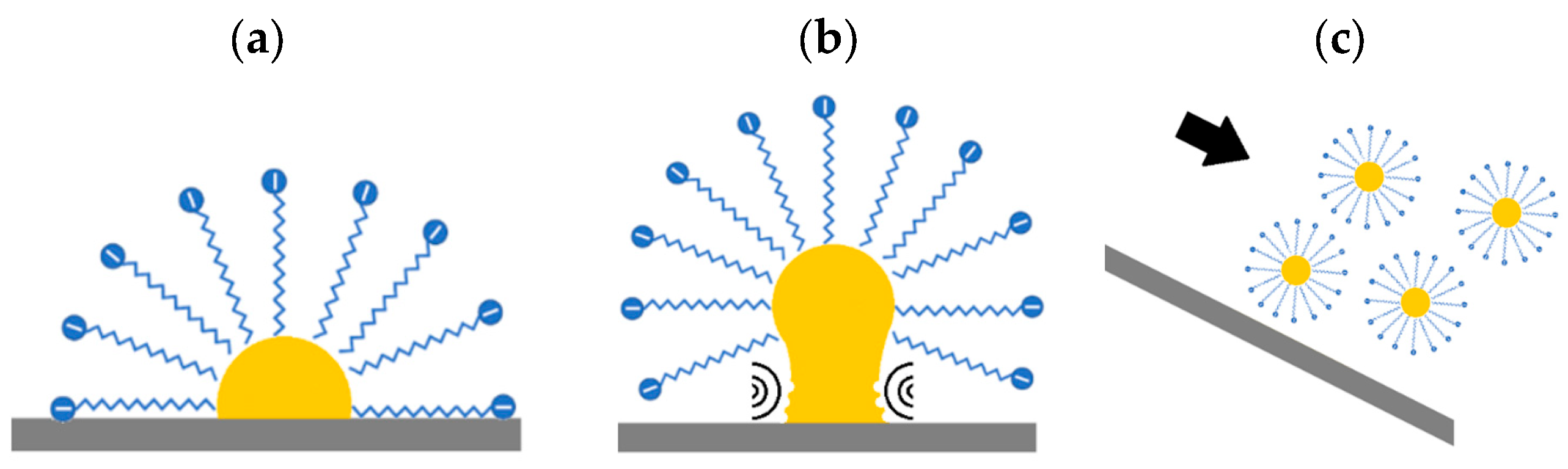
:1. Introduction
- -
- Wetting: increased ultrasonic wettability for different detergent solutions;
- -
- Cleaning: removal of soluble (hydrocarbon) and nonsoluble (silicon) grease;
- -
- Rinsing: removal of the dirty solution by gravity alone.
2. Materials and Methods
- -
- Increased wettability: ultrasound increases wettability between phases, increasing the contact area and therefore the surface area covered. To that end, the chemical surfactancy and the mechanical increase in wettability for each solution are characterised. Considering the non-return mechanism, the sliding behaviour of each solution is also analysed.
- -
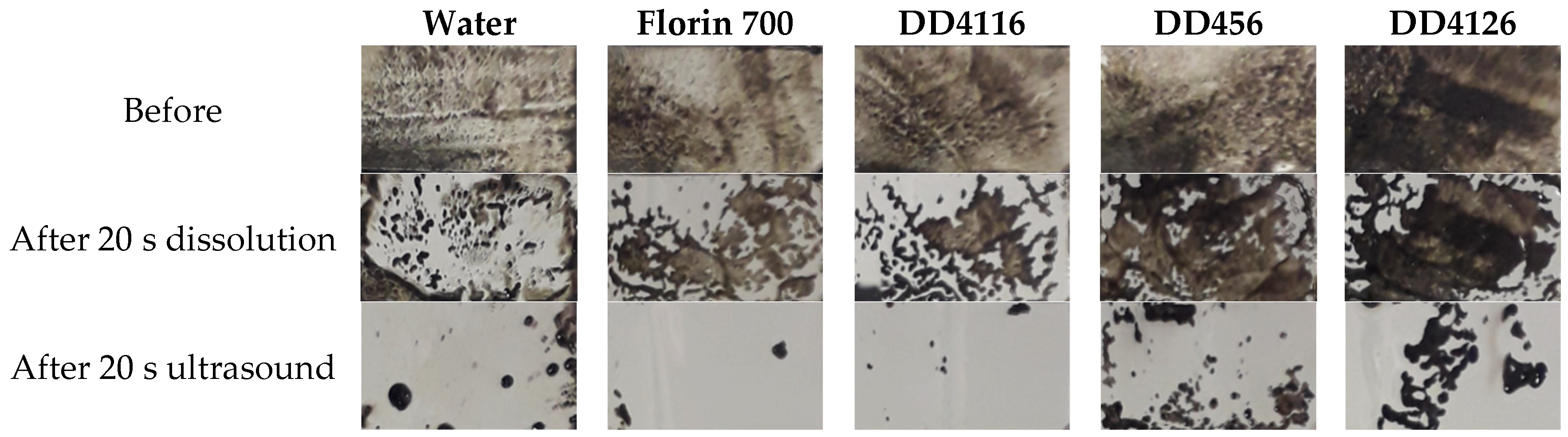
- Removal: ultrasonic cavitation accelerates the removal of dirt and its mechanical capture in micelles (Figure 2). Grease removal is measured by photographic techniques and image analysis. The method compares the effect of dissolution alone and the combination of a solution and ultrasonic cavitation.
- -
- Rinsing: once the grease is detached, it can reattach to the surface if the dirty solution is not rinsed off. The difference between using and not using detergents is studied during gravity-based rinsing.

2.1. Wettability Enhancement
2.2. Grease Removal
- -
- Homogeneous soiling: the grease was homogeneously distributed by drawing a glass microscope holder over the surface of the mirror. A 25 g weight was placed on the holder to ensure constant pressure on the grease during the spreading process.
- -
- Concentrated drop: 0.1 mL of grease was deposited on the surface of the mirror by means of a pipette.
- -
- Degreasing due to the inherent surfactant properties of the detergents;
- -
- Degreasing in combination with ultrasonic cavitation;
- -
- Rinsing after cleaning.

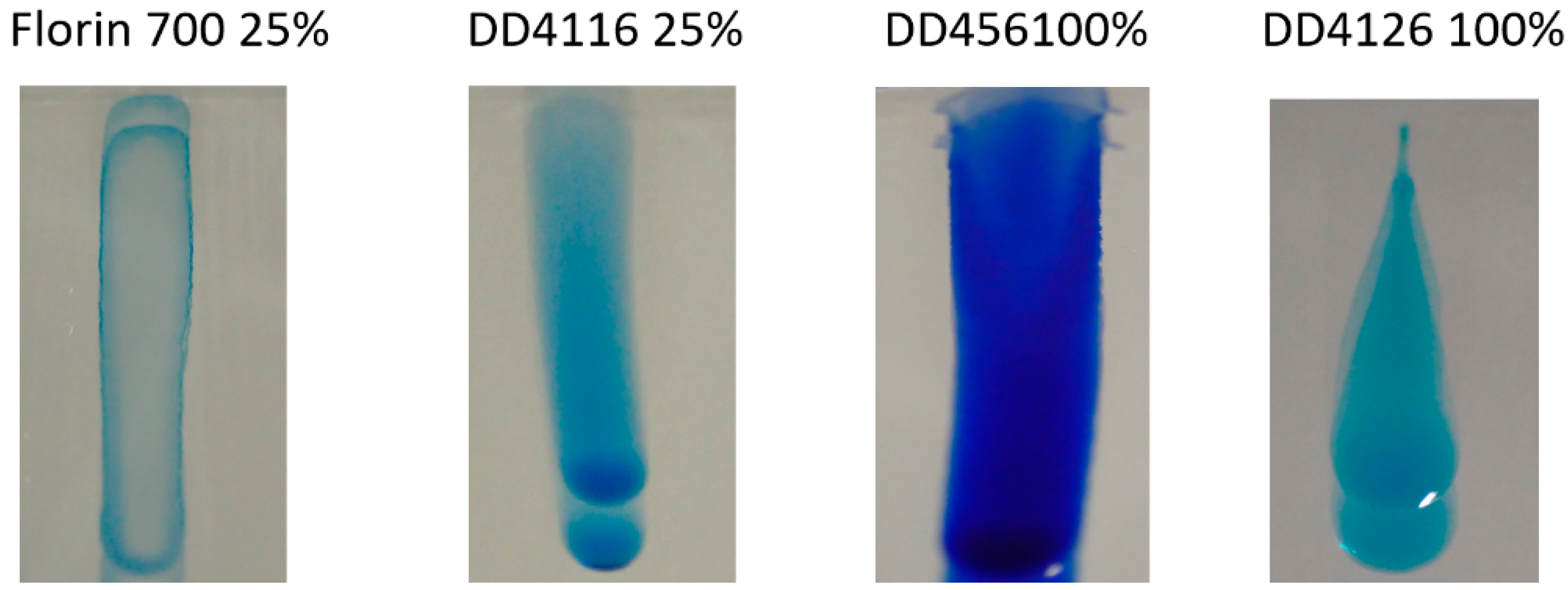
2.3. Rinsing
3. Results
3.1. Wettability Enhancement
- -
- 20 kHz frequency;
- -
- 60 W of power, equivalent to 3 µm of average amplitude (6 µm peak–peak);
- -
- 3 s of exposure.
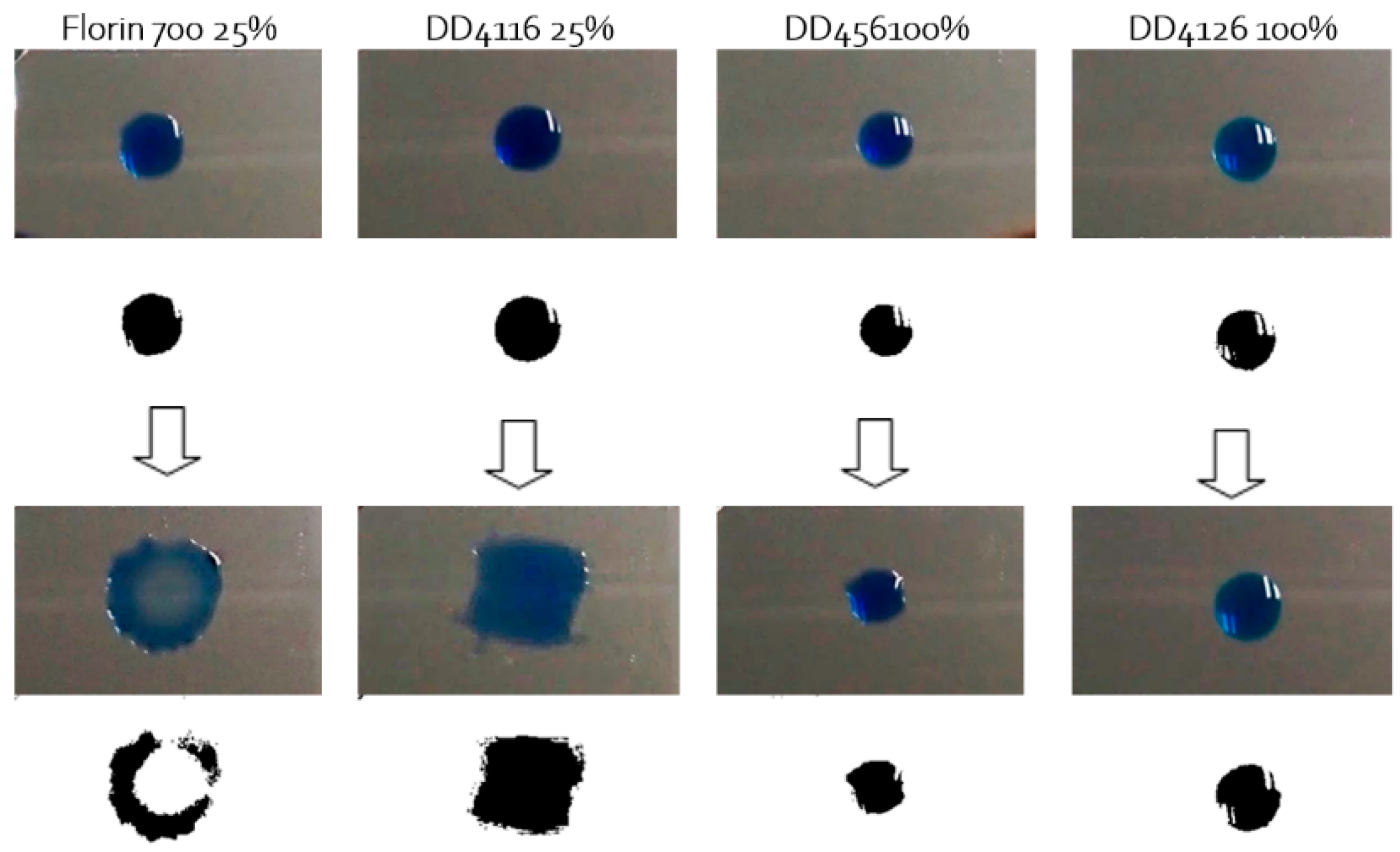
3.2. Grease Removal
- -
- 20 s of solution (without ultrasound) with a 1 mL detergent layer homogeneously distributed over the entire surface;
- -
- 20 s of ultrasonic vibration at 60 W.
3.3. Rinsing
4. Conclusions
- -
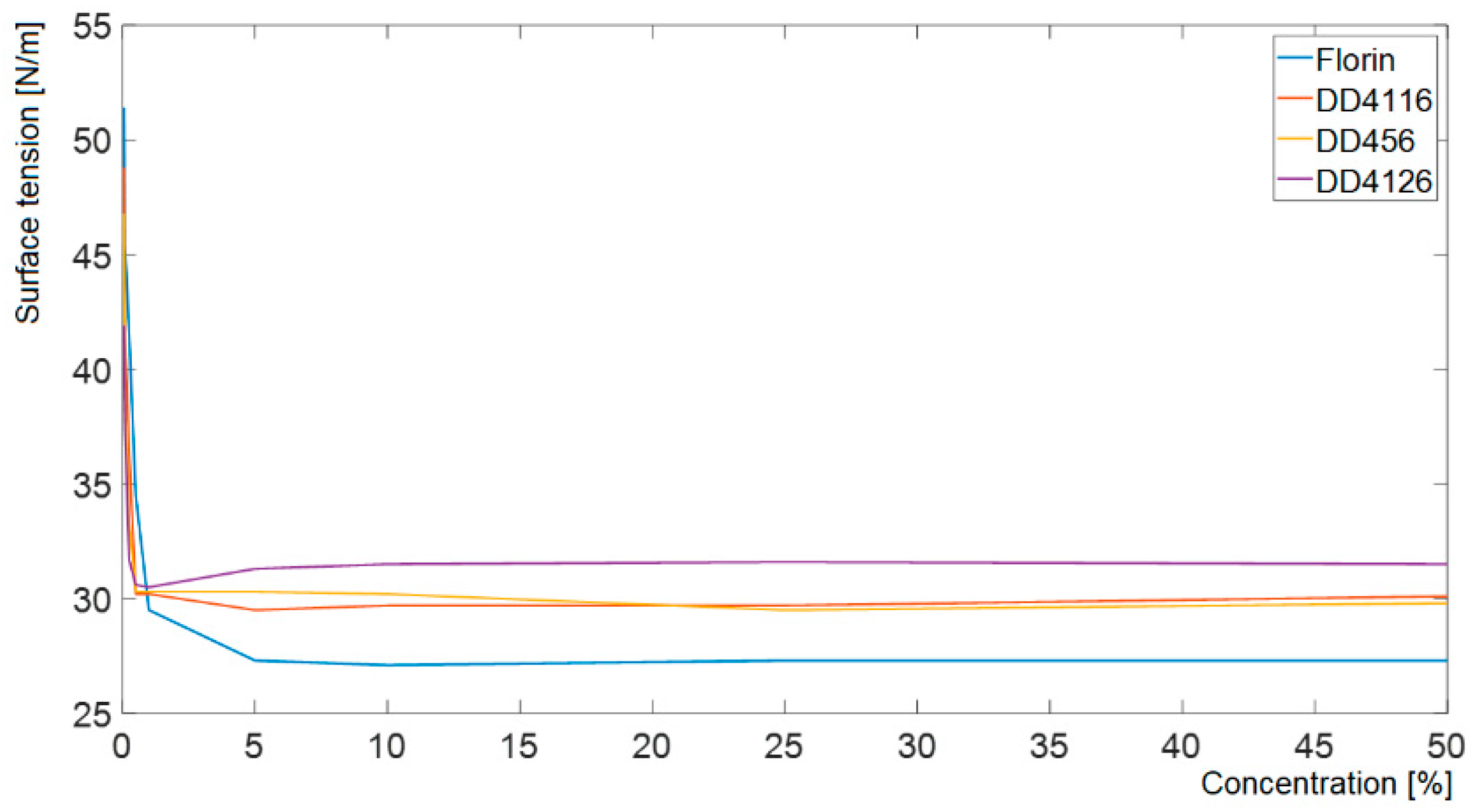
- All four detergent references studied reduce the surface tension of distilled water to similar values. However, the dynamic behaviour of each detergent with respect to ultrasound is very different, and sliding behaviour qualitatively coincides with it. Thus, it is the fluids with the highest wetting rate in the face of ultrasound (Florin 700 and ecological product DD4116) that require the smallest angle of inclination to initiate rolling and wet the surface while sliding. This is consistent with the basis of the non-return mechanism.
- -
- In the case of silicon grease, the main elimination principle is mechanical cavitation. The two substances with the best ultrasonic behaviour are, in order, the benchmark product Florin 700 (DD4116) and distilled water. Ultrasonic enhanced wetting helps cavitation to remove silicon grease through shearing forces.
- -
- The same behaviour is found in the case of hydrocarbon grease, but the enhanced wetting effect is not so evident compared to silicon grease.
- -
- Although ultrasound is the main grease removal mechanism, during gravity rinsing, there is a risk of reattachment of the surface. It is therefore necessary to use a detergent that prevents grease from reattaching.
- -
- The optimum solution is to dilute the minimum amount of DD4116 in water. In this way, cavitation and wettability are enhanced, but grease is prevented from re-adhering during rinsing. For this purpose, the concentration can be up to 10 times lower than that recommended by the manufacturer. This figure varies depending on the amount and type of grease present in the substrate.
- -
- With the proper formulation, eco-detergents combined with ultrasound can give better results than synthetic solutions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rauscher, H.; Rasmussen, K.; Riego Sintes, J.; Sala, S. Safe and Sustainable by Design Chemicals and Materials; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Ivanković, T.; Hrenović, J. Surfactants in the Environment. Arch. Ind. Hyg. Toxicol. 2010, 61, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, S.; Rakshit, A.; Acharjee, A.; Saha, B. Biodegradability and Biocompatibility: Advancements in Synthetic Surfactants. J. Mol. Liq. 2021, 324, 115105. [Google Scholar] [CrossRef]
- Chamanfar, A.; Monajati, H.; Rosenbaum, A.; Jahazi, M.; Bonakdar, A.; Morin, E. Microstructure and Mechanical Properties of Surface and Subsurface Layers in Broached and Shot-Peened Inconel-718 Gas Turbine Disc Fir-Trees. Mater. Charact. 2017, 132, 53–68. [Google Scholar] [CrossRef]
- Yi, X.; Wei, Y.; Zhai, W.; Wang, P.; Liu, D.; Zhou, Z. Effects of Three Surfactants on the Degradation and Environmental Risk of Metolachlor in Aquatic Environment. Chemosphere 2022, 300, 134295. [Google Scholar] [CrossRef]
- Kierkegaard, A.; Sundbom, M.; Yuan, B.; Armitage, J.M.; Arnot, J.A.; Droge, S.T.J.; McLachlan, M.S. Bioconcentration of Several Series of Cationic Surfactants in Rainbow Trout. Environ. Sci. Technol. 2021, 55, 8888–8897. [Google Scholar] [CrossRef] [PubMed]
- Rafi, S.; Ravikanth, S.; Basha, D.; Shaik, M.; Rao, K. Impact of detergent toxicity on energy metabolism in liver and gill of the fish channa punctatus. Trends Fish. Res. 2020, 9, 1–6. [Google Scholar]
- Azuokwu, A.A.; Yerima, Y.; Azike, R.U. Production and Performance Evaluation of Biodetergents as an Alternative to Conventional Drilling Detergent. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 2–4 August 2021. [Google Scholar]
- Pott, R.W.M.; Von Johannides, J. Process Development in Biosurfactant Production. In Biosurfactants for the Biobased Economy; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Fayaz, F.; Alara, O.R. Biosurfactants—A New Frontier for Social and Environmental Safety: A Mini Review. Biotechnol. Res. Innov. 2018, 2, 81–90. [Google Scholar] [CrossRef]
- Inui, A.; Honda, A.; Yamanaka, S.; Ikeno, T.; Yamamoto, K. Effect of Ultrasonic Frequency and Surfactant Addition on Microcapsule Destruction. Ultrason. Sonochem. 2021, 70, 105308. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.; Belwal, T.; Ruyuan, Z.; Xu, Y.; Li, L.; Luo, Z. Optimization and Mechanism of Phytochemicals Extraction from Camellia Oleifera Shells Using Novel Biosurfactant Nanobubbles Solution Coupled with Ultrasonication. Food Bioprocess Technol. 2022, 15, 1101–1114. [Google Scholar] [CrossRef]
- Lauterborn, W.; Kurz, T.; Mettin, R.; Ohl, C.D. Experimental and Theoretical Bubble Dynamics. Adv. Chem. Phys. 1999, 110, 295–380. [Google Scholar]
- Lauterborn, W.; Kurz, T.; Geisler, R.; Schanz, D.; Lindau, O. Acoustic Cavitation, Bubble Dynamics and Sonoluminescence. Ultrason. Sonochem. 2007, 14, 484–491. [Google Scholar] [CrossRef]
- Tervo, J.T.; Mettin, R.; Lauterborn, W. Bubble Cluster Dynamics in Acoustic Cavitation. Acta Acust. United Acust. 2006, 92, 178–180. [Google Scholar]
- Mason, T.J. Ultrasonic Cleaning: An Historical Perspective. Ultrason. Sonochem. 2016, 29, 519–523. [Google Scholar] [CrossRef]
- Trapuzzano, M.A. Controlled Wetting Using Ultrasonic Vibration. Doctoral Dissertation, University of South Florida, Tampa, FL, USA, 2019. [Google Scholar]
- Vukasinovic, B.; Smith, M.K.; Glezer, A. Dynamics of a Sessile Drop in Forced Vibration. J. Fluid Mech. 2007, 587, 395. [Google Scholar] [CrossRef]
- Sarasua, J.A.; Ruiz, L.; Aranzabe, E.; Vilas, J.L. Energetic Study of Ultrasonic Wettability Enhancement. Ultrason. Sonochemistry 2021, 79, 105768. [Google Scholar] [CrossRef]
- Trapuzzano, M.; Tejada-Martínez, A.; Guldiken, R.; Crane, N. Volume and Frequency-Independent Spreading of Droplets Driven by Ultrasonic Surface Vibration. Fluids 2020, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Manor, O.; Dentry, M.; Friend, J.R.; Yeo, L.Y. Substrate Dependent Drop Deformation and Wetting under High Frequency Vibration. Soft Matter 2011, 7, 7976–7979. [Google Scholar] [CrossRef]
- Sarasua Miranda, J.A.; Ruiz-Rubio, L.; Aranzabe Basterrechea, E.; Vilas-Vilela, J.L. Non-Immersion Ultrasonic Cleaning: An Efficient Green Process for Large Surfaces with Low Water Consumption. Processes 2021, 9, 585. [Google Scholar] [CrossRef]
- ElSherbini, A.I.; Jacobi, A.M. Liquid Drops on Vertical and Inclined Surfaces: I. An Experimental Study of Drop Geometry. J. Colloid Interface Sci. 2004, 273, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Nanzai, B.; Suzuki, S.; Okitsu, K. Sonochemical Degradation of Surfactants with Different Charge Types: Effect of the Critical Micelle Concentration in the Interfacial Region of the Cavity. Ultrason. Sonochem. 2021, 71, 105354. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Kunjappu, J.T. Micelle Formation by Surfactants. In Surfactants and Interfacial Phenomena; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 123–201. [Google Scholar]
- Shaeiwitz, J.; Chan, A.-C.; Cussler, E.; Evans, D. The Mechanism of Solubilization in Detergent Solutions. J. Colloid Interface Sci. 1981, 84, 47–56. [Google Scholar] [CrossRef]
- Nik Him, N.R.; Hidzir, S.N.M.; Wan Hassan, W.H. Application of CMC from Oil Palm Biomass as Anti-Redeposition Agent in Laundry Detergent. Key Eng. Mater. 2019, 797, 224–229. [Google Scholar] [CrossRef]
- Thompson, C.J.; Ainger, N.; Starck, P.; Mykhaylyk, O.O.; Ryan, A.J. Shampoo Science: A Review of the Physiochemical Processes behind the Function of a Shampoo. Macromol. Chem. Phys. 2023, 224, 2200420. [Google Scholar] [CrossRef]
- Yusof, N.S.M.; Ashokkumar, M. Ultrasonic Transformation of Micelle Structures: Effect of Frequency and Power. Ultrason. Sonochem. 2015, 24, 8–12. [Google Scholar] [CrossRef]
- James, G.K.; Walz, J.Y. Experimental and Theoretical Investigation of the Depletion and Structural Forces Produced by Ionic Micelles. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 441, 406–419. [Google Scholar] [CrossRef]
- Chen, A.; Li, S.-W.; Jing, D.; Xu, J.-H. Interactions between Colliding Oil Drops Coated with Non-Ionic Surfactant Determined Using Optical Tweezers. Chem. Eng. Sci. 2019, 193, 276–281. [Google Scholar] [CrossRef]
- Toth, S.; Muller, M.; Miller, D.C.; Moutinho, H.; To, B.; Micheli, L.; Linger, J.; Engtrakul, C.; Einhorn, A.; Simpson, L. Soiling and Cleaning: Initial Observations from 5-Year Photovoltaic Glass Coating Durability Study. Sol. Energy Mater. Sol. Cells 2018, 185, 375–384. [Google Scholar] [CrossRef]
- Sarasua, J.A.; Sandá, A.; Arguelles-Arizcun, D.; Fernández-García, A. Integration of a Non-Immersion Ultrasonic Cleaning System in a Solar Concentrating Field. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2019; Volume 2126, p. 30050. [Google Scholar]




| Description | Solution | |
|---|---|---|
| Florin 700 | Synthetic benchmark product. Contains anionic and nonionic surfactants, phosphates, odorants, dyes and potassium hydroxide. pH = 12; η = 2.082 cP | Dilute to 25% in water |
| DD4116 | A&B Ecological detergent. Contains nonionic surfactants, alkaline salts, organic chelators and enzymes. pH = 11; η = 3.36 cP | Dilute to 25% in water |
| DD456 | A&B Ecological Enzymatic cleaner. Contains nonionic surfactants, alcohols, metasilicate and enzymes. pH = 10; η = 1.3 cP | Ready for use |
| DD4126 | A&B Ecological detergent. Contains nonionic surfactants, alcohols, alkaline salts and preservatives. pH = 11.4; η = 5.46 cP | Ready for use |
| Name | Description | Reference | Impregnation |
|---|---|---|---|
| Silicon grease | Silicon-based lubricant for machinery. White. | RENOLIT SI 400 M | Homogeneous |
| Hydrocarbon grease | Hydrocarbon-based lubricant for machinery. Degraded. Black. | Shell Rimula R3X40 | Homogeneous |
| Hydrocarbon grease | Hydrocarbon-based lubricant for machinery. Degraded. Black. | Shell Rimula R3X40 | Concentrated drop |
| Concentration [%] | Florin 700 25% γ [N/m] | DD4116 25% γ [N/m] | DD456 100% γ [N/m] | DD4126 100% γ [N/m] |
|---|---|---|---|---|
| 100 (nominal) | 28.4 | 27.7 | 30.4 | 32.3 |
| 50 | 27.3 | 30.1 | 29.8 | 31.5 |
| 25 | 27.3 | 29.7 | 29.5 | 31.6 |
| 10 | 27.1 | 29.7 | 30.2 | 31.5 |
| 5 | 27.3 | 29.5 | 30.3 | 31.3 |
| 1 | 29.5 | 30.2 | 30.3 | 30.5 |
| 0.5 | 34.5 | 30.2 | 30.3 | 30.6 |
| 0.25 | 41.6 | 36.5 | 33.4 | 31.7 |
| 0.1 | 45.4 | 41.4 | 39.1 | 37.4 |
| 0.05 | 51.4 | 48.8 | 46.8 | 41.9 |
| Repetition | Florin 700 25% | DD4116 25% | DD456 100% | DD4126 100% |
|---|---|---|---|---|
| 1 | 25° | 19° | 45° | 20° |
| 2 | 22° | 18° | 40° | 22° |
| 3 | 24° | 19° | 42° | 22° |
| Average | 23.7° | 18.7° | 42.3° | 21.3° |
| SD | 1.5° | 0.6° | 2.5° | 1.2° |
| Water | Florin 700 | DD4116 | DD456 | DD4126 | |
|---|---|---|---|---|---|
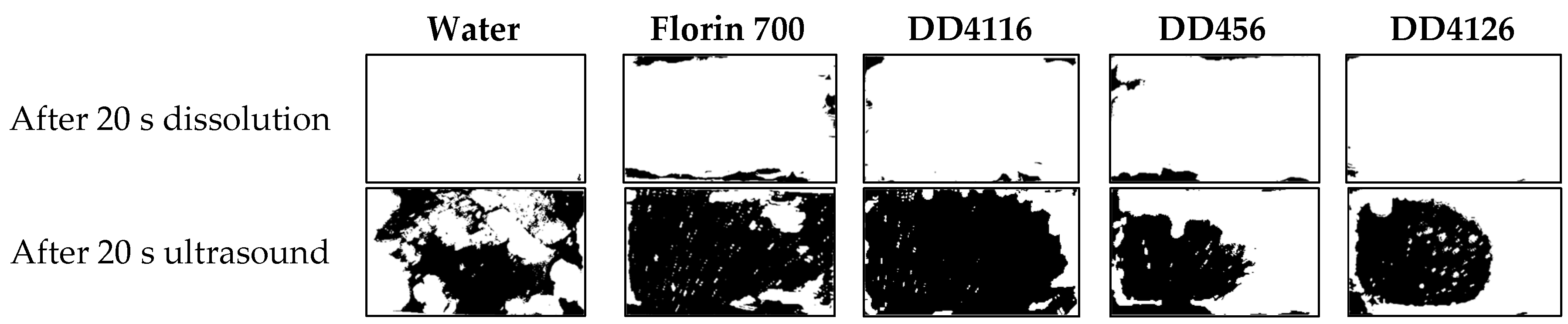
| 20 s dissolution | 0.1 | 9.7 | 3.9 | 7 | 0.9 |
| 20 s ultrasound | 59.3 | 88.6 | 85.7 | 40.7 | 50 |
| Water | Florin 700 | DD4116 | DD456 | DD4126 | |
|---|---|---|---|---|---|
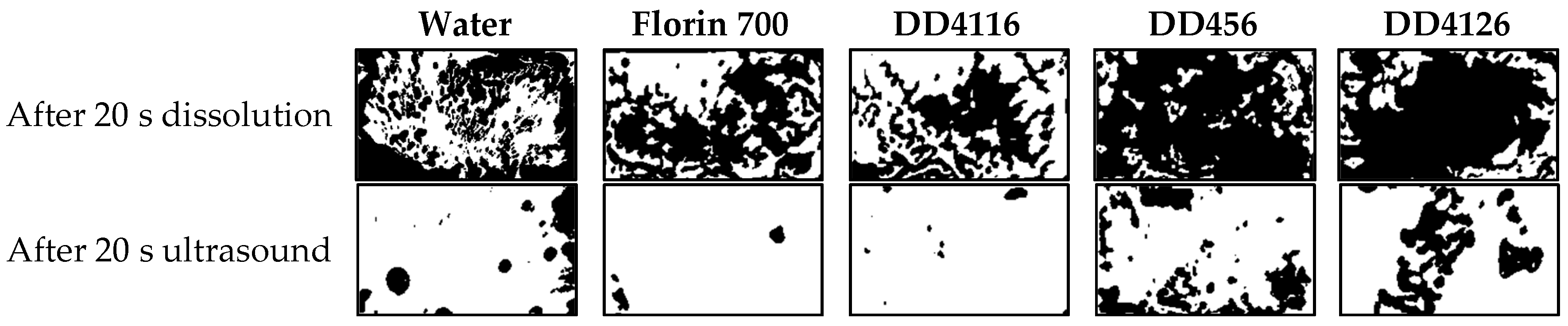
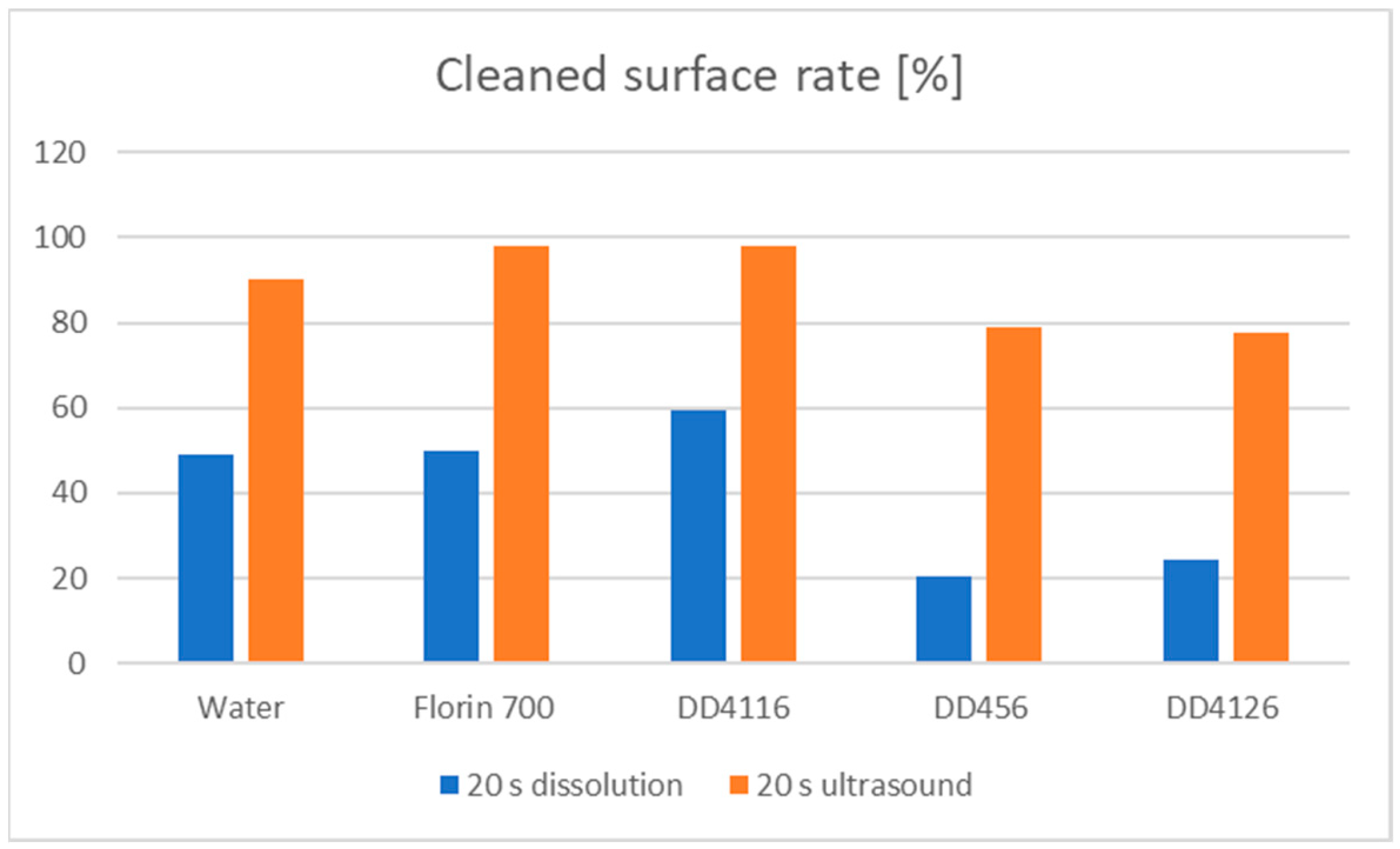
| 20 s dissolution | 49.1 | 49.9 | 59.4 | 20.6 | 24.5 |
| 20 s ultrasound | 90.4 | 98.0 | 98.2 | 78.8 | 77.7 |
| Initial | After Rubbing | With Water | |
|---|---|---|---|
| Capacitance [nF] | 0.191 | 0.201 | 0.172 |
| Voltage [mV] | 0 | 0 | 0 |
| Charge [C] | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarasua Miranda, J.A.; Ruiz Rubio, L.; Trinidad Cristobal, A.; Vilas Vilela, J.L.; Izaguirre Goyoaga, J.K.; Barbero Mangas, F.; Aranzabe Basterrechea, E. Ultrasound and Eco-Detergents for Sustainable Cleaning. Processes 2023, 11, 2082. https://doi.org/10.3390/pr11072082
Sarasua Miranda JA, Ruiz Rubio L, Trinidad Cristobal A, Vilas Vilela JL, Izaguirre Goyoaga JK, Barbero Mangas F, Aranzabe Basterrechea E. Ultrasound and Eco-Detergents for Sustainable Cleaning. Processes. 2023; 11(7):2082. https://doi.org/10.3390/pr11072082
Chicago/Turabian StyleSarasua Miranda, Jon Ander, Leire Ruiz Rubio, Ander Trinidad Cristobal, Jose Luis Vilas Vilela, Jon Kepa Izaguirre Goyoaga, Francisca Barbero Mangas, and Estibaliz Aranzabe Basterrechea. 2023. "Ultrasound and Eco-Detergents for Sustainable Cleaning" Processes 11, no. 7: 2082. https://doi.org/10.3390/pr11072082






