Abstract
Orthopedics has been identified as a major clinical medicine branch since the 18th century for musculoskeletal disease diagnosis and therapeutics. Along with technological progress, the surgical treatment of bone disorders became available in the 19th century, while its growth faced several obstacles due to a lack of proper biocompatible material and alternative structures. Therefore, tissue engineering has emerged as a key building block to overcome these challenges, providing the capability for bone growth, and fabricating scaffolds with enriched desirable cellular compatibility as well as mechanical properties. Among various structures, the electrospun layer has implied high porosity and fine pore sizes, and succeeded in cell growth and proliferation. Collagen nanofibers have represented a wide potential for mineralization, bone regeneration, and forming processes. Despite this, such scaffolds have accosted bone remodeling limitations due to inadequate osteoinductivity and mechanical strength. Hence, the tendency to fabricate efficient collagen-based nanofibrous layers enriched with organic and inorganic materials has been extensively declared. Embedding these materials leads to engineering a membrane with appropriate physical, degradability, and mechanical properties, as well as proper mineralization and biological activity required for better replicating the bone organ’s natural microenvironment. This paper highlighted a wide overview of the natural resources, electrospinning strategies, and collagen-based electrospun composites for bone regeneration. Accordingly, future prospects could be developed for generating novel 3D-scaffold formations, benefiting from organic and inorganic substances to boost the biological and mechanical properties, simultaneously.
1. Introduction
Collagen is assumed as a major constituent of the extracellular matrix (ECM) in both hard and soft tissues, forming 25 to 33% of the body’s protein mass in mammals. Generally, 28 collagen types have been recognized so far, disseminating in various tissues, such as bone, teeth, tendon, skin, and so forth. Collagens could be synthesized through different body cells based on their localizations. As an example, in the connective tissues, fibroblast cells, as well as bone osteoblast cells support collagen production. Along with the structural characteristics of collagen, proper hemostatic capacity, low iminium genicity, and appropriate dimensional stability are considered their unique features [1,2,3,4].
According to the literature, a different hierarchical conformation of collagen has been identified, including amino acid-triplet, alfa-helix, triple-helix, and collagen fibrils. Moreover, three types of collagens are the most common among the collagen family, differing in the size and length of the helix. Type I collagen is recognized as the most vital family member, which could be found in skin, tendons, and bone. Additionally, collagen type II is commonly extracted from cartilage, while type III is frequent in the vascular system [5,6,7,8]. The above-mentioned characteristics of collagen and its great compatibility with the body have resulted in promising applications in biomedical and tissue engineering fields, containing cosmetic surgeries, corneal bandages, contact lenses, implants, sutures, dental composites, and drug delivery systems [9,10].
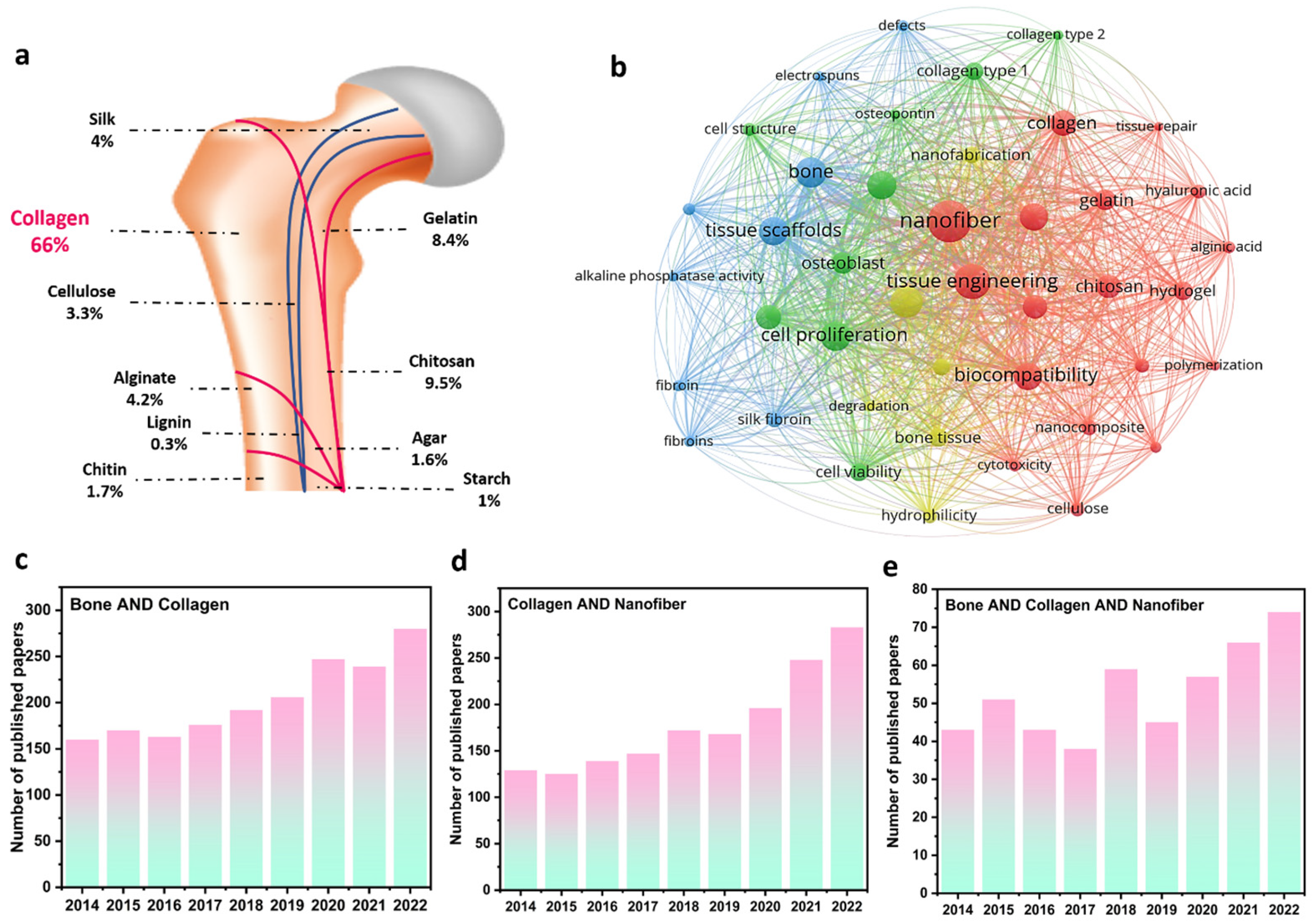
As a critical organ, bone provides fate-bearing sustainment, motion assistance, and physical protection of the brain and heart. Meanwhile, the crucial roles of bones could be adversely influenced by injuries, fractures, diseases, accidents, and functional disorders. Correspondingly, various treatment strategies have been introduced, in which employing implants is considered a major great solution for bone tissue function [11]. In this era, material selection, matrix fabrication, and biological interactions are three factors affecting the final efficiency of engineered implants [12]. Regarding the material parameter, extracted biopolymers from natural resources, such as collagen, chitosan, alginate, silk, cellulose, chitin, agar, starch, and lignin, as well as their derivatives (e.g., gelatin) are the most applicable substances through revealing proper biocompatibility, biodegradability, and great cell response in bone tissue engineering. As can be seen in Figure 1a and based on the published papers in the Scopus database, collagen structures comprise the largest portion of published papers regarding bone tissue regeneration. Figure 1b also depicts the VOSviewer visualization of the polymeric bone-related papers, showing the remarkable role of collagen and electrospun nanofibers in regenerating bone tissues. From the point of view of matrix fabrication, electrospun layers have represented highlighted properties of highly porous structure, tiny and interconnected pores, large surface-to-volume ratio, and so on, providing an outstanding condition for cell growth and proliferation. According to Figure 1c–e, the number of Scopus-indexed published papers with the keywords of “bone & collagen”, “collagen & nanofiber”, and “bone & collagen & nanofiber”, is presented. Accordingly, the dedicated efforts to exploring and designing electrospun collagen networks and their applications in bone regeneration have been dramatically enhanced in recent years. So far, numerous papers have overviewed the role of collagen structures in tissue engineering, while few attempts have considered evaluating the collagen-based bone scaffolds fabricated through the electrospinning methodology. Additionally, the synergetic effect of additives and electrospun nanofibers toward designing a bone tissue scaffold with boosted cell adhesion, growth, and proliferation is less highlighted in the performed review papers, which is comprehensively focused on in this evaluation.
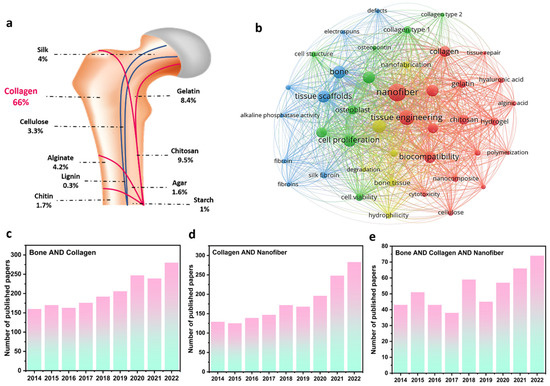
Figure 1.
Collagen’s role in bone regeneration: (a) the ratio of different natural-based polymers in bone tissue regeneration, (b) VOSviewer visualization map of the polymer-based bone tissues, and the number of Scopus-indexed papers related to (c) “bone & collagen”, (d) “collagen & nanofiber”, and (e) “bone & collagen & nanofiber” keywords.
2. Bone Regeneration Techniques
The treatment method for bone defects is divided into major bone grafting and transplant strategies, in which transplants include autograft, allograft, and xenograft [13]. Bone graft is known as an implanted material in the body, resulting in osteogenesis, osteoinduction, and osteoconduction [14]. In the autograft method, an iliac or fibular bone graft from the patient’s body is used, causing several disadvantages, such as essential secondary surgery, donor limitation, high cost, infection, and pain [13,15]. Allograft also applies fresh or transplanted tissue from another person, possibly facing some difficulties, including the rejection or transmission of diseases, infections (e.g., HIV), and high cost [13]. Meanwhile, xenograft is a bone graft from the body of other organisms (e.g., animals), suffering from the transmission of common diseases between animals and humans, immune response, and poor outcomes [14]. As a result, many efforts are looking for suitable alternatives to fix defects related to bone. Material selection, matrix design, and biological interactions are key factors toward approaching an ideal structure for bone regeneration. Inorganic and organic biomaterials are the main substances used for mimicking the structural characteristics of natural bone tissues.
Tissue engineering knowledge is aimed at providing an appropriate scaffold that can mimic the natural bone environment to respond to bone damage. In this case, the designed scaffolds are expected to have sufficient mechanical strength and porous architecture. These features ameliorate cell migration and differentiation to scaffolds [16]. Various biomaterials have been utilized as scaffolds for bone tissue engineering. Among them, the combination of type I collagen and different types of bioceramics, such as hydroxyapatite (HA), are frequently suggested. This could be linked with the point that natural bones are mostly assembled from type I collagen with a tiny amount of type V to form a network with nanosized bioceramics crystals [2,17]. The HA bioceramic has received extensive attention in bone tissue engineering due to its excellent features, such as biocompatibility, osteoinductivity, and osteoconductivity. Therefore, in the design of suitable networks for bone reconstruction, benefiting from the synergistic effect of collagen along with additives could be a valuable strategy [17].
Besides material type and synthesis process in the determination of biomaterial characteristics, fabrication methods have a key role in morphological, physiochemical, mechanical, and biological features. In 1988, Dahlin et al. used a physical substrate for bone tissue regeneration for the first time [18,19]. Then, different configurations, including hydrogels, films, sponges, micro- and nano-spheres, and electrospun layers were declared as other promising approaches for bone tissue engineering. Among the mentioned fabrication techniques, cross-linking, 3D printing, and electric-field techniques have caught many interests, leading to the formation of hydrogels, proper membranes, and nanofibrous structures, respectively.
The cross-linking procedure could successfully generate various hydrogel configurations. Hydrogels are hydrophilic polymeric materials, capable of holding large amounts of water in their three-dimensional structure. In other words, they are formed by the interaction of one or more monomers that produce a cross-linked network structure with the ability to swell while not being dissolved in water [20]. Collagen could be prepared in hydrogel forms for use in bone tissue engineering. Sotome et al. [21,22] produced a hydrogel composed of collagen/HA and alginate that is automatically cross-linked by the simultaneous titration coprecipitation method in 30 min. The proposed hydrogel was also employed as a carrier of recombinant human bone morphogenetic protein 2 (rh-BMP2), resulting in successful implantation after 5 weeks without structural deformation. Wang et al. [23] developed chitosan and collagen hydrogels with different ratios using beta-glycerophosphate as an initiator, loaded by adult human bone marrow-derived stem cells. The presence of collagen in the hydrogel increased cell expansion and proliferation, the hydrogel density, and stiffness (Figure 2a–c).
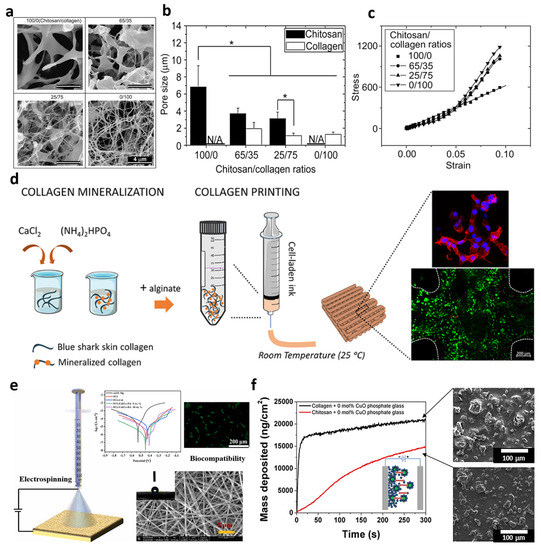
Figure 2.
Characteristics of the collagen/chitosan hydrogel: (a) SEM images, (b) average pore sizes, in which “*” symbol shows a significant difference between the data obtained, and (c) mechanical behavior. Reproduced from reference [13] with permission from Elsevier. The 3D printed Collagen/HA/alginate: (d) schematic of the synthesis process and image of the cultured cells. Reproduced from reference [14] with permission from the American Chemical Society. Electrospun collagen/PCL embedded with cerium/HA: (e) schematic of the electrospinning process, the result of the corrosion resistance, and the biocompatibility examination. Reproduced from reference [15] with permission from Elsevier. Electrophoretic phosphate glass doped with copper via collagen_chitosan: (f) mass deposition, as well as the SEM images. Reproduced from reference [16] with permission from Elsevier.
Figure 2.
Characteristics of the collagen/chitosan hydrogel: (a) SEM images, (b) average pore sizes, in which “*” symbol shows a significant difference between the data obtained, and (c) mechanical behavior. Reproduced from reference [13] with permission from Elsevier. The 3D printed Collagen/HA/alginate: (d) schematic of the synthesis process and image of the cultured cells. Reproduced from reference [14] with permission from the American Chemical Society. Electrospun collagen/PCL embedded with cerium/HA: (e) schematic of the electrospinning process, the result of the corrosion resistance, and the biocompatibility examination. Reproduced from reference [15] with permission from Elsevier. Electrophoretic phosphate glass doped with copper via collagen_chitosan: (f) mass deposition, as well as the SEM images. Reproduced from reference [16] with permission from Elsevier.
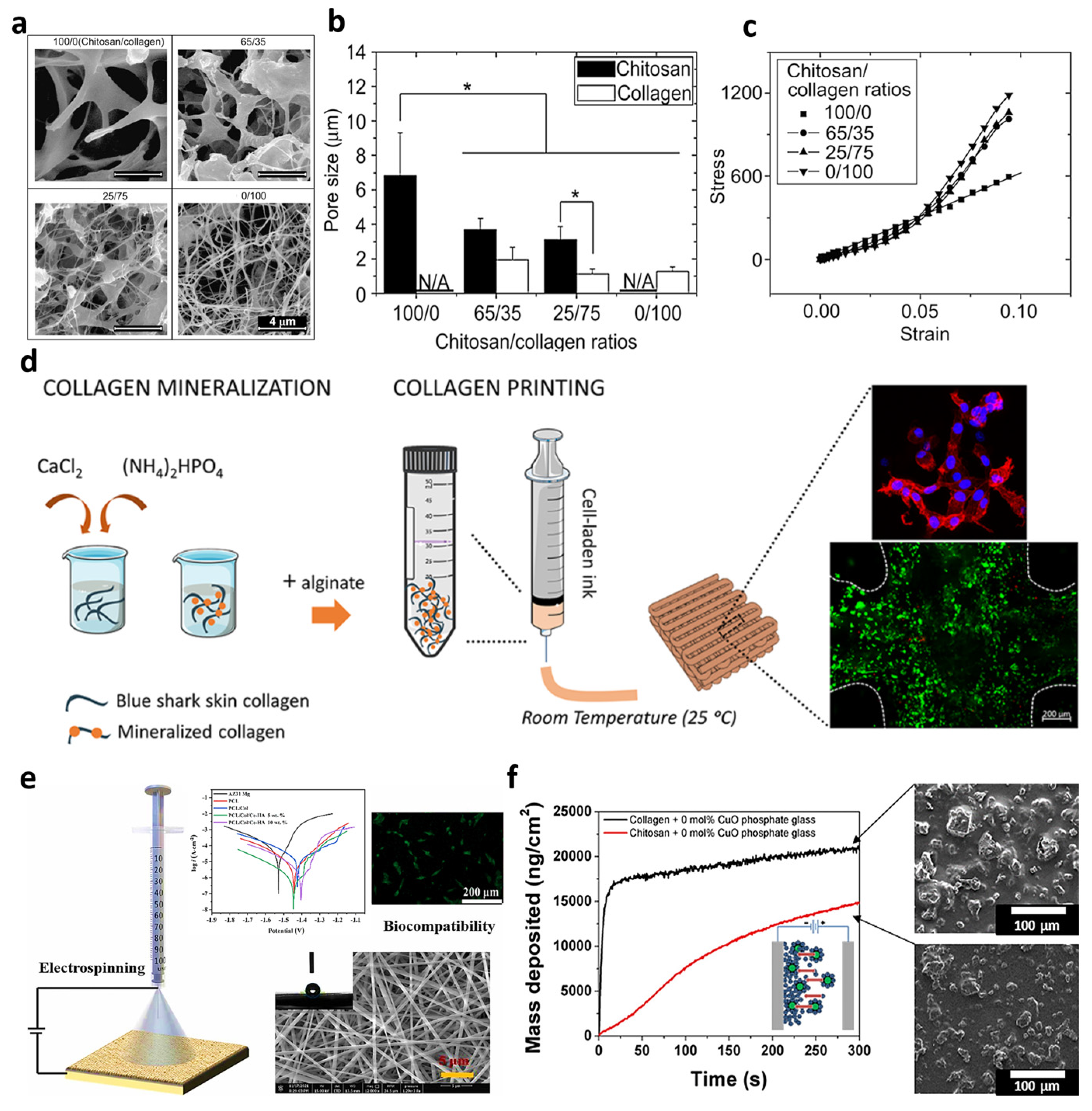
Another technique used to fabricate collagen structures is 3D printing. For the first time in 1980, 3D printer technology was used to produce segments of different sizes, shapes, and materials. It is also able to synthesize porous scaffolds with layer-by-layer techniques, which are suitable for tissue applications, especially for bone tissue engineering [17]. Kim et al. [18] designed a 3D-printed polycaprolactone (PCL) scaffold reinforced with different percentages of carbonated HA coated with marine atelocollagen (MC). The obtained results of the culturing of MC3T3-E1 cells on the 10% CHA/MC/PCL scaffold had a 1044% higher osteogenic differentiation than the pure PCL. Diogo et al. [14] also designed and fabricated a 3D printable cell-laden hydrogel, consisting of blue shark (Prionace glauca (PG))/Collagen/HA/alginate. The presence of collagen in the scaffold made the fibroblast cell line viable during and after successful printing (see Figure 2d).
Electrospinning is a fabrication technique, in which a polymer solution or melt is stretched in an electrostatic field, resulting in the assembling of fibers with distinct diameters onto a conductive collector. This simple procedure could mimic the porous structure of natural ECM due to the production of a large surface area and highly porous substrate with multi-scale fibers [19,20]. Electrospraying is another method that has been investigated for bone tissue engineering, in which droplets are created instead of fibers [21]. This route is similar to electrospinning, and so can be adjusted by manipulating the electrospinning conditions, such as voltage, polymer concentration, and syringe-to-collector distance. Based on the literature, bioceramic nanoparticles are able to coat a layer over the electrospun membranes via the electrospray procedure. Benefiting from the electrospinning procedure, Chen et al. [15] depicted that the electrospun PCL/collagen fibers incorporating 5% cerium/HA could result in uniform fibers with an average diameter of 396 nm, as well as a 30-times-greater corrosion rate than the bare Mg alloy substrate (see Figure 2e).
Electrophoretic deposition is supposed to be another fabrication technique in the electric field category, which is utilized for thick-film coating. Accordingly, charged particles (metal, polymer, ceramic, and glass) that are dispersed in a liquid phase migrate to the opposite electrode under the electric field. Unlike other solutions, the combination of organic and inorganic materials deposits to create different coatings in this technique. Deposition time, the electrical field, and concentration can influence the morphology of the produced coating. Collagen (type I), known as a natural material with a net-positive charge can move over the electrical field and deposit onto the cathode. Also, it can combine with HA or other nanoparticles (e.g., calcium phosphate) to cover the surface. In addition, chitosan as another biomaterial could co-deposit with collagen to form a film by modifying the deposition properties. Rosei et al. [16] co-deposited phosphate glass doped with copper via collagen–chitosan over a stainless-steel surface during the electrophoretic deposition method. Based on the SEM image obtained in Figure 2f, the mass deposition could be obtained in case the collagen was higher compared with chitosan, which could be a result of the superior affinity of the collagen to absorbing PG, resulting in better native cell mimicking, as well as lower rejection by the body. The combination of bioactive fragments with natural polymers was the main aim considered by this approach to closely mimic the bone tissue.
Overall, in comparison with different available fabrication methods, electrospinning has illustrated outstanding advantages, due to versatility, simplicity, cost-effectiveness, high loading capacity, and applicability at room temperature. Also, multi-scaled fibers produced via electrospinning have illustrated unique configurations with desirable surface area, porous structure, tiny and interconnected pores, pore tortuosity, diameter, and the ability to adjust the morphological features. Furthermore, a wide variety of polymer solutions can be used in this process [20]. These benefits make the electrospinning process reliable in abundant research areas, specifically in the design and engineering of versatile bone tissues. Therefore, the collagen resources, suitable conditions for its electrospinning procedure, as well as their characterizations as bone tissue scaffolds are comprehensively reviewed in the following sections.
3. Collagen Resources and Electrospun Fiber Formations
Collagen is a fibrillar protein in animals and comprises more than 25% of the total protein content of the mammalian body [22]. Collagen maintains structural integrity by forming molecular lines that reinforce tendons using large elastic sheets [23,24]. Being known as a viscoelastic material, collagen possesses high tensile strength with low extensibility. In the form of an ECM, collagen provides structural and mechanical support to connective tissues, such as skin, joints, and bones [22]. Collagen fibers have a similar structure to the fibers that make up the ECM. The triple-helix construction allows collagen molecules to form fibrils that provide mechanical strength and elasticity to tissues. It also supports the skin by providing elasticity and strength, as well as protecting it from pathogens and toxins. In addition, collagen is a key building block in cell biological functions, such as proliferation and differentiation, and contributes to the healing of damaged bones and vessels, resulting from the presence of specific sites for integrin receptors on cell surfaces, which attest to the cell’s adherence and interaction with the ECM [25]. Moreover, collagen can act as a signaling molecule through binding to specific receptors on cell surfaces and activating intracellular signaling pathways, which can regulate bioactivity behavior. Furthermore, its biodegradability and biocompatibility are vital means, enabling it to be well tolerated by the body and be broken down through natural procedures. Bones and teeth are the components, where collagen could be found in association with mineral crystals, especially HA [23].
The collagen family have consisted of polypeptidic chains with a triple-helix structure. The diameter of the three polypeptide fibrils is in the range of 10–500 nm with an estimated length of 1400 amino acids and a molecular weight of 285 kDa [25,26] (see Figure 3a). A repeating sequence of three amino acids consisting of glycine, proline, and hydroxyproline forms the primary structure [27] (see Figure 3b). In each individual chain, the atoms are linked by covalent bonds. In contrast, a triple-helical structure of three chains is generated by weaker bonds (dipole–dipole bonds, hydrogen bonds, van der Waals interactions, and ionic bonds) [28]. The stabilization of collagen within a fibril is caused by various intramolecular and intermolecular forces, while the hydrogen bonds are critical in stabilizing the triple helix of collagen. The charged groups in the collagen molecules lead to electrostatic interactions that contribute to the intramolecular structure. Thus, each collagen molecule creates a strong molecular bond with neighboring molecules [27,28].
Three types of collagens, including types I, II, and III, are the most common in the collagen family, differing in the size and length of the helix. Type I collagen, which is the most important member of the family is obtained from skin, tendons, and bone. Type II collagen is extracted from cartilage and type III from the vascular system. It is worth mentioning that type I collagen involves about 90% of the body’s collagen and is the most commonly employed type for various applications [29,30].
Collagen can be extracted from different resources, as depicted schematically in Figure 3c. Bovine, porcine, and marine collagen are the most common bases [19]. Bovine collagen, obtained from the skin and bones, is one of the major resources utilized in various industries. The bovine Achilles tendon is used to obtain type I collagen, while type II is derived from articular or nasal cartilage. The suitable biocompatibility of bovine collagen makes it a proper candidate for various applications. Generally, it is used to cover burns and extra-oral wounds of the body. The major concern about bovine collagen is the prevalence of diseases, such as bovine spongiform encephalopathy, transmissible spongiform encephalopathy, and foot and mouth disease, which are considered a threat to humans. Moreover, about 3% of people are allergic to bovine collagen, motivating researchers to seek a safer alternative for collagen sources [31].
In contrast, another widely used industrial source of collagen is porcine collagen, obtained from pigs’ skin and bones. Since porcine collagen is very similar to human collagen, it causes fewer allergic reactions. Collagen matrices made from porcine collagen have been applied for soft tissue grafting. It has been shown that porcine collagen materials can be a biocompatible alternative for autogenous transplant; however, there is still a risk of zoonosis. Moreover, religious constraints limit this collagen source in some regions and cases [32].
Given the concern about the outbreak of various diseases in terrestrial animals, marine sources have been considered for obtaining collagen. This collagen source can be considered the safest collagen base and has been classified by the FDA as GRAS (generally recognized as safe). The numerous advantages of marine collagen over land collagen could be no zoonotic risk, higher collagen content, lower molecular weight, better absorption, less inflammatory and immune responses, as well as fewer religious and ethical restrictions [19].
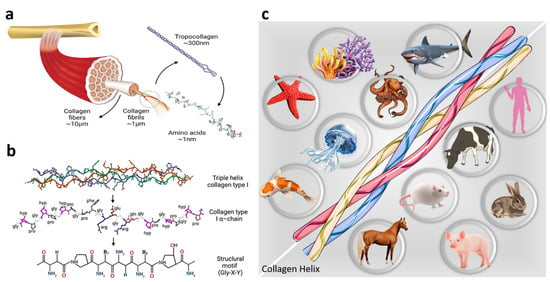
Figure 3.
Collagen resource and structure: (a,b) collagen chemical structure. Reproduced from reference [33] with MDPI Copyright, and (c) various resources employed for the extraction of collagen in different industries.
Figure 3.
Collagen resource and structure: (a,b) collagen chemical structure. Reproduced from reference [33] with MDPI Copyright, and (c) various resources employed for the extraction of collagen in different industries.
Considering several disadvantages compared to animal-derived collagen, including batch-to-batch inconsistency and the risk of inflammation and disease transmission, synthetic collagen has recently been investigated. In this type of collagen, the amino acids self-arrange into a triple-helix structure that mimics natural collagen [25]. Synthetic collagens could also be obtained using recombinant technology, in which insect, yeast, mammalian, and plant cell cultures are employed as precursors. Recombinant collagen has been successfully utilized for several medical applications in the form of sponges, gels, and fibers. Results have revealed the superior performance of the recombinant collagen than that of the collagen extracted from animals in terms of processing ability and therapeutic efficiency [34]. Despite the mentioned advantages of synthetic collagens, some shortcomings, such as low profitability and high cost, have limited their applications. In addition, the lack of enzymes essential for bioactive collagen formation leads to a decrease in demand for this product. Therefore, animal-based collagen remains the first choice for the field of research and clinical applications [24].
Reconstructive medicine is a brilliant instance where collagen-based materials have been successfully utilized and have resulted in approaching top results. Collagen matrices (especially type I) are usually used as an ECM substitute for tissue regeneration or repair [35]. Collagen-based three-dimensional scaffolds for tissue engineering have been fabricated using various methods, such as gas-forming foam [36], thermally induced phase separation [37], freeze-drying [38], 3D printing [39], and electrospinning [40].
As discussed earlier, electrospinning is a robust and potential technique for the fabrication of matrices with nanoscale and/or microscale fibers that mimic the fibrous nature of native ECM [41,42]. Electrospun fibers have a fibrous structure that is similar to the fibrous network of the ECM, providing robust mechanical support and a proper scaffold for cells to attach and grow. Additionally, they have a high surface area to volume ratio, providing more surface for cell attachment and growth, which is in line with the ECM architecture. Electrospun fibers could be fabricated using biocompatible and biodegradable substances, as well. Moreover, the properties of tissue engineering scaffolds fabricated with electrospinning can be tuned by changing the spinning parameters. The diameter and orientation of the fibers, as well as the porosity of the electrospun scaffold, can be altered during the spinning process, resulting in a wide range of scaffold architectures with the ability to mimic the ECM [43]. Moreover, various crosslinking techniques could be employed to improve the mechanical properties of collagen-based electrospun scaffolds and increase their degradation resistance [44]. For instance, UV radiation [45] and dehydrothermal treatment (DHT) [46] are physical crosslinking methods, while EDC/NHS is a common chemical crosslinking system in this field [47]. It is declared that the crosslinked samples with EDC/NHS have higher compressive modulus than the DHT-treated ones. Additionally, EDC/NHS enhances the resistance of the scaffold to degradation against collagenase [48].
Huang et al. [49] first studied the electrospinning of collagen in 2001. They employed 10 mM HCl as the solvent system and reported that no fibers could be obtained with pure collagen at a concentration of less than 2 wt.%. This could correspond to the low solubility and high viscosity of collagen in general organic solvents. So, mixing collagen with synthetic and frequent polymers, such as PCL, polyglycolide acid (PGA), polyethylene oxide (PEO), etc., has been declared as an influential strategy. For example, the addition of PEO to the solution led to uniform fiber formation with diameters ranging from 100 to 150 nm. Another way to overcome the challenging electrospinning process of collagen is by using organic versatile solvents. In 2002, Matthews et al. [50] described the prosperous electrospinning of pure collagen (type I and type III) using 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) as a solvent. Li et al. [51] investigated the influence of polymer concentration on the resulting electrospun collagen nanofibers. They concluded that increasing the concentration contributes to the rise in the diameter, and uniform fibers could be yielded above the concentration of 5%. Furthermore, the tensile modulus of collagen nanofibers was reported to be 262 ± 18 MPa. The discovery of HFIP as a strong solvent suitable for the electrospinning of numerous natural and synthetic biopolymers led to performing several attempts for various applications [52,53,54]. Meanwhile, HFIP is a highly volatile and corrosive solvent that poses serious risks to humans. There are critical concerns about the use of HFIP due to the danger to laboratory personnel and the environmental hazards of its use. Furthermore, some studies have claimed that HFIP can denature collagen into gelatin during the electrospinning process [50]. Another beneficial solution, specifically in the case of medical applications, is applying non-toxic aqueous systems. Accordingly, Dong et al. [55] described the electrospinning of type I collagen using ethanol and phosphate-buffered saline. The diameter of the resulting fibers was reported to be in the range of collagen nanofibers obtained from the HFIP solvent system. In another study, Liu et al. [56] published a paper on the electrospinning of type I collagen using 40% acetic acid. They compared the degradation of collagen in HFIP and a 40% acetic acid solvent system for electrospinning and reported greater denaturation of the scaffolds obtained from HFIP.
In addition, collagen is a suitable biopolymer for blending with other biomaterials. Therefore, the downsides of the collagen fibrous structures could be modified through fabricating collagen-based composites. Several electrospun collagen nanofiber composite systems have been extensively investigated for application in bone tissue engineering. In such cases, the poor mechanical strength of the collagen could be tackled with the presence of other substances. Considering this, Balasubramanian et al. [57] prepared the electrospun collagen/PCL nanofibers using HFIP as the solvent and a subsequent dip-coating step in 45S5 bioglass. The results demonstrated suitable osteoblast differentiation in vitro. According to tremendous studies carried out to design bone tissue composites based on the collagen polymer, the electrospun collagen-based fibrous structures could be promising as the matrix of many cell types. In the following section, the techniques used for the fabrication of nanofibrous bone tissues, as well as their performances are highlighted.
4. Collagen-Based Nanofibrous Bone Scaffolds
Considering the downsides of bone-therapeutic techniques, such as transplants and implants, the ability of bone for regeneration has received wide attention. In this era, the formation of collagens was focused more on other substances, due to their biocompatibility, biodegradability, non-toxicity, non-antigenicity, mimicking of native bone ECM, proper topology, biological renewability, ability to be cross-linked, and so forth. Collagen nanofibers have illustrated superior pros compared with other polymers because they could closely imitate the native tissues’ ultra-architectures. Additionally, collagen-based nanostructures do not activate the host iminium response in the body. Also, the cells are compatible with nano collagen structures, since collagen is present in most body parts. Among various collagen types, electrospun fibrous structures of collagen types I, II, III, and IV are more applied in bone tissue engineering. These structures could be feasibly obtained in the shapes of aligned and random nanoarrays. It is widely shown that the aligned configurations could result in better mechanical properties and more appropriate cell activity.
Meanwhile, several drawbacks have been declared for the collagen-based bone tissues, such as the negative effect of sterilization, low melting point, poor mechanical stability, challenging processes, and difficult degradability adjusting. Therefore, collagen-based structures are commonly combined with other organic materials, inorganic compounds, or both organic and inorganic structures to address the mentioned obstacles. In the following, various approaches regarding raw electrospun collagen fibers, as well as different composite structures based on the collagen nanofibers are provided.
4.1. Raw Electrospun Collagen Fibers
It is widely shown that collagen could maintain the chondrocyte phenotype in three-dimensional culture. Then, the chondrocytes are consistently distributed in the provided culture space and subsequently synthesize ECM to form new cartilage-like tissues. Shih et al. [58] extracted collagen type I from bovine skin, followed by lipid depletion with the aid of a chloroform/methanol mixture, soaking in acetic acid, and embedding with pepsin. Then, a centrifuge procedure was employed for removing the insoluble materials. After the addition of NaCl, the materials were incubated for a day and subjected to a second centrifuging phase, and the salt was removed using acetic acid. The obtained collagen was maintained in acetic acid at a temperature of 4 °C. For fabricating the collagen nanofibers, the obtained collagen was first dissolved in HFIP with concentrations of 4, 8, and 12 wt.%. The voltage and feeding rate were set at 30 kV and 200 µL·min−1, respectively. The obtained results figured out the formation of fine and homogenous fibers in the diameter ranges of 50 to 200, 200 to 500, and 500 to 1000 nm for various electrospinning solutions. Based on the attained results, the average cell viability was 30% greater on the electrospun fibers with a diameter in the range of 200–500 nm than on the 500–1000 nm collagen fibers, suggesting favorable growth conditions of the cells on the electrospun collagen matrix. The number of vinculin stains in the prepared electrospun fibers was reduced by 45%, compared with the cells on tissue culture polystyrene (TCPS), possibly resulting from the biomimetic nature of the designed matrix. The cell migration was also 56.7% (50–200 nm), 37.3% (200–500 nm), and 46.3% (500–1000 nm) greater than the TCPS.
Despite the unique characteristics of collagen, its proteins are desaturated during the electrospinning procedure due to the process condition, as well as the applied solvents. In addition, it has shown poor stability along with a fast degradation rate in the employed aqueous media. Therefore, cross-link treatments have been extensively proposed as a useful strategy. As an example, Jeha et al. [59] compared collagen electrospun fibers with nanofibrous alginate structures in various tissue engineering scaffolds. In this evaluation, HFP solvent was utilized to fabricate fine electrospun fibers. The obtained structures were vapor cross-linked via glutaraldehyde followed by blocking in glycine, rinsing in PBS, and disinfecting in alcohol. According to the obtained data, the electrospun collagen fibrils exhibited sufficient differentiation induction and HA crystal accumulation, which could have originated from the outstanding intrinsic structural motif of collagen. Meanwhile, this feature could not be found in the gelatin architecture. Barnes et al. [60] also deployed cross-linked collagen nanofibrous networks using carbodiimide in ethanol. Corresponding to the results, fine fibers with an average diameter of 180 ± 69 nm were obtained. Notably, the employed cross-linking process resulted in higher stress, modulus, and strain in the electrospun fibers, compared with the glutaraldehyde cross-linking agent. Another technique to face the shortcomings related to pristine collagen nanofibrous tissues is the synthesis of collagen-based composites by employing organic, inorganic, or both organic–inorganic components.
4.2. Integrated Collagen-Based Electrospun Composites with Organic Materials
Although collagen electrospun fibers have shown fantastic features desirable for the fabrication and engineering of bone tissue architectures, insufficient mechanical strength, and a high degradation rate have hindered their clinical applications. To overcome these, the combination of collagen with other organic materials has been broadly reported in the literature. Designing the composite structures based on the collagen polymer can be beneficial toward approaching a modified scaffold with superior physical characteristics, higher solubility of hydrophobic drugs, and better cell proliferation, migration, and adhesion. Hyaluronic acid, alginate, chitosan, and PCL are considered the major vital cases in this field, which are reviewed in this section.
Hyaluronic acid has shown a multi-faceted role in biological functions via boosting migration, proliferation, and differentiation of the cells. According to the literature, it is able to interact with cytokines, leading to a contribution to their retention in the ECM microenvironment. Hyaluronic acid is a naturally occurring glycosaminoglycan composed of repeating disaccharide units of N-acetylglucosamine and glucuronic acid. It can mimic the ECM in several ways: first, it is able to attest a physical scaffold for cells to attach and migrate on, similar to other ECM components; second, it is capable of interacting with cell-surface receptors to regulate cell behavior and signaling pathways; finally, it can serve as a reservoir for growth factors and other signaling molecules, which can be released in a controlled manner to regulate cell behavior and tissue repair processes. Immune regulation and regeneration induction are also known as other capabilities of this material. Therefore, several attempts have been declared to enhance the biological features through embedding hyaluronic acid in the structure. Niu et al. [61] designed hyaluronic acid/collagen core–shell nanofibers, showing a reduction in the inflammatory level, as well as an increase in cell proliferation. Fischer et al. [62] generated an electrospun hyaluronic acid/collagen layer, which was dissolved in 4:1 (v/v) of NaOH/DMF. Uniform fibers were obtained at a voltage of 16 kV, a distance of 5 cm, and a needle gauge of 20. Based on a water-soluble tetrazolium assay, the non-cytotoxicity of the membrane was confirmed after a 72 h incubation time. In another study by Liu et al. [63], aligned PLGA nanofibers incorporated with hyaluronic acid and collagen microparticles were developed, resulting in the well-uniform distribution of the fillers in the aligned nanofibrous structure that enhanced cell migration and proliferation.
Similarly, as an ECM component, alginate is a desirable polysaccharide, derived from brown seaweed, for approaching biocompatible elements applicable in cell transplantation, cell encapsulation, drug delivery, and tissue engineering. Several outstanding features of alginate have caused its similar behavior to ECM. Despite the fabulous features of this natural-based polymer, it has illustrated poor interaction with mammalian cells. So, it is essential to functionalize this polymer with Arg-Gly-Asp (RGD)-containing cell adhesion ligands, which could bind integrins to the membrane proteins of various cell types. To note, such ligands could be found in collagen fibrils. Therefore, it can be modified to contain cell-adhesive peptides or growth factors and mimic the chemical and biological properties of ECM. In this era, Baniasadi and Minary-Jolandan [64] proved an improvement in the rheological and indentation characteristics of an alginate hydrogel filled with collagen fibrils. A collagen-based scaffold was also fabricated in another study with a dimension of 20 mm × 20 mm × 1 mm and average pore sizes of 250 to 350 µm. The prepared membrane was then coated with an alginate solution and cross-linked with CaCl2. According to the ALP activity estimation, the prepared composite represented a gradual increase with time. Additionally, Young’s modulus was also enhanced in both dry and wet states, compared with pure collagen. Well proliferation and migration of the cells on the developed composites implied its great potential as a bone regeneration scaffold with the capacity of controllable drug delivery [65].
Chitosan is assumed as another promising biopolymer in bone regeneration because of its unique features, such as non-toxicity and immunogenicity, as well as great biocompatibility and degradability [66,67]. Various studies declared the synergetic effects of the chitosan/collagen nanofibrous structures on the collagen’s osteoinduction ability. These combinations have shown a fabulous microstructure with great degradation potential and superior mechanical strength. Collagen properties, including biological and mechanical characteristics, could be modified via the addition of chitosan, resulting from the hydrogen and ionic bonds between these elements. Chen et al. [68] studied the intramolecular interactions between the collagen and chitosan complex fibers, confirming that the performed interactions could make this combination miscible at the molecular level. Lotfi et al. [69] evaluated the osteogenic differentiation of the stem cells in two electrospun and solid-wall collagenous coatings on the collagen/chitosan membranes. It was found that the electrospun collagen coating on the prepared bilayered membrane could result in better biological properties due to the uniform mineralization and superior differentiation. Guo et al. [70] also fabricated an electrospun collagen/chitosan layer (8:2) under a voltage of 20 kV and flow rate of 0.08 mm·min−1. They illustrated that the ALP level could be significantly enhanced through employing this combined membrane after the 4th week. Li et al. [71] immersed the fabricated collagen/PCL electrospun layer in the positively charged chitosan solution (1 mg·mL−1, pH = 5) and negatively charged collagen solution (1 mg·mL−1, pH = 8) to assemble the molecules of these polymers on the fabricated layer. The integrated samples were named LBLn, in the case of LBL0.5 and LBL5.5, the outer layer was chitosan, while the LBL1 and LBL5 were denoted to the samples with collagen as an outer layer. The prepared membranes, containing the uncoated and coated specimens, are illustrated in Figure 4a. As can be seen, wrinkles appeared after the coating procedure resulting from capillarity in the synthesis step. In fact, the membranes’ robustness was increased during the coating, leading to stronger changes in the layer shape. Figure 4b,c displays the stress–strain curves of the membranes. Accordingly, high mechanical strength was obtained in the samples, due to the orientation of PCL/collagen nanofibers. In addition, both tensile strength and strain were improved by the addition of the coating layer, caused by the successful incorporation of the chitosan and collagen molecules, as well as the folds created by capillarity in the coating step. The water contact angle data exhibited an obvious hydrophilicity enhancement through the LBL modification (Figure 4d,e). Interestingly, the random layer showed a larger contact angle, compared with the oriented fibers. In the case of coating specimens, treatment with chitosan and collagen led to a water contact angle increment because it disoriented the fibers. Meanwhile, the presence of more hydroxyl groups on the outer surface resulted in the reduction of the water contact angle. According to the biocompatibility evaluations, the L929 fibroblast cells showed higher cell viability than HUVEC; however, both increased with the culture time (Figure 4f,g). Also, the LBL5 and LBL5.5 represented a noticeable improvement in the cell viability, possibly due to the biocompatibility improvement through modifying with chitosan and collagen solutions. In fact, the slight release of these polymers assists cell growth and proliferation. In other attempts, the electrospun chitosan/collagen combination with aligned PLLA fibers [72] and polypyrrole [73] has also been investigated.
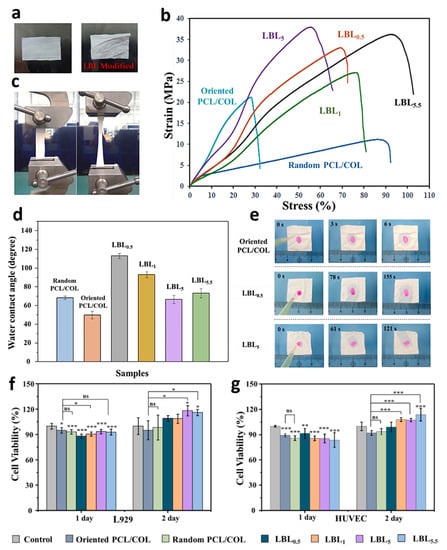
Figure 4.
Various characteristics of the electrospun PCL/collagen nanofibers soaked in chitosan and collagen solutions: (a) the optical image, (b,c) mechanical strength, (d) water contact angle, (e) wettability photographs, and (f,g) cell viability, in which significant differences are represented by *, **, and *** at various p-values of 0.05, 0.01, and 0.001, respectively. Reproduced from reference [71] with permission from Elsevier.
Figure 4.
Various characteristics of the electrospun PCL/collagen nanofibers soaked in chitosan and collagen solutions: (a) the optical image, (b,c) mechanical strength, (d) water contact angle, (e) wettability photographs, and (f,g) cell viability, in which significant differences are represented by *, **, and *** at various p-values of 0.05, 0.01, and 0.001, respectively. Reproduced from reference [71] with permission from Elsevier.
PCL is considered an approved polymer by the FDA, broadly employed in implant composites, as well as drug delivery systems. Also, it is able to incline its osteogenic functionality through the addition of efficient biopolymers, including collagen. Rather et al. [74] developed a nanofibrous collagen/PCL layer through the electrospinning of 5% type 1 collagen and PCL (1:1) dissolved in HFIP solvent. Additionally, dexamethasone and simvastatin were employed as a drug in the prepared structure. Based on the obtained results, homogenous fibers, ranging from 277 to 980 nm, were fabricated using a flow rate of 50 µL·min−1, a distance of 15 cm, and a voltage of 25 kV. The MSCs continued growing until day 17, and the cytocompatibility of the prepared layer was confirmed, which could be due to the collagen’s existence in the structure, enabling cell adhesion and proliferation (see Figure 5a–c). Based on the SEM images obtained on days 0 and 28 from the membrane in PBS, the structure was retained, corroborating its proper mechanical strength and degradation rate (Figure 5d,e). Compared to the blank specimens, the loaded membrane showed an increase in the ALP activity of cells (Figure 5f), possibly resulting from dexamethasone, which is a key building block for osteogenic differentiation and enhances the formation of HA. Lee et al. [75], in another research, designed a hybrid melt-plotting/electrospinning system for coating the melt PCL layer with the electrospun type I collagen nanofibers. Figure 5g shows a schematic illustration of the employed setup for the fabrication of the proposed composite. Two hybrid scaffolds with different pore sizes of 200 ± 20 and 300 ± 27 µm were generated. Figure 5h,i represent SEM images of the PCL and the designed composite, respectively. According to the obtained results, the produced hybrid layer showed higher water absorbency (50%) than the melt-plotted PCL membrane (32–38%). Also, greater cell proliferation was attained on the membrane with smaller pore diameters. To note, the cells were not packed on the PCL single membrane, while packed cell growth was observed in the hybrid structure. Other attempts at designing collagen-based electrospun composites by assisting the PCL polymer could be found in Baylan et al. [76], Choi et al. [77], Kim and Kim [78], and Ghavimi et al. [79].
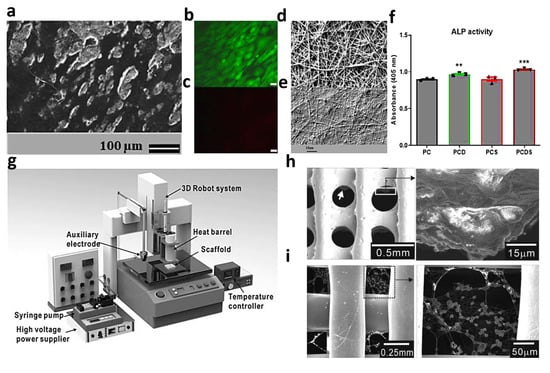
Figure 5.
PCL/collagen nanofibrous layer filled with dexamethasone and simvastatin: (a) SEM images of the cells on the designed substrate after 17 days, (b) live cells, (c) dead cells, fibers’ morphology after (d) 0 and (e) 28 days degradation in PBS, (f) ALP level of the fabricated membranes, in which a p-value of <0.05 was taken to be statistically significant, showing significant increase in ALP activity on PCD (** p = 0.0040) and a further increment on PCDS (*** p = 0.0002), Reproduced from reference [74] with permission from Elsevier. Characteristics of the electrospun collagen on the melt-plotted membrane: (g) fabrication set-up used for the sample preparation and SEM images of (h) PCL and (i) designed composite. Reproduced from reference [75] with permission from Wiley.
Figure 5.
PCL/collagen nanofibrous layer filled with dexamethasone and simvastatin: (a) SEM images of the cells on the designed substrate after 17 days, (b) live cells, (c) dead cells, fibers’ morphology after (d) 0 and (e) 28 days degradation in PBS, (f) ALP level of the fabricated membranes, in which a p-value of <0.05 was taken to be statistically significant, showing significant increase in ALP activity on PCD (** p = 0.0040) and a further increment on PCDS (*** p = 0.0002), Reproduced from reference [74] with permission from Elsevier. Characteristics of the electrospun collagen on the melt-plotted membrane: (g) fabrication set-up used for the sample preparation and SEM images of (h) PCL and (i) designed composite. Reproduced from reference [75] with permission from Wiley.
Although collagen is commonly applied in the structure due to enhancing the biocompatibility, bio adhesion, and low toxicity features in bone regeneration and remodeling, its vital other aspects are also focused in such applications. For example, Zhang et al. [80] coated a PCL/PVP layer on the collagen/chitosan polymeric film layer to control the delivery of Berberine, which is a beneficial Chinese herbal monomer for bone regeneration. The obtained results showed that 30% of the medicine was released on the first day due to the presence of PVP, while it was delivered linearly till day 7. Additionally, it continued to be released very smoothly up to day 28. Through the addition of the collagen/chitosan film into the structure, the mechanical strength was improved under elongation. According to the in vivo assays, a thicker lamellar bone with a superior density was formed in the designed membrane, compared with the single-film or electrospun layers as well as the unloaded film/fibrous membrane. Cheng et al. [81] also used type I collagen to amine-functionalize a PCL/chitosan electrospun membrane, resulting in the enhancement of cell adhesion, spreading, and proliferation, as well as the ALP level. This could be due to the mineralizing ability of collagen.
4.3. Electrospun Collagen-Based Fibers Embedded with Inorganic Materials
Bioactive materials are well known as great alternatives for bone tissues due to their mineralization capacity, leading to the nucleation and boosting of calcium phosphate growth on the scaffolds. HA, bioactive molecules and calcium phosphates are the most common substances classified in this category. The presence of the mentioned inorganic materials can integrate the cell adhesion, resulting from the surrounding bone tissue.
Collagen nanofibrous structures integrated with HA are known as promising biomaterials for bone contact and substitution. Additionally, the HA nanoparticles assist in providing sufficient mechanical compressive strength. There are two general methods for designing collagen/HA nanofibrous composites. In the first solution, the HA suspension is coated on the collagen nanofibers through an electrospray process, while the collagen/HA suspension is subjected to the electrospinning apparatus as the second route. For example, Rebiero et al. [82] used a simultaneous electrospinning/electrospraying technique to fabricate a collagen-based nanofibrous composite decorated by HA. In this attempt, a suspension of type I collagen was first prepared in acetic acid: ethyl acetate: water (40:30:30), and then the electrospinning procedure was carried out with a feeding rate of 0.1 mL·h−1 under a voltage of 20 kV. The electrospray phase was also performed using the feeding rate and voltage of 20 kV and 2 mL·h−1, respectively. According to the results, a random structure of the collagen nanofibers loaded by irregular HA nanostructures was attained. The non-cytotoxicity of the membrane was confirmed, and improved cell adhesion was declared. It was concluded that both collagen and HA interact with the cells and so enhance their adhesions. Meanwhile, Thomas et al. [83] fabricated the electrospun collagen/HA nanofibers by preparing the collagen/HA solutions in HFP, followed by an electrospinning process with 5 mL·h−1 feeding rate, 12 cm electrospinning distance, and the electrostatic field of 25 kV (see Figure 6a–d). Based on the results, the tensile strength and the modulus were increased from 1.68 to 5 MPa and 5 to 230 MPa, respectively, through the addition of 10 wt.% HA into the electrospinning solution, which could correspond to the strong adhesion of the composite elements. However, the strain declined from 55 to 3% in the integrated membrane (see Figure 6e). It is noteworthy that the incorporation of the 20 wt.% HA caused a reduction in both tensile strength and the modulus, possibly resulting from the poor interface bonding of the components, as well as a reduction of the collagen chain entanglements. Overall, the proposed membrane could not reach the ultimate tensile strength of the natural tissues (9 to 18 MPa), which could be a result of lacking hydration. A similar structure was also synthesized by Song et al. [84] in 2008, showing the cell morphology growth on the pure collagen and collagen/20 wt.% HA nanofibrous structures after 1 day of cell culture. Based on the results, the level of cell growth was increased through the addition of HA nanoparticles in the structure compared with the nanofibrous pure collagen network. This could be due to the increase in the integrin–receptor binding locations, leading to cell growth and adhesion. The osteoblastic differentiation degree was also assessed via the estimation of the alkaline phosphatase activity (ALP) on the substrates, showing an enhancement with culturing time. It was interesting that the ALP level displayed different behaviors in the prepared samples depending on the time. Therefore, the loaded substrate showed a great enhancement in the ALP level from day 7 to day 14, resulting from eliciting the osteoblast cells at a later time to a greater extent, which could be beneficial for later maturation and mineralization. To make the process environmentally friendly, Castilla et al. [85] electrospun the solution containing HA and collagen in acetic acid 90% (v/v). The fabricated fibers showed an increment in the average diameter from 142 ± 73 nm to 342 ± 67 nm through the addition of 10% (w/v) HA. After a day, 50% of the cells were well adhered to the substrate, confirming its great potential as a green-synthesized network applicable as an ECM for bone regeneration.
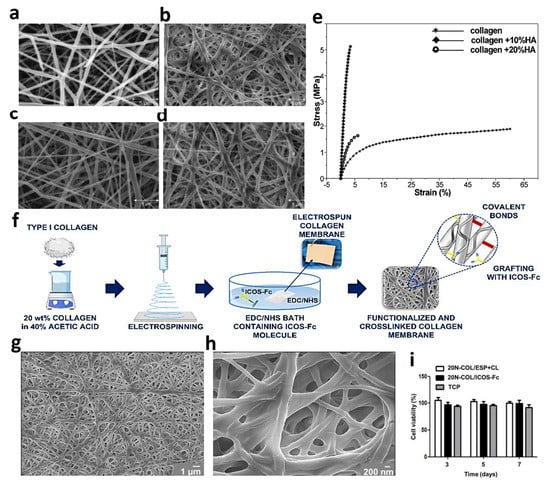
Figure 6.
Features of the electrospun collagen/HA nanofibers: (a–d) SEM images and (e) stress_strain curves. Reproduced from reference [83] with permission from the American Chemical Society. The crosslinked collagen nanofibers functionalized by ICOS-Fc bioactive molecules: (f) schematic of the synthesis procedure, (g,h) FESEM graphs of nanofibrous collagen membranes before and after crosslinking with EDC/NHS, respectively, and (i) U2OS cells viability in contact with samples and tissue culture plastic at 3, 5, and 7 days of cell culturing. Reproduced with copyright from reference [86].
Figure 6.
Features of the electrospun collagen/HA nanofibers: (a–d) SEM images and (e) stress_strain curves. Reproduced from reference [83] with permission from the American Chemical Society. The crosslinked collagen nanofibers functionalized by ICOS-Fc bioactive molecules: (f) schematic of the synthesis procedure, (g,h) FESEM graphs of nanofibrous collagen membranes before and after crosslinking with EDC/NHS, respectively, and (i) U2OS cells viability in contact with samples and tissue culture plastic at 3, 5, and 7 days of cell culturing. Reproduced with copyright from reference [86].
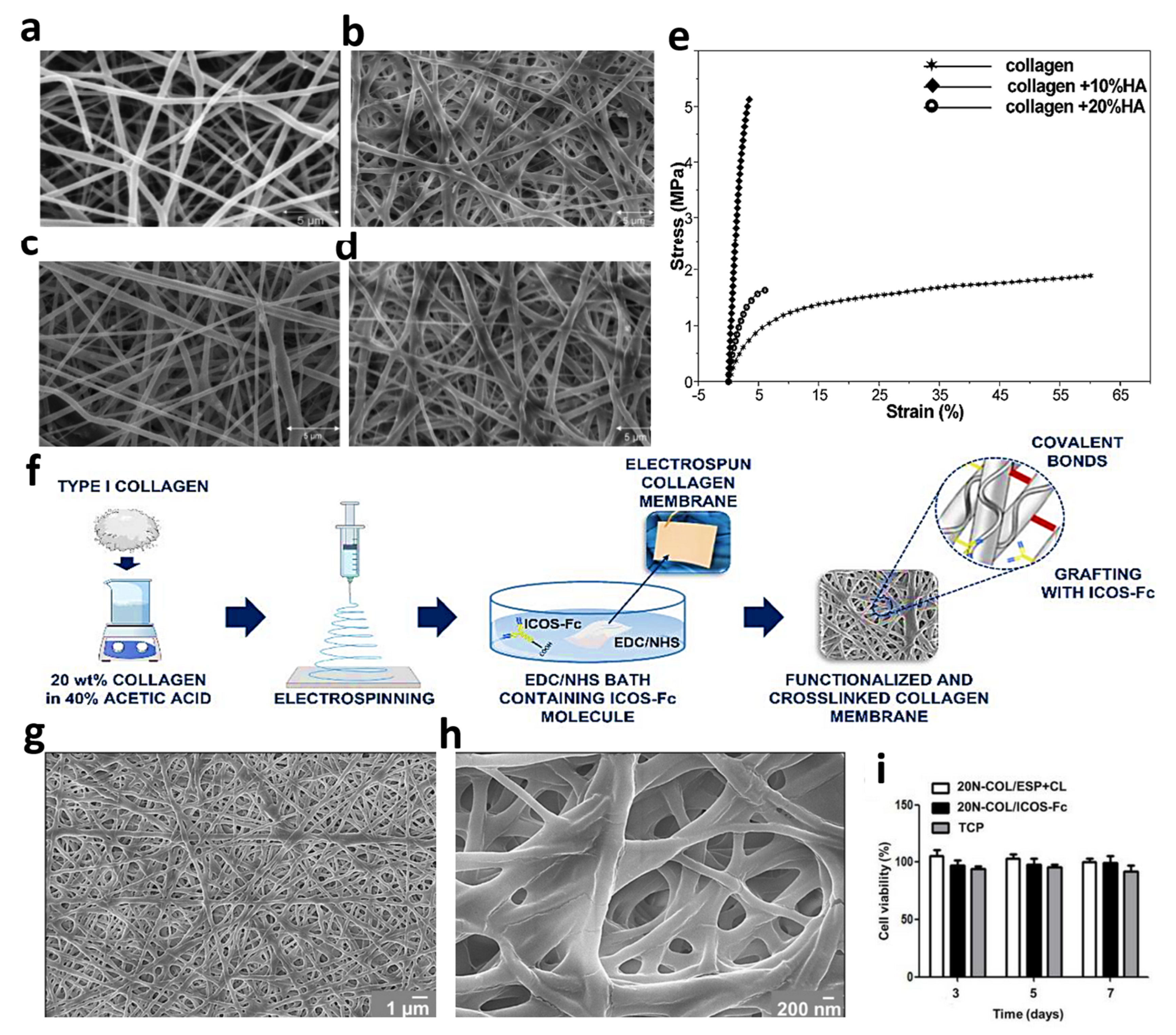
Another method for overcoming the drawbacks of the electrospun collagen networks is fibrous functionalization through encapsulation or covalent binding of the bioactive molecules. In an effort, the electrospun type I collagen fibers, extracted from a rat tail were integrated with the ICOS-Fc bioactive element. Fine fibers were formed using an electrospinning distance of 12 cm, a flow rate of 300 µL·h−1, and under a high voltage of 22 kV. Notably, the N-(3-Dimethylaminopropyl)-N′-ethyl carbodiimide hydrochloride and 1-Hydroxy-2,5-pyrrolidinedione combination in pure ethanol was utilized as the cross-linking solution. Figure 6f schematically illustrates the synthesis route employed in this study. From the results, the fiber morphology did not change after the cross-linking procedure (Figure 6g,h). Moreover, the samples did not show cytotoxicity, confirming that both electrospinning and cross-linking procedures have not influenced the membrane’s biocompatibility (see Figure 6i) [86]. Montalbano et al. [87] also incorporated mesoporous bioactive glass in electrospun type I collagen, extracted from the Achilles tendon. So, the derived collagen was dissolved in acetic acid/ethyl acetate/water, followed by sonicating the bioactive glass in the prepared solution. The electrospinning phase was also conducted at a 10–15 cm distance, 0.05–0.15 mL·h−1 feeding rate, and a high voltage of 18.5–20 kV. Accordingly, the fine fibers with an average diameter of 100 nm were successfully prepared using the highlighted non-toxic solvent mixture.
Feasible adsorbing of the collagen with metal alloys could be assumed as a fabulous feature, assisting in the preparation of an ECM-like interface, a key factor for cell attachment. Lin and Peng [88] developed a type I collagen nanofibrous structure embedded with Ti-6Al-4V alloys. In this examination, three fibrous structures, including random fibers with low density (COL (L)), random fibers with high density (COL (H)), and aligned fibers with high density (COL (A)) were generated and compared. Corresponding to the results, the cell amounts after 21 days were attained the most in the COL (H), compared with that of the other two substrates. In addition, the ALP level was increased in all samples from day 7 to day 21. Moreover, the COL (A) specimen was able to align the cells along the oriented fibrous structure, while two other membranes could not manage it. In another research, Estévez et al. [89] used superparamagnetic iron oxide nanoparticles to fabricate enriched collagen-based nanofibers. The electrospinning solution was prepared using a non-toxic acid acetic solvent, and the procedure was conducted at a distance of 12 cm, 300–400 µL·h−1, and 20–22 kV voltage. The synthesized nanoparticles and the fabricated fibers showed average diameters of 11 and 100–200 nm, respectively. To assess the biological features of the samples, the hMSCs cells were cultured on the samples. According to the results, pure and loaded fibrous networks showed higher cell viability, proliferation, and adhesion on day 5, compared with the control. Therefore, the presence of this mineral material did not affect the membrane’s biological efficiency. Faraji et al. [90] also fabricated Zeolite/collagen nanocomposite structures applicable in treating bone defects, declaring the great capability of the suggested membrane for bone defect reconstruction.
4.4. Electrospun Collagen-Based Composites with Both Organic and Inorganic Materials
There are growing appeals for collagen-based electrospun architectures from researchers in the field of bone tissue engineering due to their unique properties. To rectify the problem of such structures, it has been widely suggested to employ the synergistic impacts of both organic and inorganic materials in collagen-based nanofibers. Table 1 summarizes the attempts reported toward enriching the collagen electrospun bone tissues with organic materials, such as alginate, polyvinyl alcohol (PVA), PCL, PLGA, silk, and so on, and inorganic materials, including HA, metal alloys, bio-active glass, etc.

Table 1.
Summary of collagen-based electrospun-synthesized with organic and inorganic materials.
5. Conclusions and Future Remarks
Bone regeneration is one of the new methods in the treatment of bone diseases and defects, which can be guided by using membranes and scaffolds. The choice of the polymer type and the scaffold design seems influential in the final scaffold outcome to approach native tissue reconstruction, regarding biological and mechanical characteristics as well as proper degradation rate. Among a diverse range of features, bio absorbency is one of the major factors that must be considered to yield the least side effects. Based on this, collagen has illustrated a more validated scientific background and excellent clinical studies.
Collagen could be fabricated in different forms of textures, films, hydrogels, and electrospun layers. According to the excellent porosity, tiny pore sizes, and interconnected pores of the electrospun fibers, this review article is focused on electrospun collagen studies. The collagen electrospun scaffold attains the proper bioactivity. Nevertheless, lack of mineralization and poor mechanical strength are declared as cons linked with this polymer. Meanwhile, embedding additive organic and inorganic materials has been suggested as a beneficial solution to cope with these blocks. Organic elements are commonly employed to attest to a more desirable fabrication process, degradation rate, and mechanical strength. In contrast, inorganic compounds could enhance mineralization capability, cell adhesion, and stem cell differentiation. A comprehensive and critical discussion is briefly highlighted in this review paper regarding collagen resources, its electrospinning strategies, and the reported collagen-based electrospun composites for bone regeneration. Correspondingly, several challenging problems which arise in this domain must be considered in future studies regarding the practical usage of collagen-based nanofibers in bone tissue engineering. The electrospinning of collagen through green and eco-friendly methods has not yet been discovered. The detachment of the electrospun layers from the collectors is always a challenging procedure in electrospinning. Also, the stability and morphology of the electrospun layers in aqueous and cell-culturing media are hardly retained unchanged. Therefore, future works can design matrices synthesized by the combination of two or more fabrication methods. Additionally, the utilized cross-linking agents and the employed process are required to be modified, since they can cause an immunological response. Furthermore, future works could be devoted to designing collagen nanofibers filled with both versatile organic and inorganic components to approach the synergetic features of ideal bioactivity behavior, as well as outstanding dimensional stability while customizing degradation rate.
Funding
This work is supported by the Key Research and Development Special Project of Henan Provincial Science and Technology (No. 222102230025, 232102231015, and 232102231011), Major Science Research Project of High Education of Henan Province (No. 23B430016), and Major Science Research Project of Zhengzhou Railway Vocational & Technical College (2021KY011).
Data Availability Statement
No new data is created.
Conflicts of Interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen tissue engineering: Development of novel biomaterials and applications. Pediatr. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef]
- Smith, K.; Rennie, M.J. New approaches and recent results concerning human-tissue collagen synthesis. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 582–590. [Google Scholar] [CrossRef]
- Fratzl, P. Collagen: Structure and mechanics, an introduction. In Collagen; Springer: Boston, MA, USA, 2008; pp. 1–13. [Google Scholar]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 1–22. [Google Scholar] [CrossRef]
- Oosterlaken, B.M.; Vena, M.P.; de With, G. In vitro mineralization of collagen. Adv. Mater. 2021, 33, 2004418. [Google Scholar] [CrossRef] [PubMed]
- Banitaba, S.N.; Ebadi, S.V.; Salimi, P.; Bagheri, A.; Gupta, A.; Arifeen, W.U.; Chaudhary, V.; Mishra, Y.K.; Kaushik, A.; Mostafavi, E. Biopolymer-based electrospun fibers in electrochemical devices: Versatile platform for energy, environment, and health monitoring. Mater. Horiz. 2022, 9, 2914–2948. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef]
- Neela, S.; Bandigari, P.; Mayuri, K. Collagen—A Review. YMER 2022, 21, 100–110. [Google Scholar] [CrossRef]
- Nasari, M.; Poursharifi, N.; Fakhrali, A.; Banitaba, S.N.; Mohammadi, S.; Semnani, D. Fabrication of novel PCL/PGS fibrous scaffold containing HA and GO through simultaneous electrospinning-electrospray technique. Int. J. Polym. Mater. Polym. Biomater. 2022, 1–17. [Google Scholar] [CrossRef]
- Khademolqorani, S.; Tavanai, H.; Chronakis, I.; Boisen, A.; Ajalloueian, F. The determinant role of fabrication technique in final characteristics of scaffolds for tissue engineering applications: A focus on silk fibroin-based scaffolds. Mater. Sci. Eng. C 2021, 122, 111867. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Stegemann, J.P. Thermogelling chitosan and collagen composite hydrogels initiated with β-glycerophosphate for bone tissue engineering. Biomaterials 2010, 31, 3976–3985. [Google Scholar] [CrossRef] [PubMed]
- Diogo, G.S.; Marques, C.F.; Sotelo, C.G.; Pérez-Martín, R.I.; Pirraco, R.P.; Reis, R.L.; Silva, T.H. Cell-laden biomimetically mineralized shark-skin-collagen-based 3D printed hydrogels for the engineering of hard tissues. ACS Biomater. Sci. Eng. 2020, 6, 3664–3672. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Ouyang, Y.; Chen, Y.; Yin, X.; Liu, Y.; Ying, H.; Yang, W. Electrospinning polycaprolactone/collagen fiber coatings for enhancing the corrosion resistance and biocompatibility of AZ31 Mg alloys. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 131041. [Google Scholar] [CrossRef]
- Deen, I.; Selopal, G.S.; Wang, Z.M.; Rosei, F. Electrophoretic deposition of collagen/chitosan films with copper-doped phosphate glasses for orthopaedic implants. J. Colloid Interface Sci. 2022, 607, 869–880. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Khan, R.H.; Suman, R. 3D printing applications in bone tissue engineering. J. Clin. Orthop. Trauma 2020, 11, S118–S124. [Google Scholar] [CrossRef]
- Kim, S.-C.; Heo, S.-Y.; Oh, G.-W.; Yi, M.; Jung, W.-K. A 3D-Printed Polycaprolactone/Marine Collagen Scaffold Reinforced with Carbonated Hydroxyapatite from Fish Bones for Bone Regeneration. Mar. Drugs 2022, 20, 344. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lau, C.-S.; Liang, K.; Wen, F.; Teoh, S.H. Marine collagen scaffolds in tissue engineering. Curr. Opin. Biotechnol. 2022, 74, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Dhand, C.; Ong, S.T.; Dwivedi, N.; Diaz, S.M.; Venugopal, J.R.; Navaneethan, B.; Fazil, M.H.; Liu, S.; Seitz, V.; Wintermantel, E. Bio-inspired in situ crosslinking and mineralization of electrospun collagen scaffolds for bone tissue engineering. Biomaterials 2016, 104, 323–338. [Google Scholar] [CrossRef]
- Khademolqorani, S.; Banitaba, N. Application of Electrosprayed Nanoparticles as Targeted Drug Delivery Systems: A Mini Review. J. Appl. Sci. Nanotechnol. 2022, 2, 1–7. [Google Scholar] [CrossRef]
- Blackstone, B.N.; Gallentine, S.C.; Powell, H.M. Collagen-based electrospun materials for tissue engineering: A systematic review. Bioengineering 2021, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2017, 28, 4–9. [Google Scholar] [CrossRef]
- Bazrafshan, Z.; Stylios, G.K. Spinnability of collagen as a biomimetic material: A review. Int. J. Biol. Macromol. 2019, 129, 693–705. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Woodley, D.T.; Keene, D.R.; Atha, T.; Huang, Y.; Lipman, K.; Li, W.; Chen, M. Injection of recombinant human type VII collagen restores collagen function in dystrophic epidermolysis bullosa. Nat. Med. 2004, 10, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.L.; Hiller, J.; Haston, J.L.; Holmes, D.F.; Kadler, K.E.; Murdoch, A.; Meakin, J.R.; Wess, T.J. Analysis of collagen fibril diameter distribution in connective tissues using small-angle X-ray scattering. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2005, 1722, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Bella, J. A new method for describing the helical conformation of collagen: Dependence of the triple helical twist on amino acid sequence. J. Struct. Biol. 2010, 170, 377–391. [Google Scholar] [CrossRef]
- Miller, A. Collagen: The organic matrix of bone. Philosophical Transactions of the Royal Society of London. B Biol. Sci. 1984, 304, 455–477. [Google Scholar]
- Silvipriya, K.; Kumar, K.K.; Bhat, A.; Kumar, B.D.; John, A. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Ellingsworth, L.; DeLustro, F.; Brennan, J.; Sawamura, S.; McPherson, J. The human immune response to reconstituted bovine collagen. J. Immunol. 1986, 136, 877–882. [Google Scholar] [CrossRef]
- Herford, A.S.; Akin, L.; Cicciu, M.; Maiorana, C.; Boyne, P.J. Use of a porcine collagen matrix as an alternative to autogenous tissue for grafting oral soft tissue defects. J. Oral Maxillofac. Surg. 2010, 68, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.M.R.; Motta, A.; Fauzi, M.B. A comprehensive review on collagen type I development of biomaterials for tissue engineering: From biosynthesis to bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef]
- Wang, T.; Lew, J.; Premkumar, J.; Poh, C.L.; Win Naing, M. Production of recombinant collagen: State of the art and challenges. Eng. Biol. 2017, 1, 18–23. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Fayzullin, A.L.; Vukolova, M.N.; Rudenko, T.G.; Osipycheva, V.D.; Litvitsky, P.F. Medical applications of collagen and collagen-based materials. Curr. Med. Chem. 2019, 26, 506–516. [Google Scholar] [CrossRef]
- Lv, Q.; Feng, Q.; Hu, K.; Cui, F. Three-dimensional fibroin/collagen scaffolds derived from aqueous solution and the use for HepG2 culture. Polymer 2005, 46, 12662–12669. [Google Scholar] [CrossRef]
- Martínez-Pérez, C.A.; Olivas-Armendariz, I.; Castro-Carmona, J.S.; García-Casillas, P.E. Scaffolds for tissue engineering via thermally induced phase separation. Adv. Regen. Med. 2011, 35, 275–294. [Google Scholar]
- Lowe, C.J.; Reucroft, I.M.; Grota, M.C.; Shreiber, D.I. Production of highly aligned collagen scaffolds by freeze-drying of self-assembled, fibrillar collagen gels. ACS Biomater. Sci. Eng. 2016, 2, 643–651. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef]
- Boland, E.D.; Matthews, J.A.; Pawlowski, K.J.; Simpson, D.G.; Wnek, G.E.; Bowlin, G.L. Electrospinning collagen and elastin: Preliminary vascular tissue engineering. Front. Biosci.-Landmark 2004, 9, 1422–1432. [Google Scholar] [CrossRef]
- Meamar, R.; Ghasemi-Mobarakeh, L.; Norouzi, M.-R.; Siavash, M.; Hamblin, M.R.; Fesharaki, M. Improved wound healing of diabetic foot ulcers using human placenta-derived mesenchymal stem cells in gelatin electrospun nanofibrous scaffolds plus a platelet-rich plasma gel: A randomized clinical trial. Int. Immunopharmacol. 2021, 101, 108282. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.-R.; Ghasemi-Mobarakeh, L.; Itel, F.; Schoeller, J.; Fashandi, H.; Borzi, A.; Neels, A.; Fortunato, G.; Rossi, R.M. Emulsion electrospinning of sodium alginate/poly (ε-caprolactone) core/shell nanofibers for biomedical applications. Nanoscale Adv. 2022, 4, 2929–2941. [Google Scholar] [CrossRef]
- Khosravi, A.; Ghasemi-Mobarakeh, L.; Mollahosseini, H.; Ajalloueian, F.; Masoudi Rad, M.; Norouzi, M.R.; Sami Jokandan, M.; Khoddami, A.; Chronakis, I.S. Immobilization of silk fibroin on the surface of PCL nanofibrous scaffolds for tissue engineering applications. J. Appl. Polym. Sci. 2018, 135, 46684. [Google Scholar] [CrossRef]
- Tierney, C.M.; Haugh, M.G.; Liedl, J.; Mulcahy, F.; Hayes, B.; O’Brien, F.J. The effects of collagen concentration and crosslink density on the biological, structural and mechanical properties of collagen-GAG scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2009, 2, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Moldovan, L.; Constantin, D.; Stanciuc, A.; Boeti, P.S.; Efrimescu, I. Collagen–based scaffolds for skin tissue engineering. J. Med. Life 2011, 4, 172. [Google Scholar]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 89, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Nong, L.-M.; Zhou, D.; Zheng, D.; Jiang, Y.-Q.; Xu, N.-W.; Zhao, G.-Y.; Wei, H.; Zhou, S.-Y.; Han, H.; Han, L. The effect of different cross-linking conditions of EDC/NHS on type II collagen scaffolds: An in vitro evaluation. Cell Tissue Bank. 2019, 20, 557–568. [Google Scholar] [CrossRef]
- Kozłowska, J.; Sionkowska, A. Effects of different crosslinking methods on the properties of collagen–calcium phosphate composite materials. Int. J. Biol. Macromol. 2015, 74, 397–403. [Google Scholar] [CrossRef]
- Huang, L.; Nagapudi, K.; Apkarian, R.P.; Chaikof, E.L. Engineered collagen–PEO nanofibers and fabrics. J. Biomater. Sci. Polym. Ed. 2001, 12, 979–993. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef]
- Li, M.; Mondrinos, M.J.; Gandhi, M.R.; Ko, F.K.; Weiss, A.S.; Lelkes, P.I. Electrospun protein fibers as matrices for tissue engineering. Biomaterials 2005, 26, 5999–6008. [Google Scholar] [CrossRef] [PubMed]
- Wakuda, Y.; Nishimoto, S.; Suye, S.-I.; Fujita, S. Native collagen hydrogel nanofibres with anisotropic structure using core-shell electrospinning. Sci. Rep. 2018, 8, 6248. [Google Scholar] [CrossRef] [PubMed]
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.-M.; Park, Y.J.; Hong, S.-D.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B.-M. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006, 27, 1452–1461. [Google Scholar] [CrossRef]
- Dulnik, J.; Denis, P.; Sajkiewicz, P.; Kołbuk, D.; Choińska, E. Biodegradation of bicomponent PCL/gelatin and PCL/collagen nanofibers electrospun from alternative solvent system. Polym. Degrad. Stab. 2016, 130, 10–21. [Google Scholar] [CrossRef]
- Dong, B.; Arnoult, O.; Smith, M.E.; Wnek, G.E. Electrospinning of collagen nanofiber scaffolds from benign solvents. Macromol. Rapid Commun. 2009, 30, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Teng, W.K.; Chan, B.P.; Chew, S.Y. Photochemical crosslinked electrospun collagen nanofibers: Synthesis, characterization and neural stem cell interactions. J. Biomed. Mater. Res. Part A 2010, 95, 276–282. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Roether, J.A.; Schubert, D.W.; Beier, J.P.; Boccaccini, A.R. Bi-layered porous constructs of PCL-coated 45S5 bioactive glass and electrospun collagen-PCL fibers. J. Porous Mater. 2015, 22, 1215–1226. [Google Scholar] [CrossRef]
- Shih, Y.R.V.; Chen, C.N.; Tsai, S.W.; Wang, Y.J.; Lee, O.K. Growth of Mesenchymal Stem Cells on Electrospun Type I Collagen Nanofibers. Stem. Cells 2006, 24, 2391–2397. [Google Scholar] [CrossRef]
- Jha, B.S.; Ayres, C.E.; Bowman, J.R.; Telemeco, T.A.; Sell, S.A.; Bowlin, G.L.; Simpson, D.G. Electrospun Collagen: A Tissue Engineering Scaffold with Unique Functional Properties in a Wide Variety of Applications. J. Nanomater. 2011, 2011, 348268. [Google Scholar] [CrossRef]
- Barnes, C.P.; Pemble IV, C.W.; Brand, D.D.; Simpson, D.G.; Bowlin, G.L. Cross-linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng. 2007, 13, 1593–1605. [Google Scholar] [CrossRef]
- Niu, Y.; Stadler, F.J.; Yang, X.; Deng, F.; Liu, G.; Xia, H. HA-coated collagen nanofibers for urethral regeneration via in situ polarization of M2 macrophages. J. Nanobiotechnol. 2021, 19, 283. [Google Scholar] [CrossRef]
- Fischer, R.L.; McCoy, M.G.; Grant, S.A. Electrospinning collagen and hyaluronic acid nanofiber meshes. J. Mater. Sci. Mater. Med. 2012, 23, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jia, W.; Zhou, Y.; Zhou, H.; Liu, M.; Li, M.; Zhang, X.; Gu, G.; Chen, Z. Hyaluronic acid oligosaccharide-collagen mineralized product and aligned nanofibers with enhanced vascularization properties in bone tissue engineering. Int. J. Biol. Macromol. 2022, 206, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, M.; Minary-Jolandan, M. Alginate-collagen fibril composite hydrogel. Materials 2015, 8, 799–814. [Google Scholar] [CrossRef]
- Lee, H.-j.; Ahn, S.-H.; Kim, G.H. Three-Dimensional Collagen/Alginate Hybrid Scaffolds Functionalized with a Drug Delivery System (DDS) for Bone Tissue Regeneration. Chem. Mater. 2012, 24, 881–891. [Google Scholar] [CrossRef]
- Shin, S.Y.; Park, H.N.; Kim, K.H.; Lee, M.H.; Choi, Y.S.; Park, Y.J.; Lee, Y.M.; Ku, Y.; Rhyu, I.C.; Han, S.B. Biological evaluation of chitosan nanofiber membrane for guided bone regeneration. J. Periodontol. 2005, 76, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, X.; He, C.; Wang, H. Intermolecular interactions in electrospun collagen–chitosan complex nanofibers. Carbohydr. Polym. 2008, 72, 410–418. [Google Scholar] [CrossRef]
- Lotfi, G.; Shokrgozar, M.A.; Mofid, R.; Abbas, F.M.; Ghanavati, F.; Baghban, A.A.; Yavari, S.K.; Pajoumshariati, S. Biological evaluation (in vitro and in vivo) of bilayered collagenous coated (nano electrospun and solid wall) chitosan membrane for periodontal guided bone regeneration. Ann. Biomed. Eng. 2016, 44, 2132–2144. [Google Scholar] [CrossRef]
- Guo, S.; He, L.; Yang, R.; Chen, B.; Xie, X.; Jiang, B.; Weidong, T.; Ding, Y. Enhanced effects of electrospun collagen-chitosan nanofiber membranes on guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2020, 31, 155–168. [Google Scholar] [CrossRef]
- Li, D.; Dai, F.; Li, H.; Wang, C.; Shi, X.; Cheng, Y.; Deng, H. Chitosan and collagen layer-by-layer assembly modified oriented nanofibers and their biological properties. Carbohydr. Polym. 2021, 254, 117438. [Google Scholar] [CrossRef]
- Deepthi, S.; Nivedhitha Sundaram, M.; Deepti Kadavan, J.; Jayakumar, R. Layered chitosan-collagen hydrogel/aligned PLLA nanofiber construct for flexor tendon regeneration. Carbohydr. Polym. 2016, 153, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Rather, H.A.; Varghese, J.F.; Dhimmar, B.; Yadav, U.C.S.; Vasita, R. Polycaprolactone-collagen nanofibers loaded with dexamethasone and simvastatin as an osteoinductive and immunocompatible scaffold for bone regeneration applications. Biomater. Biosyst. 2022, 8, 100064. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yeo, M.; Ahn, S.; Kang, D.O.; Jang, C.H.; Lee, H.; Park, G.M.; Kim, G.H. Designed hybrid scaffolds consisting of polycaprolactone microstrands and electrospun collagen-nanofibers for bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Baylan, N.; Bhat, S.; Ditto, M.; Lawrence, J.G.; Lecka-Czernik, B.; Yildirim-Ayan, E. Polycaprolactone nanofiber interspersed collagen type-I scaffold for bone regeneration: A unique injectable osteogenic scaffold. Biomed. Mater. 2013, 8, 045011. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, S.J.; Christ, G.J.; Atala, A.; Yoo, J.J. The influence of electrospun aligned poly(ɛ-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, G. Rapid-prototyped collagen scaffolds reinforced with PCL/β-TCP nanofibres to obtain high cell seeding efficiency and enhanced mechanical properties for bone tissue regeneration. J. Mater. Chem. 2012, 22, 16880–16889. [Google Scholar] [CrossRef]
- Ghavimi, M.A.; Negahdari, R.; Bani Shahabadi, A.; Sharifi, S.; Kazeminejad, E.; Shahi, S.; Maleki Dizaj, S. Preparation and study of starch/ collagen/ polycaprolactone nanofiber scaffolds for bone tissue engineering using electrospinning technique. Eurasian Chem. Commun. 2020, 2, 122–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Li, J.; Cui, X.; Jiang, M.; Zhang, M.; Wang, X.; Zhang, W.; Liu, Z. Bilayer membrane composed of mineralized collagen and chitosan cast film coated with berberine-loaded PCL/PVP electrospun nanofiber promotes bone regeneration. Front. Bioeng. Biotechnol. 2021, 9, 684335. [Google Scholar] [CrossRef]
- Cheng, Y.; Ramos, D.; Lee, P.; Liang, D.; Yu, X.; Kumbar, S.G. Collagen functionalized bioactive nanofiber matrices for osteogenic differentiation of mesenchymal stem cells: Bone tissue engineering. J. Biomed. Nanotechnol. 2014, 10, 287–298. [Google Scholar] [CrossRef]
- Ribeiro, N.; Sousa, S.R.; Van Blitterswijk, C.A.; Moroni, L.; Monteiro, F.J. A biocomposite of collagen nanofibers and nanohydroxyapatite for bone regeneration. Biofabrication 2014, 6, 035015. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.; Dean, D.R.; Jose, M.V.; Mathew, B.; Chowdhury, S.; Vohra, Y.K. Nanostructured biocomposite scaffolds based on collagen coelectrospun with nanohydroxyapatite. Biomacromolecules 2007, 8, 631–637. [Google Scholar] [CrossRef]
- Song, J.-H.; Kim, H.-E.; Kim, H.-W. Electrospun fibrous web of collagen–apatite precipitated nanocomposite for bone regeneration. J. Mater. Sci. Mater. Med. 2008, 19, 2925–2932. [Google Scholar] [CrossRef]
- Castilla-Casadiego, D.A.; Maldonado, M.; Sundaram, P.; Almodovar, J. “Green” electrospinning of a collagen/hydroxyapatite composite nanofibrous scaffold. MRS Commun. 2016, 6, 402–407. [Google Scholar] [CrossRef]
- Melo, P.; Montalbano, G.; Boggio, E.; Gigliotti, C.L.; Dianzani, C.; Dianzani, U.; Vitale-Brovarone, C.; Fiorilli, S. Electrospun Collagen Scaffold Bio-Functionalized with Recombinant ICOS-Fc: An Advanced Approach to Promote Bone Remodelling. Polymers 2022, 14, 3780. [Google Scholar] [CrossRef]
- Montalbano, G.; Tomasina, C.; Fiorilli, S.; Camarero-Espinosa, S.; Vitale-Brovarone, C.; Moroni, L. Biomimetic Scaffolds Obtained by Electrospinning of Collagen-Based Materials: Strategies to Hinder the Protein Denaturation. Materials 2021, 14, 4360. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Peng, Z.-X. Nanofibers grafted on titanium alloy: The effects of fiber alignment and density on osteoblast mineralization. J. Mater. Sci. Mater. Med. 2017, 28, 149. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Montalbano, G.; Gallo-Cordova, A.; Ovejero, J.G.; Izquierdo-Barba, I.; González, B.; Tomasina, C.; Moroni, L.; Vallet-Regí, M.; Vitale-Brovarone, C. Incorporation of Superparamagnetic Iron Oxide Nanoparticles into Collagen Formulation for 3D Electrospun Scaffolds. Nanomaterials 2022, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Faraji, D.; Jahandideh, A.; Asghari, A.; Akbarzadeh, A.; Hesaraki, S. Effect of zeolite and zeolite/collagen nanocomposite scaffolds on healing of segmental femur bone defect in rabbits. Iran. J. Vet. Surg. 2017, 12, 63–70. [Google Scholar]
- Song, W.; Markel, D.C.; Wang, S.; Shi, T.; Mao, G.; Ren, W. Electrospun polyvinyl alcohol–collagen–hydroxyapatite nanofibers: A biomimetic extracellular matrix for osteoblastic cells. Nanotechnology 2012, 23, 115101. [Google Scholar] [CrossRef]
- Khandaker, M.; Riahinezhad, S.; Sultana, F.; Morris, T.; Wolf, R.; Vaughan, M. Effect of collagen-polycaprolactone nanofibers matrix coating on the in vitro cytocompatibility and in vivo bone responses of titanium. J. Med. Biol. Eng. 2018, 38, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Ngiam, M.; Liao, S.; Patil, A.J.; Cheng, Z.; Chan, C.K.; Ramakrishna, S. The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering. Bone 2009, 45, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, S.; Zheng, Z.; Li, J. Fabrication of Biologically Inspired Electrospun Collagen/Silk fibroin/bioactive glass composited nanofibrous scaffold to accelerate the treatment efficiency of bone repair. Regen. Ther. 2022, 21, 122–138. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, S.; Ma, Y.; Xu, W.; Meng, W.; Lin, X.; Wang, W.; Wang, S.; Zhang, J. Innovative biodegradable poly(L-lactide)/collagen/hydroxyapatite composite fibrous scaffolds promote osteoblastic proliferation and differentiation. Int. J. Nanomed. 2017, 12, 7577. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M. Polycaprolactone, β-tricalcium phosphate, and collagen nanofibers: Fabrication, physical properties, and in vitro cell activity for bone tissue regeneration. Biomacromolecules 2011, 12, 502–510. [Google Scholar] [CrossRef]
- Matei, E.; Gaidau, C.; Râpă, M.; Stefan, L.M.; Ditu, L.-M.; Predescu, A.M.; Stanca, M.; Pantilimon, M.C.; Berechet, M.D.; Predescu, C. Sustainable Coated Nanostructures Based on Alginate and Electrospun Collagen Loaded with Antimicrobial Agents. Coatings 2021, 11, 121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).