Tailing Ash for the Removal of Methylene Blue from Aqueous Solutions by Batch Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Method
2.3. Floatation Process
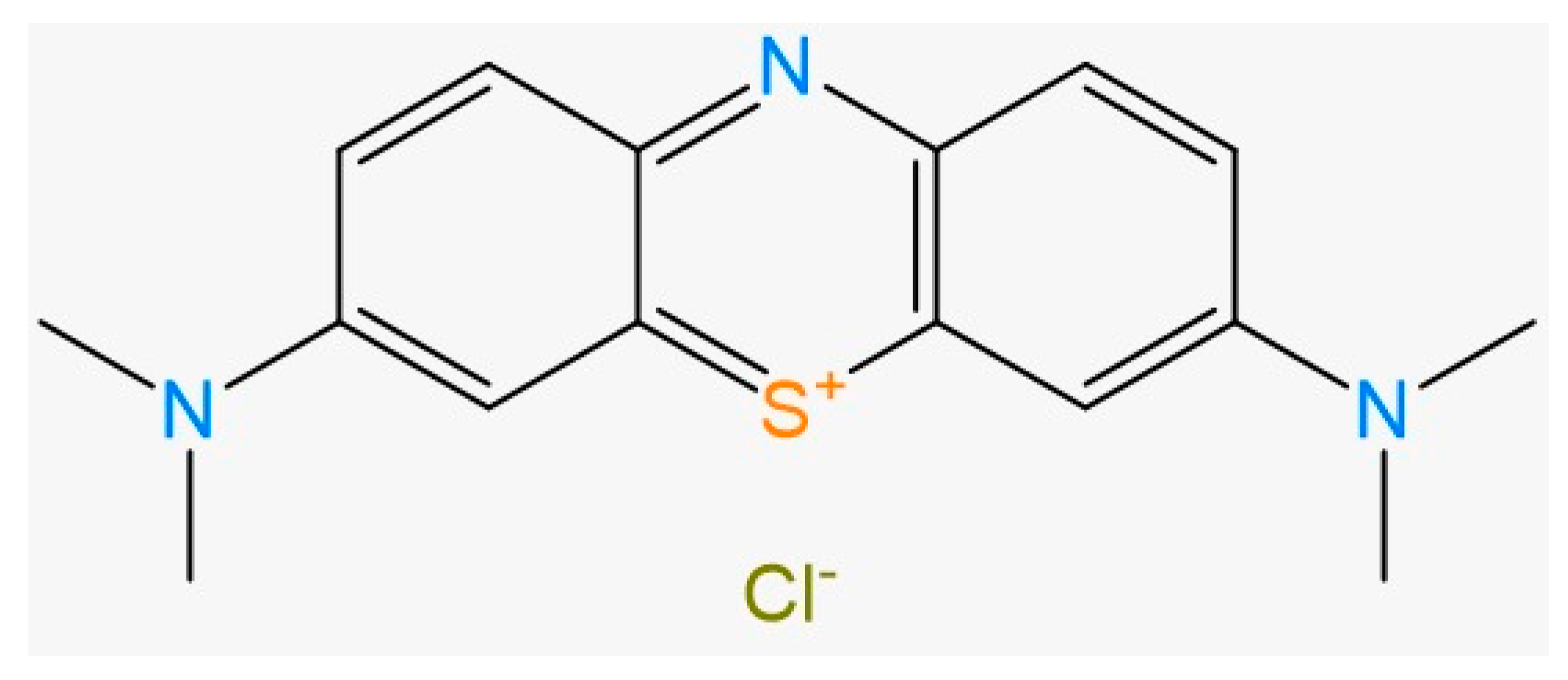
2.4. Methylene Blue Concentration Determination
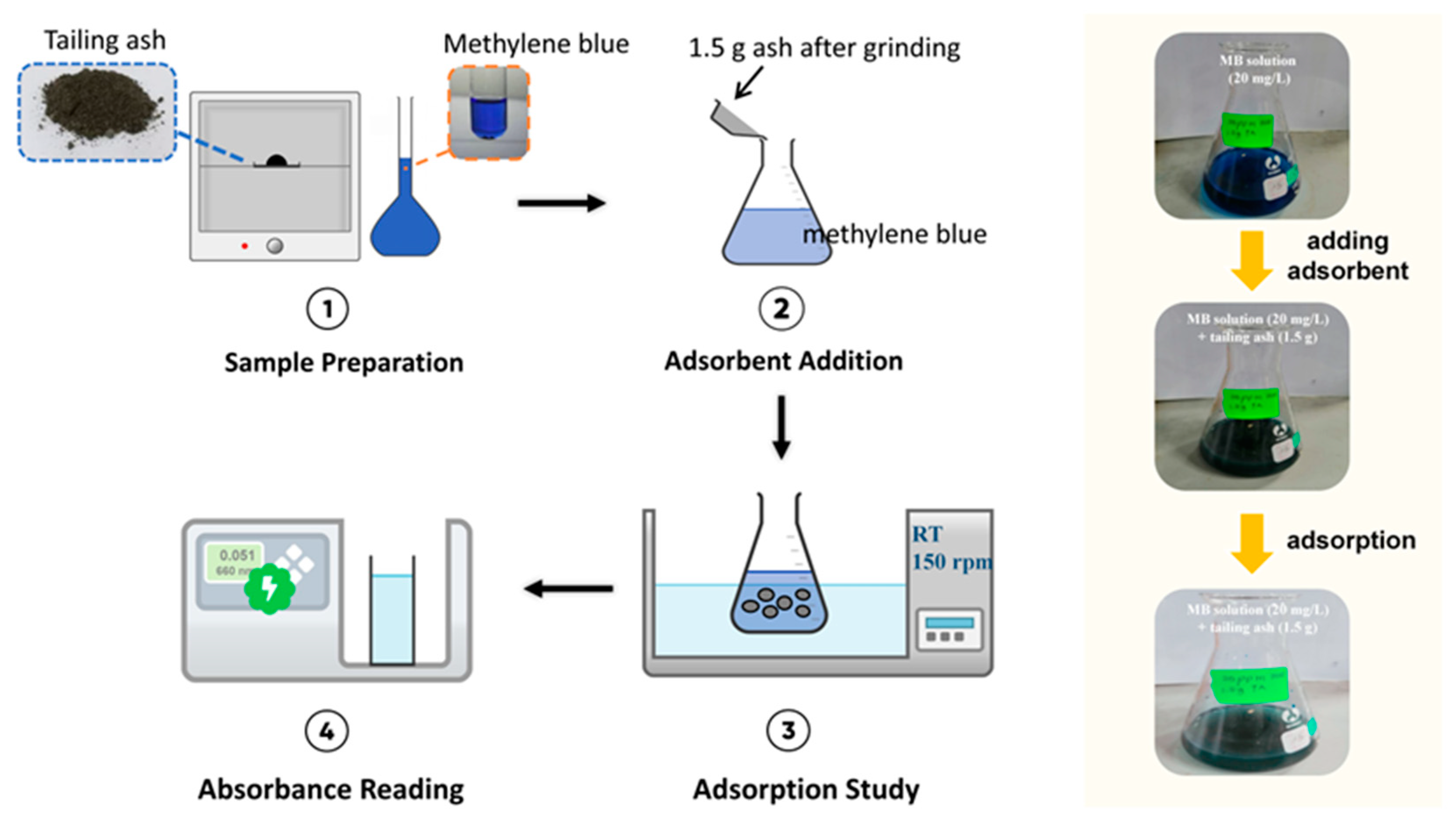
2.5. Adsorption Study
2.6. Material Development
2.6.1. Tailing Ash Pretreated with H2O2
2.6.2. Thermal Tailing Ash
3. Results
3.1. Characterization
3.1.1. XRF Analysis Results
3.1.2. SEM Analysis Results
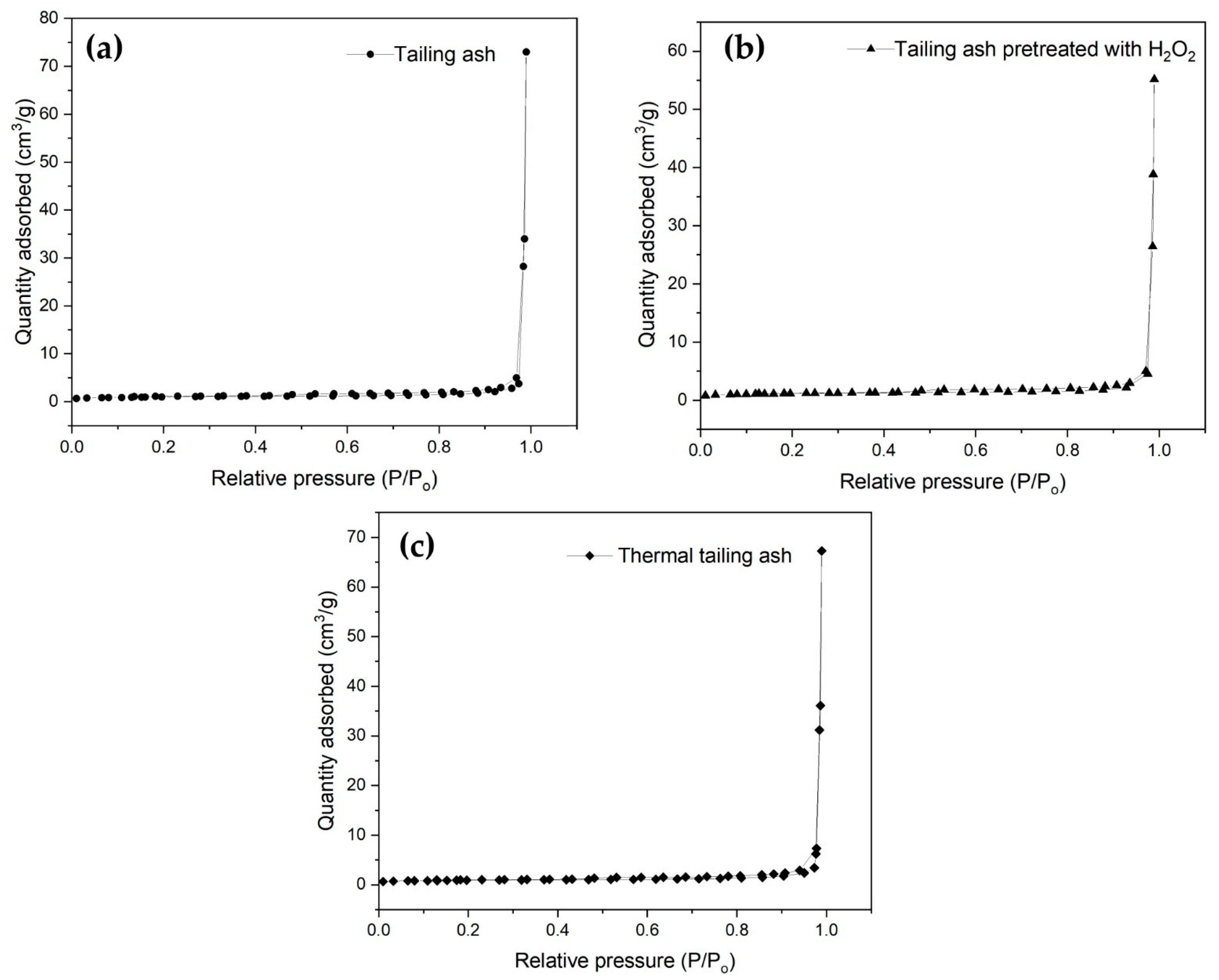
3.1.3. BET Analysis Results
3.2. Adsorption Study
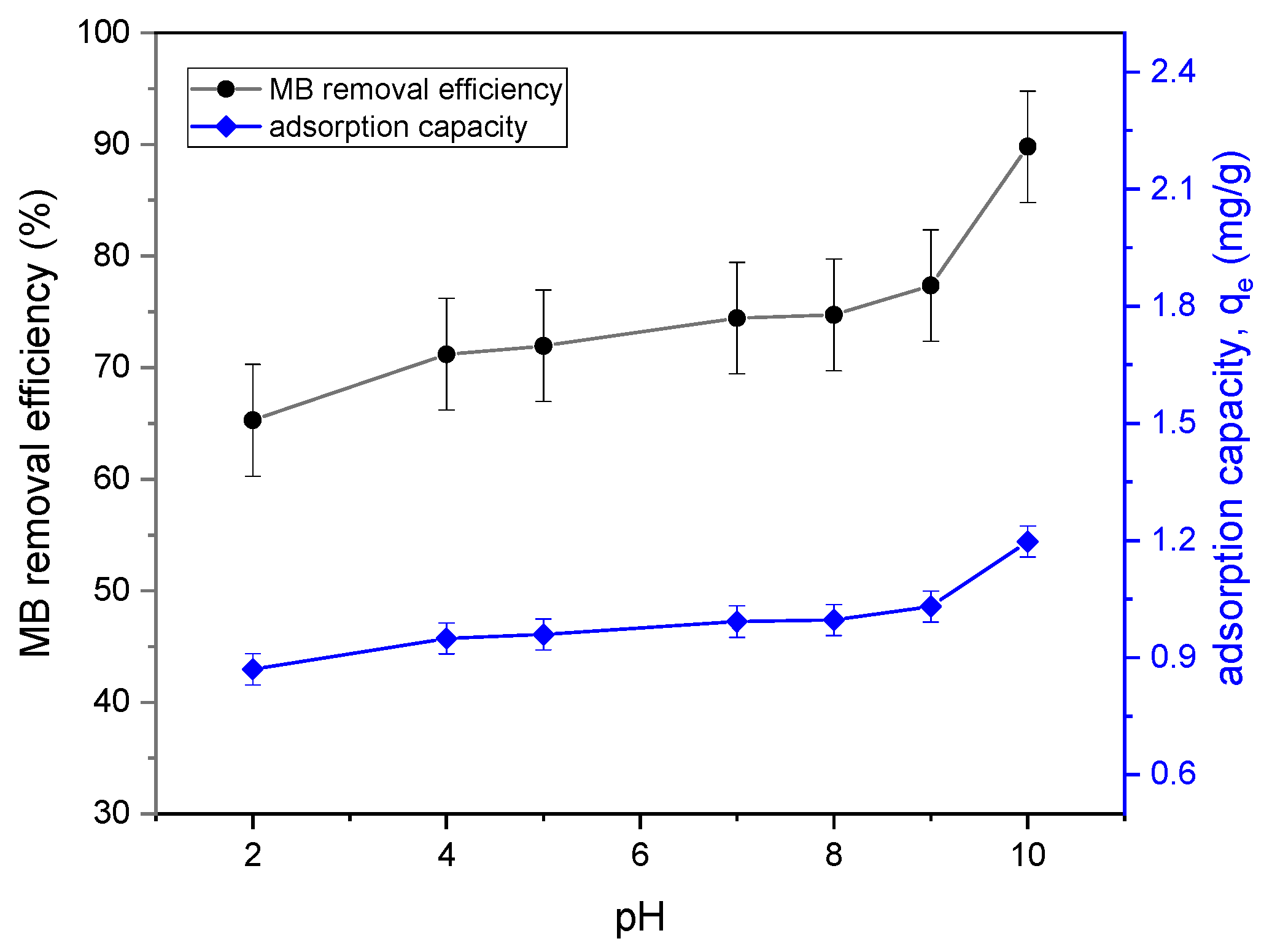
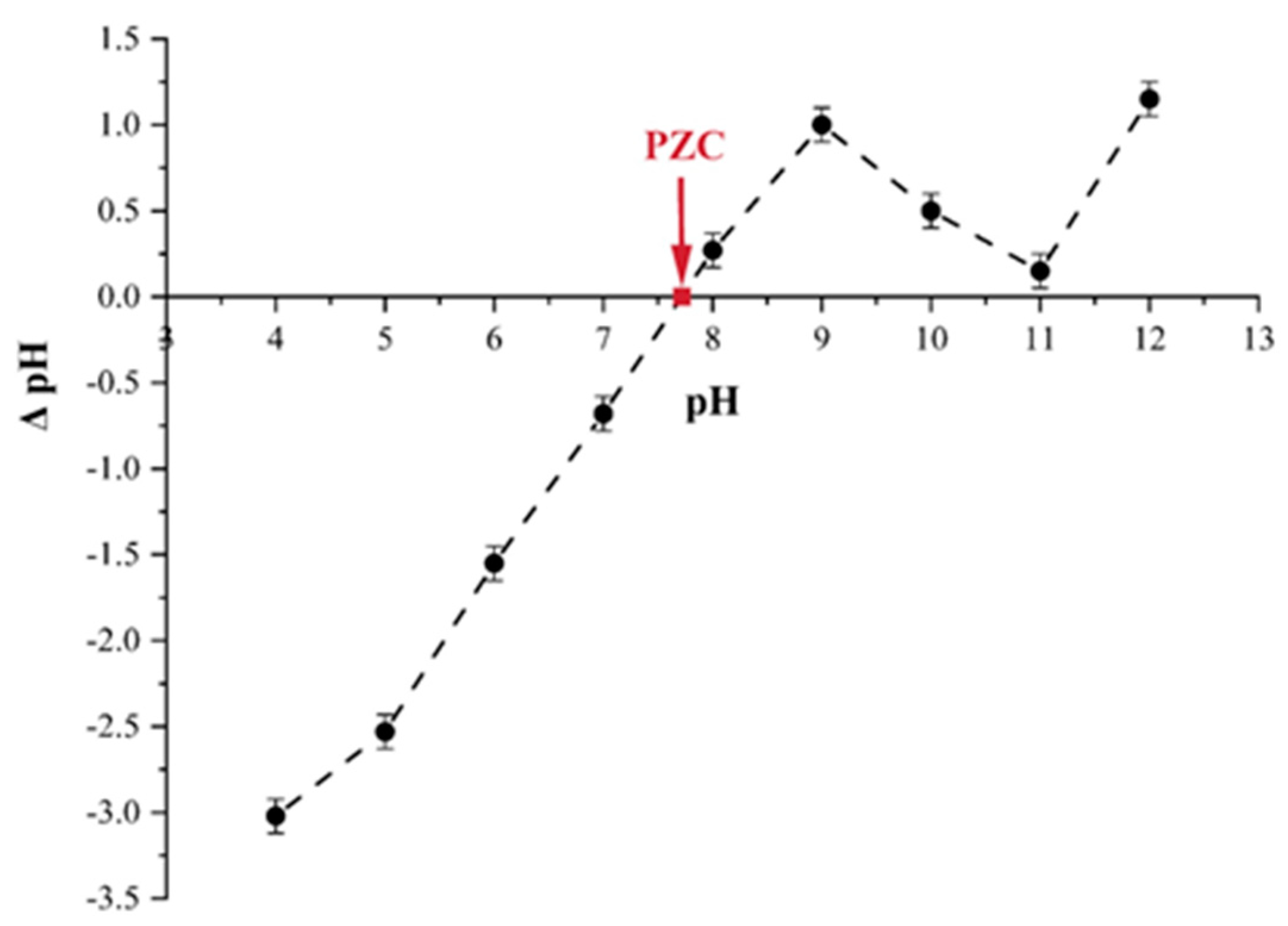
3.2.1. Effect of pH and Determination of pH Point of Zero Charge (pHpzc) of Tailing Ash
3.2.2. Effect of Stirring Speed
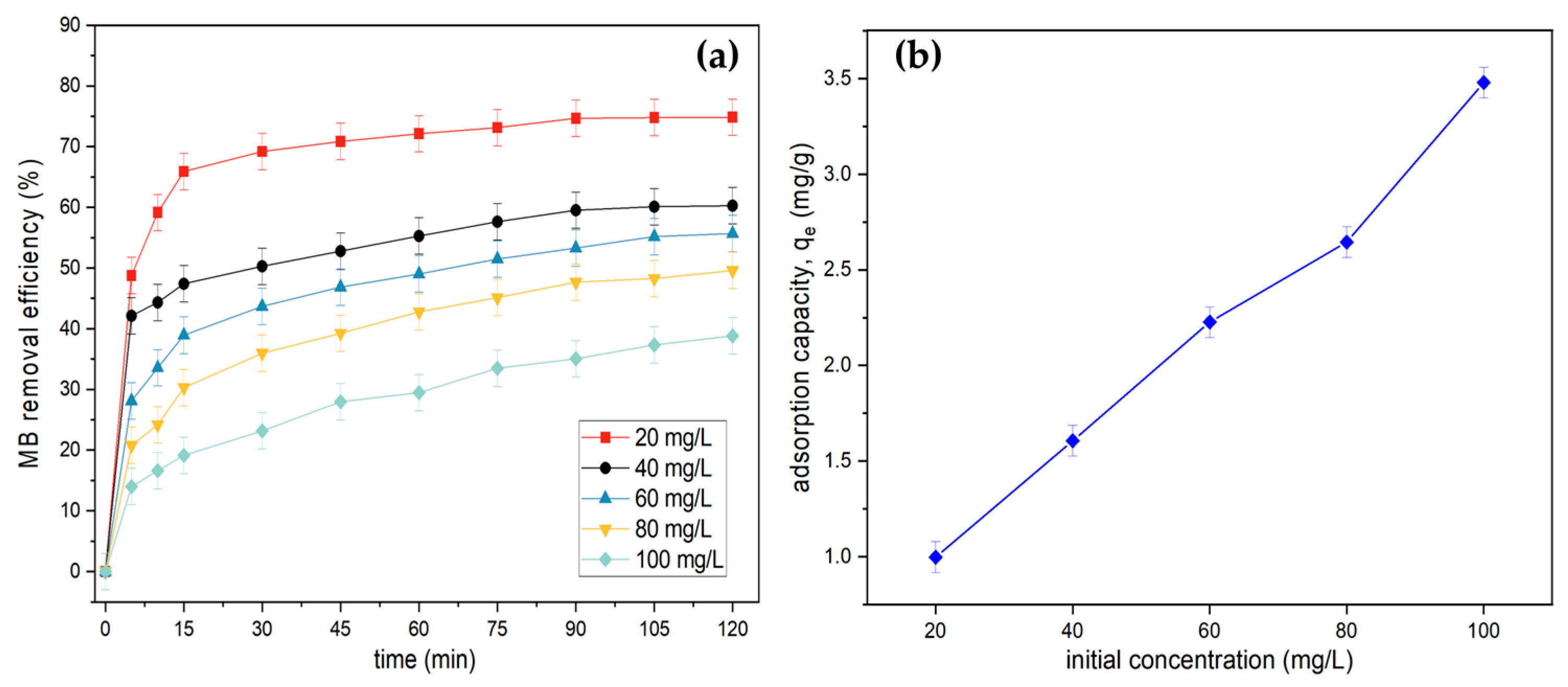
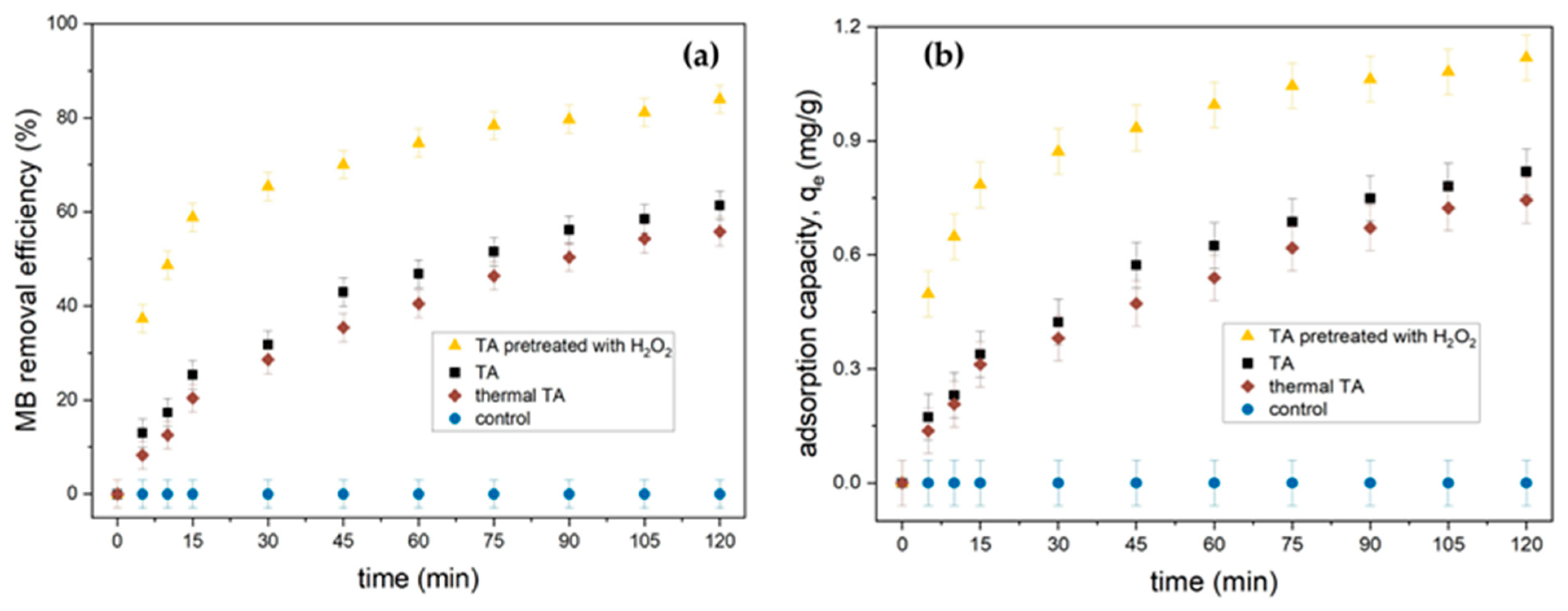
3.2.3. Effect of Initial Dye Concentration and Contact Time
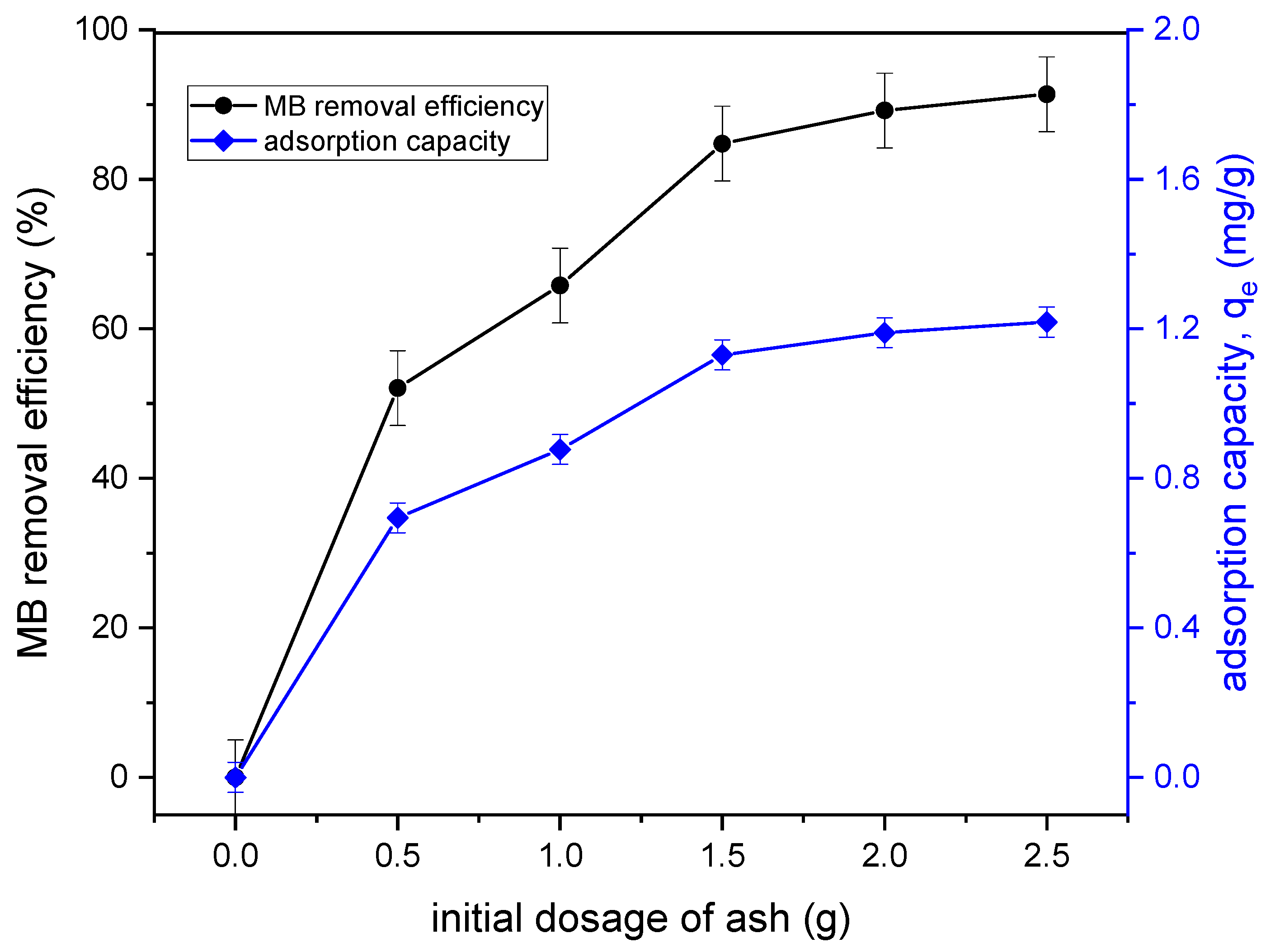
3.2.4. Effect of Adsorbent Dosage
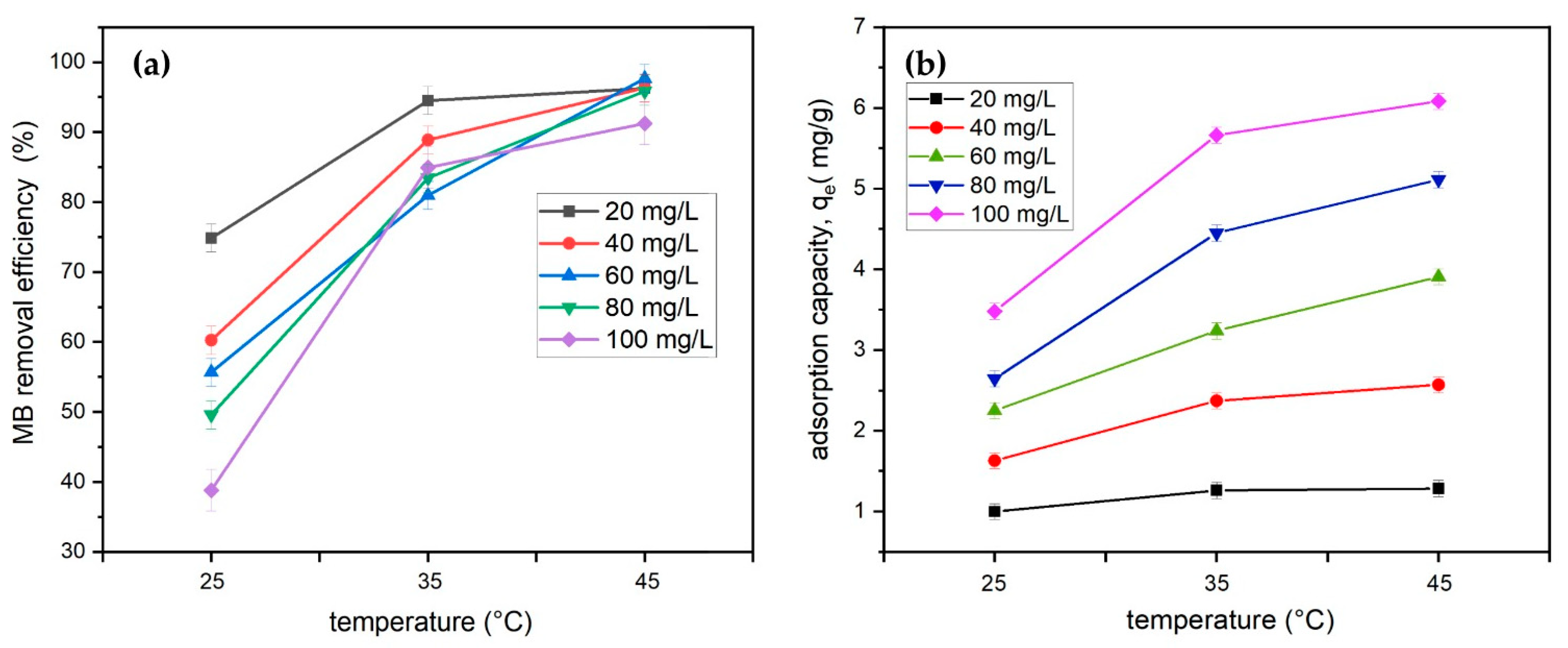
3.2.5. Effect of Temperature
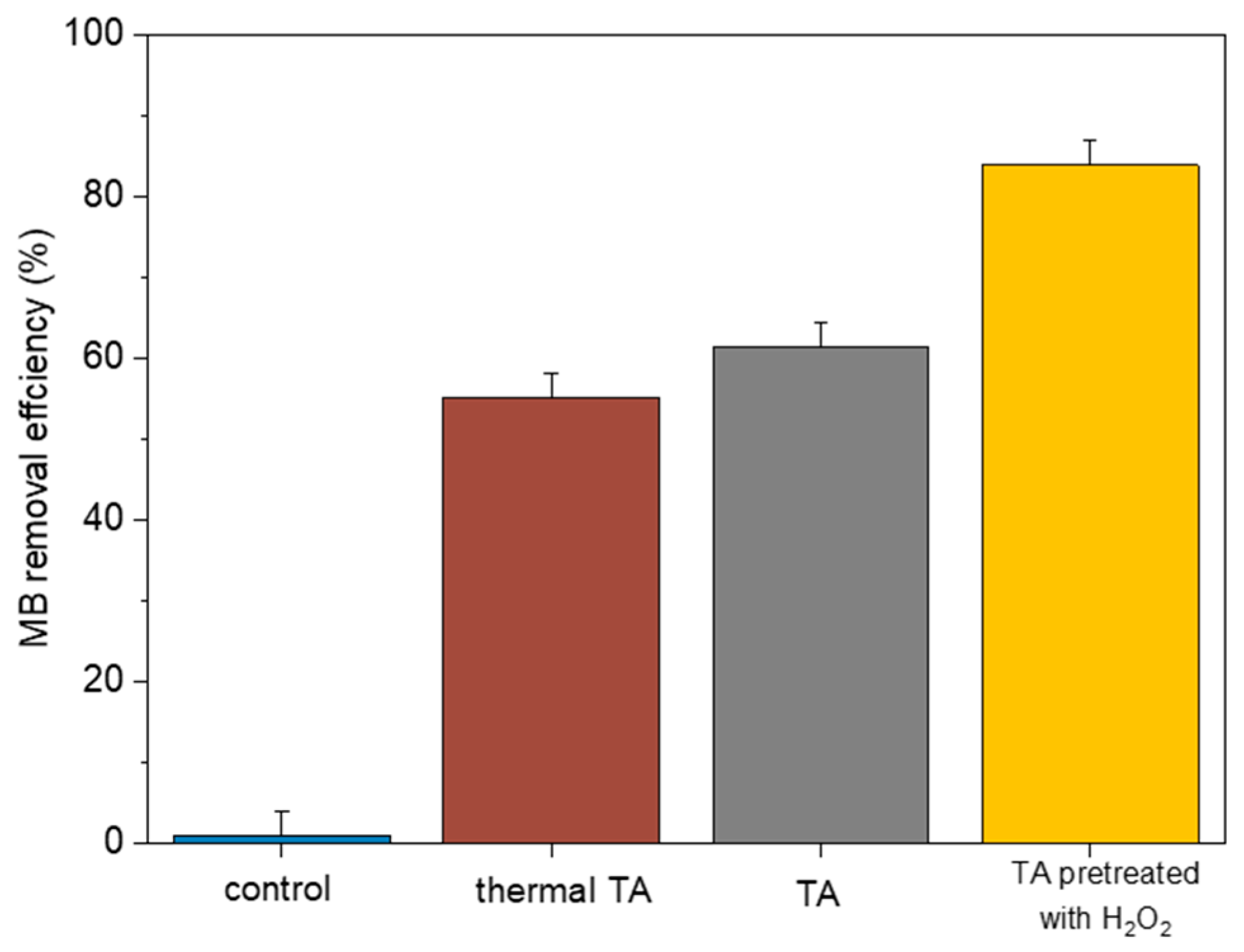
3.3. Modification of Adsorbent
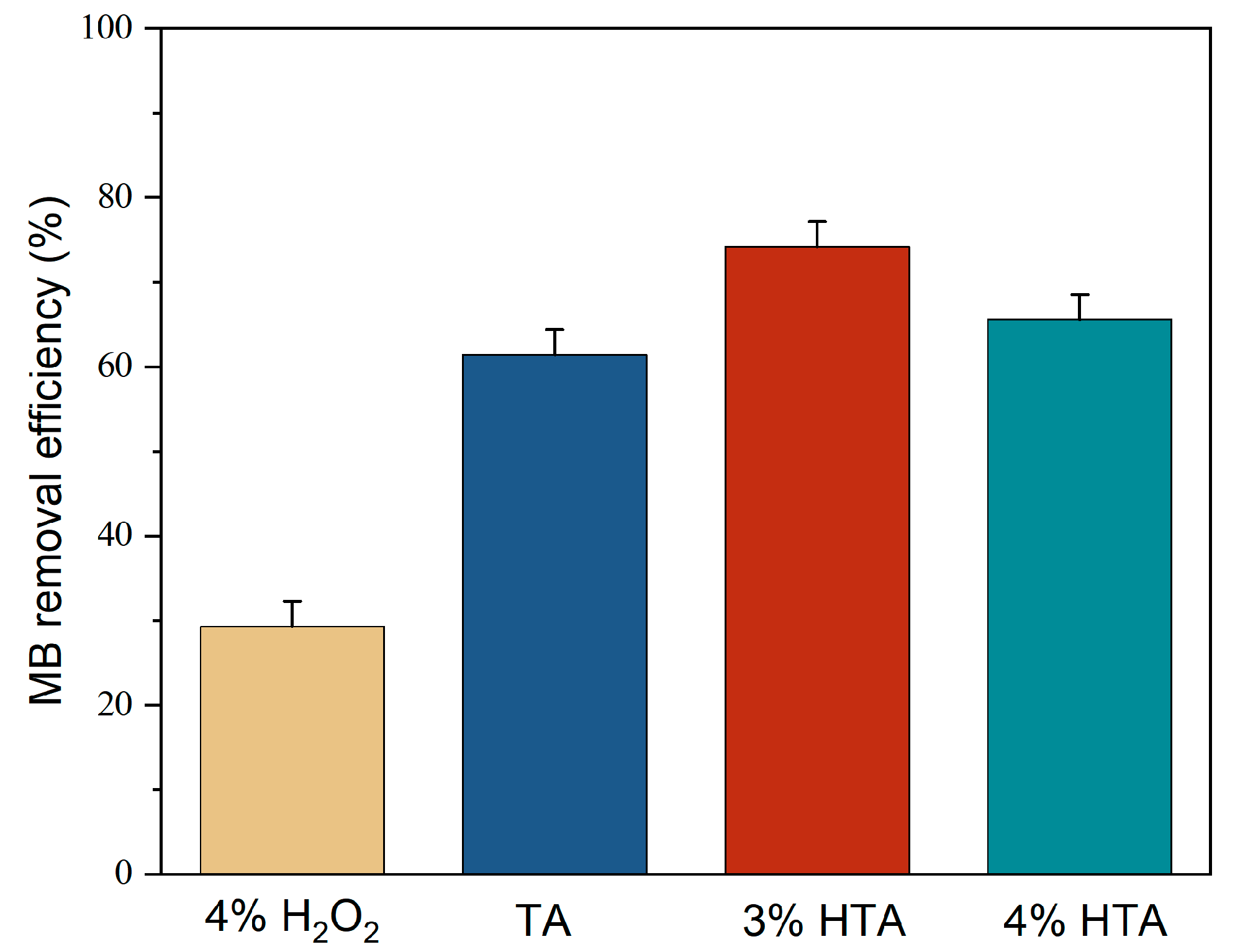
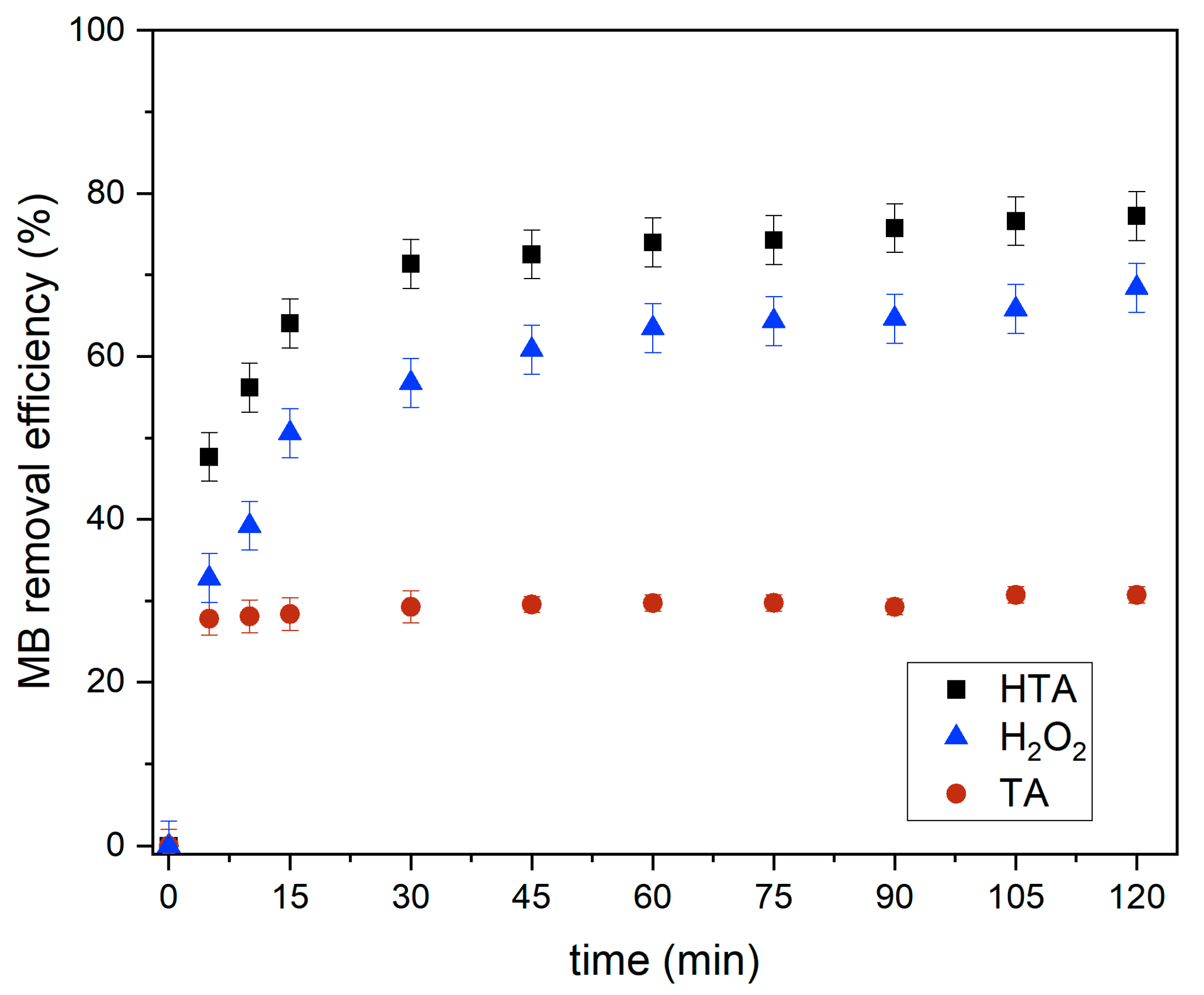
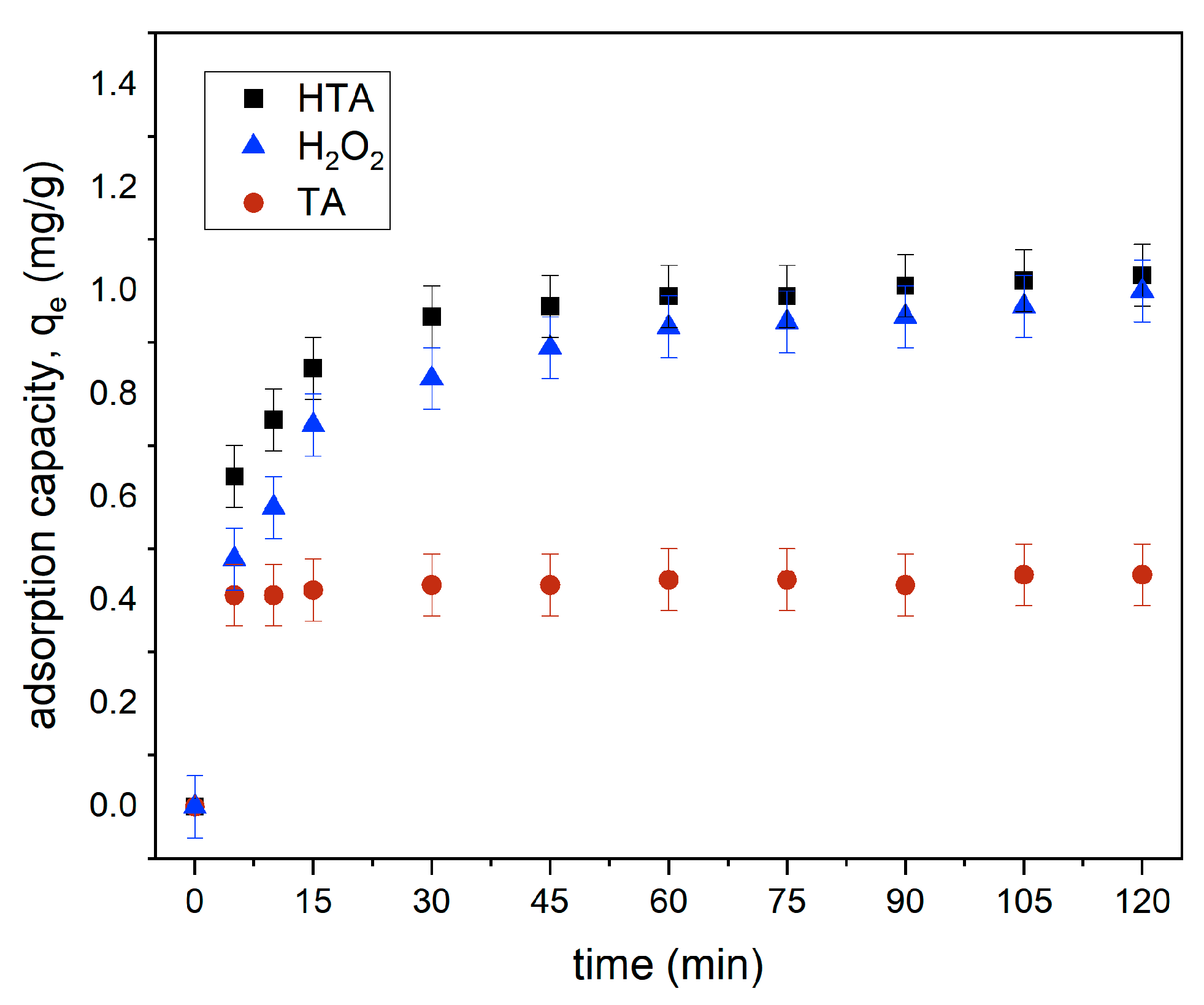
3.4. Functioning of H2O2
Effect of Contact Time by TA, H2O2, and HTA for MB Removal
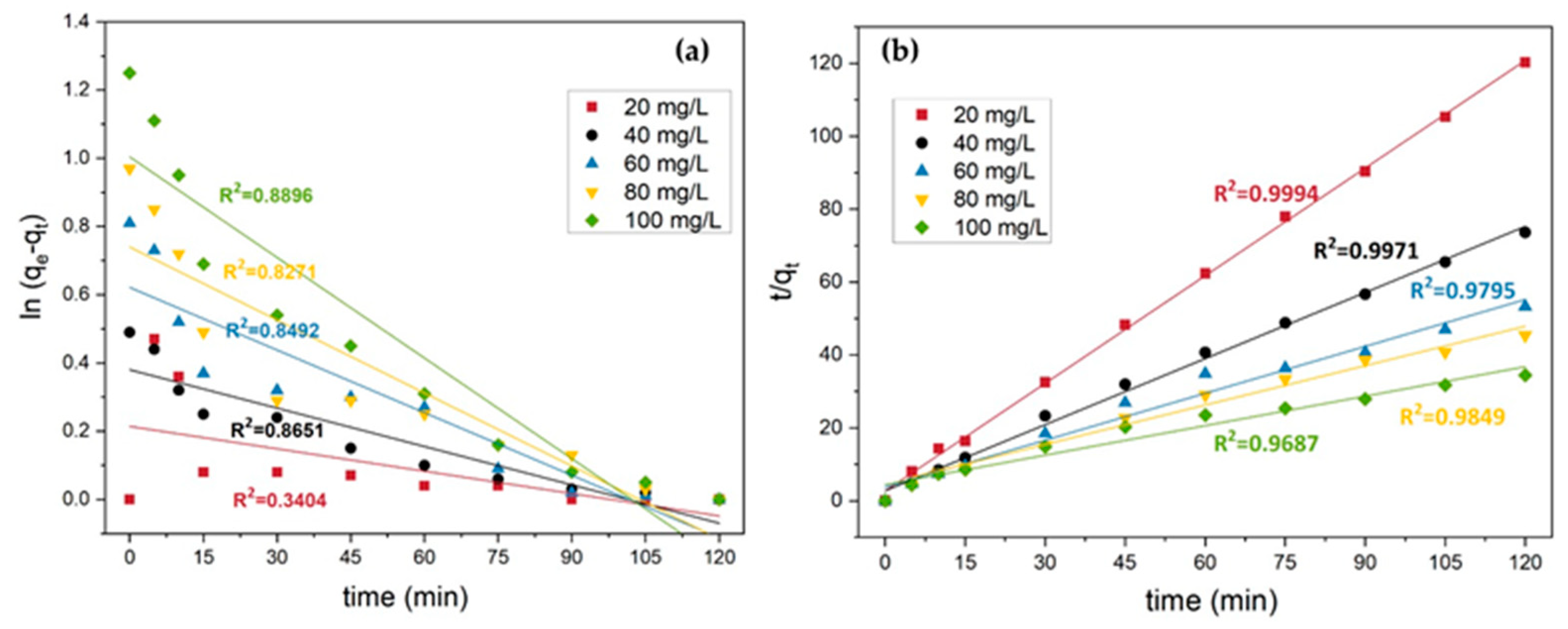
3.5. Adsorption Kinetics and Isotherm
3.5.1. Adsorption Kinetics
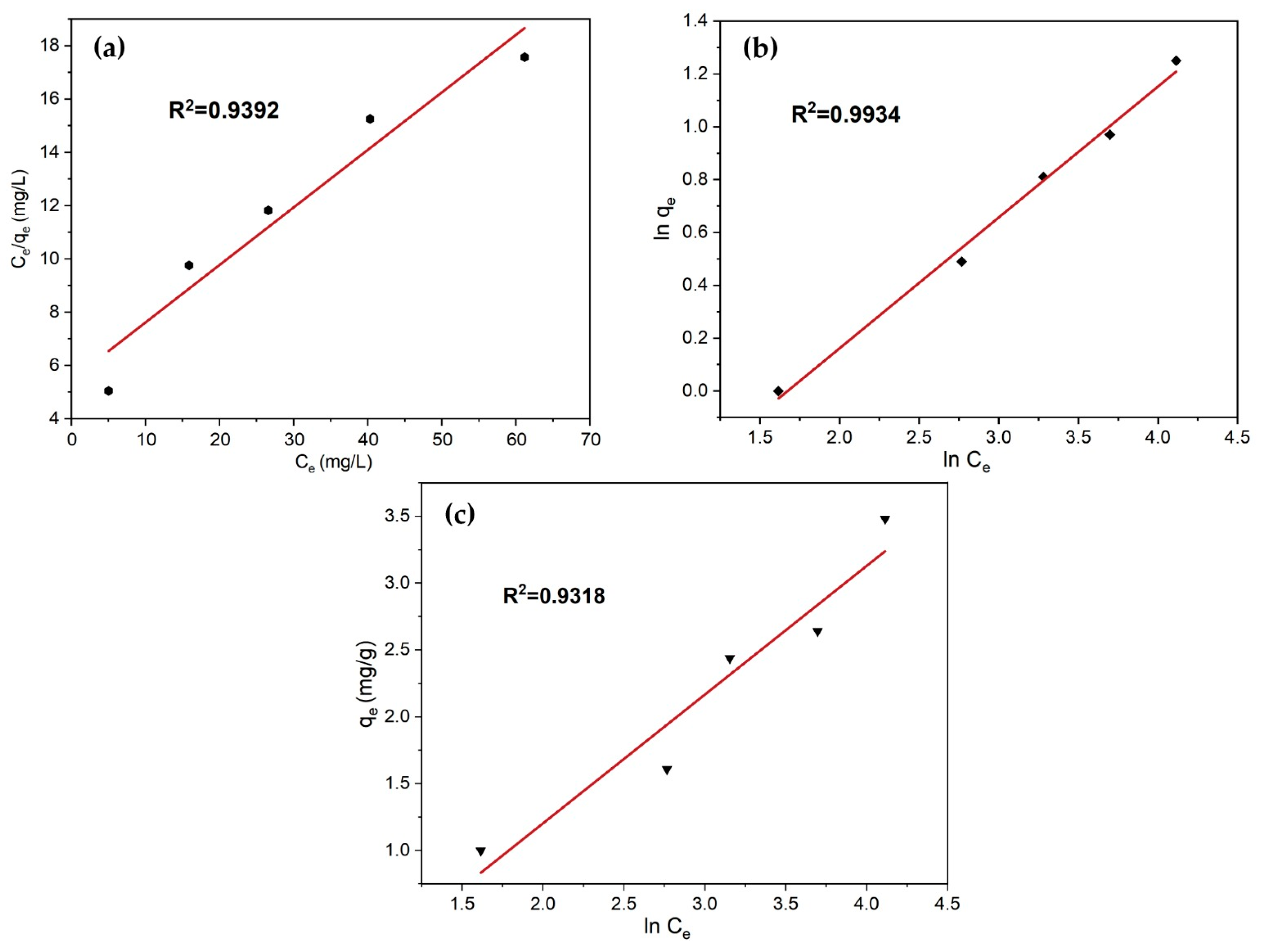
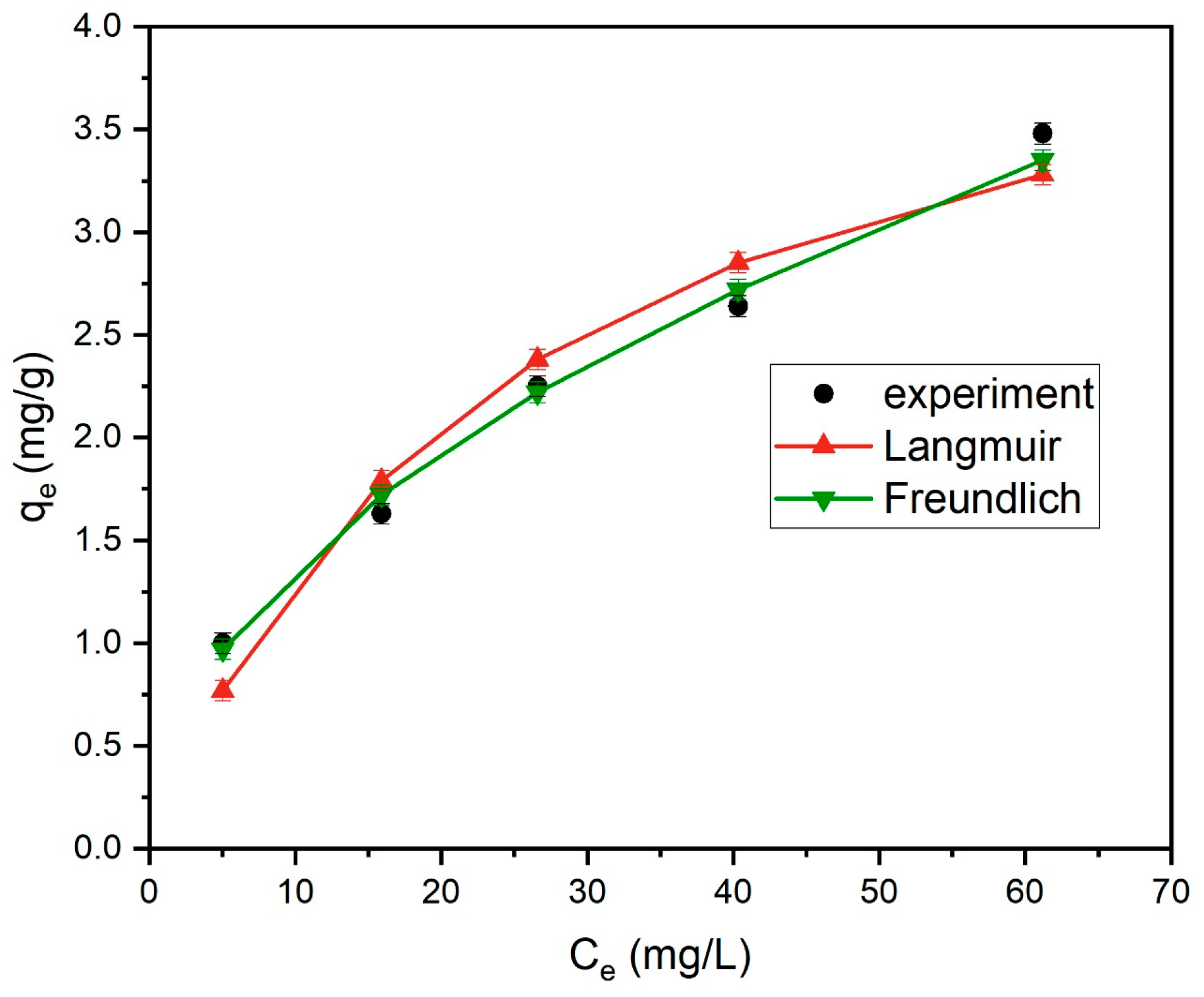
3.5.2. Adsorption Isotherm Study
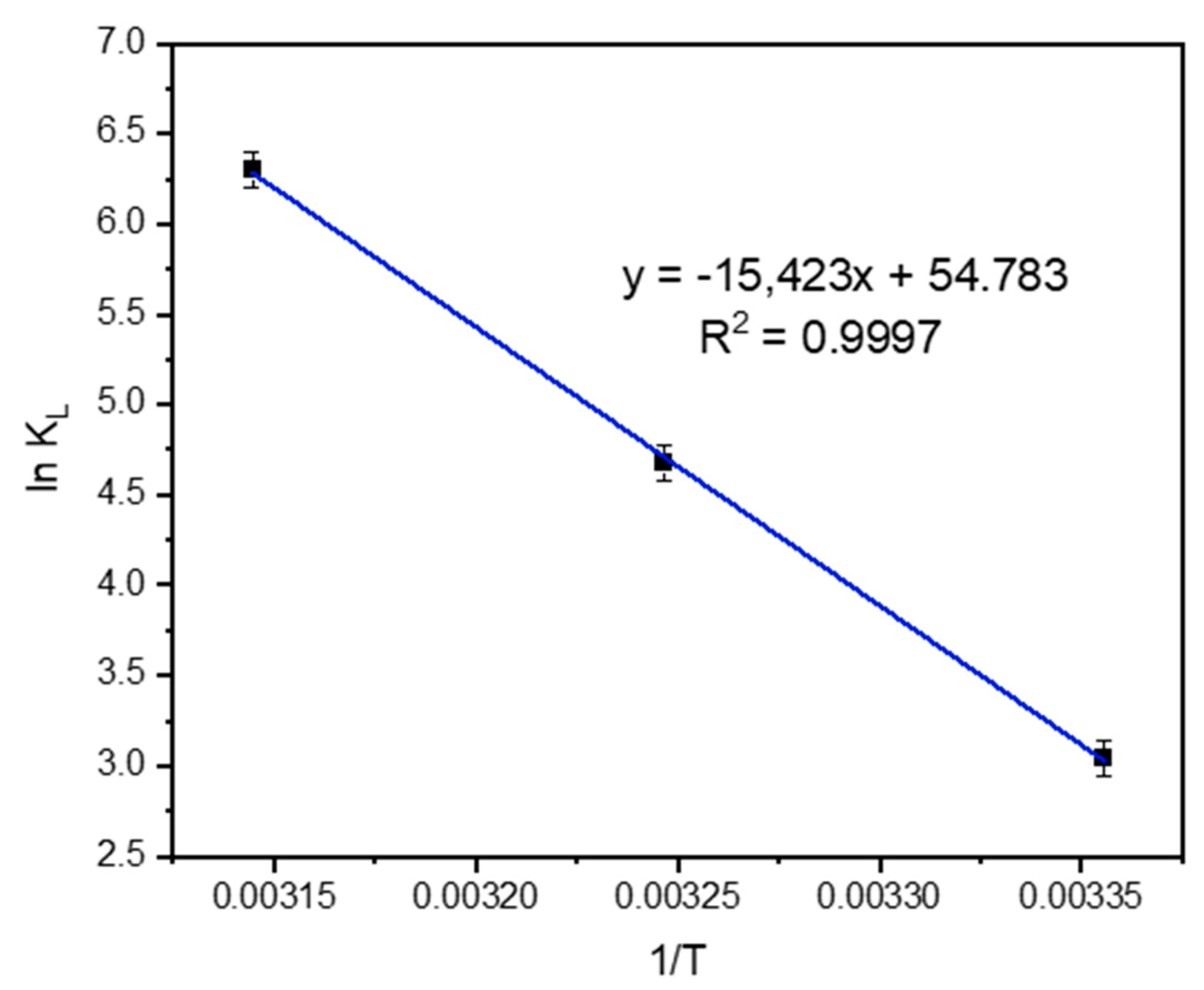
3.5.3. Thermodynamic Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heidrich, C.; Feuerborn, H.; Weir, A. Coal Combustion Products: A Global Perspective. In Proceedings of the 2013 World of Coal Ash (WOCA) Conference, Lexington, KY, USA, 22–25 April 2013. [Google Scholar]
- Reynolds-Clausen, K.; Singh, N. South Africa’s Power Producer’s Revised Coal Ash Strategy and Implementation Progress. Coal Combust. Gasif. Prod. 2019, 11, 10–17. [Google Scholar] [CrossRef]
- Sarkar, M.; Ehsan, M.; Islam, M. Issues relating to energy conservation and renewable energy in Bangladesh. Energy Sustain. Dev. 2003, 7, 77–87. [Google Scholar] [CrossRef]
- Abuelgasim, R.; Rashid, A.S.; Bouassida, M.; Shien, N.; Abdullah, M.H. Geotechnical Characteristics of Tanjung Bin Coal Bottom Ash. IOP Conf. Ser. Mater. Sci. Eng. 2020, 932, 012055. [Google Scholar] [CrossRef]
- Badenhorst, C.; Wagner, N.; Valentim, B.; Santos, A.C.; Guedes, A.; Białecka, B.; Mozco, J.C.; Popescu, L.; Cruceru, M.; Predeanu, G.; et al. Char from Coal Ash as a Possible Precursor for Synthetic Graphite—Recent Developments of the Charphite Project. In Proceedings of the World of Coal Ash (WOCA), St. Louis, MO, USA, 13–16 May 2019; pp. 1–32. [Google Scholar]
- Gupta, V.K.; Carrott, P.J.M.; Carrott, M.M.L.R. Suhas Low-Cost Adsorbents: Growing Approach to Wastewater Treatment—A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 783–842. [Google Scholar] [CrossRef]
- Gupta, V.K. Suhas Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Gorme, J.B.; Maniquiz, M.C.; Kim, S.-S.; Son, Y.-G.; Kim, Y.-T.; Kim, L.-H. Characterization of Bottom Ash as an Adsorbent of Lead from Aqueous Solutions. Environ. Eng. Res. 2010, 15, 207–213. [Google Scholar] [CrossRef]
- Shim, Y.-S.; Kim, Y.-K.; Kong, S.-H.; Rhee, S.-W.; Lee, W.-K. The adsorption characteristics of heavy metals by various particle sizes of MSWI bottom ash. Waste Manag. 2003, 23, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Inglezakis, V.J.; Poulopoulos, S.G.; Kazemian, H. Insights into the S-shaped sorption isotherms and their dimensionless forms. Microporous Mesoporous Mater. 2018, 272, 166–176. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of Methylene Blue in Water onto Activated Carbon by Surfactant Modification. Water 2020, 12, 587. [Google Scholar] [CrossRef] [Green Version]
- Kusmiyati, K.; Listyanto, P.A.; Vitasary, D.; Indra, R.; Islamica, D. Hadiyanto Coal Bottom Ash and Activated Carbon for Removal of Vertigo Blue Dye in Batik Textile Waste Water: Adsorbent Characteristic, Isotherms, and Kinetics Studies. Walailak J. Sci. Technol. 2017, 14, 427–439. [Google Scholar]
- Hannan, N.I.R.R.; Shahidan, S.; Ali, N.; Maarof, M.Z. A Comprehensive Review on the Properties of Coal Bottom Ash in Concrete as Sound Absorption Material. MATEC Web Conf. 2017, 103, 1005. [Google Scholar] [CrossRef]
- Boumediene, M.; Benaïssa, H.; George, B.; Molina, S.; Merlin, A. Effects of PH and Ionic Strength on Methylene Blue Removal from Synthetic Aqueous Solutions by Sorption onto Orange Peel and Desorption Study. J. Mater. Environ. Sci. 2018, 9, 1700–1711. [Google Scholar]
- Altaher, H.; Khalil, T.E.; Abubeah, R. The effect of dye chemical structure on adsorption on activated carbon: A comparative study. Color. Technol. 2014, 130, 205–214. [Google Scholar] [CrossRef]
- Aworanti, A.; Agarry, S.E. Kinetics, Isothermal and Thermodynamic Modelling Studies of Hexavalent Chromium Ions Adsorption from Simulated Wastewater onto Parkia Biglobosa-Sawdust Derived Acid-Steam Activated. Appl. J. Environ. Eng. Sci. 2017, 3, 58–76. [Google Scholar]
- Idan, I.J.; Abdullah, L.C.; Choong, T.S.; Jamil, S.N.A.B.M. Equilibrium, kinetics and thermodynamic adsorption studies of acid dyes on adsorbent developed from kenaf core fiber. Adsorpt. Sci. Technol. 2017, 36, 694–712. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mittal, A.; Jhare, D.; Mittal, J. Batch and bulk removal of hazardous colouring agent Rose Bengal by adsorption techniques using bottom ash as adsorbent. RSC Adv. 2012, 2, 8381–8389. [Google Scholar] [CrossRef]
- Park, S.-J.; Jang, Y.-S. Pore Structure and Surface Properties of Chemically Modified Activated Carbons for Adsorption Mechanism and Rate of Cr(VI). J. Colloid Interface Sci. 2002, 249, 458–463. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, D.; Hu, J.; Shen, F.; Long, L.; Zhang, Y.; Yang, G.; Zeng, Y.; Zhang, J.; He, J.; et al. Functionalizing bottom ash from biomass power plant for removing methylene blue from aqueous solution. Sci. Total Environ. 2018, 634, 760–768. [Google Scholar] [CrossRef]
- Mall, I.D.; Srivastava, V.C.; Agarwal, N.K. Removal of Orange-G and Methyl Violet dyes by adsorption onto bagasse fly ash—Kinetic study and equilibrium isotherm analyses. Dyes Pigments 2006, 69, 210–223. [Google Scholar] [CrossRef]
- Mittal, J.; Jhare, D.; Vardhan, H.; Mittal, A. Utilization of bottom ash as a low-cost sorbent for the removal and recovery of a toxic halogen containing dye eosin yellow. Desalination Water Treat. 2013, 52, 4508–4519. [Google Scholar] [CrossRef]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour. Ind. 2016, 15, 14–27. [Google Scholar] [CrossRef] [Green Version]
- Agarry, S.; Ogunleye, O. Chemically treated kola nut pod as low-cost natural adsorbent for the removal of 2,4-dinitrophenol from synthetic wastewater: Batch equilibrium, kinetic, and thermodynamic modelling studies. Turk. J. Eng. Environ. Sci. 2014, 38, 11–40. [Google Scholar] [CrossRef]
- Taher, T.; Rohendi, D.; Mohadi, R.; Lesbani, A. Congo red dye removal from aqueous solution by acid-activated bentonite from sarolangun: Kinetic, equilibrium, and thermodynamic studies. Arab. J. Basic Appl. Sci. 2019, 26, 125–136. [Google Scholar] [CrossRef]
- Keleşoğlu, S.; Kes, M.; Sütçü, L.; Polat, H. Adsorption of Methylene Blue from Aqueous Solution on High Lime Fly Ash: Kinetic, Equilibrium, and Thermodynamic Studies. J. Dispers. Sci. Technol. 2012, 33, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Nicola, R.; Muntean, S.-G.; Nistor, M.-A.; Putz, A.-M.; Almásy, L.; Săcărescu, L. Highly efficient and fast removal of colored pollutants from single and binary systems, using magnetic mesoporous silica. Chemosphere 2020, 261, 127737. [Google Scholar] [CrossRef] [PubMed]
- Esa, Y.A.M.; Sapawe, N. Removal of methylene blue from aqueous solution using silica nanoparticle extracted from skewer coconut leaves. Mater. Today Proc. 2020, 31, 398–401. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Xu, Z.; Zhang, M. Synthetic zeolite from coal bottom ash and its application in cadmium and nickel removal from acidic wastewater. Desalination Water Treat. 2016, 57, 26089–26100. [Google Scholar] [CrossRef]
- Dinh, N.T.; Vo, L.N.H.; Tran, N.T.T.; Phan, T.D.; Nguyen, D.B. Enhancing the removal efficiency of methylene blue in water by fly ash via a modified adsorbent with alkaline thermal hydrolysis treatment. RSC Adv. 2021, 11, 20292–20302. [Google Scholar] [CrossRef] [PubMed]
- Nsami, J.N.; Mbadcam, J.K. The Adsorption Efficiency of Chemically Prepared Activated Carbon from Cola Nut Shells by ZnCl2 on Methylene Blue. J. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Lelifajri, L.; Rahmi, R.; Supriatno, S.; Susilawati, S.; Indarum, A.S. Study on methylene blue dye adsorption in aqueous solution by heat-treated Gnetum gnemon shell waste particles as low-cost adsorbent. AIP Conf. Proc. 2020, 2243, 020012. [Google Scholar] [CrossRef]


| Component | wt (%) |
|---|---|
| SiO2 | 32.52 |
| Al2O3 | 10.96 |
| Fe2O3 | 15.95 |
| CaO | 16.93 |
| MgO | 6.83 |
| K2O | 0.71 |
| Na2O | 0.63 |
| TiO2 | 0.83 |
| SO3 | 0.58 |
| Cl | - |
| P2O5 | 0.21 |
| BaO | 0.25 |
| MnO | 0.14 |
| SrO | 0.17 |
| ZrO2 | 0.03 |
| V2O5 | - |
| Cr2O3 | 0.02 |
| CeO2 | 0.03 |
| ZnO | 0.01 |
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| Tailing ash | 3.5420 | 0.078078 | 38.5800 |
| Tailing ash pretreated with H2O2 | 4.0471 | 0.098927 | 55.899 |
| Thermal tailing ash | 3.2220 | 0.096678 | 60.0112 |
| C0 (mg/L) | qe (mg/g) Exp. | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| k1 (1/min) | qe (mg/g) Cal. | R2 | k2 (g/mg/min) | qe (mg/g) Cal. | R2 | ||
| 20 | 0.99 | 0.003 | 1.32 | 0.5548 | 0.077 | 1.03 | 0.9994 |
| 40 | 1.63 | 0.003 | 1.42 | 0.8796 | 0.027 | 1.68 | 0.9971 |
| 60 | 2.25 | 0.006 | 1.76 | 0.8574 | 0.007 | 2.41 | 0.9795 |
| 80 | 2.64 | 0.006 | 1.96 | 0.8339 | 0.006 | 2.78 | 0.9849 |
| 100 | 3.48 | 0.009 | 2.53 | 0.8999 | 0.002 | 3.94 | 0.9687 |
| Model | Isotherm Parameters | R2 |
|---|---|---|
| Langmuir | qmax = 4.63 mg/g KL = 0.183 L/mg | 0.9392 |
| Freundlich | Kf = 0.44 mg/g 1/n = 0.496 | 0.9934 |
| Temkin | B = 0.73 b = −3.4 kJ/mol A = 1.24 L/g | 0.9845 |
| ∆H° | ∆S° | ∆G° KJ/mol | ||
|---|---|---|---|---|
| KJ/mol | KJ/(K mol) | 298 | 308 | 318 |
| −0.4555 | −128.226 | −7.5403 | −11.9745 | −16.6550 |
| Low-Cost Adsorbent | MB Removal, % | Qmax (mg/g) | pH | Ref. |
|---|---|---|---|---|
| Tailing ash | 96.23 | 6.08 | 10 | (this study) |
| Fly ash (modified with alkali) | 97.11 | 28.65 | 7 | [30] |
| Activated carbon (Cola nuts shell) | 90 | 87.37 | 3.5 | [31] |
| Orange peel | 98.76 | 98 | 2 | [14] |
| High-lime fly ash | 96.99 | 6.06 | 9–10 | [26] |
| Heat-treated gnetum gnemon shell | 95.80 | 14.37 | 6 | [32] |
| Mesoporous silica nanoparticles | 95 | 31 | 7 | [28] |
| Magnetic mesoporous silica | 96.35 | 208.31 | 10 | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahya, C.J.F.b.; Choong, T.S.Y.; Li, F.; Ghani, W.A.W.A.K.; Aziz, F.N.A.A.; Jamil, S.N.A.M. Tailing Ash for the Removal of Methylene Blue from Aqueous Solutions by Batch Adsorption. Processes 2023, 11, 2282. https://doi.org/10.3390/pr11082282
Yahya CJFb, Choong TSY, Li F, Ghani WAWAK, Aziz FNAA, Jamil SNAM. Tailing Ash for the Removal of Methylene Blue from Aqueous Solutions by Batch Adsorption. Processes. 2023; 11(8):2282. https://doi.org/10.3390/pr11082282
Chicago/Turabian StyleYahya, Cik Jamla Farhan bt, Thomas Shean Yaw Choong, Fan Li, Wan Azlina Wan Ab Karim Ghani, Farah Nora Aznieta Abd Aziz, and Siti Nurul Ain Md. Jamil. 2023. "Tailing Ash for the Removal of Methylene Blue from Aqueous Solutions by Batch Adsorption" Processes 11, no. 8: 2282. https://doi.org/10.3390/pr11082282





