Prediction of Physical Properties and Phase Characteristics of Ethane and Ethane Mixture in the Ethane Pipeline
Abstract
:1. Introduction
2. PVT EOSs Based on Thermodynamics
2.1. Cubic-Type PVT EOS
2.2. Non-Cubic PVT EOS
- (1)
- BWRS EOS
- (2)
- LKP EOS
- (3)
- GERG-2008 EOS
3. EOS Evaluation for Predicting Physical Properties
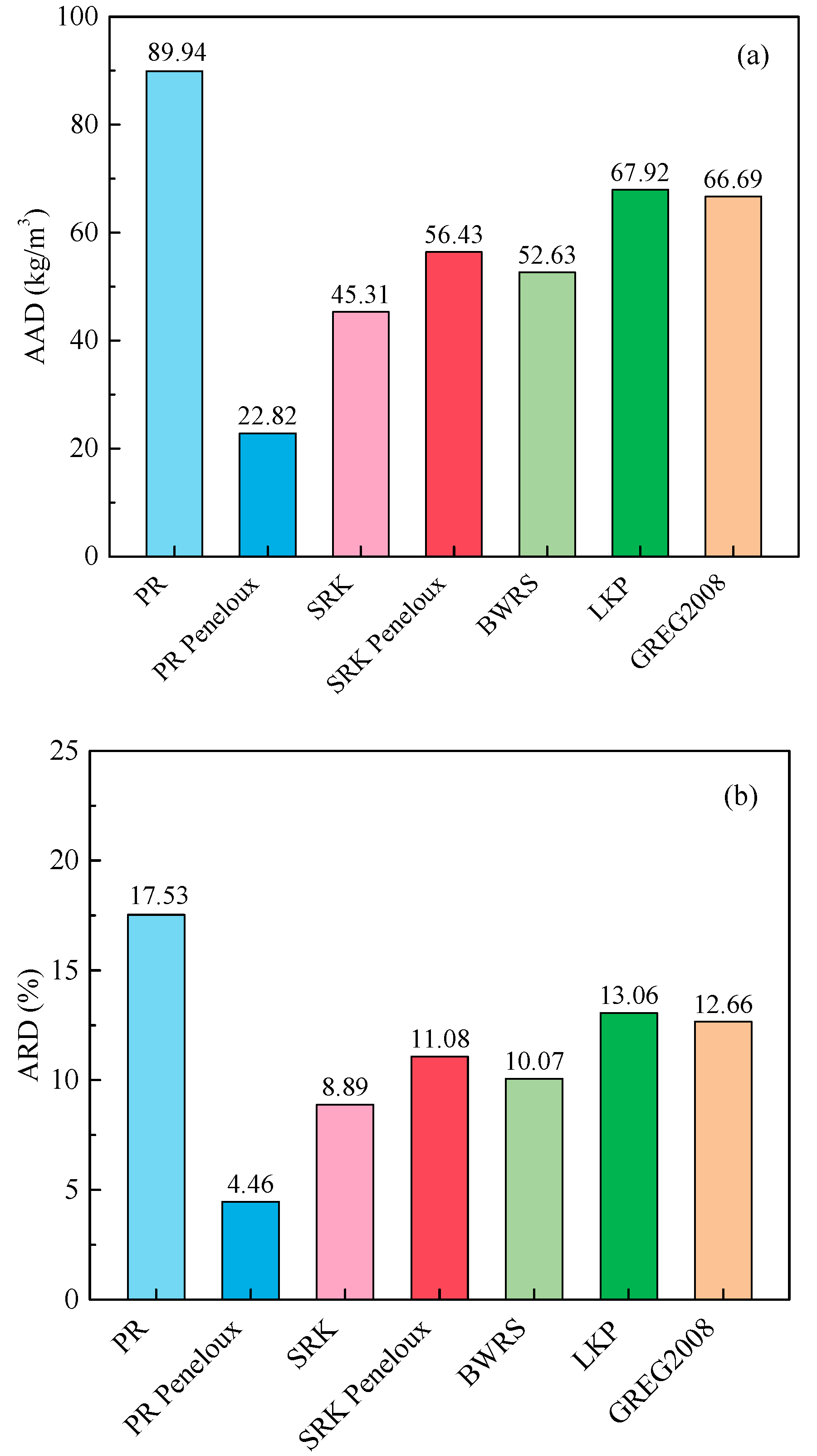
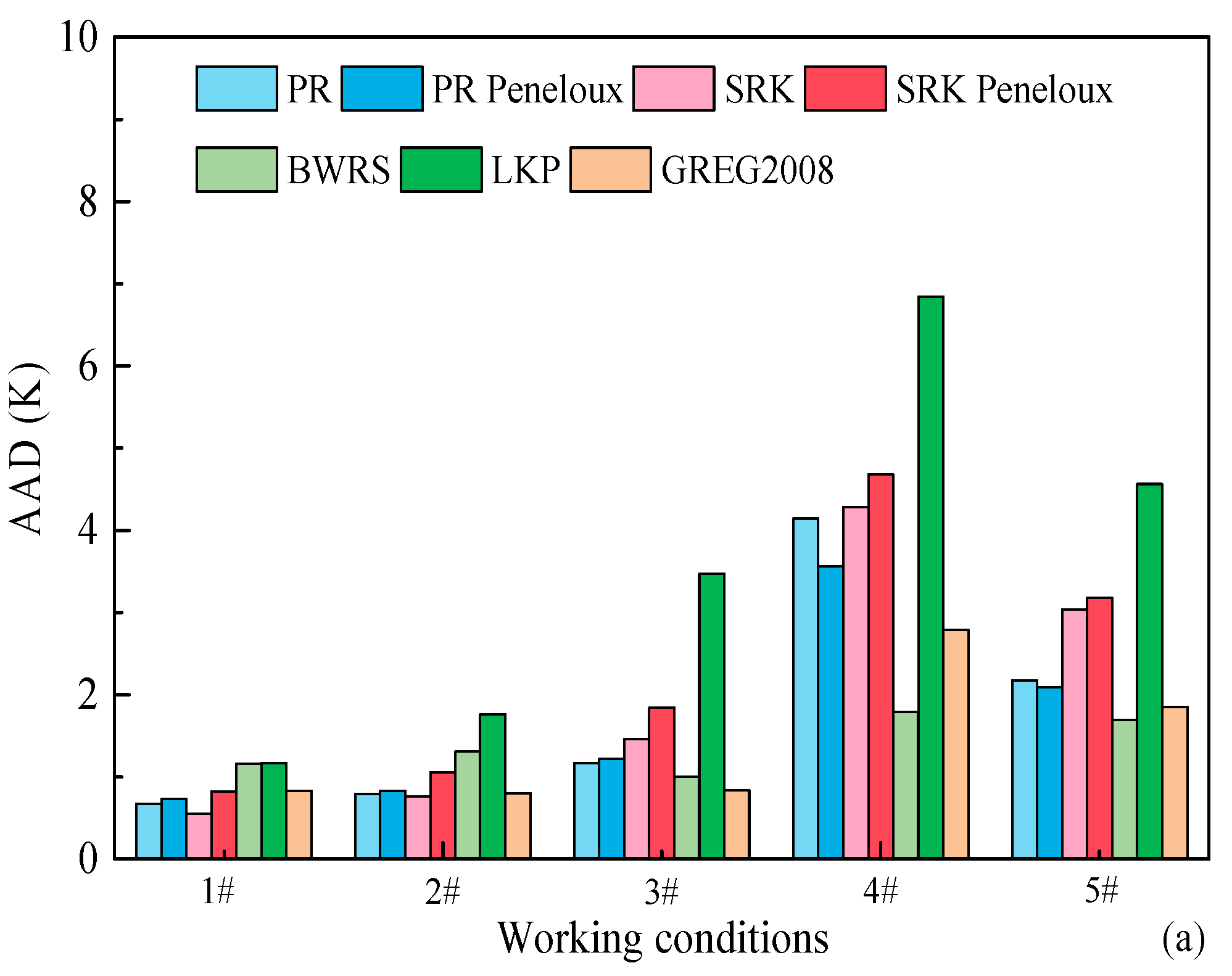
3.1. Evaluation of EOSs for Predicting Density
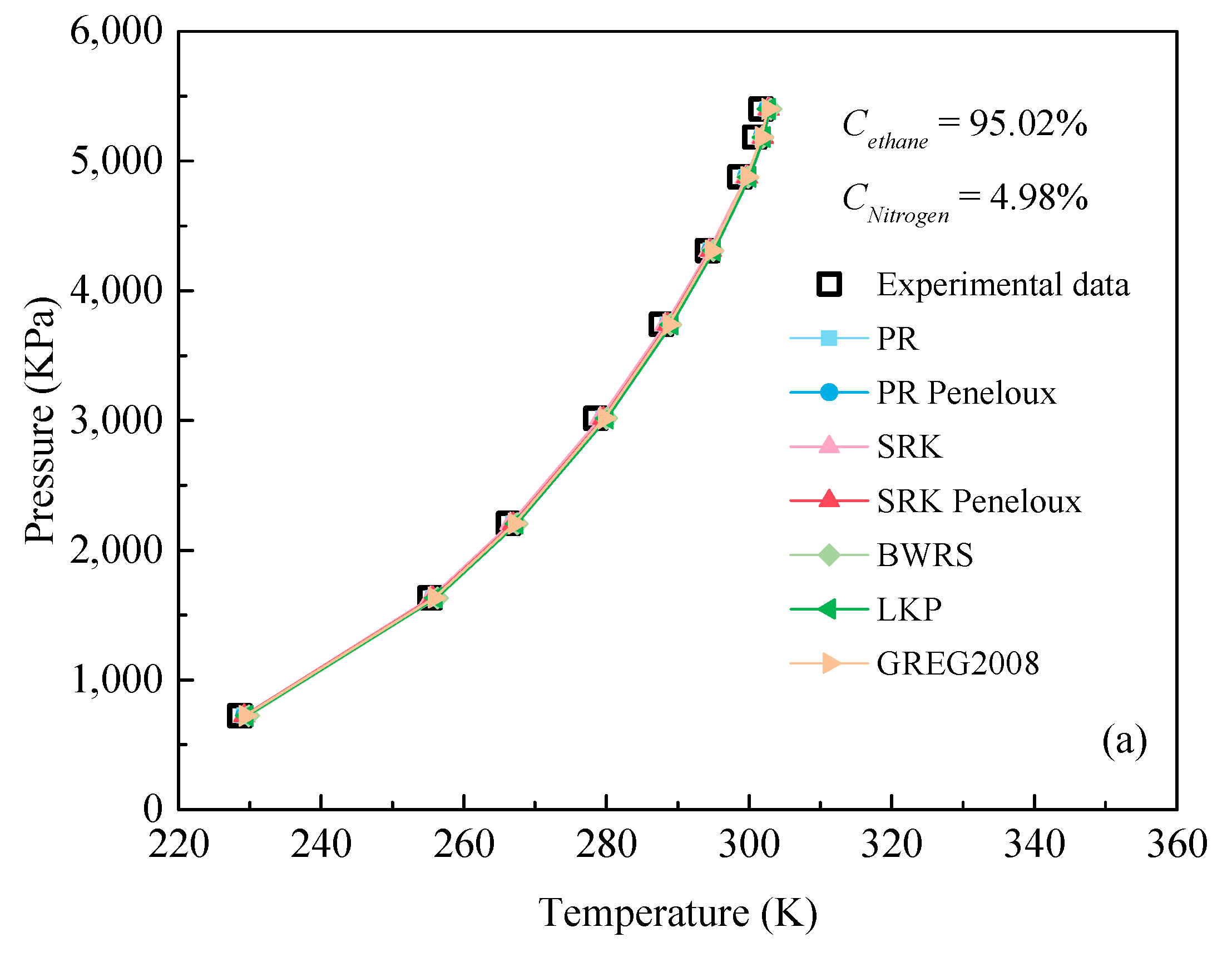
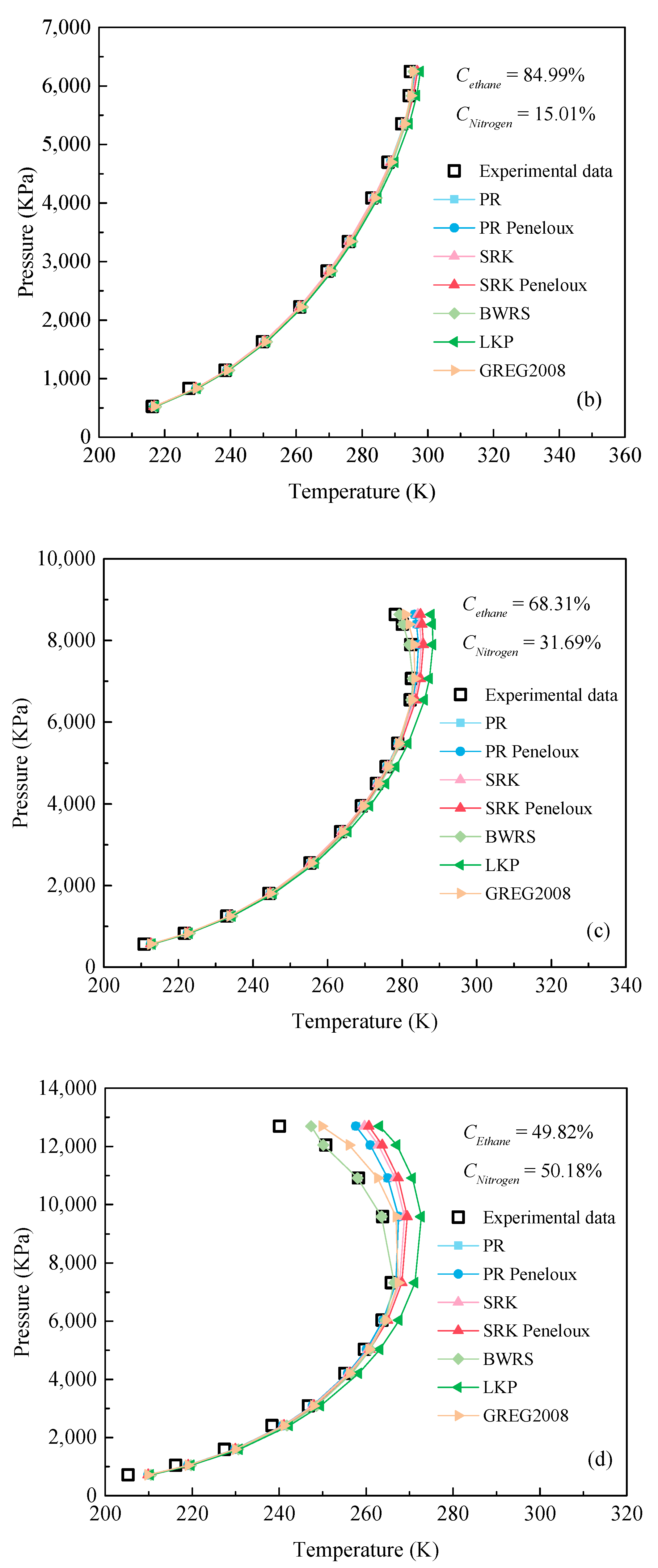
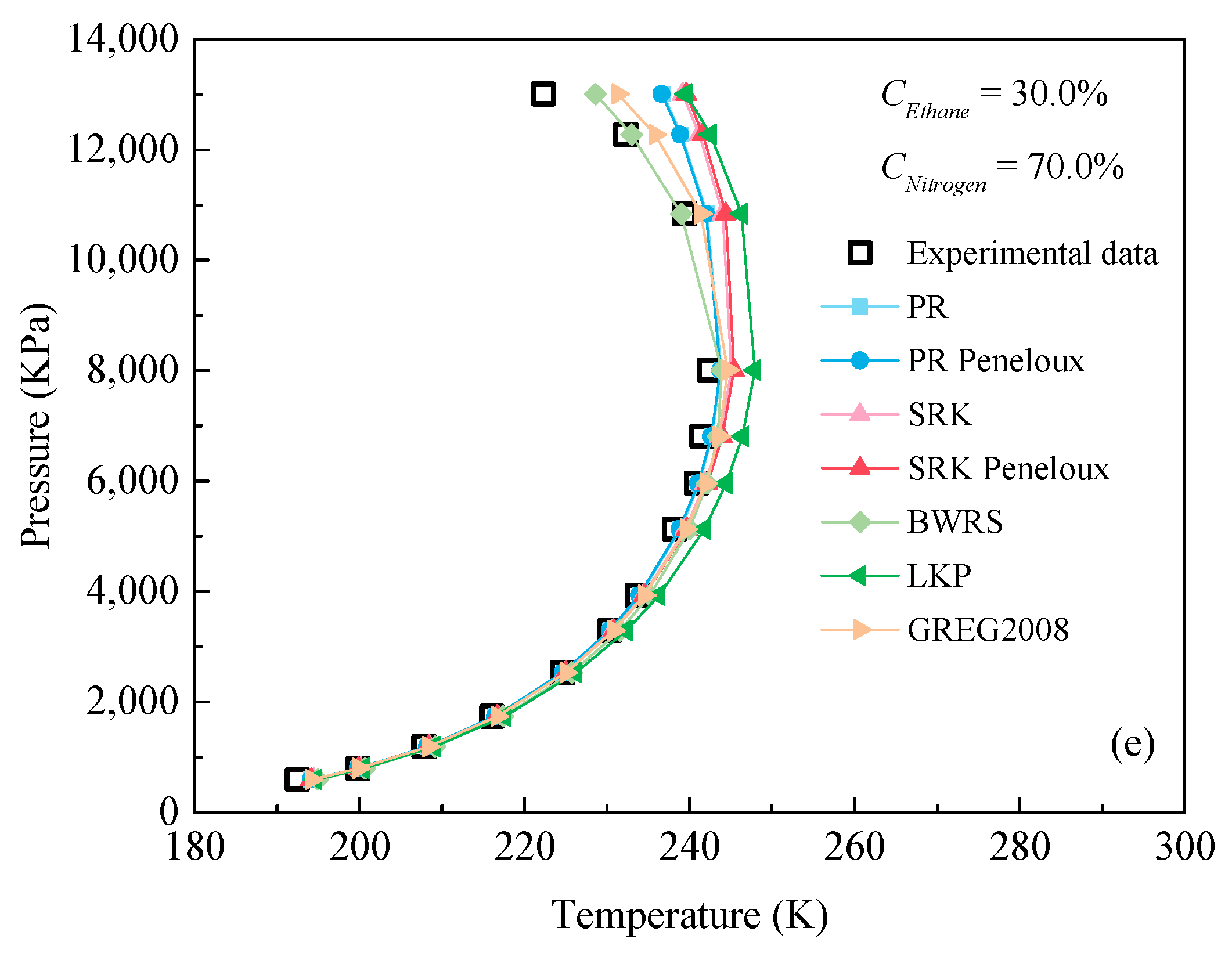
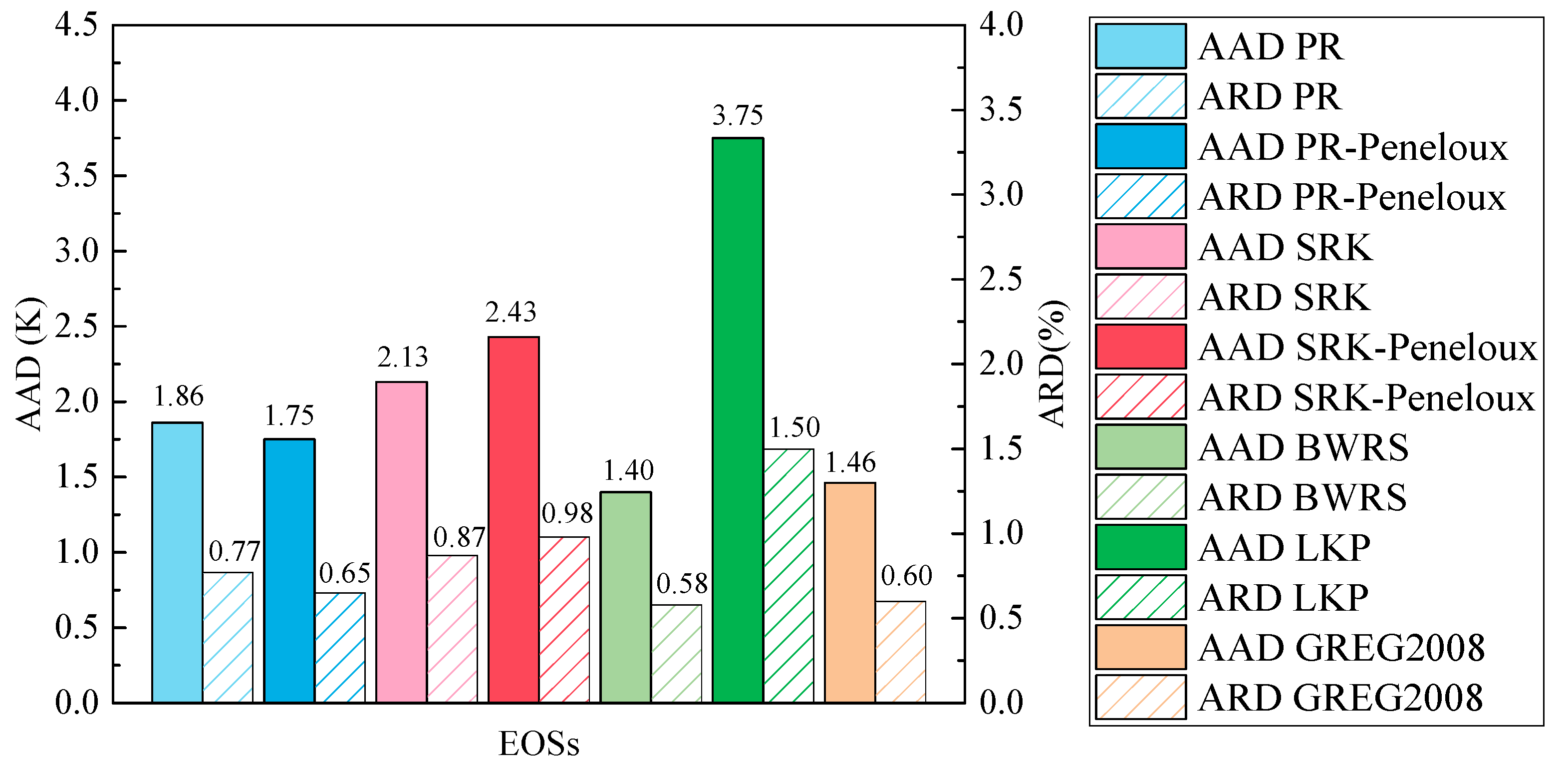
3.2. Evaluation of EOSs for Predicting Dew Point
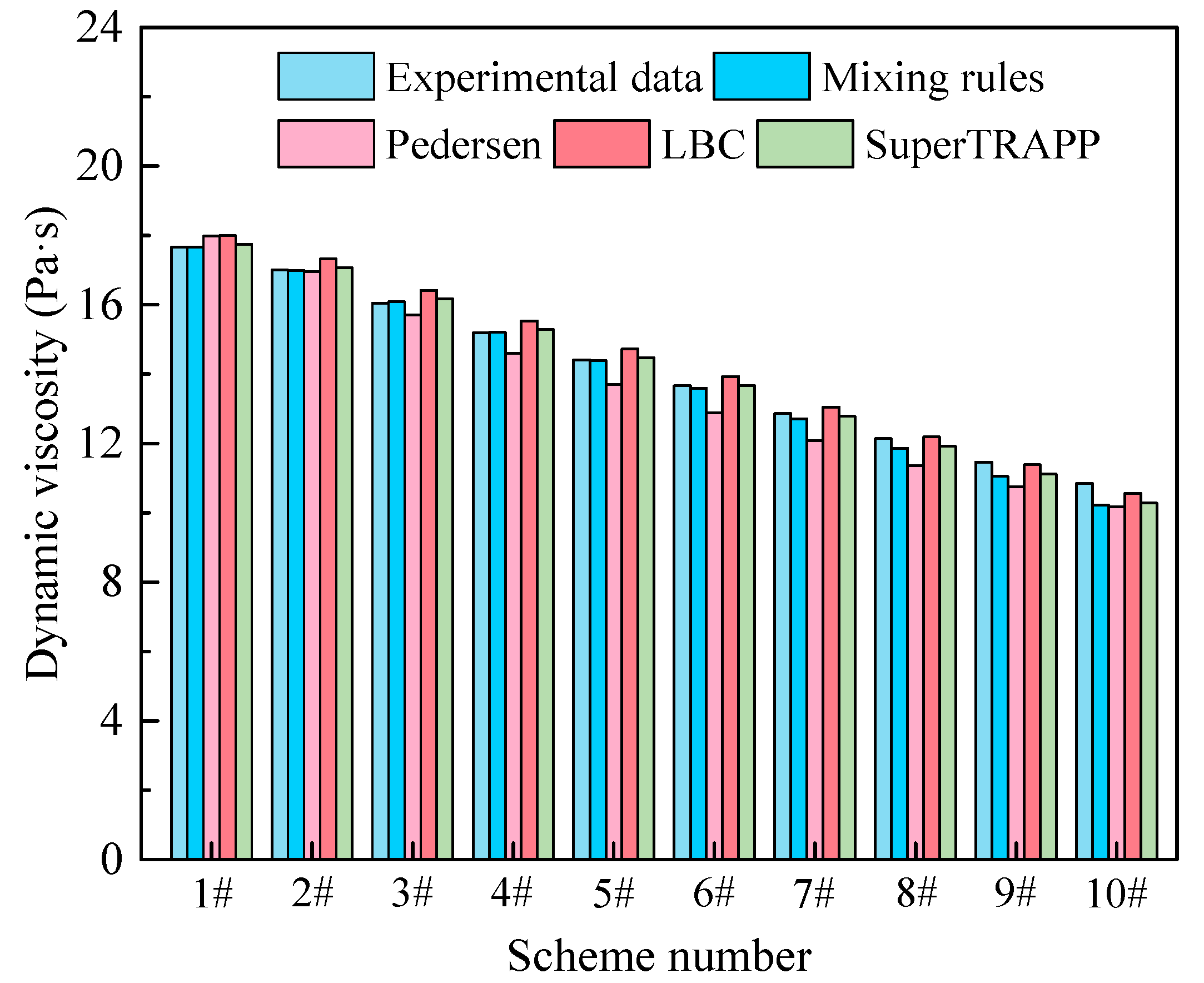
3.3. Evaluation of EOSs for Predicting Dynamic Viscosity
3.4. Phase Characteristic of C2H6 or N2 Component
3.5. Phase Characteristic of C2H6-N2 Mixed Component
4. Conclusions
- (1)
- PR-Peneloux EOS, SRK EOS, BWRS EOS, SRK-Peneloux EOS, GREG-2008 EOS, LKP EOS and PR EOS can efficiently predict the density of CH4-C2H6-N2 mixture with the ARD values within 20%. The prediction accuracy is in the order of PR-Peneloux EOS > SRK EOS > BWRS EOS > SRK-Peneloux EOS > GREG-2008 EOS > LKP EOS > PR EOS.
- (2)
- All EOSs can accurately predict the dew point of the C2H6-N2 mixture when the concentration of ethane is greater than 84%. The BWRS EOS has the best performance with an average ARD value of 0.58%, regardless of the mixed ratio of C2H6 and N2.
- (3)
- Mixing rules, the Pedersen formula, LBC formula and SuperTRAPP formula can predict dynamic viscosity with good accuracy under the conditions of atmospheric pressure and temperature of 300 K. SuperTRAPP formula has the smallest calculation error, with AAD and ARD values of 0.16 and 1.33%.
- (4)
- The critical temperature and pressure of commercial ethane in the oilfield of China are 32.79 °C and 4.97 MPa, respectively. It is difficult to avoid gas-liquid two-phase flow during the production process of long-distance liquefied ethane pipelines, so further research is needed for this special working condition to ensure the smooth production and operation of the pipeline.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclatures
| bivariate interaction coefficient | |
| molar fraction of component i | |
| critical temperature of natural gas, K | |
| volume translation coefficient | |
| pressure ratio | |
| molar volume ratio | |
| temperature ratio | |
| molar volume, m3/mol | |
| gas constant, 8.314 J/(mol·k) | |
| Z | compression factor |
| gas constant, 8.314 J/(mol·k) | |
| molar fraction of each component in the mixture, % | |
| calculated value | |
| experimental data | |
| Greek Symbols | |
| density variable | |
| temperature variable | |
| Abbreviation | |
| AAD | average absolute deviation |
| ARD | average relative deviation |
| C2H6 | ethane |
| EOS | equation of state |
| N2 | Nitrogen |
References
- Ma, Y.S.; Cai, X.Y.; Zhao, P.R. China’s shale gas exploration and development: Understanding and practice. Petrol. Explor. Dev. 2018, 45, 589–603. [Google Scholar] [CrossRef]
- Van Goethem, M.W.M.; Barendregt, S.; Grievink, J.; Verheijen, P.J.T.; Dente, M.; Ranzi, E. A kinetic modelling study of ethane cracking for optimal ethylene yield. Chem. Eng. Res. Des. 2013, 91, 1106–1110. [Google Scholar] [CrossRef]
- Zandhaghighi, S.; Iranshahi, D.; Shakeri, M.; Bagherpour-Ardakani, E. Applying a new configuration for thermal integration of ethane cracking and CLC processes to enhance the ethylene and hydrogen productions. Chem. Eng. Res. Des. 2022, 186, 672–684. [Google Scholar] [CrossRef]
- Jung, W.; Lee, J.; Ha, K.S. A combined production technology for ethylene and hydrogen with an ethane cracking center and dielectric barrier discharge plasma reactor. Chem. Eng. J. 2023, 462, 142155. [Google Scholar] [CrossRef]
- Teh, C.J.; Barifcani, A.; Pack, D.; Tade, M.O. The importance of ground temperature to a liquid carbon dioxide pipeline. Int. J. Greenh. Gas. Con. 2015, 39, 463–469. [Google Scholar] [CrossRef]
- Lu, H.F.; Ma, X.; Huang, K.; Fu, L.D.; Azimi, M. Carbon dioxide transport via pipelines: A systematic review. J. Clean. Prod. 2020, 266, 121994. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Atiku, F.A.; Pirouzfar, V.; Su, C.H. Technical, economic and thermodynamic analysis for loading, storing, unloading and transporting of Ethane fluid. J. Taiwan Inst. Chem. E. 2021, 120, 218–228. [Google Scholar] [CrossRef]
- Raed, Z.; Carlos, A. Transport of ethane-rich gases using an extensive gas pipeline system. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 26–29 September 2004. [Google Scholar]
- Mcguire, P.L.; Okuno, R.; Gould, T.L. Ethane-based EOR: An innovative and profitable EOR opportunity for a low-price environment. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OH, USA, 11–13 April 2016. [Google Scholar]
- Sicotte, D.M. From cheap ethane to a plastic planet: Regulating an industrial global production network. Energy Res. Social Sci. 2020, 66, 101479. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.S.; Hao, Z.R.; Wang, Y.; Ren, W.L. Prediction of the instability for simply supported pipes conveying gas-liquid two-phase slug flow. Ocean Eng. 2022, 244, 110388. [Google Scholar] [CrossRef]
- Liu, H.S.; Duan, J.M.; Gu, K.C.; Li, J.; Yan, H.; Wang, J. Slug flow hydrodynamics modelling for gas-liquid two-phase flow in a pipe. Energies 2022, 15, 533. [Google Scholar] [CrossRef]
- Jia, W.L.; Zhang, Y.R.; Li, C.J.; Wu, X.; Song, S.S.; Yang, F. Optimal diameter of liquid-phase ethane transportation pipeline considering the liquid-vapor phase change. J. Nat. Gas. Sci. Eng. 2022, 107, 104797. [Google Scholar] [CrossRef]
- Lozano-Martín, D.; Khanipour, P.; Kipphardt, H.; Tuma, D.; Chamorro, C.R. Thermodynamic characterization of the (H2 + C3H8) system significant for the hydrogen economy: Experimental (p, ρ, T) determination and equation of state modelling. Int. J. Hydrog. Energy 2023, 48, 8645–8667. [Google Scholar] [CrossRef]
- Vitali, M.; Leporini, M.; Masi, O.; Speranza, A.; Corvaro, F.; Marchetti, B. Net zero Flow Assurance—Validation of various equations of state for the prediction of VLE and density of CO2-rich mixtures for CCUS applications. Int. J. Greenh. Gas Control 2023, 125, 103877. [Google Scholar] [CrossRef]
- Seo, J.; Kim, J.S.; Kim, K.H. Improved prediction of the thermodynamic properties of JP-10 using an extended Redlich-Kwong-Peng-Robinson equation of state. J. Ind. Eng. Chem. 2023, 123, 88–103. [Google Scholar] [CrossRef]
- Redlich, O.; Kwong, J.N.S. On the thermodynamics of solutions (V): An equation of state. Fugacities of gaseous solutions. Chem. Rev. 1949, 44, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Soave, G. Equilibrium constants for modified Redlich-Kwongequation of state. Chem. Eng. Sci. 1972, 27, 1197–1203. [Google Scholar] [CrossRef]
- Peng, D.Y.; Robinson, D.B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Péneloux, A.; Rauzy, E.; Fréze, R. A consistent correction for Redlich-Kwong-Soave volumes. Fluid Phase Equilibr. 1982, 8, 7–23. [Google Scholar] [CrossRef]
- Starling, K.E. A new approach for determining equation-of-state parameters using phase equilibria data. SPE J. 1966, 6, 363–371. [Google Scholar] [CrossRef]
- Lee, B.I.; Kesler, M.G. A generalized thermodynamic correlation based on three-parameter corresponding states. AIChE J. 1975, 21, 510–527. [Google Scholar] [CrossRef]
- Kunz, O.; Wagner. The GERG-2008 wide-range equation of state for natural gases and other mixtures: An expansion of GERG-2004. J. Chem. Eng. Data 2012, 57, 3032–3091. [Google Scholar] [CrossRef]
- Patel, N.C.; Abovsky, V.; Watanasiri, S. Mixing rules for van-der-Waals type equation of state based on thermodynamic perturbation theory. Fluid Phase Equilibr. 1998, 152, 219–233. [Google Scholar] [CrossRef]
- Funke, M.; Kleinrahm, R.; Wagner, W. Measurement and correlation of the (p, ρ, T) relation of ethane II. Saturated-liquid and saturated-vapour densities and vapour pressures along the entire coexistence curve. J. Chem. Thermodyn. 2002, 34, 2017–2039. [Google Scholar] [CrossRef]
- Bier, K.; Kunze, J.; Maurer, G.; Sand, H. Experimental results for heat capacity and Joule-Thomson coefficient of ethane at zero pressure. J. Chem. Eng. Data 1976, 21, 5–7. [Google Scholar] [CrossRef]
- Syed, T.H.; Hughes, T.J.; Marsh, K.N.; May, E.F. Isobaric heat capacity measurements of liquid methane, ethane, and propane by differential scanning calorimetry at high pressures and low temperatures. J. Chem. Eng. Data 2012, 57, 3573–3580. [Google Scholar] [CrossRef]
- Jossi, J.A.; Stiel, L.I.; Thodos, G. The viscosity of pure substances in the dense gaseous and liquid phases. AIChE J. 1962, 8, 59–63. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Fredenslund, A. An improved corresponding states model for the prediction of oil and gas viscosities and thermal conductivities. Chem. Eng. Sci. 1987, 42, 182–186. [Google Scholar] [CrossRef]
- Carr, N.L.; Kobayashi, R.; Burrows, D. Viscosity of hydrocarbon cases under pressure. Part I: Correlation of the effect of pressure, temperature, and composition on the viscosity of hydrocarbons and their mixtures. J. Pet. Technol. 1954, 10, 47–55. [Google Scholar] [CrossRef]







| Equation of State | Functional Form | Pure Component | |
|---|---|---|---|
| a | b | ||
| RK [17] | |||
| SRK [18] | |||
| PR [19] | |||
| SRK-Peneloux [20] | |||
| PR-Peneloux [20] | |||
| Equation of State | Functional Form | Mixed Component | |
|---|---|---|---|
| a | b | ||
| RK [17] | |||
| SRK [18] | |||
| PR [19] | |||
| SRK-Peneloux [20] | |||
| PR-Peneloux [20] | |||
| Working Conditions | Temperature (°C) | Pressure (MPa) | Molar Concentration of CH4 (%) | Molar Concentration of C2H6 (%) | Molar Concentration of N2 (%) | Density (kg/m3) |
|---|---|---|---|---|---|---|
| 1# | −181.15 | 0.1383 | - | 96.70 | 3.30 | 650.50 |
| 2# | −181.15 | 1.0394 | - | 95.20 | 4.80 | 651.30 |
| 3# | −181.15 | 0.1591 | 88.40 | - | 11.60 | 451.00 |
| 4# | −181.05 | 0.2737 | 29.10 | 54.50 | 16.40 | 586.40 |
| 5# | −181.05 | 0.276 | 82.60 | 9.30 | 8.10 | 491.10 |
| 6# | −181.05 | 0.2785 | 87.90 | 5.40 | 6.70 | 471.20 |
| 7# | −181.05 | 1.0988 | 60.10 | 29.30 | 10.60 | 527.20 |
| 8# | −181.05 | 1.1005 | 75.20 | 6.20 | 18.60 | 478.30 |
| 9# | −179.15 | 0.274 | 40.50 | 50.60 | 8.90 | 569.70 |
| 10# | −179.15 | 0.2764 | 68.00 | 19.30 | 12.70 | 455.10 |
| 11# | −179.15 | 0.543 | 20.60 | 61.80 | 17.60 | 614.60 |
| 12# | −179.15 | 0.5685 | 72.40 | 10.60 | 17.00 | 475.30 |
| 13# | −179.15 | 0.5687 | 62.00 | 24.40 | 13.60 | 517.30 |
| 14# | −179.15 | 0.8281 | 75.60 | 8.20 | 16.20 | 474.70 |
| 15# | −179.15 | 1.0976 | 41.10 | 15.90 | 43.00 | 606.80 |
| 16# | −177.15 | 0.106 | 17.70 | 80.30 | 2.00 | 607.50 |
| 17# | −177.15 | 0.164 | 48.00 | 45.80 | 6.20 | 565.30 |
| 18# | −177.15 | 0.2763 | 79.40 | 18.20 | 2.40 | 471.00 |
| 19# | −177.15 | 1.1081 | 56.90 | 20.60 | 22.50 | 521.50 |
| 20# | −177.15 | 1.1088 | 68.60 | 6.70 | 24.70 | 472.50 |
| No. | 1# | 2# | 3# | 4# | 5# | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| K | KPa | K | KPa | K | KPa | K | KPa | K | KPa | |
| 1 | 228.62 | 724.96 | 216.24 | 522.80 | 210.83 | 563.73 | 205.25 | 713.51 | 192.52 | 598.32 |
| 2 | 255.31 | 1631.37 | 227.40 | 833.31 | 221.65 | 830.49 | 216.24 | 1045.72 | 199.84 | 798.01 |
| 3 | 266.30 | 2203.25 | 238.39 | 1143.73 | 232.98 | 1250.02 | 227.40 | 1595.90 | 207.87 | 1194.13 |
| 4 | 278.51 | 3015.41 | 249.73 | 1628.64 | 244.32 | 1800.29 | 238.39 | 2407.46 | 216.06 | 1742.86 |
| 5 | 287.75 | 3738.96 | 261.07 | 2222.48 | 255.31 | 2546.48 | 246.76 | 3086.99 | 224.61 | 2531.43 |
| 6 | 294.21 | 4308.61 | 269.44 | 2836.66 | 263.51 | 3313.09 | 255.14 | 4202.31 | 230.37 | 3296.84 |
| 7 | 298.74 | 4877.33 | 275.89 | 3340.95 | 269.09 | 3947.68 | 259.67 | 5032.49 | 233.68 | 3930.33 |
| 8 | 300.84 | 5183.39 | 283.05 | 4085.26 | 273.10 | 4494.36 | 263.68 | 6036.71 | 238.22 | 5130.91 |
| 9 | 301.71 | 5400.01 | 287.93 | 4697.72 | 275.72 | 4909.62 | 265.78 | 7323.28 | 240.83 | 5960.14 |
| 10 | - | - | 292.12 | 5353.43 | 278.86 | 5477.66 | 263.86 | 9588.27 | 241.53 | 6810.23 |
| 11 | - | - | 294.38 | 5833.88 | 282.17 | 6546.91 | 258.28 | 10,914.64 | 242.40 | 8009.01 |
| 12 | - | - | 294.73 | 6248.03 | 282.52 | 7069.99 | 250.78 | 12,043.93 | 239.44 | 10,840.03 |
| 13 | - | - | - | - | 282.35 | 7897.85 | 240.14 | 12,692.36 | 232.29 | 12,274.54 |
| 14 | - | - | - | - | 280.08 | 8397.88 | - | - | 222.34 | 13,010.48 |
| 15 | - | - | - | - | 278.16 | 8636.62 | - | - | - | - |
| No. | Molar Concentration of C2H6 (%) | Molar Concentration of N2 (%) | Dynamic Viscosity (Pa·s) |
|---|---|---|---|
| 1# | 1.62 | 98.38 | 17.67 |
| 2# | 9.33 | 90.67 | 17.00 |
| 3# | 19.88 | 80.12 | 16.05 |
| 4# | 30.22 | 69.78 | 15.19 |
| 5# | 39.76 | 60.24 | 14.41 |
| 6# | 49.29 | 50.71 | 13.66 |
| 7# | 59.84 | 40.16 | 12.86 |
| 8# | 70.18 | 29.82 | 12.14 |
| 9# | 79.92 | 20.08 | 11.46 |
| 10# | 90.06 | 9.94 | 10.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, J.; Li, C.; Yang, W.; Zhang, W.; Yuan, X.; Xu, Y. Prediction of Physical Properties and Phase Characteristics of Ethane and Ethane Mixture in the Ethane Pipeline. Processes 2023, 11, 2283. https://doi.org/10.3390/pr11082283
Ban J, Li C, Yang W, Zhang W, Yuan X, Xu Y. Prediction of Physical Properties and Phase Characteristics of Ethane and Ethane Mixture in the Ethane Pipeline. Processes. 2023; 11(8):2283. https://doi.org/10.3390/pr11082283
Chicago/Turabian StyleBan, Jiuqing, Changjun Li, Wei Yang, Wei Zhang, Xiaoyun Yuan, and Yingying Xu. 2023. "Prediction of Physical Properties and Phase Characteristics of Ethane and Ethane Mixture in the Ethane Pipeline" Processes 11, no. 8: 2283. https://doi.org/10.3390/pr11082283




