Review of Thermal Runaway Monitoring, Warning and Protection Technologies for Lithium-Ion Batteries
Abstract
1. Introduction
2. Lithium-Ion Battery Thermal Runaway Monitoring Technology
2.1. Monitoring Techniques Based on External Battery Parameters
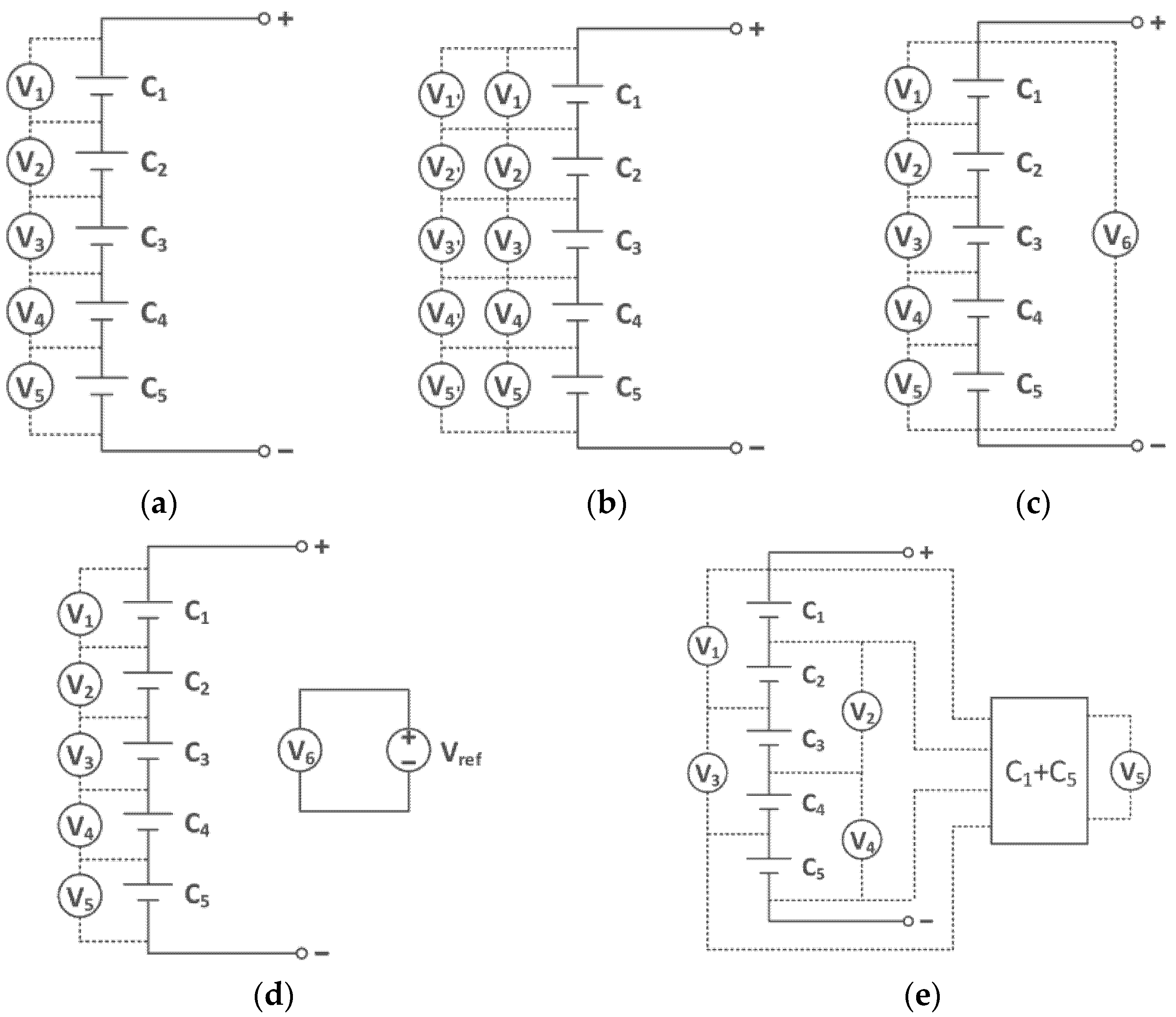
2.1.1. Voltage
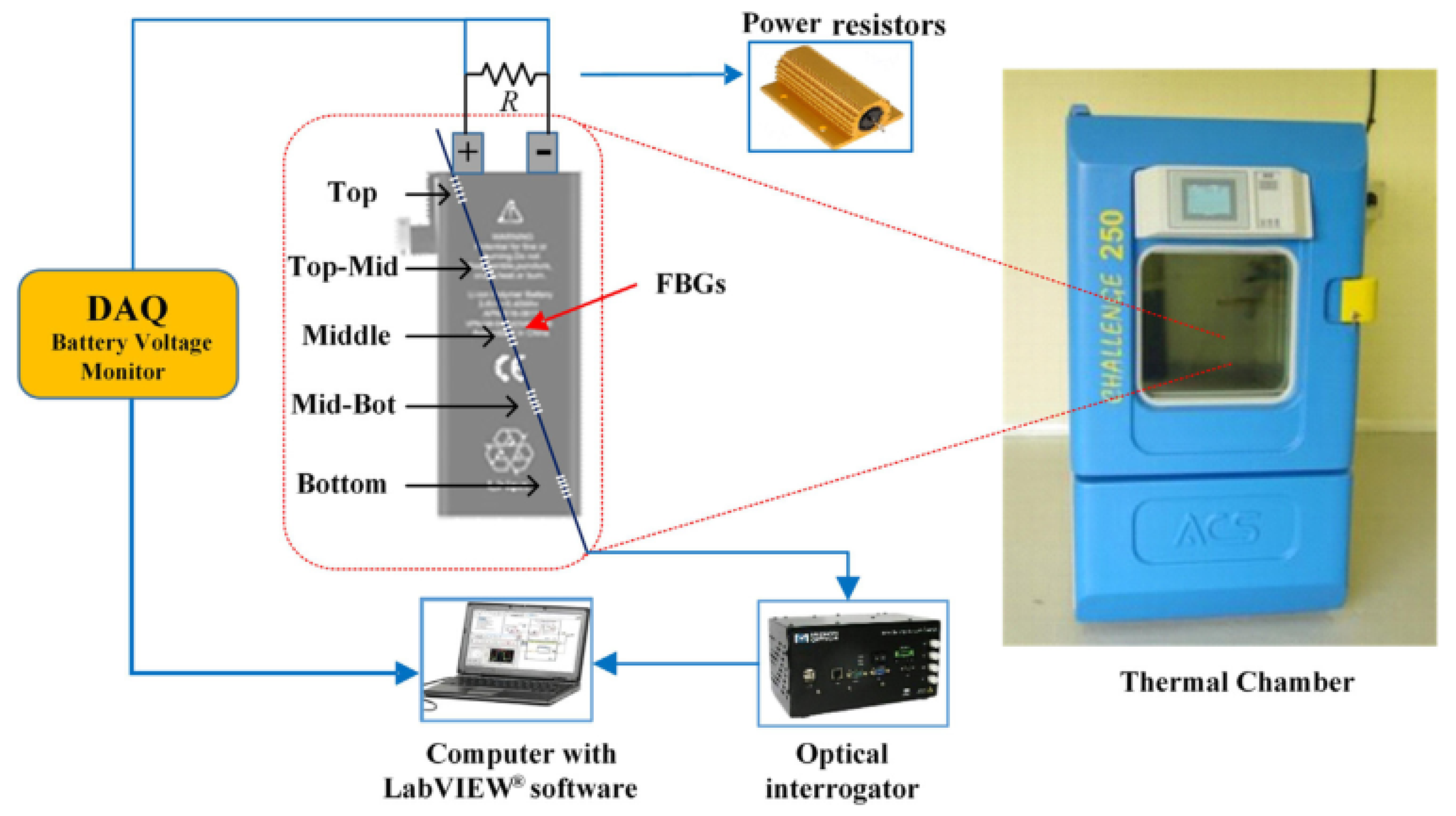
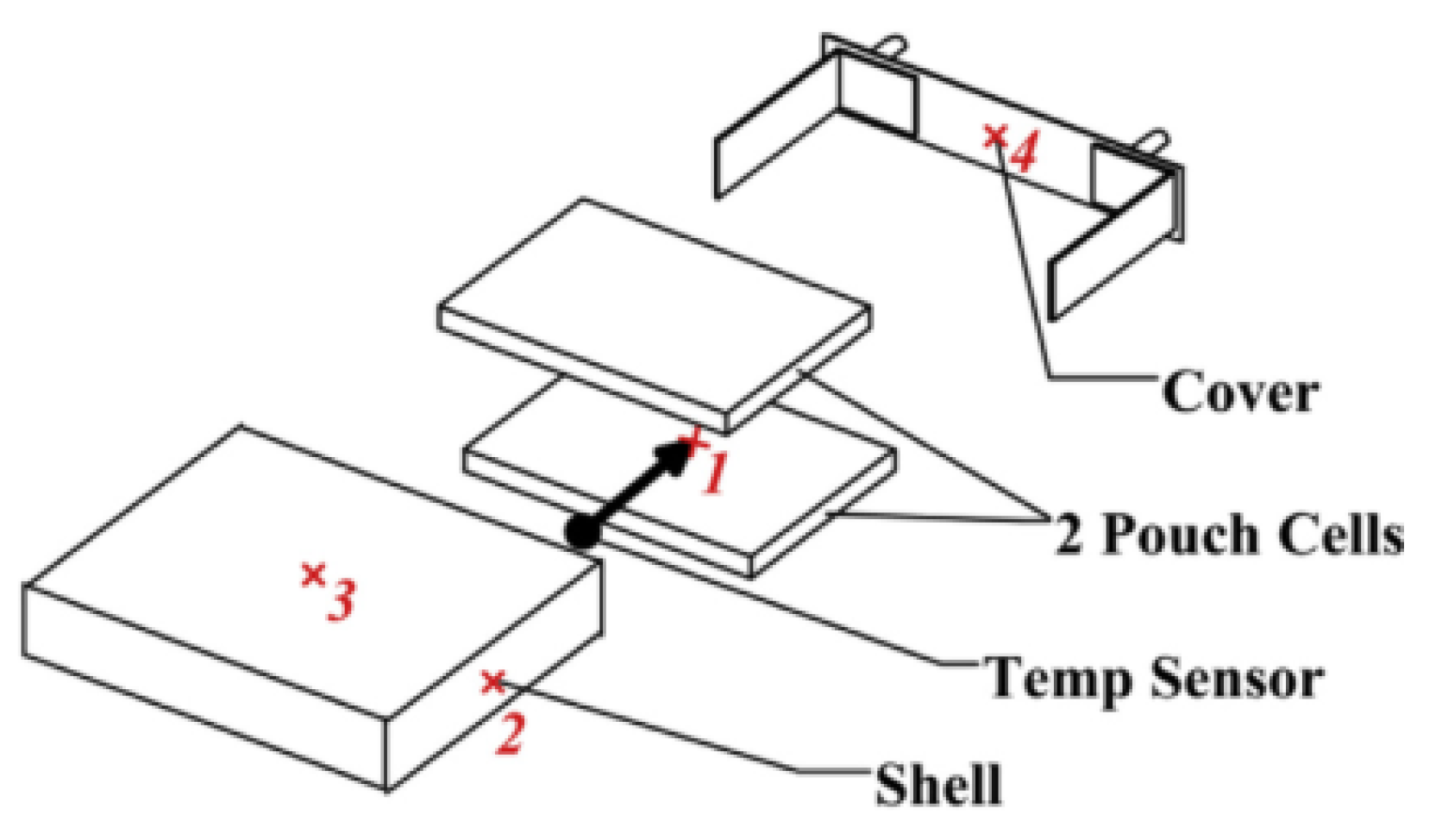
2.1.2. Temperature
2.2. Monitoring Technology Based on Internal Battery Parameters
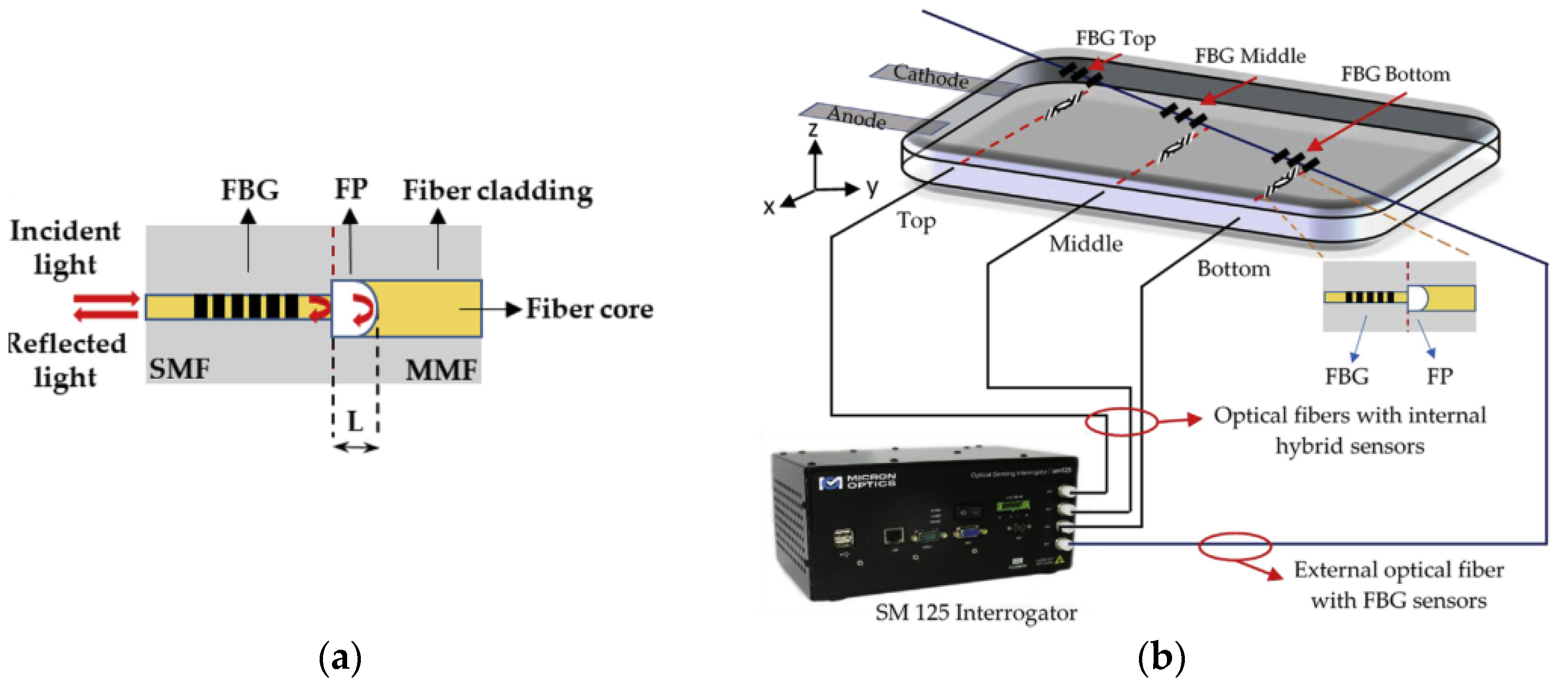
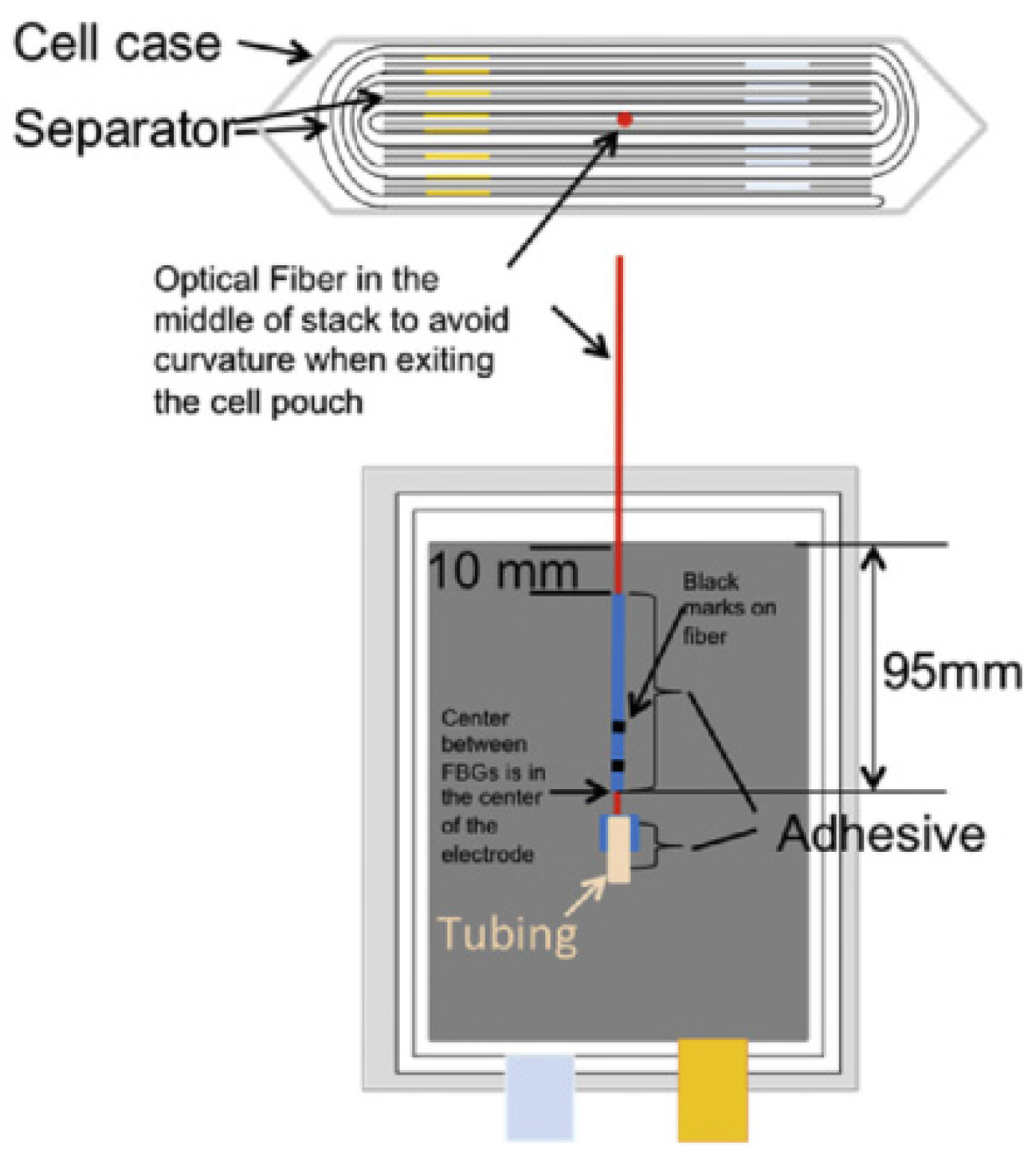
2.2.1. Embedded Fiber Optic Sensor
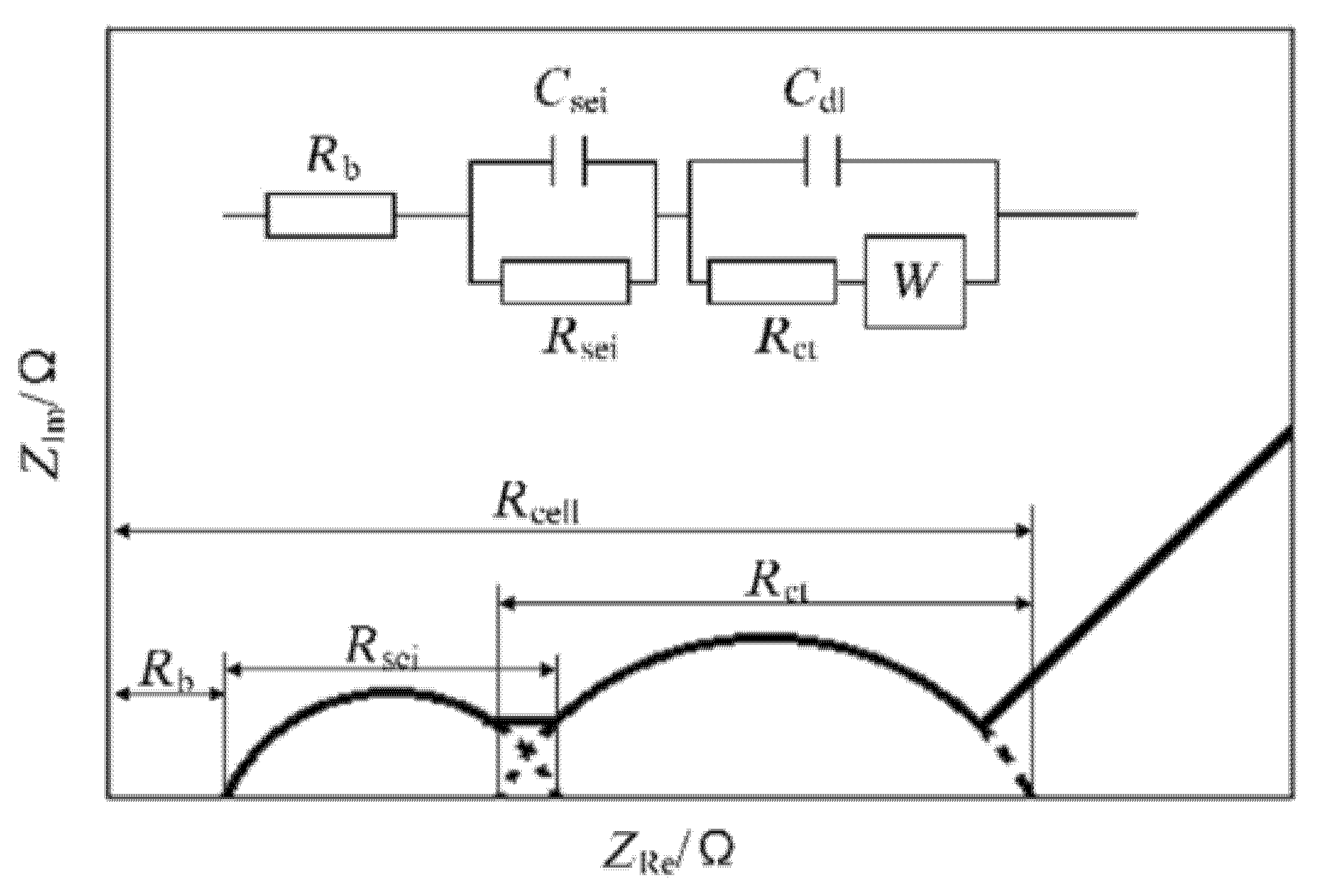
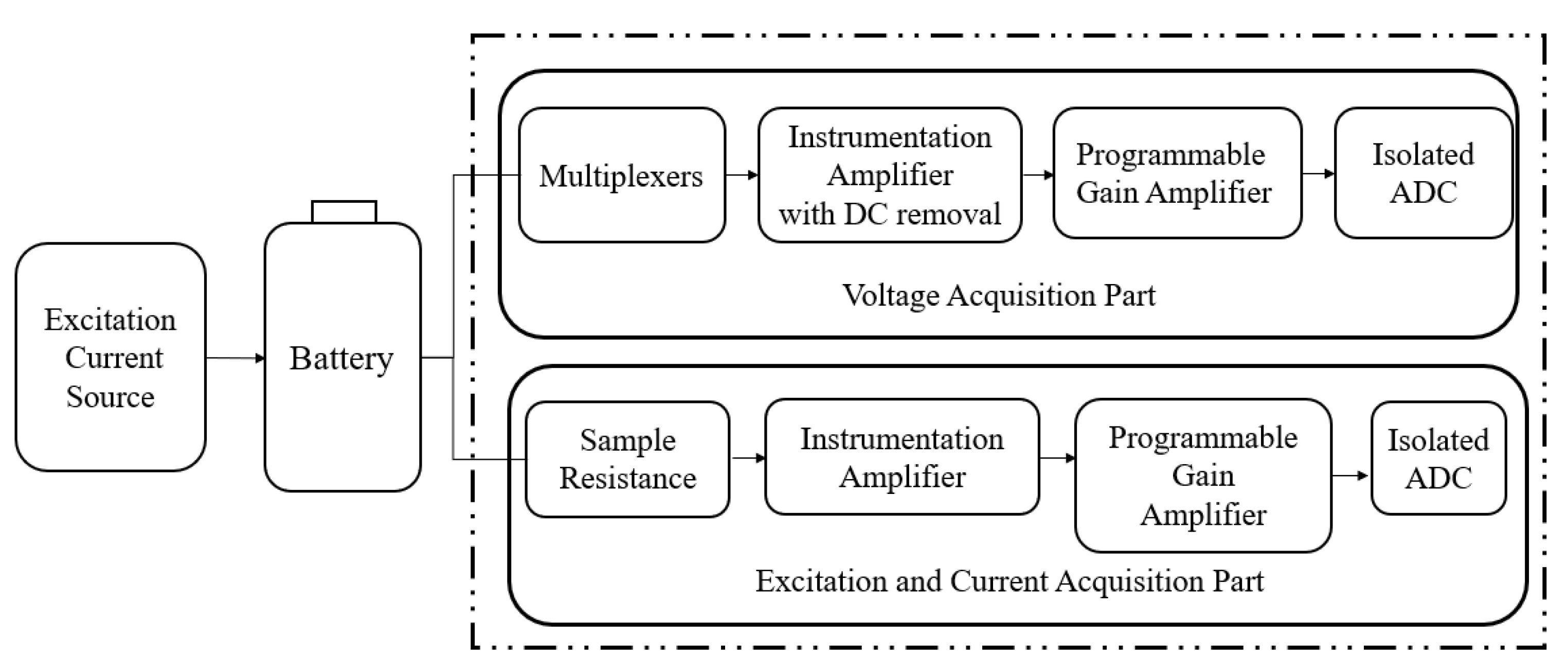
2.2.2. Electrochemical Impedance Spectroscopy (EIS)
2.3. Summary
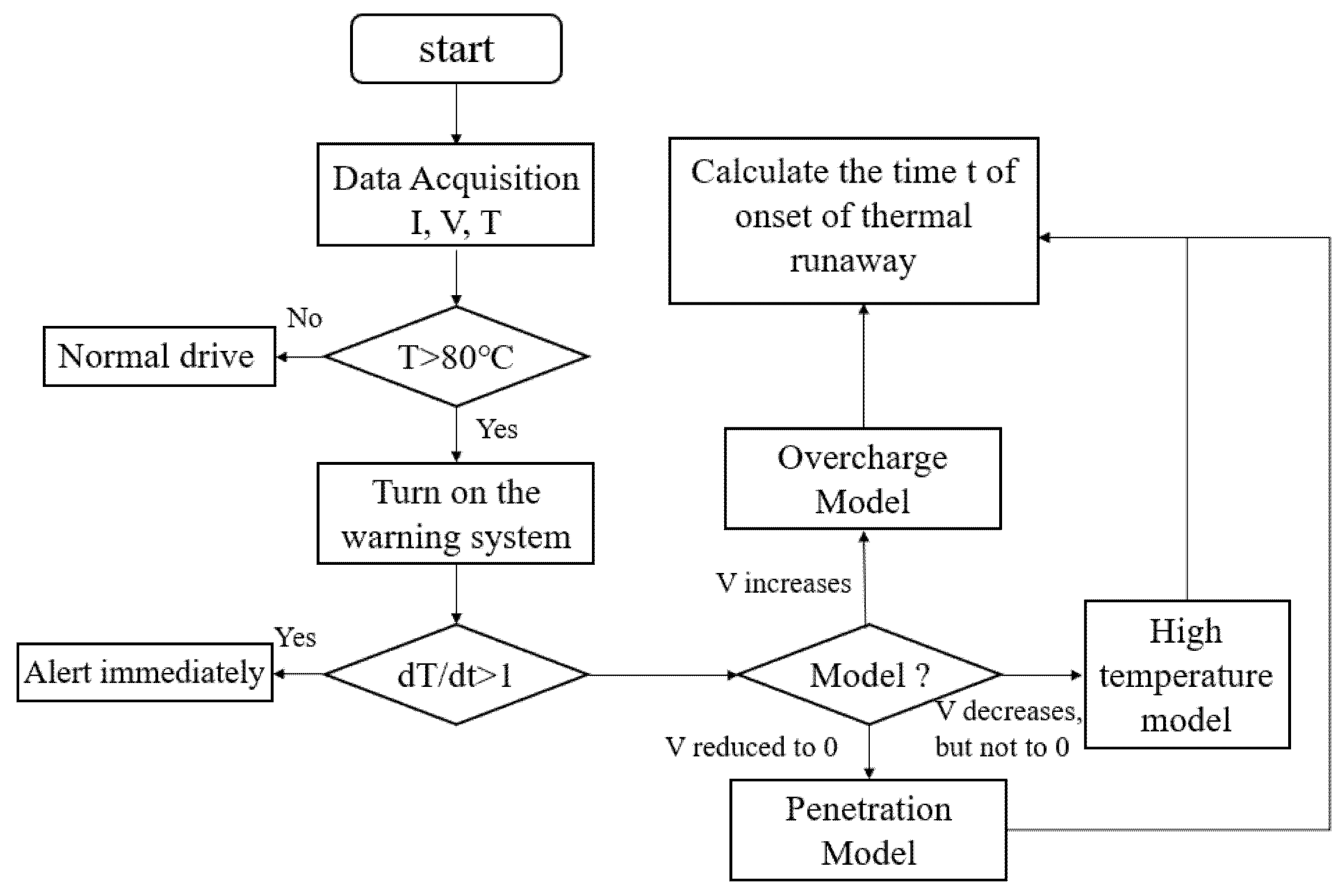
3. Lithium-Ion Battery Thermal Runaway Warning Technology
3.1. Thermal Runaway Warning Technology Based on Lithium-Ion Battery Voltage
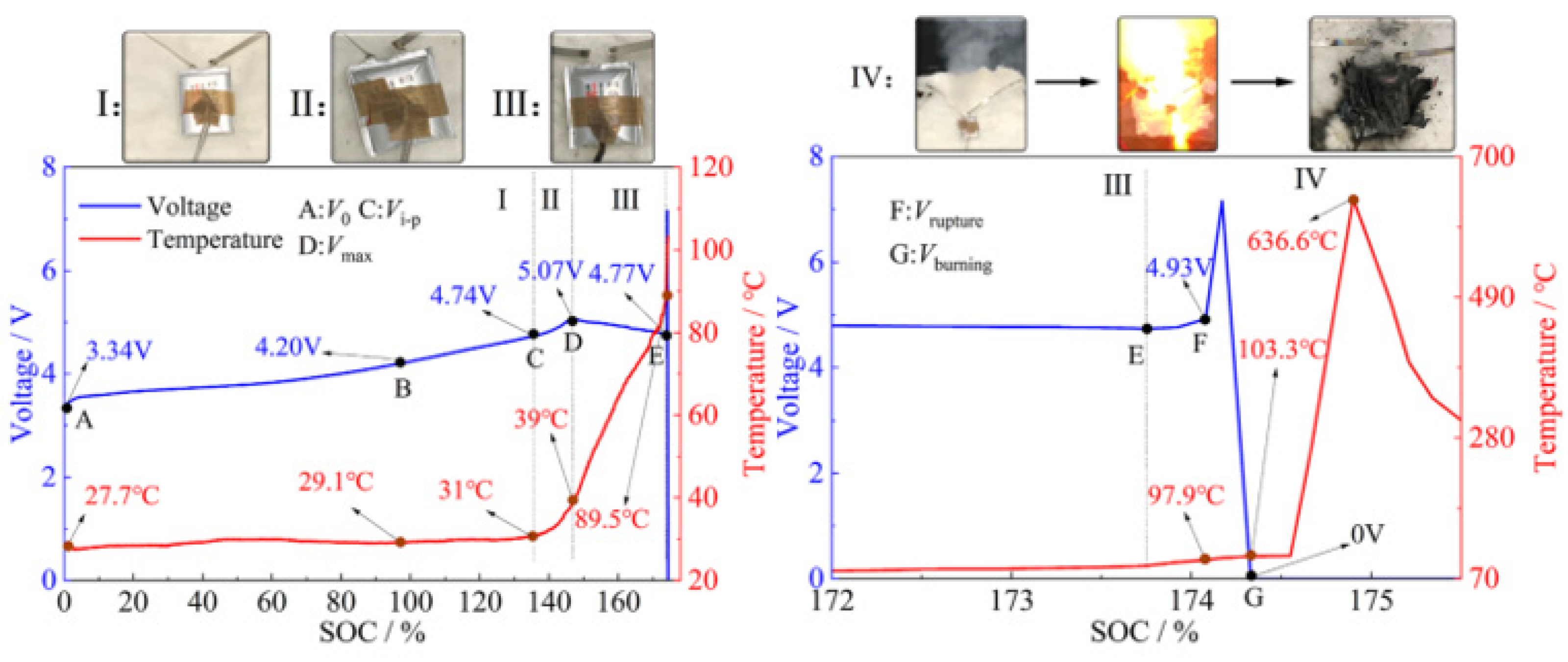
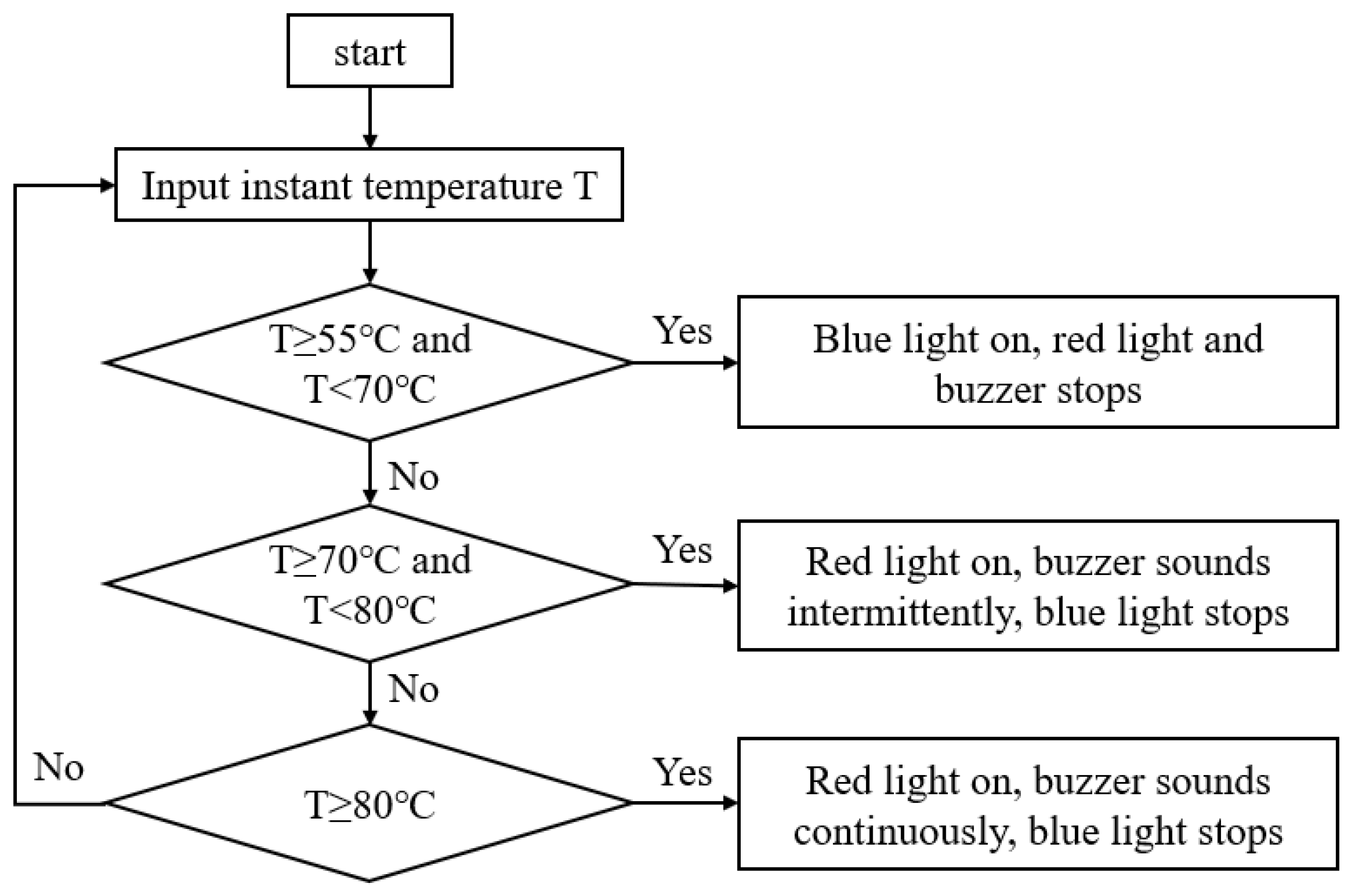
3.2. Thermal Runaway Warning Technology Based on Lithium-Ion Battery Temperature
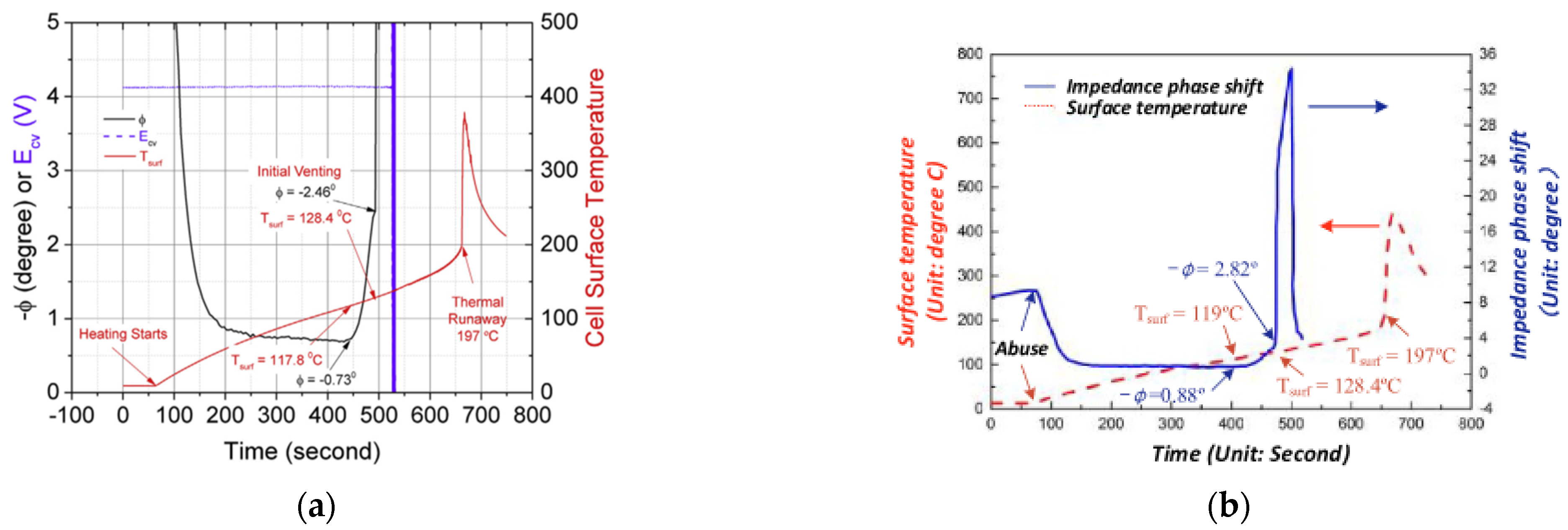
3.3. Thermal Runaway Warning Technology Based on EIS
3.4. Summary
4. Lithium-Ion Battery Thermal Runaway Protection Technology
4.1. Lithium-Ion Battery Thermal Runaway Internal Protection Technology
4.1.1. Electrolyte
- Improve the thermal stability of lithium salts
- 2.
- An additive for flame retardancy
- 3.
- Non-flammable electrolyte
- 4.
- Solid electrolyte
4.1.2. Separator
- High performance of polyolefin separator
- 2.
- Surface modification of polyolefin separator
4.1.3. Electrodes
4.2. Lithium-Ion Battery Thermal Runaway External Protection Technology
4.2.1. Battery Management Technology
4.2.2. Cooling Technology
4.2.3. Blocking Technology
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Yamanaka, T.; Takagishi, Y.; Tozuka, Y.; Yamaue, T. Modeling lithium ion battery nail penetration tests and quantitative evaluation of the degree of combustion risk. J. Power Sources 2019, 416, 132–140. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, M.; Liu, J.; Wei, R.; Weng, J.; Wang, J. Investigation of a commercial lithium-ion battery under overcharge/over-discharge failure conditions. RSC Adv. 2018, 8, 33414–33424. [Google Scholar] [CrossRef]
- Henriksen, M.; Vågsæther, K.; Lundberg, J.; Forseth, S.; Bjerketvedt, D. Explosion characteristics for Li-ion battery electrolytes at elevated temperatures. J. Hazard. Mater. 2019, 371, 1–7. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.; Zhou, T.; Yang, K.; Wei, S.; Tang, N.; Dang, N.; Li, H.; Qiu, X.; Chen, L. Toxicity, a serious concern of thermal runaway from commercial Li-ion battery. Nano Energy 2016, 27, 313–319. [Google Scholar] [CrossRef]
- ISO 12405-3; Electrically Propelled Road Vehicles–Test Specification for Lithium-Ion Traction Battery Packs and Systems–Part 3, Safety Performance Requirements. ISO: Geneva, Switzerland, 2014.
- IEC 62133.2-2017; Secondary Cells and Batteries Containing Alkaline or Other Non—Acid Electrolytes–Safety Requirements for Portable Sealed Secondary Cells, and for Batteries Made from Them, for Use in Portable Applications—Part 2, Lithium Systems. IEC: Geneva, Switzerland, 2017.
- Liu, K.; Li, K.; Peng, Q.; Zhang, C. A brief review on key technologies in the battery management system of electric vehicles. Front. Mech. Eng. 2019, 14, 47–64. [Google Scholar] [CrossRef]
- Xia, B.; Mi, C. A fault-tolerant voltage measurement method for series connected battery packs. J. Power Sources 2016, 308, 83–96. [Google Scholar] [CrossRef]
- Nascimento, M.; Ferreira, M.S.; Pinto, J.L. Real time thermal monitoring of lithium batteries with fiber sensors and thermocouples: A comparative study. Measurement 2017, 111, 260–263. [Google Scholar] [CrossRef]
- Nascimento, M.; Ferreira, M.S.; Pinto, J.L. Temperature fiber sensing of Li-ion batteries under different environmental and operating conditions. Appl. Therm. Eng. 2019, 149, 1236–1243. [Google Scholar] [CrossRef]
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Drake, S.J.; Wetz, D.A.; Ostanek, J.K.; Miller, S.P.; Heinzel, J.M.; Jain, A. Measurement of anisotropic thermophysical properties of cylindrical Li-ion cells. J. Power Sources 2014, 252, 298–304. [Google Scholar] [CrossRef]
- Srinivasan, R.; Carkhuff, B.G. Empirical analysis of contributing factors to heating in lithium-ion cells: Anode entropy versus internal resistance. J. Power Sources 2013, 241, 560–566. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Arnold, S.; Loges, A.; Werner, D.; Wetzel, T.; Ivers-Tiffée, E. Measurement of the internal cell temperature via impedance: Evaluation and application of a new method. J. Power Sources 2013, 243, 110–117. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Manka, D.; Klotz, D.; Ivers-Tiffée, E. Investigation of the thermal properties of a Li-ion pouch-cell by electrothermal impedance spectroscopy. J. Power Sources 2011, 196, 8140–8146. [Google Scholar] [CrossRef]
- Belov, D.; Yang, M.H. Investigation of the kinetic mechanism in overcharge process for Li-ion battery. Solid State Ion. 2008, 179, 1816–1821. [Google Scholar] [CrossRef]
- Du, C.; Zhang, W.S.; Liu, L.S.; He, L.M. Measuring Device for Internal Temperature of Lithium-Ion Battery and Measuring Method. Chem. Commun. 2011, 47, 12578–12591. [Google Scholar]
- Nascimento, M.; Novais, S.; Ding, M.S.; Ferreira, M.S.; Koch, S.; Passerini, S.; Pinto, J.L. Internal strain and temperature discrimination with optical fiber hybrid sensors in Li-ion batteries. J. Power Sources 2019, 410, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Zhu, J.; Gao, Y. A Critical Review of Thermal Runaway Prediction and Early-Warning Methods for Lithium-Ion Batteries. Energy Mater. Adv. 2023, 4, 0008. [Google Scholar] [CrossRef]
- Barsoukov, E.; Jang, J.H.; Lee, H. Thermal impedance spectroscopy for Li-ion batteries using heat-pulse response analysis. J. Power Sources 2002, 109, 313–320. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Chrobak, T.; Ender, M.; Illig, J.; Klotz, D.; Ivers-Tiffée, E. Studies on LiFePO4 as cathode material using impedance spectroscopy. J. Power Sources 2011, 196, 5342–5348. [Google Scholar] [CrossRef]
- Srinivasan, R.; Carkhuff, B.G.; Butler, M.H.; Baisden, A.C. Instantaneous measurement of the internal temperature in lithium-ion rechargeable cells. Electrochim. Acta 2011, 56, 6198–6204. [Google Scholar] [CrossRef]
- Suresh, P.; Shukla, A.K.; Munichandraiah, N. Temperature dependence studies of ac impedance of lithium-ion cells. J. Appl. Electrochem. 2002, 32, 267–273. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. Electrochemical impedance study on the low temperature of Li-ion batteries. Electrochim. Acta 2004, 49, 1057–1061. [Google Scholar] [CrossRef]
- Zhu, J.G.; Sun, Z.C.; Wei, X.Z.; Dai, H.F. A new lithium-ion battery internal temperature on-line estimate method based on electrochemical impedance spectroscopy measurement. J. Power Sources 2015, 274, 990–1004. [Google Scholar] [CrossRef]
- Lin, X.; Perez, H.E.; Mohan, S.; Siegel, J.B.; Stefanopoulou, A.G.; Ding, Y.; Castanier, M.P. A lumped-parameter electro-thermal model for cylindrical batteries. J. Power Sources 2014, 257, 1–11. [Google Scholar] [CrossRef]
- Raijmakers, L.H.; Danilov, D.L.; Van Lammeren, J.P.; Lammers, M.J.; Notten, P.H. Sensorless battery temperature measurements based on electrochemical impedance spectroscopy. J. Power Sources 2014, 247, 539–544. [Google Scholar] [CrossRef]
- Richardson, R.R.; Ireland, P.T.; Howey, D.A. Battery internal temperature estimation by combined impedance and surface temperature measurement. J. Power Sources 2014, 265, 254–261. [Google Scholar] [CrossRef]
- Love, C.T.; Swider-Lyons, K. Impedance diagnostic for overcharged lithium-ion batteries. Electrochem. Solid-State Lett. 2012, 15, A53. [Google Scholar] [CrossRef]
- Love, C.T.; Virji, M.B.; Rocheleau, R.E.; Swider-Lyons, K.E. State-of-health monitoring of 18650 4S packs with a single-point impedance diagnostic. J. Power Sources 2014, 266, 512–519. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Li, Z.; Ren, Y.; Xie, J.; He, H.; Xu, F. Failure study of commercial LiFePO4 cells in over-discharge conditions using electrochemical impedance spectroscopy. J. Electrochem. Soc. 2014, 161, A620. [Google Scholar] [CrossRef]
- Kassem, M.; Bernard, J.; Revel, R.; Pelissier, S.; Duclaud, F.; Delacourt, C. Calendar aging of a graphite/LiFePO4 cell. J. Power Sources 2012, 208, 296–305. [Google Scholar] [CrossRef]
- Stiaszny, B.; Ziegler, J.C.; Krauß, E.E.; Schmidt, J.P.; Ivers-Tiffée, E. Electrochemical characterization and post-mortem analysis of aged LiMn2O4–Li (Ni0.5Mn0.3Co0.2)O2/graphite lithium ion batteries. Part I: Cycle aging. J. Power Sources 2014, 251, 439–450. [Google Scholar] [CrossRef]
- Spinner, N.S.; Love, C.T.; Rose-Pehrsson, S.L.; Tuttle, S.G. Expanding the operational limits of the single-point impedance diagnostic for internal temperature monitoring of lithium-ion batteries. Electrochim. Acta 2015, 174, 488–493. [Google Scholar] [CrossRef]
- Schwarz, R.; Semmler, K.; Wenger, M.; Lorentz, V.R.; März, M. Sensorless battery cell temperature estimation circuit for enhanced safety in battery systems. In Proceedings of the IECON 2015-41st Annual Conference of the IEEE Industrial Electronics Society, Yokohama, Japan, 9–12 November 2015; pp. 001536–001541. [Google Scholar]
- Beelen, H.P.; Raijmakers, L.H.; Donkers, M.C.F.; Notten, P.H.; Bergveld, H.J. A comparison and accuracy analysis of impedance-based temperature estimation methods for Li-ion batteries. Appl. Energy 2016, 175, 128–140. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Liu, L.; Hsu, H.; Lu, L.; Wang, L.; He, X.; Ouyang, M. Investigating the relationship between internal short circuit and thermal runaway of lithium-ion batteries under thermal abuse condition. Energy Storage Mater. 2021, 34, 563–573. [Google Scholar] [CrossRef]
- Xiong, R.; Li, L.; Tian, J. Towards a smarter battery management system: A critical review on battery state of health monitoring methods. J. Power Sources 2018, 405, 18–29. [Google Scholar] [CrossRef]
- Mao, N.; Zhang, T.; Wang, Z.; Cai, Q. A systematic investigation of internal physical and chemical changes of lithium-ion batteries during overcharge. J. Power Sources 2022, 518, 230767. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, K.; Chen, X.Y.; Wang, Z.R.; Wang, S.P. The research and explosion of early warning device of type 18650 lithium ion battery fire. Fire Sci. Technol. 2018, 37, 939–942. [Google Scholar]
- Li, B.; Parekh, M.H.; Adams, R.A.; Adams, T.E.; Love, C.T.; Pol, V.G.; Tomar, V. Lithium-ion battery thermal safety by early internal detection, prediction and prevention. Sci. Rep. 2019, 9, 13255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.F.; Huang, H.; Cao, L.Y.; Hu, C. Study on the lithium battery thermal runaway characteristics under overheating and the detection mode on battery fires. Fire Sci. Technol. 2018, 37, 55–58. [Google Scholar]
- Grandjean, T.; Barai, A.; Hosseinzadeh, E.; Guo, Y.; McGordon, A.; Marco, J. Large format lithium ion pouch cell full thermal characterisation for improved electric vehicle thermal management. J. Power Sources 2017, 359, 215–225. [Google Scholar] [CrossRef]
- Parhizi, M.; Ahmed, M.B.; Jain, A. Determination of the core temperature of a Li-ion cell during thermal runaway. J. Power Sources 2017, 370, 27–35. [Google Scholar] [CrossRef]
- Raghavan, A.; Kiesel, P.; Sommer, L.W.; Schwartz, J.; Lochbaum, A.; Hegyi, A.; Schuh, A.; Arakaki, K.; Saha, B.; Ganguli, A.; et al. Embedded fiber-optic sensing for accurate internal monitoring of cell state in advanced battery management systems part 1, Cell embedding method and performance. J. Power Sources 2017, 341, 466–473. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, Z.; Wei, X.; Dai, H. Battery internal temperature estimation for LiFePO4 battery based on impedance phase shift under operating conditions. Energies 2017, 10, 60. [Google Scholar] [CrossRef]
- Srinivasan, R.; Demirev, P.A.; Carkhuff, B.G. Rapid monitoring of impedance phase shifts in lithium-ion batteries for hazard prevention. J. Power Sources 2018, 405, 30–36. [Google Scholar] [CrossRef]
- Carkhuff, B.G.; Demirev, P.A.; Srinivasan, R. Impedance-based battery management system for safety monitoring of lithium-ion batteries. IEEE Trans. Ind. Electron. 2018, 65, 6497–6504. [Google Scholar] [CrossRef]
- Raijmakers, L.H.; Danilov, D.L.; Van Lammeren, J.P.M.; Lammers, T.J.; Bergveld, H.J.; Notten, P.H. Non-zero intercept frequency: An accurate method to determine the integral temperature of li-ion batteries. IEEE Trans. Ind. Electron. 2016, 63, 3168–3178. [Google Scholar] [CrossRef]
- Richardson, R.R.; Howey, D.A. Sensorless battery internal temperature estimation using a Kalman filter with impedance measurement. IEEE Trans. Sustain. Energy 2015, 6, 1190–1199. [Google Scholar] [CrossRef]
- Lyu, N.; Jin, Y.; Xiong, R.; Miao, S.; Gao, J. Real-time overcharge warning and early thermal runaway prediction of Li-ion battery by online impedance measurement. IEEE Trans. Ind. Electron. 2021, 69, 1929–1936. [Google Scholar] [CrossRef]
- Ma, L.K.; Ma, X.L.; Chen, C.; Yang, H.W.; Zhang, J.W. Research progress of modification of polyolefin separators for power lithium ion battery. Power Technol. 2018, 42, 452–454. [Google Scholar]
- Venugopal, G.; Moore, J.; Howard, J.; Pendalwar, S. Characterization of microporous separators for lithium-ion batteries. J. Power Sources 1999, 77, 34–41. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, L.; Yu, Y.; Sun, J. Progress of enhancing the safety of lithium ion battery from the electrolyte aspect. Nano Energy 2019, 55, 93–114. [Google Scholar] [CrossRef]
- Li, F.; Gong, Y.; Jia, G.; Wang, Q.; Peng, Z.; Fan, W.; Bai, B. A novel dual-salts of LiTFSI and LiODFB in LiFePO4-based batteries for suppressing aluminum corrosion and improving cycling stability. J. Power Sources 2015, 295, 47–54. [Google Scholar] [CrossRef]
- Chen, X.; Xu, W.; Engelhard, M.H.; Zheng, J.; Zhang, Y.; Ding, F.; Qian, J.; Zhang, J.G. Mixed salts of LiTFSI and LiBOB for stable LiFePO 4-based batteries at elevated temperatures. J. Mater. Chem. A 2014, 2, 2346–2352. [Google Scholar] [CrossRef]
- Wang, J.; Yamada, Y.; Sodeyama, K.; Chiang, C.H.; Tateyama, Y.; Yamada, A. Superconcentrated electrolytes for a high-voltage lithium-ion battery. Nat. Commun. 2016, 7, 12032. [Google Scholar] [CrossRef]
- Gond, R.; van Ekeren, W.; Mogensen, R.; Naylor, A.J.; Younesi, R. Non-flammable liquid electrolytes for safe batteries. Mater. Horiz. 2021, 8, 2913–2928. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yamada, Y.; Sodeyama, K.; Watanabe, E.; Takada, K.; Tateyama, Y.; Yamada, A. Fire-extinguishing organic electrolytes for safe batteries. Nat. Energy 2018, 3, 22–29. [Google Scholar] [CrossRef]
- Liang, H.; Zuo, X.; Zhang, L.; Huang, W.; Chen, Q.; Zhu, T.; Liu, J.; Nan, J. Nonflammable LiTFSI-Ethylene Carbonate/1, 2-Dimethoxyethane Electrolyte for High-Safety Li-ion Batteries. J. Electrochem. Soc. 2020, 167, 090520. [Google Scholar] [CrossRef]
- Liu, K.; Liu, W.; Qiu, Y.; Kong, B.; Sun, Y.; Chen, Z.; Zhuo, D.; Lin, D.; Cui, Y. Electrospun core-shell microfiber separator with thermal-triggered flame-retardant properties for lithium-ion batteries. Sci. Adv. 2017, 3, e1601978. [Google Scholar] [CrossRef]
- Pham, H.Q.; Lee, H.Y.; Hwang, E.H.; Kwon, Y.G.; Song, S.W. Non-flammable organic liquid electrolyte for high-safety and high-energy density Li-ion batteries. J. Power Sources 2018, 404, 13–19. [Google Scholar] [CrossRef]
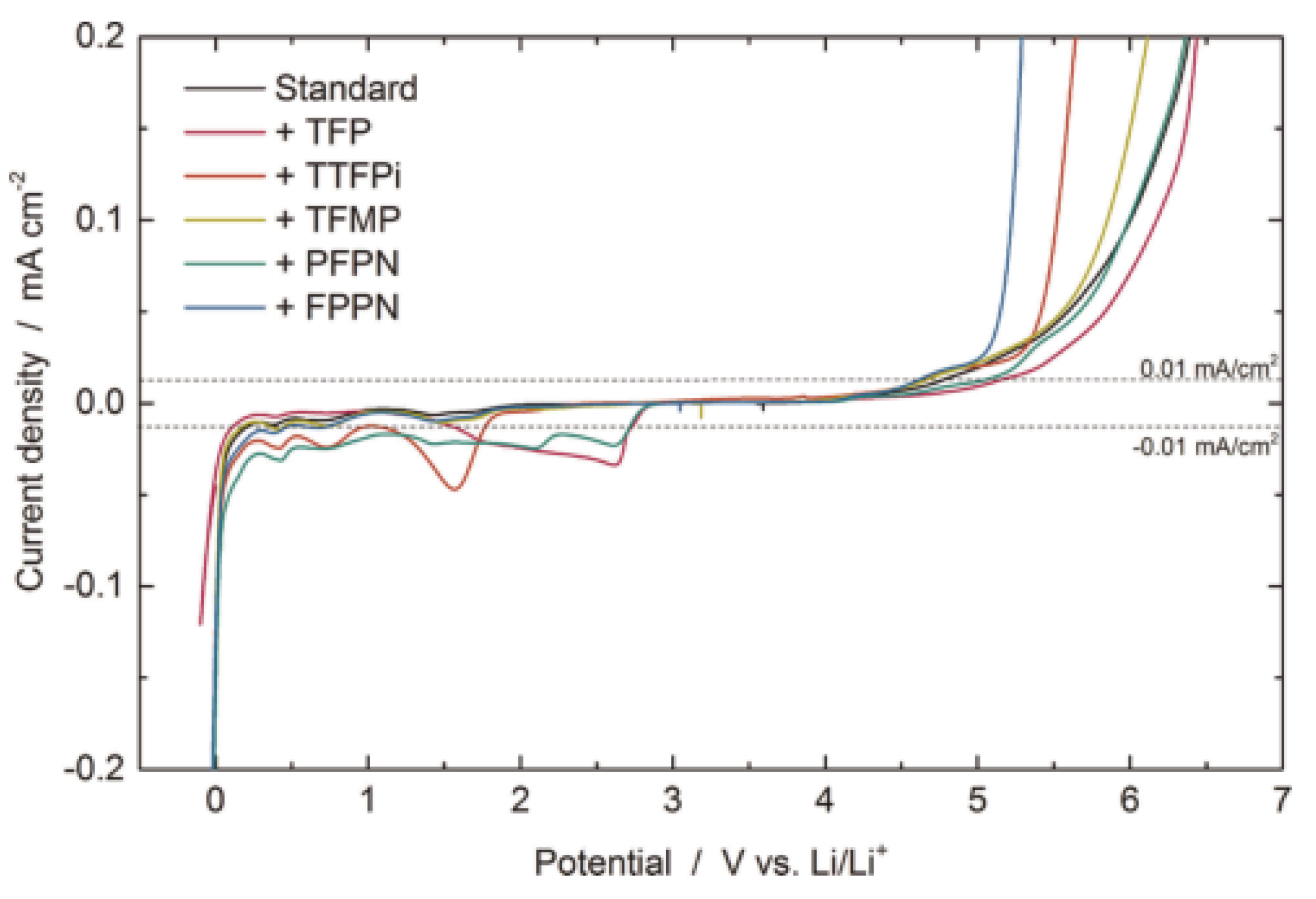
- Pires, J.; Castets, A.; Timperman, L.; Santos-Peña, J.; Dumont, E.; Levasseur, S.; Tessier, C.; Dedryvère, R.; Anouti, M. Tris (2, 2, 2-trifluoroethyl) phosphite as an electrolyte additive for high-voltage lithium-ion batteries using lithium-rich layered oxide cathode. J. Power Sources 2015, 296, 413–425. [Google Scholar] [CrossRef]
- Jin, Z.; Gao, H.; Kong, C.; Zhan, H.; Li, Z. A novel phosphate-based flame retardant and film-forming electrolyte additive for lithium ion batteries. ECS Electrochem. Lett. 2013, 2, A66. [Google Scholar] [CrossRef]
- Zeng, Z.; Murugesan, V.; Han, K.S.; Jiang, X.; Cao, Y.; Xiao, L.; Ai, X.; Yang, H.; Zhang, J.G.; Sushko, M.L.; et al. Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries. Nat. Energy 2018, 3, 674–681. [Google Scholar] [CrossRef]
- Gu, Y.; Fang, S.; Yang, L.; Hirano, S.I. A non-flammable electrolyte for long-life lithium ion batteries operating over a wide-temperature range. J. Mater. Chem. A 2021, 9, 15363–15372. [Google Scholar] [CrossRef]
- Dagger, T.; Rad, B.R.; Schappacher, F.M.; Winter, M. Comparative performance evaluation of flame retardant additives for lithium ion batteries–I. Safety, chemical and electrochemical stabilities. Energy Technol. 2018, 6, 2011–2022. [Google Scholar] [CrossRef]
- Xiang, H.F.; Xu, H.Y.; Wang, Z.Z.; Chen, C.H. Dimethyl methylphosphonate (DMMP) as an efficient flame retardant additive for the lithium-ion battery electrolytes. J. Power Sources 2007, 173, 562–564. [Google Scholar] [CrossRef]
- Nagasubramanian, G.; Fenton, K. Reducing Li-ion safety hazards through use of non-flammable solvents and recent work at Sandia National Laboratories. Electrochim. Acta 2013, 101, 3–10. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A review of recent progress in phosphorus-based flame retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Liu, S.; Mao, J.; Zhang, Q.; Wang, Z.; Pang, W.K.; Zhang, L.; Du, A.; Sencadas, V.; Zhang, W.; Guo, Z. An intrinsically non-flammable electrolyte for high-performance potassium batteries. Angew. Chem. Int. Ed. 2020, 59, 3638–3644. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Ding, M.S.; Zhang, S.; Allen, J.L.; Jow, T.R. An attempt to formulate nonflammable lithium ion electrolytes with alkyl phosphates and phosphazenes. J. Electrochem. Soc. 2002, 149, A622. [Google Scholar] [CrossRef]
- Fang, S.; Wang, G.; Qu, L.; Luo, D.; Yang, L.; Hirano, S.I. A novel mixture of diethylene glycol diethylether and non-flammable methyl-nonafluorobutyl ether as a safe electrolyte for lithium ion batteries. J. Mater. Chem. A 2015, 3, 21159–21166. [Google Scholar] [CrossRef]
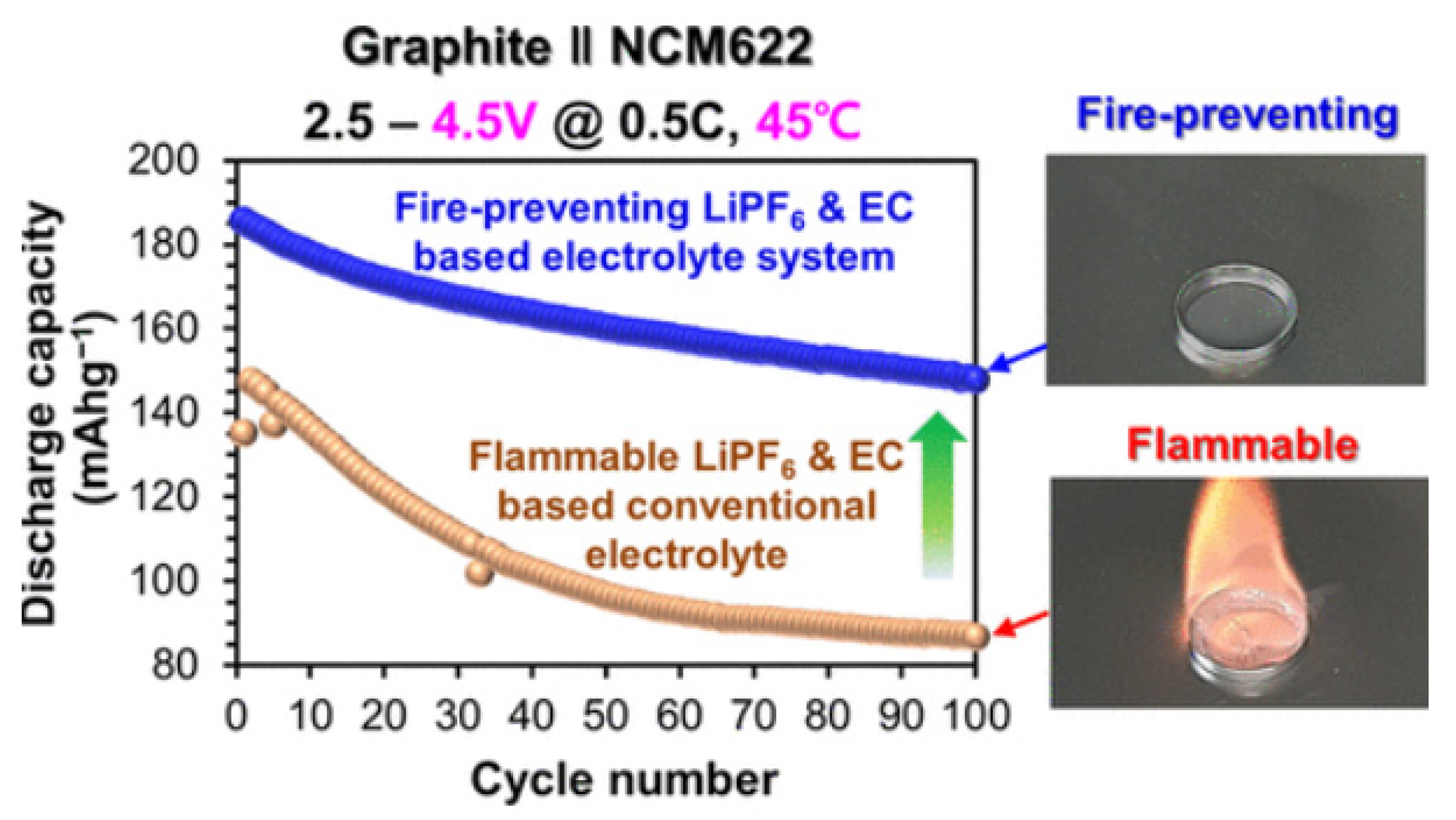
- Chung, G.J.; Han, J.; Song, S.W. Fire-preventing LiPF6 and ethylene carbonate-based organic liquid electrolyte system for safer and outperforming lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 42868–42879. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Song, J.; Zhang, Y.; Wang, X.; Jiang, Z.; Sun, T.; Zhao, J. Revealing the mechanisms of lithium-ion transport and conduction in composite solid polymer electrolytes. Cell Rep. Phys. Sci. 2023, 4, 101321. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef]
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-liquid-based polymer electrolytes for battery applications. Angew. Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef]
- Stephan, A.M. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Huang, L.L.; Lu, L.G.; Liu, L.S.; Zhan, J.H. Recent progress on failure mechanism and preventive measures in lithium ion batteries. Power Technol. 2021, 45, 1087–1090+1099. [Google Scholar]
- Zou, Y.N. Research progress of modification for power lithium-ion battery membrane. Synth. Resins Plast. 2016, 33, 87–90. [Google Scholar]
- Feng, Y.F.; Liu, L.; Chen, Z.P.; Huang, S.X.; Zhong, L.S.; Zhu, H.B. Influence of Extrusion and Calendering Process on Pore-forming Properties of Lithium Battery Separator. Insul. Mater. 2019, 52, 17–20. [Google Scholar]
- Huang, S.X.; Wei, J.T.; Chen, Z.P.; Feng, Y.F.; Zhong, L.S.; Liu, L. Effect of Synchronous Biaxial Stretching Process on the Film-forming Property of Lithium Battery Separator. Insul. Mater. 2018, 51, 15–19. [Google Scholar]
- Jiang, W.; Lin, Y.; Zeng, L.X.; Qian, Q.R.; Chen, Q.H. Performance Parameters and Their Measurement for Lithium-ion Battery Separators. Insul. Mater. 2018, 51, 7–14+20. [Google Scholar]
- Li, H.; Chen, Z.K.; Hou, X.H.; Yu, S.J. Effect of separator heat treating on the performance of Li-ion battery. Battery 2010, 40, 87–89. [Google Scholar]
- Wang, B.; Li, X.; Zhang, X.; Luo, B.; Jin, M.; Liang, M.; Dayeh, S.A.; Picraux, S.T.; Zhi, L. Adaptable silicon–carbon nanocables sandwiched between reduced graphene oxide sheets as lithium ion battery anodes. ACS Nano 2013, 7, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.M. Effects of separator on safety performance of lithium batteries. Power Technol. 2019, 43, 1767–1770. [Google Scholar]
- Hu, W.; Yang, J.; He, X.Y.; Zhang, D.S.; Wu, L.; Li, W.Y.; Wu, A.P.; Wu, Q.Y.; Zhang, J.A.; Wu, M.Y.; et al. Fabrication, Pore Formation and Working Mechanism Analysis of Three-Layer Composite Separator for Vehicle Power Lithium Batteries. Anhui Chem. 2019, 45, 35–37+42. [Google Scholar]
- Lei, J.; Zhu, D.; Rong, L.B.; Zhang, G.H. High rate performance of Li-ion battery prepared with Al2O3 coating PE separators. Battery 2018, 48, 341–343. [Google Scholar]
- Yao, W.B.; Chen, P.; Zhou, Y.; Wang, C.X.; Xie, J. Effect of ceramic-coating separators on the performance of Li-ion batteries. Battery Ind. 2013, 18, 124–127. [Google Scholar]
- An, Y.Q.; Zhang, H.H.; Wu, C.D.; Yang, Y.; Xu, Y. Research Progress of Low Moisture Bomsite Coating Separators. Guangdong Chem. 2019, 46, 106–107+105. [Google Scholar]
- Wang, Z.H.; Peng, D.C.; Sun, K.N. Research progress of separator materials for lithium ion batteries. J. Chem. Eng. 2018, 69, 282–294. [Google Scholar]
- An, Y.Q.; Zhang, H.H.; Wu, C.D. Advance in Water-based PVDF Coating Separator for Lithium-ion Battery. Guangdong Chem. 2019, 46, 100–101. [Google Scholar]
- Xiong, M.; Tang, H.; Wang, Y.; Pan, M. Ethylcellulose-coated polyolefin separators for lithium-ion batteries with improved safety performance. Carbohydr. Polym. 2014, 101, 1140–1146. [Google Scholar] [CrossRef]
- Song, J.; Ryou, M.H.; Son, B.; Lee, J.N.; Lee, D.J.; Lee, Y.M.; Choi, J.W.; Park, J.K. Co-polyimide-coated polyethylene separators for enhanced thermal stability of lithium ion batteries. Electrochim. Acta 2012, 85, 524–530. [Google Scholar] [CrossRef]
- An, M.Y.; Kim, H.T.; Chang, D.R. Multilayered separator based on porous polyethylene layer, Al 2 O 3 layer, and electro-spun PVdF nanofiber layer for lithium batteries. J. Solid State Electrochem. 2014, 18, 1807–1814. [Google Scholar] [CrossRef]
- Jeong, H.S.; Hong, S.C.; Lee, S.Y. Effect of microporous structure on thermal shrinkage and electrochemical performance of Al2O3/poly (vinylidene fluoride-hexafluoropropylene) composite separators for lithium-ion batteries. J. Membr. Sci. 2010, 364, 177–182. [Google Scholar] [CrossRef]
- Belharouak, I.; Sun, Y.K.; Liu, J.; Amine, K. Li (Ni1/3Co1/3Mn1/3) O2 as a suitable cathode for high power applications. J. Power Sources 2003, 123, 247–252. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Wu, Y.; Manthiram, A. High Capacity, Surface-Modified Layered Li[Li(1−x)∕3Mn(2−x)∕3Nix∕3Cox∕3]O2 Cathodes with Low Irreversible Capacity Loss. Electrochem. Solid-State Lett. 2006, 9, A221. [Google Scholar] [CrossRef]
- Sun, Y.K.; Lee, M.J.; Yoon, C.S.; Hassoun, J.; Amine, K.; Scrosati, B. The role of AlF3 coatings in improving electrochemical cycling of Li-enriched nickel-manganese oxide electrodes for Li-ion batteries. Adv. Mater. 2012, 24, 1192–1196. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, L.; Sun, J. A multi-component additive to improve the thermal stability of Li (Ni1/3Co1/3Mn1/3) O2-based lithium ion batteries. Energies 2016, 9, 424. [Google Scholar] [CrossRef]
- Lin, C.H.; Jarvis, D.L. Utility of temporally distinct baculovirus promoters for constitutive and baculovirus-inducible transgene expression in transformed insect cells. J. Biotechnol. 2013, 165, 11–17. [Google Scholar] [CrossRef]
- Xia, L.; Li, S.L.; Ai, X.P.; Yang, H.X.; Cao, Y.L. Temperature-sensitive cathode materials for safer lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2845–2848. [Google Scholar] [CrossRef]
- Liu, K.; Pei, A.; Lee, H.R.; Kong, B.; Liu, N.; Lin, D.; Liu, Y.; Liu, C.; Hsu, P.C.; Bao, Z.; et al. Lithium metal anodes with an adaptive “solid-liquid” interfacial protective layer. J. Am. Chem. Soc. 2017, 139, 4815–4820. [Google Scholar] [CrossRef]
- Zheng, G.; Lee, S.W.; Liang, Z.; Lee, H.W.; Yan, K.; Yao, H.; Wang, H.; Li, W.; Chu, S.; Cui, Y. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnol. 2014, 9, 618–623. [Google Scholar] [CrossRef]
- Shao, Q.; Yu, P.; Huang, H.; Hu, Z.X.; Li, X.; Wang, C.M. Design of Early Warning Mechanism for Lithium Battery Thermal Runaway. Intern. Combust. Engine Accessories 2020, 9, 232–234. [Google Scholar]
- Niu, M.; Jiang, J.C.; Guo, H.Y. Controlling strategy research on HEV batteries balance. Microprocessor 2010, 31, 125–127. [Google Scholar]
- Speltino, C.; Stefanopoulou, A.; Fiengo, G. Cell Equalization in Battery Stacks through State of Charge Estimation Polling. In Proceedings of the 2010 American Control Conference, Baltimore, MD, USA, 30 June–5 July 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 5050–5055. [Google Scholar]
- Sun, H.; Dixon, R. Development of cooling strategy for an air cooled lithium-ion battery pack. J. Power Sources 2014, 272, 404–414. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J. Design a J-type air-based battery thermal management system through surrogate-based optimization. Appl. Energy 2019, 252, 113426. [Google Scholar] [CrossRef]
- Wang, T.; Tseng, K.J.; Zhao, J.; Wei, Z. Thermal investigation of lithium-ion battery module with different cell arrangement structures and forced air-cooling strategies. Appl. Energy 2014, 134, 229–238. [Google Scholar] [CrossRef]
- Peng, X.; Cui, X.; Liao, X.; Garg, A. A thermal investigation and optimization of an air-cooled lithium-ion battery pack. Energies 2020, 13, 2956. [Google Scholar] [CrossRef]
- Fan, Y.; Bao, Y.; Ling, C.; Chu, Y.; Tan, X.; Yang, S. Experimental study on the thermal management performance of air cooling for high energy density cylindrical lithium-ion batteries. Appl. Therm. Eng. 2019, 155, 96–109. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, X.; Li, G.; Hua, D. Assessment of the forced air-cooling performance for cylindrical lithium-ion battery packs: A comparative analysis between aligned and staggered cell arrangements. Appl. Therm. Eng. 2015, 80, 55–65. [Google Scholar] [CrossRef]
- Hirano, H.; Tajima, T.; Hasegawa, T.; Sekiguchi, T.; Uchino, M. Boiling Liquid Battery Cooling for Electric Vehicle. In Proceedings of the 2014 IEEE Conference and Expo Transportation Electrification Asia-Pacific (ITEC Asia-Pacific), Beijing, China, 31 August–3 September 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 1–4. [Google Scholar]
- Smith, J.; Hinterberger, M.; Hable, P.; Koehler, J. Simulative method for determining the optimal operating conditions for a cooling plate for lithium-ion battery cell modules. J. Power Sources 2014, 267, 784–792. [Google Scholar] [CrossRef]
- Mondal, B.; Lopez, C.F.; Mukherjee, P.P. Exploring the efficacy of nanofluids for lithium-ion battery thermal management. Int. J. Heat Mass Transf. 2017, 112, 779–794. [Google Scholar] [CrossRef]
- Tang, A.; Li, J.; Lou, L.; Shan, C.; Yuan, X. Optimization design and numerical study on water cooling structure for power lithium battery pack. Appl. Therm. Eng. 2019, 159, 113760. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, J.; Fang, X.; Zhang, Z.; Xu, T.; Gao, X.; Wang, S. Experimental and numerical investigation of the application of phase change materials in a simulative power batteries thermal management system. Appl. Energy 2014, 121, 104–113. [Google Scholar] [CrossRef]
- Wu, X.H.; Wang, K.; Ma, X.F.; Gao, L.; Liu, H. Research on high temperature heat dissipation performance of power battery based on composite system of phase change material and liquid cooling. Cryog. Supercond. 2020, 48, 73–79. [Google Scholar]
- GB/T 36276-2018; State Administration of Market Supervision and Administration, National Standardization Management Committee. Lithium-ion batteries for power storage. China Standards Press: Beijing, China, 2018.
- Chen, X.C.; Niu, H.C.; Li, Z.; Li, L.; Mo, S.; Huang, X. Thermal runaway propagation mitigation of lithium-ion battery by epoxy resin board. Energy Storage Sci. Technol. 2019, 8, 532–537. [Google Scholar]
- Berdichevsky, E.M.; Cole, P.D.; Hebert, A.J.; Hermann, W.A.; Kelty, K.R.; Kohn, S.I.; Lyons, D.F.; Straubel, J.B.; Mendez, N.J.; Tesla Motor Inc. Mitigation of Propagation of Thermal Runaway in a Multi-Cell Battery Pack. U.S. Patent 7,433,794, 7 October 2008. [Google Scholar]
- Larsson, F.; Anderson, J.; Andersson, P.; Mellander, B.E. Thermal modelling of cell-to-cell fire propagation and cascading thermal runaway failure effects for lithium-ion battery cells and modules using fire walls. J. Electrochem. Soc. 2016, 163, A2854. [Google Scholar] [CrossRef]
- Mehta, V.H.; Prilutsky, A.; Hermann, W.A. Cell Thermal Runaway Propagation Resistance Using an Internal Layer of Intumescent Material. U.S. Patent 7,781,097, 24 August 2010. [Google Scholar]
- Mehta, V.H.; Prilutsky, A. Cell Thermal Runaway Propagation Resistance Using Dual Intumescent Material Layers. U.S. Patent 7,763,381, 27 July 2010. [Google Scholar]
- Mehta, V.H.; Hermann, W.A.; Kalayjian, N.R. Cell Thermal Runaway Propagation Resistant Battery Pack. U.S. Patent 7,820,319, 26 October 2010. [Google Scholar]
- Zhang, Y.H. Research on Thermal Runaway and Chain Reaction for Li-ion Batteries. Ship Electr. Technol. 2018, 38, 16–20. [Google Scholar]
- Wilke, S.; Schweitzer, B.; Khateeb, S.; Al-Hallaj, S. Preventing thermal runaway propagation in lithium ion battery packs using a phase change composite material: An experimental study. J. Power Sources 2017, 340, 51–59. [Google Scholar] [CrossRef]
- Schweitzer, B.; Wilke, S.; Khateeb, S.; Al-Hallaj, S. Experimental validation of a 0-D numerical model for phase change thermal management systems in lithium-ion batteries. J. Power Sources 2015, 287, 211–219. [Google Scholar] [CrossRef]
- Yi, X.Y.; Li, Z.K.; Liu, Q.Y.; Wei, C.Y.; Liu, T. Effectiveness of aerogel blanket in blocking thermal runaway propagation of lithium-ion batteries. J. Shandong Univ. Sci. Technol. 2021, 40, 43–51. [Google Scholar]
- Yuan, S.; Chang, C.; Yan, S.; Zhou, P.; Qian, X.; Yuan, M.; Liu, K. A review of fire-extinguishing agent on suppressing lithium-ion batteries fire. J. Energy Chem. 2021, 62, 262–280. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, J. Research progress of water mist fire extinguishing technology and its application in battery fires. Process Saf. Environ. Prot. 2021, 149, 559–574. [Google Scholar] [CrossRef]





| Method | Advantages | Disadvantages | |
|---|---|---|---|
| External parameters | Terminal voltage | Monitor the voltage in real- time Capable of locating faulty battery Easy to operate, low cost | Complex topology of voltage sensors, high cost |
| Surface temperature | Monitor the surface temperature in real time Easy to operate, low cost | Significant temperature difference from the internal temperature, Inaccurate for determining the state of the battery | |
| Internal parameters | Embedded optical fiber sensors | Monitor the internal core temperature of the battery directly | High cost Higher requirements for battery packaging |
| Electrochemical impedance spectroscopy analysis | Predict the internal core temperature of the battery without complex hardware Online monitoring of battery status | Fail to monitor large-scale batteries quickly and effectively | |
| Method | Advantages | Disadvantages |
|---|---|---|
| Based on battery voltage | Voltage can be monitored in real time Faulty batteries can be located Predict the state of charge of the battery in real time | Voltage variations are complex and poorly regulated Thermal runaway warning is lagging behind Influenced by other external factors |
| Based on battery temperature | Surface temperature can be monitored in real time Predict the state of health of the battery in real time Directly measure internal battery temperature | Large temperature difference from internal temperature Low accuracy of thermal runaway prediction Thermal runaway warning is lagging behind Used in combination with EIS technology or embedded fiber optic sensor technology |
| Based on EIS | Sensitive to temperature changes Independent of SOC The original structure of the battery will not be destroyed | Influenced by other external factors Complex calibration process as different lithium-ion battery systems have different parameters of impedance Relies on more complex mathematical models |
| Models | Illustrations | Advantages | Disadvantages |
|---|---|---|---|
| U-type |  | Small pressure drop, low energy consumption | Bad temperature field consistency, higher maximum temperature |
| Z-type |  | Good temperature field consistency | High pressure drop, high energy consumption |
| J-type |  | Small pressure drop, low energy consumption Good temperature field consistency | Complex structure, high sealing requirements |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, S.; Liu, J.; Cong, B. Review of Thermal Runaway Monitoring, Warning and Protection Technologies for Lithium-Ion Batteries. Processes 2023, 11, 2345. https://doi.org/10.3390/pr11082345
Yin S, Liu J, Cong B. Review of Thermal Runaway Monitoring, Warning and Protection Technologies for Lithium-Ion Batteries. Processes. 2023; 11(8):2345. https://doi.org/10.3390/pr11082345
Chicago/Turabian StyleYin, Sumiao, Jianghong Liu, and Beihua Cong. 2023. "Review of Thermal Runaway Monitoring, Warning and Protection Technologies for Lithium-Ion Batteries" Processes 11, no. 8: 2345. https://doi.org/10.3390/pr11082345
APA StyleYin, S., Liu, J., & Cong, B. (2023). Review of Thermal Runaway Monitoring, Warning and Protection Technologies for Lithium-Ion Batteries. Processes, 11(8), 2345. https://doi.org/10.3390/pr11082345






