A Review of Dendrophthoe pentandra (Mistletoe): Phytomorphology, Extraction Techniques, Phytochemicals, and Biological Activities
Abstract
1. Introduction
2. Phytomorphology
2.1. Leaves
2.2. Flowers
2.3. Fruits and Seeds
2.4. Roots
3. Extraction Techniques
3.1. Maceration
3.2. Reflux
3.3. Soxhlet
3.4. Microwave-Assisted Extraction
3.5. Accelerated Solvent Extraction
3.6. Supercritical Fluid Extraction
3.7. Ultrasonic-Assisted Extraction
4. Phytochemical Constituents
4.1. Phenolics and Polyphenolics
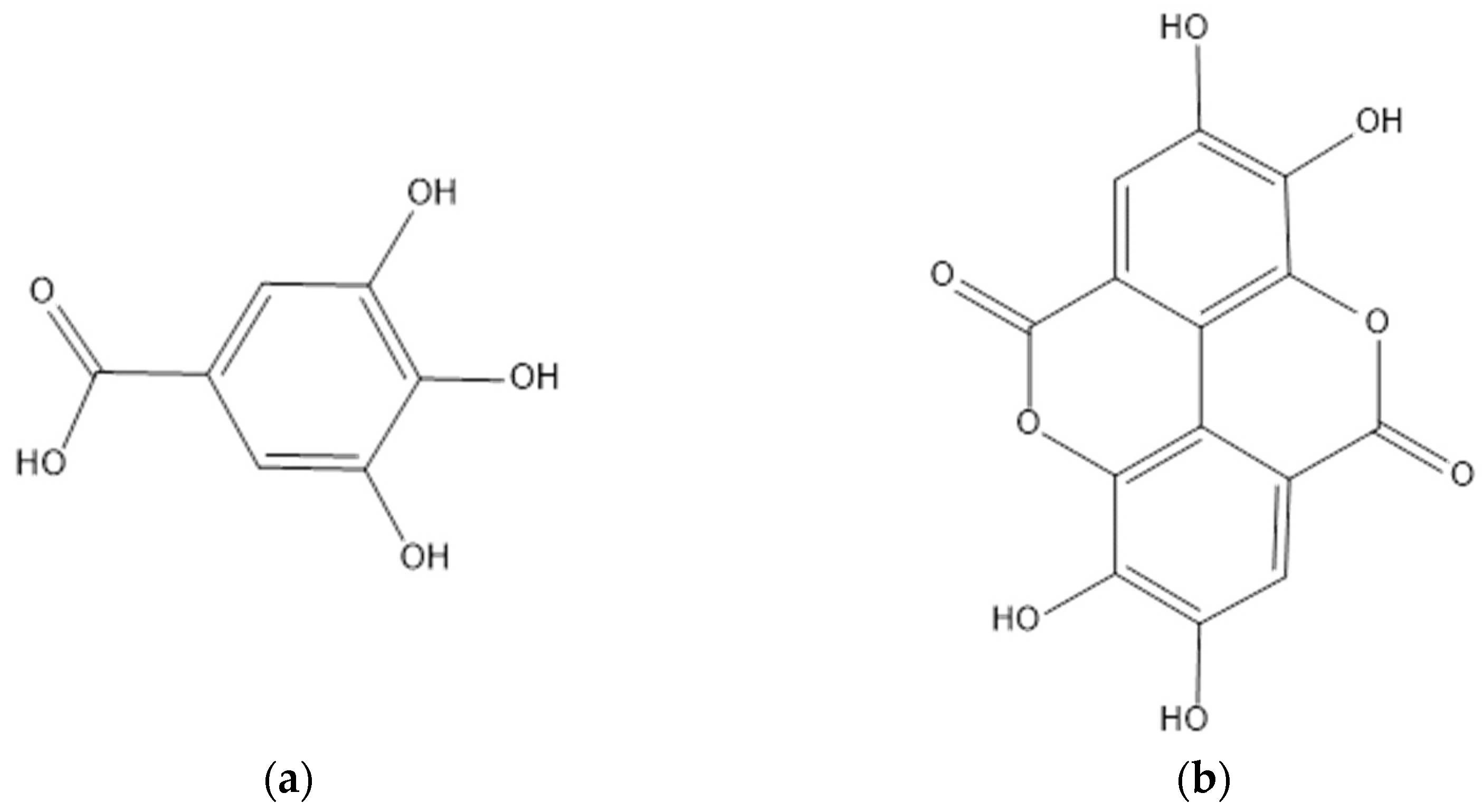
4.2. Tannins
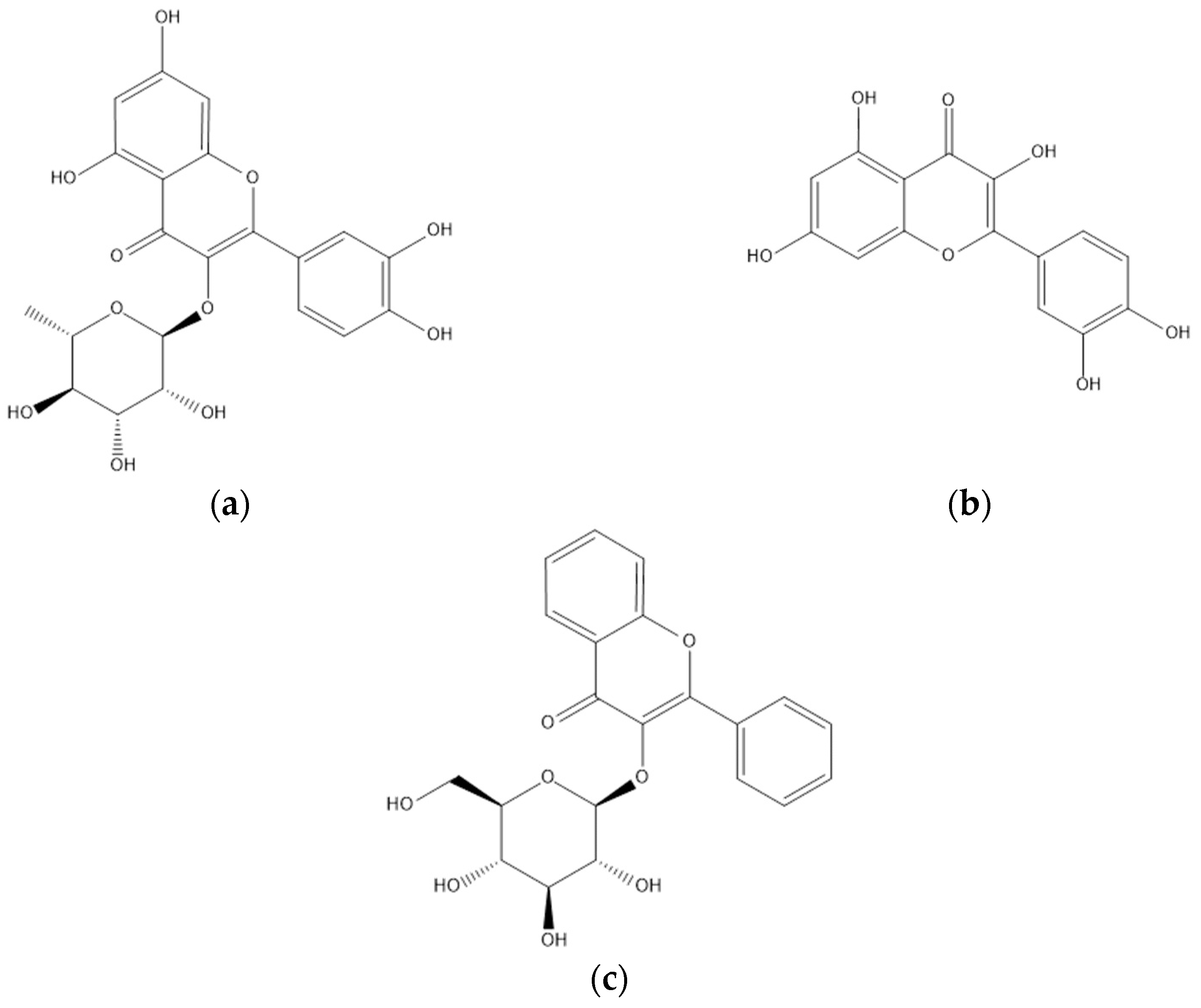
4.3. Flavonoids
4.4. Alkaloids
4.5. Terpenoids
4.6. Saponins
5. Biological Activities
5.1. Antioxidant Activity
5.2. Antibacterial Activity
5.3. Anticancer and Antiproliferative Activities
5.4. Antidiabetic and Antihyperglycaemic Activities
5.5. Other Biological Activities
6. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASE | Accelerated solvent extraction |
| CO2 | Carbon dioxide |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| IC50 | Half maximal inhibitory concentration |
| LC50 | Lethal concentration 50 |
| MAE | Microwave-assisted extraction |
| miRNAs | microRNAs |
| ROS | Reactive oxygen species |
| SFE | Supercritical fluid extraction |
| UAE | Ultrasonic-assisted extraction |
| ZOI | Zone of inhibition |
References
- Artanti, N.; Firmansyah, T.; Darmawan, A. Bioactivities evaluation of Indonesian mistletoes (Dendrophthoe pentandra (L.) Miq.) leaves extracts. J. Appl. Pharm. Sci. 2012, 2, 24–27. [Google Scholar]
- Jayanti, E.D. Uji Aktivitas Antibakteri Ekstrak Etanol dan Fraksi Daun Benalu Mangga Gadung (Dendrophthoe pentandra (L.) Miq.) terhadap Staphylococcus aureus ATCC 6538 Dan Escherichia coli ATCC 25922. Undergraduate’s Thesis, Universitas Jember, Kabupaten Jember, Jawa Timur, Indonesia, 2018. [Google Scholar]
- Těšitel, J.; Li, A.-R.; Knotková, K.; McLellan, R.; Bandaranayake, P.C.G.; Watson, D.M. The bright side of parasitic plants: What are they good for? Plant Physiol. 2021, 185, 1309–1324. [Google Scholar] [CrossRef]
- Artanti, N.; Ma’arifa, Y.; Hanafi, M. Isolation and identification of active antioxidant compound from star fruit (Averrhoa carambola) mistletoe (Dendrophthoe pentandra (L.) Miq.) ethanol extract. J. Appl. Sci. 2006, 6, 1659–1663. [Google Scholar] [CrossRef][Green Version]
- Alharits, L.; Handayani, W.; Yasman; Hemelda, N.M. Phytochemical analysis and antioxidant activity of leaves and flowers extracts of mistletoe (Dendrophthoe pentandra (L.) Miq.), collected from UI Campus, Depok. AIP Conf. Proc. 2019, 2168, 020101. [Google Scholar] [CrossRef]
- Hardiyanti, R.; Marpaung, L.; Adnyana, I.K.; Simanjuntak, P. Antioxidant and antibacterial activities of various extracts of duku’s mistletoe leaf (Dendrophthoe pentandra (L.) Miq) collected from Medan, Indonesia. Asian J. Pharm. Clin. Res. 2018, 11, 526–529. [Google Scholar] [CrossRef]
- Hardiyanti, R.; Marpaung, L.; Adnyana, I.K.; Simanjuntak, P. Isolation of quercitrin from Dendrophthoe pentandra (L.) Miq leaves and it’s antioxidant and antibacterial activities. Rasāyan J. Chem. 2019, 12, 1822–1827. [Google Scholar] [CrossRef]
- Widowati, W.; Mozef, T.; Risdian, C.; Ratnawati, H.; Tjahjani, S.; Sandra, F. The comparison of antioxidative and proliferation inhibitor properties of Piper betle L., Catharanthus roseus [L] G.Don, Dendrophtoe petandra L., Curcuma mangga Val. extracts on T47D cancer cell line. Int. Res. J. Biochem. Bioinform. 2011, 1, 22–28. [Google Scholar]
- Yismairai, E.; Hemelda, N.M.; Yasman; Handayani, W. Antioxidant activity of extract of mistletoe, Dendrophthoe pentandra (L.) Miq., lived in three different host plants, collected from Kampus UI, Depok. AIP Conf. Proc. 2019, 2168, 020100. [Google Scholar]
- Kristiningrum, N.; Wulandari, L.; Zuhriyah, A. Phytochemical screening, total phenolic content, and antioxidant activity of water, ethyl acetate, and n-hexane fractions from mistletoe Moringa oleifera Lam. (Dendrophthoe pentandra (L.) Miq.). Asian J. Pharm. Clin. Res. 2018, 11, 104–106. [Google Scholar] [CrossRef]
- Zainuddin, N.A.S.N.; Zakaria, Y.; Sul’ain, M.D. Dendrophthoe pentandra induced apoptosis and cell cycle arrest at G1/S in human breast adenocarcinoma cells, MCF-7 via up-regulation of p53. J. Appl. Pharm. Sci. 2018, 8, 130–141. [Google Scholar] [CrossRef]
- Endharti, A.T.; Wulandari, A.; Listyana, A.; Norahmawati, E.; Permana, S. Dendrophthoe pentandra (L.) Miq extract effectively inhibits inflammation, proliferation and induces p53 expression on colitis-associated colon cancer. BMC Complement. Altern. Med. 2016, 16, 374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zamani, A.; Mat Jusoh, S.A.; Al-Jamal, H.A.N.; Sul’ain, M.D.; Johan, M.F. Anti-proliferative effects of Dendrophthoe pentandra methanol extract on BCR/ABL-positive and imatinib-resistant leukemia cell lines. Asian Pac. J. Cancer Prev. 2016, 17, 4857–4861. [Google Scholar] [CrossRef] [PubMed]
- Elsyana, V.; Bintang, M.; Priosoeryanto, B.P. Cytotoxicity and antiproliferative activity assay of clove mistletoe (Dendrophthoe pentandra (L.) Miq.) leaves extracts. Adv. Pharmacol. Sci. 2016, 2016, 3242698. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zainuddin, N.A.S.N.; Sul’ain, M.D. Antiproliferative effect of Dendrophthoe pentandra extracts towards human breast adenocarcinoma cells (MCF-7). J. Teknol. 2015, 77, 35–39. [Google Scholar] [CrossRef][Green Version]
- Yee, L.S.; Mohd Fauzi, N.F.; Nik Mat Daud, N.N.N.; Sul’ain, M.D. Study of Dendrophthoe pentandra ethyl acetate extract as potential anticancer candidate on safety and toxicity aspects. J. Anal. Pharm. Res. 2017, 6, 00167. [Google Scholar] [CrossRef]
- Endharti, A.T.; Wahyuningtyas, T.E.; Hardini; Handono, K.; Widjajanto, E.; Permana, S. Dendrophthoe pentandra leaves extract promotes apoptotic effects of doxorubicin in human breast cancer cell via modulation of intracellular calcium and survivin. J. Appl. Pharm. Sci. 2018, 8, 39–43. [Google Scholar] [CrossRef][Green Version]
- Endharti, A.T.; Sulastri, E.; Umanailo, R.; Yunialce; Nurseta, T.; Handono, K. Mango mistletoe Dendrophthoe pentandra leaf extract acts synergistically with 5-fluorouracil to induce apoptosis and increase p21 expression in human cervical adenocarcinoma HeLa cells by reducing survivin expression. J. Appl. Pharm. Sci. 2018, 8, 10–15. [Google Scholar] [CrossRef][Green Version]
- Hasan, M.; Ali, M.T.; Khan, R.; Palit, P.; Islam, A.; Seidel, V.; Akter, R.; Nahar, L. Hepatoprotective, antihyperglycemic and antidiabetic effects of Dendrophthoe pentandra leaf extract in rats. Clin. Phytoscience 2018, 4, 16. [Google Scholar] [CrossRef]
- Endharti, A.T.; Permana, S. Extract from mango mistletoes Dendrophthoe pentandra ameliorates TNBS-induced colitis by regulating CD4+ T cells in mesenteric lymph nodes. BMC Complement. Altern. Med. 2017, 17, 468. [Google Scholar] [CrossRef]
- Ang, H.Y.; Subramani, T.; Yeap, S.K.; Omar, A.R.; Ho, W.Y.; Abdullah, M.P.; Alitheen, N.B. Immunomodulatory effects of Potentilla indica and Dendrophthoe pentandra on mice splenocytes and thymocytes. Exp. Ther. Med. 2014, 7, 1733–1737. [Google Scholar] [CrossRef]
- Handono, K.; Pratama, M.Z.; Yuniati, M.G.; Sermoati, I.A.; Norahmawati, E.; Endharti, A.T.; Irwanto, Y.; Hidayat, S.; Solikhin, M.B.; Indahwati, L.; et al. The effect of mango mistletoes (Dendrophthoe pentandra) leaves extract toward immunosenescence on old BALB/c mice. AIP Conf. Proc. 2023, 2634, 020010. [Google Scholar]
- Kristiningrum, N.; Ridlo, M.; Pratoko, D.K. Phytochemical screening and determination of total phenolic content of Dendophthoe pentandra L. leaves ethanolic extract on mango host. Ann. Trop. Med. Public Health 2020, 23, 98–107. [Google Scholar] [CrossRef]
- Abd Aziz, N.A.; Hasham, R.; Sarmidi, M.R.; Suhaimi, S.H.; Idris, M.K.H. A review on extraction techniques and therapeutic value of polar bioactives from Asian medicinal herbs: Case study on Orthosiphon aristatus, Eurycoma longifolia and Andrographis paniculata. Saudi Pharm. J. 2021, 29, 143–165. [Google Scholar] [CrossRef]
- Yong, J.W.H.; Wei, J.W.; Khew, J.Y.T.; Rong, S.C.; San, W.W. A Guide to the Common Epiphytes and Mistletoes of Singapore, 1st ed.; Cengage Learning Asia: Singapore, 2014; ISBN 9789814609777. [Google Scholar]
- Nickrent, D.L.; Musselman, L.J. Introduction to parasitic flowering plants. Plant Health Instr. 2004. [Google Scholar] [CrossRef]
- Nisa, R.N.; Nasrullah, N. Evaluation of roadside green belt trees damaged by mistletoes parasite plant in Medan Merdeka Road, Central Jakarta, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 203, 012031. [Google Scholar] [CrossRef]
- Sunaryo, S.; Rachman, E.; Uji, T. Morphological damage of plants collections in Purwodadi Botanic Gardens by mistletoe (Loranthaceae and Viscaceae). Ber. Biol. 2006, 8, 129–139. [Google Scholar]
- Pramestya, C.D.; Yasman, Y.; Hemelda, N.M.; Handayani, W. Organo-specific distribution of untargeted metabolomics of mistletoe’s extract, Dendrophthoe pentandra (L.) Miq., from FKM UI, Kampus UI Depok. AIP Conf. Proc. 2019, 2168, 020089. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Anuar, A.; Awang, M.A.; Tan, H.F. Impact of solvent selection on the extraction of total phenolic content and total flavonoid content from kaffir lime leaves: Ultrasonic assisted extraction (UAE) and microwave assisted extraction (MAE). AIP Conf. Proc. 2021, 2347, 020020. [Google Scholar] [CrossRef]
- Awang, M.A.; Chua, L.S.; Abdullah, L.C.; Pin, K.Y. Drying kinetics and optimization of quercetrin extraction from Melastoma malabathricum leaves. Chem. Eng. Technol. 2021, 44, 1214–1220. [Google Scholar] [CrossRef]
- Handa, S.S. An overview of extraction techniques for medicinal and aromatic plants. In Extraction Technologies for Medicinal and Aromatic Plants; Handa, S.S., Khanuja, S.P.S., Longo, G., Rakesh, D.D., Eds.; International Centre for Science and High Technology: Trieste, Italy, 2008; pp. 21–52. [Google Scholar]
- Chua, L.S.; Latiff, N.A.; Mohamad, M. Reflux extraction and cleanup process by column chromatography for high yield of andrographolide enriched extract. J. Appl. Res. Med. Aromat. Plants 2016, 3, 64–70. [Google Scholar] [CrossRef]
- Wang, D.-G.; Liu, W.-Y.; Chen, G.-T. A simple method for the isolation and purification of resveratrol from Polygonum cuspidatum. J. Pharm. Anal. 2013, 3, 241–247. [Google Scholar] [CrossRef]
- Awang, M.A.; Aziz, R.; Sarmidi, M.R.; Abdullah, L.C.; Yong, P.K.; Musa, N.F. Comparison of different solvents on the extraction of Melastoma malabathricum leaves using Soxhlet extraction method. Der Pharm. Lett. 2016, 8, 153–157. [Google Scholar]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 1000196. [Google Scholar] [CrossRef]
- De Boer, J. Polychlorinated biphenyls. In Encyclopedia of Analytical Science; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 214–225. ISBN 9781351369183. [Google Scholar]
- Trusheva, B.; Trunkova, D.; Bankova, V. Different extraction methods of biologically active components from propolis: A preliminary study. Chem. Cent. J. 2007, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on phenolic compounds stability during microwave-assisted extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef]
- Rahmalia, W.; Fabre, J.-F.; Mouloungui, Z. Effects of cyclohexane/acetone ratio on bixin extraction yield by accelerated solvent extraction method. Procedia Chem. 2015, 14, 455–464. [Google Scholar] [CrossRef]
- Tan, L.; Ji, T.; Jiang, G.; Hu, F. Simultaneous identification and quantification of five flavonoids in the seeds of Rheum palmatum L. by using accelerated solvent extraction and HPLC–PDA–ESI/MSn. Arab. J. Chem. 2019, 12, 1345–1352. [Google Scholar] [CrossRef]
- Xynos, N.; Papaefstathiou, G.; Psychis, M.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A.-L. Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves. J. Supercrit. Fluids 2012, 67, 89–93. [Google Scholar] [CrossRef]
- Basa’ar, O.; Fatema, S.; Alrabie, A.; Mohsin, M.; Farooqui, M. Supercritical carbon dioxide extraction of Triognella foenum graecum Linn seeds: Determination of bioactive compounds and pharmacological analysis. Asian Pac. J. Trop. Biomed. 2017, 7, 1085–1091. [Google Scholar] [CrossRef]
- Liza, M.S.; Abdul Rahman, R.; Mandana, B.; Jinap, S.; Rahmat, A.; Zaidul, I.S.M.; Hamid, A. Supercritical carbon dioxide extraction of bioactive flavonoid from Strobilanthes crispus (Pecah Kaca). Food Bioprod. Process. 2010, 88, 319–326. [Google Scholar] [CrossRef]
- Naudé, Y.; de Beer, W.H.J.; Jooste, S.; van der Merwe, L.; van Rensburg, S.J. Comparison of supercritical fluid extraction and Soxhlet extraction for the determination of DDT, DDD and DDE in sediment. Water SA 1998, 24, 205–214. [Google Scholar]
- Romdhane, M.; Gourdon, C. Investigation in solid-liquid extraction: Influence of ultrasound. Chem. Eng. J. 2002, 87, 11–19. [Google Scholar] [CrossRef]
- Easmin, M.S.; Sarker, M.Z.I.; Ferdosh, S.; Shamsudin, S.H.; Yunus, K.B.; Uddin, M.S.; Sarker, M.M.R.; Akanda, M.J.H.; Hossain, M.S.; Khalil, H.P.S.A. Bioactive compounds and advanced processing technology: Phaleria macrocarpa (Sheff.) Boerl, a review. J. Chem. Technol. Biotechnol. 2015, 90, 981–991. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Mochamad, L.; Hermanto, B.; Hestianah, E.P. Determination of progesterone compounds in the crude methanol extract of benalu duku leaves. Vet. World 2019, 12, 358–366. [Google Scholar] [CrossRef]
- Randhir, R.; Lin, Y.-T.; Shetty, K. Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 2004, 39, 637–646. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Akinyemi, A.J.; Henle, T.; Saliu, J.A.; Schwarzenbolz, U. Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro. J. Funct. Foods 2012, 4, 450–458. [Google Scholar] [CrossRef]
- Demarque, D.P.; Callejon, D.R.; de Oliveira, G.G.; Silva, D.B.; Carollo, C.A.; Lopes, N.P. The role of tannins as antiulcer agents: A fluorescence-imaging based study. Rev. Bras. Farmacogn. 2018, 28, 425–432. [Google Scholar] [CrossRef]
- Kosińska, A.; Karamać, M.; Penkacik, K.; Urbalewicz, A.; Amarowicz, R. Interactions between tannins and proteins isolated from broad bean seeds (Vicia faba Major) yield soluble and non-soluble complexes. Eur. Food Res. Technol. 2011, 233, 213–222. [Google Scholar] [CrossRef]
- Mena, P.; Calani, L.; Bruni, R.; Del Rio, D. Bioactivation of high-molecular-weight polyphenols by the gut microbiome. In Diet-Microbe Interactions in the Gut: Effects on Human Health and Disease; Tuohy, K., Del Rio, D., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 73–101. ISBN 9780124079410. [Google Scholar]
- Okuda, T.; Ito, H. Tannins of constant structure in medicinal and food plants-hydrolyzable tannins and polyphenols related to tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Min, B.R.; Pinchak, W.E.; Anderson, R.C.; Callaway, T.R. Effect of tannins on the in vitro growth of Escherichia coli O157:H7 and in vivo growth of generic Escherichia coli excreted from steers. J. Food Prot. 2007, 70, 543–550. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Dewi, R.T.; Ekapratiwi, Y.; Sundowo, A.; Ariani, N.; Yolanda, T.; Filaila, E. Bioconversion of quercetin glucosides from Dendrophthoe pentandra leaf using Aspergillus acueletus LS04-3. AIP Conf. Proc. 2019, 2175, 020048. [Google Scholar] [CrossRef]
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. Int. J. Biol. Sci. 2018, 14, 341–357. [Google Scholar] [CrossRef]
- Qurrat-Ul-Ain; Khan, H.; Mubarak, M.S.; Pervaiz, A. Plant alkaloids as antiplatelet agent: Drugs of the future in the light of recent developments. Front. Pharmacol. 2016, 7, 292. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in chemical structures and biological properties of plant alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent Advances in Natural Products Analysis; Silva, A.S., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567. ISBN 9780128164556. [Google Scholar]
- Katerova, Z.; Todorova, D.; Tasheva, K.; Sergiev, I. Influence of ultraviolet radiation on plant secondary metabolite production. Genet. Plant Physiol. 2012, 2, 113–144. [Google Scholar]
- Khwaja, T.A.; Dias, C.B.; Pentecost, S. Recent studies on the anticancer activities of mistletoe (Viscum album) and its alkaloids. Oncology 1986, 43, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kienle, G.S.; Kiene, H. Influence of Viscum album L (European mistletoe) extracts on quality of life in cancer patients: A systematic review of controlled clinical studies. Integr. Cancer Ther. 2010, 9, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.G.; Lathe, R. Terpenes, hormones and life: Isoprene rule revisited. J. Endocrinol. 2019, 242, R9–R22. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Chen, X.; Chen, Y. Plant noncoding RNAs: Hidden players in development and stress responses. Annu. Rev. Cell Dev. Biol. 2019, 35, 407–431. [Google Scholar] [CrossRef] [PubMed]
- Jones-Rhoades, M.W.; Bartel, D.P.; Bartel, B. MicroRNAs and their regulatory roles in plants. Annu. Rev. Plant Biol. 2006, 57, 19–53. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Adolf, J.; Melzig, M.F. Identification of Viscum album L. miRNAs and prediction of their medicinal values. PLoS ONE 2017, 12, e0187776. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Siddhuraju, P.; Becker, K. Saponins. In Plant Secondary Metabolites; Makkar, H.P.S., Siddhuraju, P., Becker, K., Eds.; Humana Press: Totowa, NJ, USA, 2002; Volume 393, pp. 93–100. ISBN 978-1-59745-425-4. [Google Scholar]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Danish, P.; Ali, Q.; Hafeez, M.M.; Malik, A. Antifungal and antibacterial activity of Aloe vera plant extract. Biol. Clin. Sci. Res. J. 2020, 2020, 4. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Naveen, J.; Baskaran, V. Antidiabetic plant-derived nutraceuticals: A critical review. Eur. J. Nutr. 2018, 57, 1275–1299. [Google Scholar] [CrossRef] [PubMed]
- Kwanda, N.; Noikotr, K.; Sudmoon, R.; Tanee, T.; Chaveerach, A. Medicinal parasitic plants on diverse hosts with their usages and barcodes. J. Nat. Med. 2013, 67, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Rajabalaya, R.; David, S.R. A hidden treasure: The Borneo mistletoes. Pharmacogn. Rev. 2017, 11, 153–157. [Google Scholar] [PubMed]
- Kunwar, R.M.; Adhikari, N.; Devkota, M.P. Indigenous use of mistletoes in tropical and temperate region of Nepal. Banko Janakari 1970, 15, 38–42. [Google Scholar] [CrossRef]





| Host Plant | Extraction Technique | Solvents Used | Targeted Compound | Yield (%) | Ref. |
|---|---|---|---|---|---|
| Annona squamosa | Maceration | M, A | – | – | [1] |
| Camellia sinensis | |||||
| Spondias dulcis | |||||
| Stelechocarpus burahol | |||||
| Averrhoa carambola | Maceration | E | Quercitrin and flavanol glycoside | – | [4] |
| Bauhinia purpurea | Maceration | M | – | – | [5] |
| Duku | Maceration | M, H, EA | – | – | [6] |
| Lansium domesticum | Maceration | M, H, EA | Quercitrin | – | [7] |
| – | Maceration | E | – | – | [8] |
| Bauhinia purpurea | Maceration | M | – | 27.1 | [9] |
| Mangifera indica | 19.6 | ||||
| Stelechocarpus burahol | 29.5 | ||||
| Moringa oleifera | Maceration | M, A | – | 12.2 | [10] |
| M, EA | 10.2 | ||||
| M, H | 26.7 | ||||
| – | Maceration | M | – | 35.8 | [11] |
| Mango | Maceration | E | Quercetin | – | [12] |
| – | Maceration | M | – | – | [13] |
| Clove | Reflux | A | – | – | [14] |
| Maceration | E | ||||
| – | Maceration | C | – | – | [15] |
| DE | |||||
| EA | |||||
| M | |||||
| PE | |||||
| Lansium parasiticum | Maceration | C | – | 70.0 | [16] |
| DE | 12.5 | ||||
| EA | 5.0 | ||||
| PE | 12.5 | ||||
| Mango | Maceration | E | – | – | [17] |
| Mango | Maceration | E | Quercetin | – | [18] |
| – | Maceration | M | – | – | [19] |
| Mango | Maceration | E | Quercetin | – | [20] |
| – | Maceration | E | – | 8.7 | [21] |
| Mango | Maceration | E | – | – | [22] |
| Mango | Maceration | E | – | 11.0–16.0 | [23] |
| Bauhinia purpurea | Maceration | M | – | 10.7–33.1 | [29] |
| Lansium domesticum | Maceration | M | Progesterone | – | [51] |
| Biological Activities | Host Plant | Solvents Used | Results | +/− Control | Ref. | |
|---|---|---|---|---|---|---|
| Antioxidant Activity | Stelechocarpus burahol | M | IC50= | 21.5 µg/mL | – | [1] |
| A | 299.0 µg/mL | |||||
| Spondias dulcis | M | 30.9 µg/mL | ||||
| A | 445.0 µg/mL | |||||
| Annona squamosa | M | 22.9 µg/mL | ||||
| A | 741.0 µg/mL | |||||
| Camellia sinensis | M | 84.9 µg/mL | ||||
| A | 303.0 µg/mL | |||||
| Averrhoa carambola | E | IC50= | 9.05–24.72 µg/mL | – | [4] | |
| Bauhinia purpurea | M | IC50= | 6.99–13.58 µg/mL | 9.57 µg/mL | [5] | |
| Duku | M | IC50= | 2.89–13.21 µg/mL | – | [6] | |
| Lansium domesticum | M | IC50= | 3.59 ppm | 5.10 ppm | [7] | |
| – | E | IC50= | 4.74 µg/mL | 3.24 µg/mL | [8] | |
| Bauhinia purpurea | M | IC50= | 15.30 µg/mL | 3.40 µg/mL | [9] | |
| Mangifera indica | M | 21.50 µg/mL | ||||
| Stelechocarpus burahol | M | 10.33 µg/mL | ||||
| Moringa oleifera | A | IC50= | 29.46 µg/mL | 3.46 µg/mL | [10] | |
| EA | 7.08 µg/mL | |||||
| H | 10.90 µg/mL | |||||
| Antibacterial Activity | Duku | M | ZOI= | 8.13–9.12 mm | – | [6] |
| H | 7.85–8.97 mm | |||||
| EA | 7.75–9.00 mm | |||||
| Lansium domesticum | M, H, EA | ZOI= | 7.23–9.54 mm | NZ | [7] | |
| Anticancer and Antiproliferative Activities | – | E | IC50= | 728.05 µg/mL | NS | [8] |
| – | M | IC50= | 10.65 µg/mL (MCF-7 cell) | S | [11] | |
| Mango | E | Dose: | 250 mg/kg BW | NS | [12] | |
| – | M | IC50= | 192–500 µg/mL | NS | [13] | |
| Clove | H | IA= | 38.69% (K562 cell) | S | [14] | |
| 41.50% (MCM-B2 cell) | ||||||
| – | EA | IC50= | 14.42 µg/mL | S | [15] | |
| M | 17.70 µg/mL | |||||
| C | 82.33 µg/mL | NS | ||||
| DE | 101.57 µg/mL | |||||
| PE | 89.70 µg/mL | |||||
| Lansium parasiticum | EA | IC50= | 4.72 µg/mL (MCF-7 cell) | S | [16] | |
| IC50= | 18.12 µg/mL (L929 cell) | |||||
| – | E | Dose: | 25 & 50 µg/mL + 5 µg/mL (doxorubicin) | S | [17] | |
| Mango | E | Dose: | 50 µg/mL + 5 µg/mL (5-fluorouracil) | S | [18] | |
| Antidiabetic and Antihyperglycaemic Activities | Stelechocarpus burahol | M | IC50= | 31.8 µg/mL | – | [1] |
| A | 29.4 µg/mL | |||||
| Spondias dulcis | M | 41.2 µg/mL | ||||
| A | 34.1 µg/mL | |||||
| Annona squamosa | M | 50.9 µg/mL | ||||
| A | 13.9 µg/mL | |||||
| Camellia sinensis | M | 17.6 µg/mL | ||||
| A | 11.8 µg/mL | |||||
| – | M | Dose: | 400 mg/kg BW | S | [19] | |
| Anti-inflammatory Activity | Mango | E | Dose: | 600 mg/kg BW | S | [20] |
| Cytotoxicity Activity | Stelechocarpus burahol | M | LC50= | >1000 µg/mL | – | [1] |
| A | >1000 µg/mL | |||||
| Spondias dulcis | M | >1000 µg/mL | ||||
| A | >1000 µg/mL | |||||
| Annona squamosa | M | >1000 µg/mL | ||||
| A | >1000 µg/mL | |||||
| Camellia sinensis | M | >1000 µg/mL | ||||
| A | >1000 µg/mL | |||||
| Clove | A | LC50= | >1000 µg/mL | – | [14] | |
| E | >1000 µg/mL | |||||
| E | >1000 µg/mL | |||||
| EA | 649.12 µg/mL | |||||
| H | 55.32 µg/mL | |||||
| Lansium parasiticum | EA | LC50= | >1000 ppm | NS | [16] | |
| Hepatoprotective Activity | – | M | Dose: | 400 mg/kg BW | S | [19] |
| Immunomodulatory Activity | – | E | Dose: | 100 µg/mL | S | [21] |
| Anti-ageing Activity | Mango | E | Dose: | 600 mg/kg BW | S | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awang, M.A.; Nik Mat Daud, N.N.N.; Mohd Ismail, N.I.; Abdullah, F.I.; Benjamin, M.A.Z. A Review of Dendrophthoe pentandra (Mistletoe): Phytomorphology, Extraction Techniques, Phytochemicals, and Biological Activities. Processes 2023, 11, 2348. https://doi.org/10.3390/pr11082348
Awang MA, Nik Mat Daud NNN, Mohd Ismail NI, Abdullah FI, Benjamin MAZ. A Review of Dendrophthoe pentandra (Mistletoe): Phytomorphology, Extraction Techniques, Phytochemicals, and Biological Activities. Processes. 2023; 11(8):2348. https://doi.org/10.3390/pr11082348
Chicago/Turabian StyleAwang, Mohd Azrie, Nik Nurul Najihah Nik Mat Daud, Nurul Izzati Mohd Ismail, Farah Izana Abdullah, and Mohammad Amil Zulhilmi Benjamin. 2023. "A Review of Dendrophthoe pentandra (Mistletoe): Phytomorphology, Extraction Techniques, Phytochemicals, and Biological Activities" Processes 11, no. 8: 2348. https://doi.org/10.3390/pr11082348
APA StyleAwang, M. A., Nik Mat Daud, N. N. N., Mohd Ismail, N. I., Abdullah, F. I., & Benjamin, M. A. Z. (2023). A Review of Dendrophthoe pentandra (Mistletoe): Phytomorphology, Extraction Techniques, Phytochemicals, and Biological Activities. Processes, 11(8), 2348. https://doi.org/10.3390/pr11082348







