Hydrogen/Deuterium Exchange in Ambrox Could Improve the Long-Term Scent and Shelf Life of Perfumes
Abstract
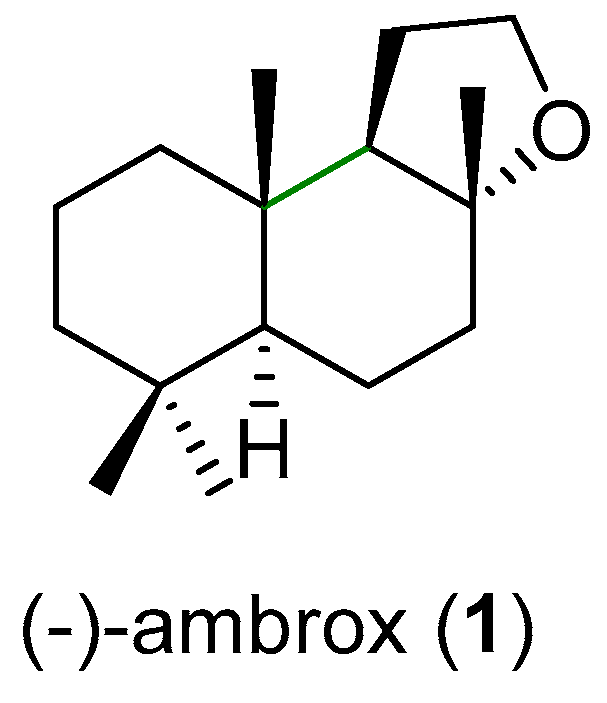
:1. Introduction
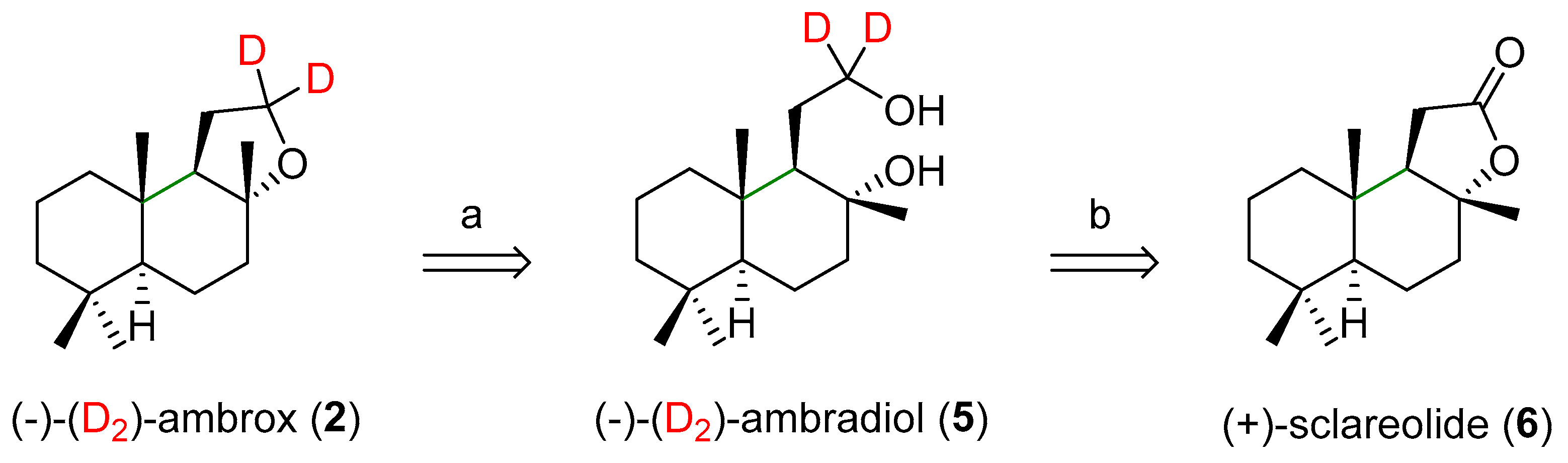
2. Future Perspectives: Synthesis Proposals for the Deuteration of Ambrox (1)
3. Modified Yamamoto’s Synthesis of Ambrox (1)
4. Modified Schaub’s Synthesis of Ambrox (1)
5. Modified Rosales Martínez’s Synthesis of Ambrox (1)
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teo, W.J.; Yang, X.; Poon, Y.Y.; Ge, S. Cobalt-catalyzed deoxygenative triborylation of allylic ethers to access 1,1,3-triborylalkanes. Nat. Commun. 2020, 11, 51932. [Google Scholar] [CrossRef]
- Matt, C.; Kern, C.; Streuff, J. Zirconium-Catalyzed Remote Defunctionalization of Alkenes. ACS Catal. 2020, 10, 6409–6413. [Google Scholar] [CrossRef]
- Weweler, J.; Younas, S.L.; Streuff, J. Titanium(III)-Catalyzed Reductive Decyanation of Geminal Dinitriles by a Non-Free-Radical Mechanism. Angew. Chem. Int. Ed. 2019, 58, 17700–17703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-Q.; Poppel, C.; Panfilova, A.; Bohle, F.; Grimme, S.; Gansäuer, A. SN2 Reactions at Tertiary Carbon Centers in Epoxides. Angew. Chem. Int. Ed. 2017, 56, 9719–9722. [Google Scholar] [CrossRef] [PubMed]
- Fra, L.; Millán, A.; Souto, J.A.; Muñiz, K. Indole Synthesis Based On A Modified Koser Reagent. Angew. Chem. Int. Ed. 2014, 53, 7349–7353. [Google Scholar] [CrossRef] [PubMed]
- Rosales, A.; Muñoz-Bascón, J.; Lopez-Sanchez, C.; Alvarez-Corral, M.; Muñoz-Dorado, M.; Rodriguez-Garcia, I.; Oltra, J.E. Ti-catalyzed homolytic opening of ozonides: A sustainable C-C bond-forming reaction. J. Org. Chem. 2012, 77, 4171–4176. [Google Scholar] [CrossRef]
- Cuerva, J.M.; Campaña, A.G.; Justicia, J.; Rosales, A.; Oller-Lopez, J.L.; Robles, R.; Cárdenas, D.J.; Buñuel, E.; Oltra, J.E. Water: The ideal hydrogen-atom source in free-radical chemistry mediated by TiIII and other single-electron-transfer metals? Angew. Chem. Int. Ed. 2006, 45, 5522–5526. [Google Scholar] [CrossRef]
- Hartmann, B.; Müller, M.; Seyler, L.; Bäuerle, T.; Wilferth, T.; Avdievitch, N.; Ruhm, L.; Henning, A.; Lesiv, P.; Ivashkin, P.; et al. Feasibility of deuterium magnetic resonance spectroscopy of 3-O-Methylglucose at 7 Tesla. PLoS ONE 2021, 16, e0252935. [Google Scholar] [CrossRef]
- Kostyukevich, Y.; Acter, T.; Zherebker, A.; Ahmed, A.; Kim, S.; Nikolaev, E. Hydrogen/deuterium exchange in mass spectrometry. Mass Spec. Rev. 2018, 37, 811–853. [Google Scholar] [CrossRef]
- MacCarthy, P. Infrared Spectroscopy of Deuterated Compounds. J. Chem. Educ. 1985, 62, 633–634. [Google Scholar] [CrossRef]
- Hewavitharana, A.K. Matrix matching in liquid chromatography-mass spectrometry with stable isotope labelled internal standards—Is it necessary? J. Chromatogr. A 2011, 1218, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.-K.; Shi, H. Catalytic Hydrogen Isotope Exchange Reactions in Late-Stage Functionalization. Synlett 2022, 33, 329–338. [Google Scholar]
- Atzrodt, J.; Derdau, V.; Kerr, W.J.; Reid, M. Deuterium- and Tritium-Labelled Compounds: Applications in the Life Sciences. Angew. Chem. 2018, 130, 1774–1802. [Google Scholar] [CrossRef]
- Prakash, G.; Paul, N.; Oliver, G.A.; Werz, D.B.; Maiti, D. C–H deuteration of organic compounds and potential drug candidates. Chem. Soc. Rev. 2022, 51, 3123–3163. [Google Scholar] [CrossRef] [PubMed]
- Pirali, T.; Serafini, M.; Cargnin, S.; Genazzani, A. Applications of Deuterium in Medicinal Chemistry. J. Med. Chem. 2019, 62, 5276–5297. [Google Scholar] [CrossRef] [PubMed]
- Cargnin, S.; Serafini, M.; Pirali, T. A primer of deuterium in drug design. Future Med. Chem. 2019, 16, 2039–2042. [Google Scholar] [CrossRef]
- Mullard, A. Deuterated drugs draw heavier backing. Nat. Rev. Drug Discov. 2016, 15, 219–221. [Google Scholar] [CrossRef]
- Murray, A., III; Williams, D.L. Organic Syntheses with Isotopes; Part II; Interscience Publishers: New York, NY, USA; London, UK, 1958. [Google Scholar]
- Ma, S.; Villa, G.; Thuy-Boun, P.S.; Homs, A.; Yu, J.-Q. Palladium-Catalyzed ortho-Selective C-H Deuteration of Arenes: Evidence for Superior Reactivity of Weakly Coordinated Palladacycles. Angew. Chem. Int. Ed. 2014, 53, 734–737. [Google Scholar] [CrossRef]
- Takakahashi, M.; Oshima, K.; Matsubara, S. Ruthenium catalyzed deuterium labelling of alpha-carbon in primary alcohol and primary/secondary amine in D2O. Chem. Lett. 2005, 34, 192–193. [Google Scholar] [CrossRef]
- Zhou, J.; Hartwig, J.F. Iridium-catalyzed H/D exchange at vinyl roups without olefin isomerization. Angew. Chem. Int. Ed. 2008, 47, 5783–5787. [Google Scholar] [CrossRef]
- Yung, C.M.; Skaddan, M.B.; Bergman, R.G. Stoichiometric and Catalytic H/D Incorporation by Cationic Iridium Complexes: A Common Monohydrido-Iridium Intermediate. J. Am. Chem. Soc. 2004, 126, 13033–13043. [Google Scholar] [CrossRef]
- Rosales, A.; Rodríguez-García, I. Cp2TiCl/D2 O/Mn, a formidable reagent for the deuteration of organic compounds. Beilstein J. Org. Chem. 2016, 12, 1585–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosales Martínez, A.; Pozo Morales, L.; Díaz Ojeda, E.; Castro Rodríguez, M.; Rodríguez-García, I. The Proven Versatility of Cp2TiCl. J. Org. Chem. 2021, 86, 1311–1329. [Google Scholar] [CrossRef] [PubMed]
- Szostak, M.; Spain, M.; Procter, D.J. Selective Synthesys of α,α-Dideuterio Alcohols by the Reduction of Carboxylic Acids Using SmI2 and D2 O as Deuterium Source under SET Conditions. Org. Lett. 2014, 16, 5052–5055. [Google Scholar] [CrossRef]
- Henriques, D.S.G.; Rojo-Wiechel, E.; Klare, S.; Mika, R.; Höthker, S.; Schacht, J.H.; Schmickler, N.; Gansäuer, A. Titanocene(III)-Catalyzed Precision Deuteration of Epoxides. Angew. Chem. Int. Ed. 2022, 61, e202114198. [Google Scholar] [CrossRef] [PubMed]
- Frater, G.; Bajgrowicz, J.A.; Kraft, P. Fragrance chemistry. Tetrahedron 1998, 54, 7633–7703. [Google Scholar] [CrossRef]
- Lederer, E.; Marx, F.; Mercier, D.; Pérot, G. Sur les constituants de l’ambre gris II. Ambréine et Coprostanone. Helv. Chim. Acta. 1946, 29, 1354–1365. [Google Scholar] [CrossRef]
- Stoll, M.; Hinder, M. Odeur et Constitution III. Les substances bicyclohomofarnésiques. Helv. Chim. Acta 1950, 33, 1251–1260. [Google Scholar] [CrossRef]
- Ohloff, G. In fragrance chemistry. In The Science of the Sense of Smell; Theimer, E.T., Ed.; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Herrmann, A. The Chemistry and Biology of Volatiles; John Wiley & Sons: Chichester, UK, 2010. [Google Scholar]
- Epstein, J.L.; Castaldi, M.; Patel, G.; Telidecki, P.; Karakkatt, K. Using Flavor Chemistry to Design and Synthesize Artificial Scents and Flavors. J. Chem. Educ. 2014, 92, 954–957. [Google Scholar] [CrossRef]
- Francl, M. Scents and sensibility. Nat. Chem. 2015, 7, 265–266. [Google Scholar]
- Goss, K.-U. The physical chemistry of odors—Consequences for the work with detection dogs. Forensic Sci. Int. 2019, 296, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kukkar, D.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Potential Use of Polymers and their Complexes as Media for Storage and Delivery of Fragrances. J. Control. Release 2018, 285, 81–95. [Google Scholar] [CrossRef]
- Rodrigues, S.N.; Martins, I.M.; Fernandes, I.P.; Gomes, P.B.; Mata, V.G.; Barreiro, M.F.; Rodrigues, A.E. Scentfashion®: Microencapsulated Perfumes for Textile Application. Chem. Eng. J. 2009, 149, 463–472. [Google Scholar] [CrossRef]
- Martins, I.M.; Barreiro, M.F.; Coelho, M.; Rodrigues, A.E. Microencapsulation of Essential Oils with Biodegradable Polymeric Carriers for Cosmetic Applications. Chem. Eng. J. 2014, 245, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Hofmeister, I.; Landfester, K.; Taden, A. Controlled Formation of Polymer Nanocapsules with High Diffusion-Barrier Properties and Prediction of Encapsulation Efficiency. Angew. Chem. Int. Ed. 2015, 54, 327–330. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M. Sol-gel Microencapsulation of Odorants and Flavors: Opening the Route to Sustainable Fragrances and Aromas. Chem. Soc. Rev. 2013, 42, 9243–9250. [Google Scholar] [CrossRef]
- López-Sánchez, J.; Alajarin, M.; Pastor, A.; Berna, J. Mechanically Interlocked Profragances for the Controlled Release of Scents. J. Org. Chem. 2021, 86, 15045–15054. [Google Scholar] [CrossRef]
- Saura-Sanmartín, A.; Andreu-Ardil, L. Recent Advances in the Preparation of Delivery Systems for the Controlled Release of Scents. Int. J. Mol. Sci. 2023, 24, 4685. [Google Scholar] [CrossRef]
- Rosales, A.; Foley, L.A.R.; Padial, N.M.; Muñoz-Bascón, J.; Sancho-Sanz, I.; Roldán-Molina, E.; Pozo-Morales, L.; Irías-Álvarez, A.; Rodríguez-Maecker, R.; Rodríguez-García, I.; et al. Diastereoselective Synthesis of (±)-ambrox by Titanium(III)-Catalyzed Radical Tandem Cyclization. Synlett 2016, 27, 369–374. [Google Scholar] [CrossRef]
- Chapuis, C.; Cantatore, C.; Fankhauser, P.; Challand, R.; Riedhauser, J.-J. Synthesis of Deuterium-Labeled Perfume Ingredients as Internal Standards for Their GC/MS Quantification. Helv. Chim. Acta 2009, 92, 1782–1799. [Google Scholar] [CrossRef]
- Gane, S.; Georganakis, D.; Maniati, K.; Vamvakias, M.; Ragoussis, N.; Skoulakis, E.M.C.; Turin, L. Molecular Vibration-Sensing Component in Human Olfaction. PLoS ONE 2013, 8, e55780. [Google Scholar] [CrossRef]
- Ncube, E.N.; Steenkamp, L.; Dubery, I.A. Ambrafuran (AmbroxTM) Synthesis from Natural Plant Product Precursors. Molecules 2020, 25, 3851. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tian, H.; Sun, B.; Liu, Y.; Hao, Y.; Lv, Y. One-pot synthesis of (-)-Ambrox. Sci. Rep. 2016, 6, 32650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, J.; Blüthner, W.-D. Medicinal, Aromatic and Stimulant Plants. In Handbook of Plant Breeding; Chapter 12; Springer Nature: Basel, Switzerland, 2020. [Google Scholar]
- Ishihara, K.; Ishibashi, H.; Yamamoto, H. Enantio- and Diastereoselective Stepwise Cyclization of Polyprenoids Induced by Chiral and Achiral LBAs. A New Entry to (−)-Ambrox, (+)-Podocarpa-8,11,13-triene Diterpenoids, and (−)-Tetracyclic Polyprenoid of Sedimentary Origin. J. Am. Chem. Soc. 2002, 124, 3647–3655. [Google Scholar] [CrossRef] [PubMed]
- Zubar, V.; Lichtenberger, N.; Schelwies, M.; Oeser, T.; Hashmi, A.S.K.; Schaub, T. Manganese-Catalyzed Hydrogenation of Sclareolide to Ambradiol. ChemCatChem 2022, 14, e202101443. [Google Scholar] [CrossRef]
- D’Acunto, M.; Monica, C.D.; Izzo, I.; De Petrocellis, L.; di Marzo, V.; Spinella, A. Enantioselective synthesis of 3(S)-hydroxy polygodial derivatives and evaluation of their vanilloid activity. Tetrahedron 2010, 66, 9785–9789. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales Martínez, A.; Rodríguez-García, I. Hydrogen/Deuterium Exchange in Ambrox Could Improve the Long-Term Scent and Shelf Life of Perfumes. Processes 2023, 11, 2358. https://doi.org/10.3390/pr11082358
Rosales Martínez A, Rodríguez-García I. Hydrogen/Deuterium Exchange in Ambrox Could Improve the Long-Term Scent and Shelf Life of Perfumes. Processes. 2023; 11(8):2358. https://doi.org/10.3390/pr11082358
Chicago/Turabian StyleRosales Martínez, Antonio, and Ignacio Rodríguez-García. 2023. "Hydrogen/Deuterium Exchange in Ambrox Could Improve the Long-Term Scent and Shelf Life of Perfumes" Processes 11, no. 8: 2358. https://doi.org/10.3390/pr11082358
APA StyleRosales Martínez, A., & Rodríguez-García, I. (2023). Hydrogen/Deuterium Exchange in Ambrox Could Improve the Long-Term Scent and Shelf Life of Perfumes. Processes, 11(8), 2358. https://doi.org/10.3390/pr11082358








