Pulsed Laser Deposition of Carbon-Based Materials: A Focused Review of Methods and Results
Abstract
:1. Introduction
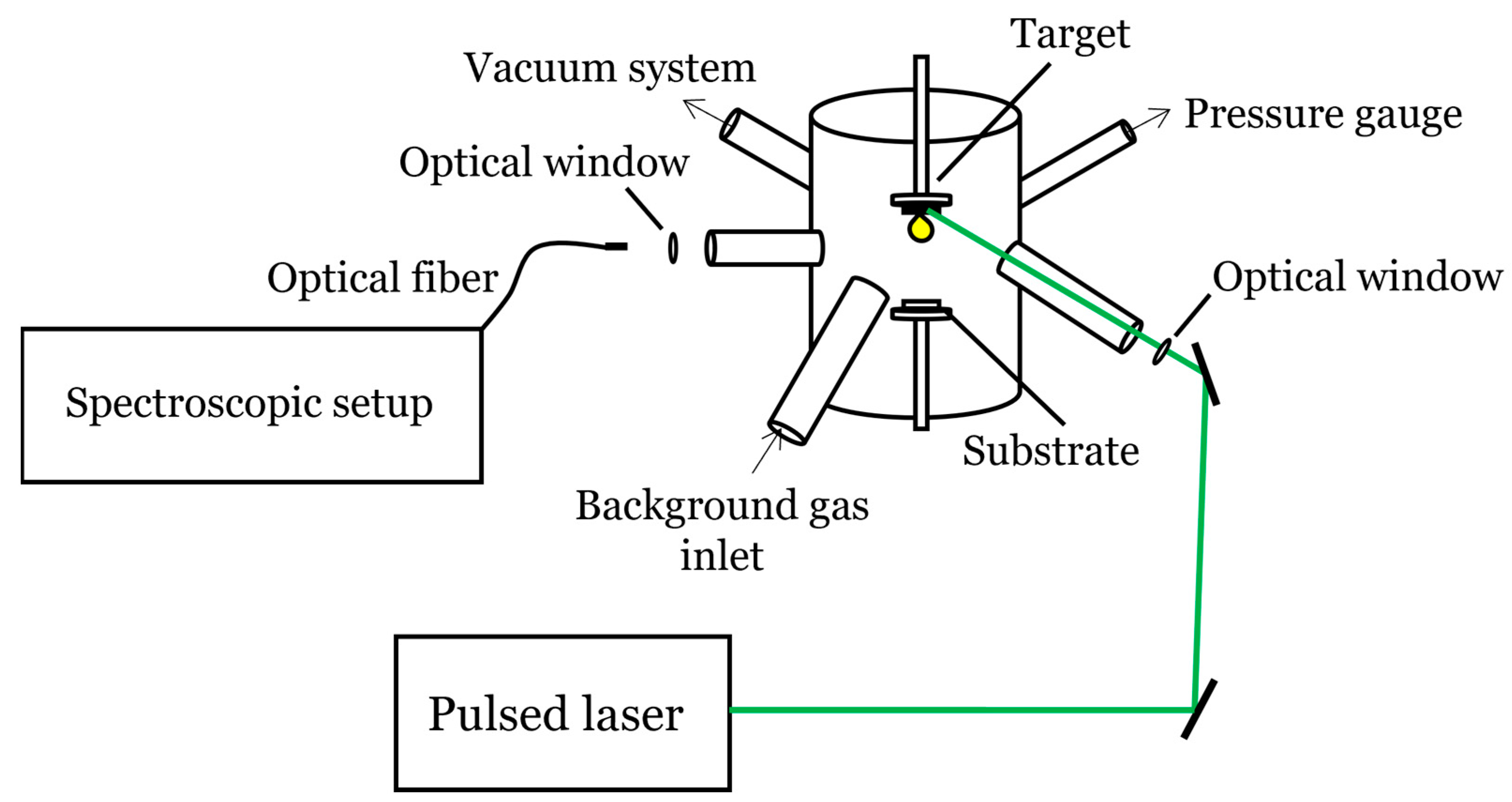
2. Fundamental Concepts of PLD
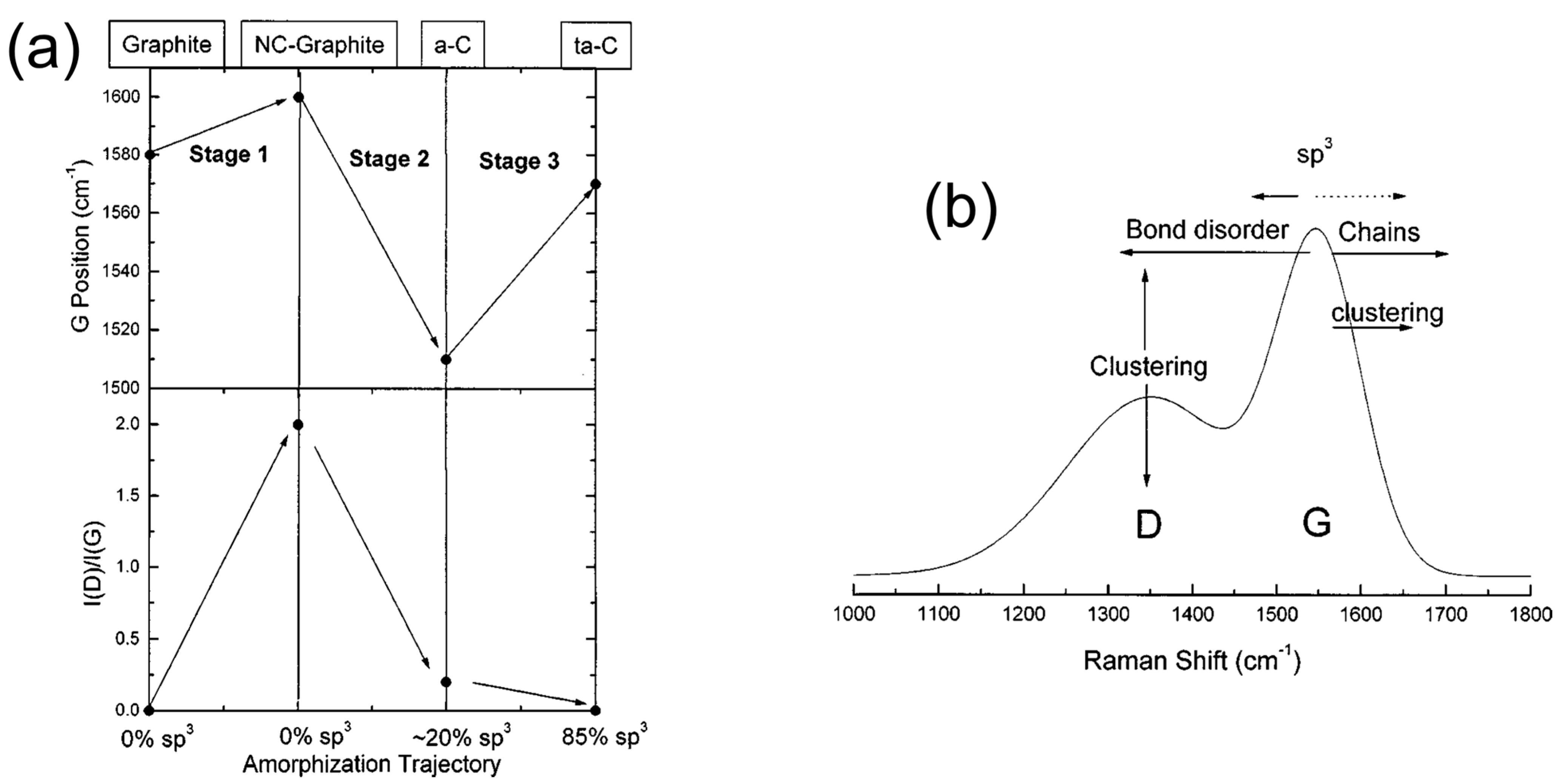
3. PLD of Diamond-like Carbon
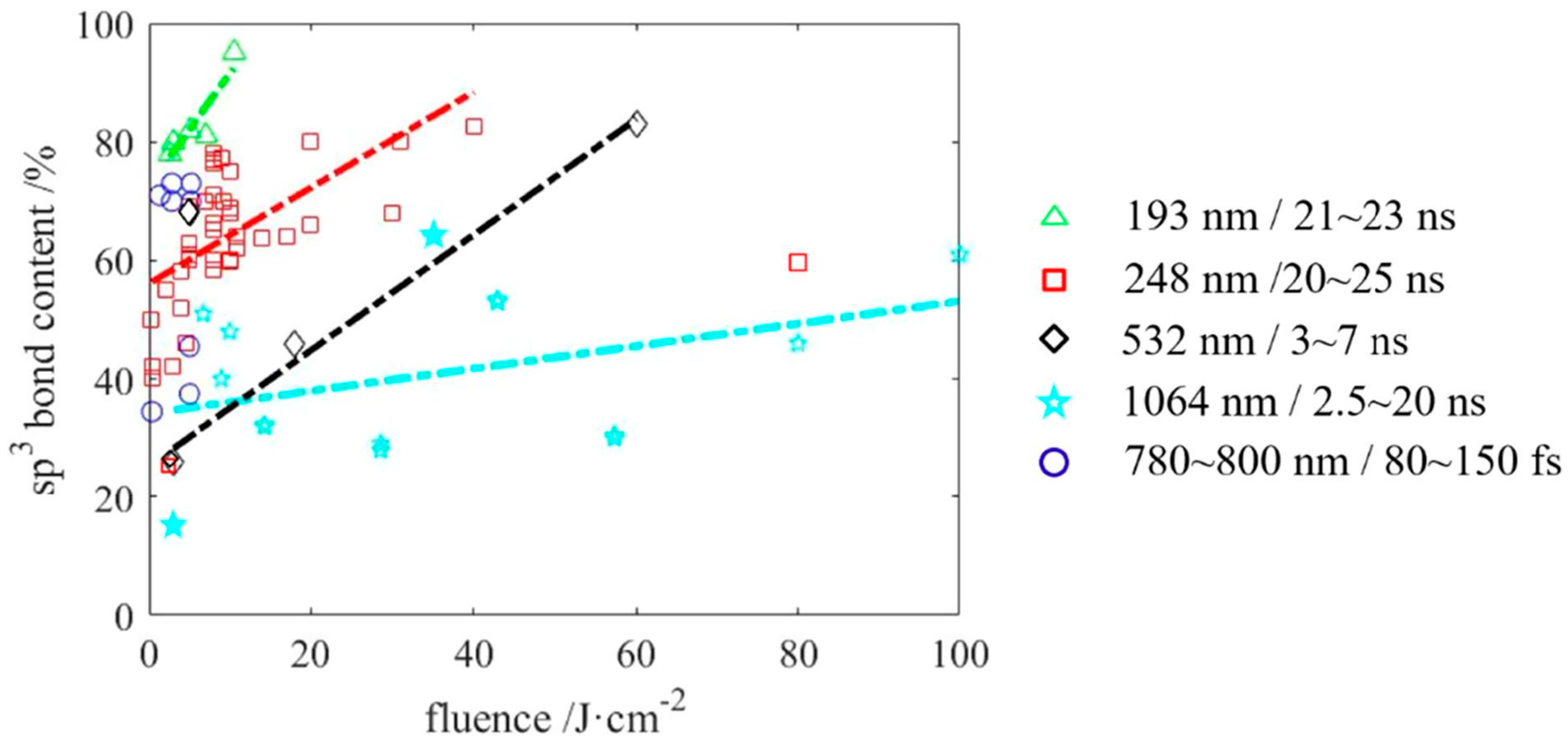
3.1. Laser Wavelength, Fluence and Pulse Width
3.2. Additional Sources of Energy
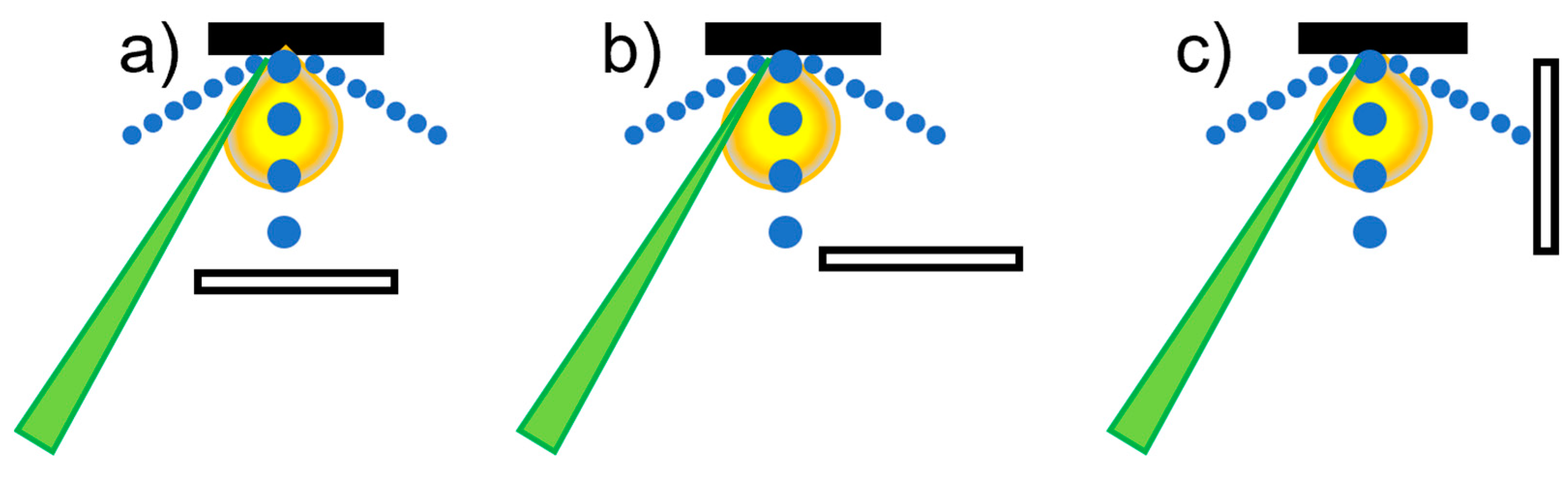
3.3. Type and Pressure of Background Gas
3.4. Effect of Target and Substrate Quality, Distance and Relative Orientation
4. PLD of Graphene
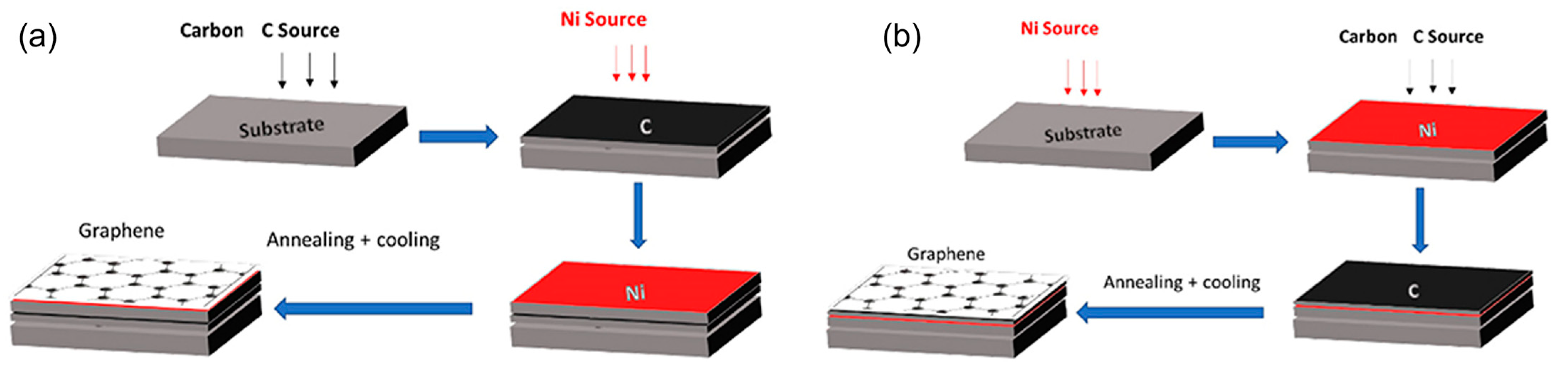
4.1. PLD of Graphene with and without Metal Catalysts
4.2. Effect of Laser Parameters and Deposition Conditions
4.3. PLD of Graphene Derivatives
5. PLD of Carbyne/Carbon-Atom Wires (CAW)
6. Conclusions and Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Levi, P. The Periodic Table; Penguin Publishing: London, UK, 1975. [Google Scholar]
- Thapliyal, V.; Alabdulkarim, M.E.; Whelan, D.R.; Mainali, B.; Maxwell, J.L. A concise review of the Raman spectra of carbon allotropes. Diam. Relat. Mater. 2022, 127, 109180. [Google Scholar] [CrossRef]
- dos Santos, M.C.; Maynart, M.C.; Aveiro, L.R.; da Paz, E.C.; dos Santos Pinheiro, V. Carbon-Based Materials: Recent Advances, Challenges, and Perspectives. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Saba, N.; Jawaid, M.; Fouad, H.; Alothman, O.Y. Nanocarbon: Preparation, properties, and applications. In Nanocarbon and Its Composites; Khan, A., Jawaid, M.I., Asiri, A.M., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2019; pp. 327–354. [Google Scholar]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Chalifoux, W.A.; Tykwinski, R.R. Synthesis of extended polyynes: Toward carbyne. Comptes Rendus Chimie 2009, 12, 341–358. [Google Scholar] [CrossRef]
- Chalifoux, W.A.; Tykwinski, R.R. Synthesis of polyynes to model the sp-carbon allotrope carbyne. Nat. Chem. 2010, 2, 967–971. [Google Scholar] [CrossRef]
- Vetter, J. 60 years of DLC coatings: Historical highlights and technical review of cathodic arc processes to synthesize various DLC types, and their evolution for industrial applications. Surf. Coat. Technol. 2014, 257, 213–240. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.-M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef] [PubMed]
- Sivamaran, V.; Balasubramanian, V.; Gopalakrishnan, M.; Viswabaskaran, V.; Gourav Rao, A.; Selvamani, S. Carbon nanotubes, nanorings, and nanospheres: Synthesis and fabrication via chemical vapor deposition—A review. Nanomater. Nanotechnol. 2022, 12, 18479804221079495. [Google Scholar] [CrossRef]
- Wang, X.; Sui, X.; Zang, S.; Yan, M.; Yang, J.; Hao, J.; Liu, W. Effect of deposition pressures on uniformity, mechanical and tribological properties of thick DLC coatings inside of a long pipe prepared by PECVD method. Surf. Coat. Technol. 2019, 375, 150–157. [Google Scholar] [CrossRef]
- Li, A.; Li, X.; Wang, Y.; Lu, Z.; Wang, Y.; Zhang, G.; Wu, Z. Investigation of mechanical and tribological properties of super-thick DLC films with different modulation ratios prepared by PECVD. Mater. Res. Express 2019, 6, 086433. [Google Scholar] [CrossRef]
- Devi, M.; Rawat, S.; Sharma, S. A comprehensive review of the pyrolysis process: From carbon nanomaterial synthesis to waste treatment. Oxf. Open Mater. Sci. 2021, 1, itab014. [Google Scholar] [CrossRef]
- Aguilar-Elguézabal, A.; Antúnez, W.; Alonso, G.; Paraguay Delgado, F.; Espinosa, F.; Miki-Yoshida, M. Study of carbon nanotubes synthesis by spray pyrolysis and model of growth. Diam. Relat. Mater. 2006, 15, 1329–1335. [Google Scholar] [CrossRef]
- Illakkiya, J.T.; Rajalakshmi, P.U.; Oommen, R. Nebulized spray pyrolysis: A new method for synthesis of graphene film and their characteristics. Surf. Coat. Technol. 2016, 307, 65–72. [Google Scholar] [CrossRef]
- Cai, J.; Han, X.; Wang, X.; Meng, X. Atomic Layer Deposition of Two-Dimensional Layered Materials: Processes, Growth Mechanisms, and Characteristics. Matter 2020, 2, 587–630. [Google Scholar] [CrossRef] [Green Version]
- Vervuurt, R.H.J.; Kessels, W.M.M.; Bol, A.A. Atomic Layer Deposition for Graphene Device Integration. Adv. Mater. Interfaces 2017, 4, 1700232. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Kisslinger, K.; Monikandan, R. Atomic Layer Deposition of Nanolayered Carbon Films. C 2021, 7, 67. [Google Scholar] [CrossRef]
- Marichy, C.; Pinna, N. Carbon-nanostructures coated/decorated by atomic layer deposition: Growth and applications. Coordin. Chem. Rev. 2013, 257, 3232–3253. [Google Scholar] [CrossRef]
- Chowdhury, S.; Laugier, M.T.; Rahman, I.Z. Characterization of DLC coatings deposited by rf magnetron sputtering. J. Mater. Process. Technol. 2004, 153–154, 804–810. [Google Scholar] [CrossRef]
- Fiaschi, G.; Rota, A.; Ballestrazzi, A.; Marchetto, D.; Vezzalini, E.; Valeri, S. A Chemical, Mechanical, and Tribological Analysis of DLC Coatings Deposited by Magnetron Sputtering. Lubricants 2019, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Stankus, V.; Vasiliauskas, A.; Guobienė, A.; Andrulevičius, M.; Meškinis, Š. Direct synthesis of graphene on silicon by reactive magnetron sputtering deposition. Surf. Coat. Technol. 2022, 437, 128361. [Google Scholar] [CrossRef]
- Nakajima, Y.; Murata, H.; Saitoh, N.; Yoshizawa, N.; Suemasu, T.; Toko, K. Low-Temperature (400 °C) Synthesis of Multilayer Graphene by Metal-Assisted Sputtering Deposition. ACS Omega 2019, 4, 6677–6680. [Google Scholar] [CrossRef] [PubMed]
- Presel, F.; Tetlow, H.; Bignardi, L.; Lacovig, P.; Tache, C.A.; Lizzit, S.; Kantorovich, L.; Baraldi, A. Graphene growth by molecular beam epitaxy: An interplay between desorption, diffusion and intercalation of elemental C species on islands. Nanoscale 2018, 10, 7396–7406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, J.M.J. MBE Growth of Graphene. In Molecular Beam Epitaxy: Materials and Applications for Electronics and Optoelectronics; Asahi, H., Horikoshi, Y., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 395–409. [Google Scholar]
- Albar, J.D.; Summerfield, A.; Cheng, T.S.; Davies, A.; Smith, E.F.; Khlobystov, A.N.; Mellor, C.J.; Taniguchi, T.; Watanabe, K.; Foxon, C.T.; et al. An atomic carbon source for high temperature molecular beam epitaxy of graphene. Sci. Rep. 2017, 7, 6598. [Google Scholar] [CrossRef] [Green Version]
- Chrisey, D.B.; Hubler, G.K. (Eds.) Pulsed Laser Deposition of Thin Films; John Wiley & Sons: Hoboken, NJ, USA, 1994. [Google Scholar]
- Eason, R. (Ed.) Pulsed Laser Deposition of Thin Films: Applications-Led Growth of Functional Materials; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Ashfold, M.N.R.; Claeyssens, F.; Fuge, G.M.; Henley, S.J. Pulsed laser ablation and deposition of thin films. Chem. Soc. Rev. 2004, 33, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepelin, N.A.; Tehrani, Z.P.; Ohannessian, N.; Schneider, C.W.; Pergolesi, D.; Lippert, T. A practical guide to pulsed laser deposition. Chem. Soc. Rev. 2023, 52, 2294–2321. [Google Scholar] [CrossRef]
- Masood, K.B.; Kumar, P.; Malik, M.A.; Singh, J. A comprehensive tutorial on the pulsed laser deposition technique and developments in the fabrication of low dimensional systems and nanostructures. Emergent Mater. 2021, 4, 737–754. [Google Scholar] [CrossRef]
- Haider, A.J.; Alawsi, T.; Haider, M.J.; Taha, B.A.; Marhoon, H.A. A comprehensive review on pulsed laser deposition technique to effective nanostructure production: Trends and challenges. Opt. Quant. Electron. 2022, 54, 488. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Tite, T.; Loir, A.-S.; Maddi, C.; Donnet, C.; Garrelie, F. Review of Graphene Growth from a Solid Carbon Source by Pulsed Laser Deposition (PLD). Front. Chem. 2018, 6, 572. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Huang, G.; Wang, S.; Mi, C.; Wei, S.; Tian, F.; Li, W.; Cao, H.; Cheng, Y. A review on diamond-like carbon films grown by pulsed laser deposition. Appl. Surf. Sci. 2021, 541, 148573. [Google Scholar] [CrossRef]
- Casari, C.S.; Tommasini, M.; Tykwinski, R.R.; Milani, A. Carbon-atom wires: 1-D systems with tunable properties. Nanoscale 2016, 8, 4414–4435. [Google Scholar] [CrossRef] [Green Version]
- Radziemski, L.J.; Cremers, D.A. (Eds.) Laser-Induced Plasma and Applications; Marcel Dekker: New York, NY, USA, 1989. [Google Scholar]
- Phipps, C. (Ed.) Laser Ablation and Its Applications; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Wen, S.B.; Mao, X.; Greif, R.; Russo, R.E. Laser ablation induced vapor plume expansion into a background gas. I. Analysis. J. Appl. Phys. 2007, 101, 023114. [Google Scholar] [CrossRef]
- Wen, S.B.; Mao, X.; Greif, R.; Russo, R.E. Laser ablation induced vapor plume expansion into a background gas. II. Experimental analysis. J. Appl. Phys. 2007, 101, 023115. [Google Scholar] [CrossRef]
- Arnold, N.; Gruber, J.; Heitz, J. Spherical expansion of the vapor plume into ambient gas: An analytical model. Appl. Phys. A 1999, 69, S87–S93. [Google Scholar] [CrossRef]
- Aragón, C.; Aguilera, J.A. Characterization of laser induced plasmas by optical emission spectroscopy: A review of experiments and methods. Spectrochim. Acta B 2008, 63, 893–916. [Google Scholar] [CrossRef]
- Hahn, D.W.; Omenetto, N. Laser-Induced Breakdown Spectroscopy (LIBS), Part I: Review of Basic Diagnostics and Plasma–Particle Interactions: Still-Challenging Issues within the Analytical Plasma Community. Appl. Spectrosc. 2010, 64, 335A–366A. [Google Scholar] [CrossRef] [Green Version]
- Hahn, D.W.; Omenetto, N. Laser-induced breakdown spectroscopy (LIBS), part II: Review of instrumental and methodological approaches to material analysis and applications to different fields. Appl. Spectrosc. 2012, 66, 347–419. [Google Scholar] [CrossRef]
- Gaudiuso, R.; Dell’Aglio, M.; De Pascale, O.; Senesi, G.S.; De Giacomo, A. Laser Induced Plasma Spectroscopy for elemental analysis in environmental, cultural heritage and space applications: A review of methods and results. Sensors 2010, 10, 7434. [Google Scholar] [CrossRef] [Green Version]
- Musazzi, S.; Perrini, U. Laser-Induced Breakdown Spectroscopy: Theory and Applications; Springer Series in Optical Sciences; Springer: Berlin/Heidelberg, Germany, 2014; Volume 182. [Google Scholar]
- Gaudiuso, R.; Melikechi, N.; Abdel-Salam, Z.A.; Harith, M.A.; Palleschi, V.; Motto-Ros, V.; Busser, B. Laser-induced breakdown spectroscopy for human and animal health: A review. Spectrochim. Acta B 2019, 152, 123–148. [Google Scholar] [CrossRef]
- Jantzi, S.C.; Motto-Ros, V.; Trichard, F.; Markushin, Y.; Melikechi, N.; DeGiacomo, A. Sample Treatment and Preparation for Laser-Induced Breakdown Spectroscopy. Spectrochim. Acta B 2016, 115, 52–63. [Google Scholar] [CrossRef]
- Wang, Z.; Afgan, M.S.; Gu, W.; Song, Y.; Wang, Y.; Hou, Z.; Song, W.; Li, Z. Recent advances in laser-induced breakdown spectroscopy quantification: From fundamental understanding to data processing. Trends Anal. Chem. 2021, 143, 116385. [Google Scholar] [CrossRef]
- Singh, J.P.; Thakur, S.N. Laser-Induced Breakdown Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- De Giacomo, A.; Dell’Aglio, M.; Gaudiuso, R.; Amoruso, S.; De Pascale, O. Effects of the background environment on formation, evolution and emission spectra of laser-induced plasmas. Spectrochim. Acta B 2012, 78, 1. [Google Scholar] [CrossRef]
- Effenberger, A.J., Jr.; Scott, J.R. Effect of Atmospheric Conditions on LIBS Spectra. Sensors 2010, 10, 4907–4925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yogesh, G.K.; Shukla, S.; Sastikumar, D.; Koinkar, P. Progress in pulsed laser ablation in liquid (PLAL) technique for the synthesis of carbon nanomaterials: A review. Appl. Phys. A 2021, 127, 810. [Google Scholar] [CrossRef]
- De Giacomo, A.; De Bonis, A.; Dell’Aglio, M.; De Pascale, O.; Gaudiuso, R.; Orlando, S.; Santagata, A.; Senesi, G.S.; Taccogna, F.; Teghil, R. Laser Ablation of Graphite in water in a range of pressure from 1 to 146 atm using single and double pulse techniques for the production of carbon nanostructures. J. Phys. Chem. C 2011, 115, 5123. [Google Scholar] [CrossRef]
- Santagata, A.; De Bonis, A.; De Giacomo, A.; Dell’Aglio, M.; Laurita, A.; Senesi, G.S.; Gaudiuso, R.; Orlando, S.; Teghil, R.; Parisi, G. Carbon-Based Nanostructures Obtained in Water by Ultrashort Laser Pulses. J. Phys. Chem. C 2011, 115, 5160. [Google Scholar] [CrossRef]
- Russo, P.; Hu, A.; Compagnini, G.; Duley, W.W.; Zhou, N.Y. Femtosecond laser ablation of highly oriented pyrolytic graphite: A green route for large-scale production of porous graphene and graphene quantum dots. Nanoscale 2014, 6, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Compagnini, G.; Mita, V.; Cataliotti, R.S.; Puglisi, O. Short polyyne chains produced by pulsed laser ablation of graphite in water. Carbon 2007, 45, 2456–2458. [Google Scholar] [CrossRef]
- Yang, G.W. Laser ablation in liquids: Applications in the synthesis of nanocrystals. Prog. Mater. Sci. 2007, 52, 648–698. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Gaudiuso, R.; De Pascale, O.; De Giacomo, A. Mechanisms and processes of pulsed laser ablation in liquids during nanoparticle production. Appl. Surf. Sci. 2015, 348, 4–9. [Google Scholar] [CrossRef]
- Fazio, E.; Gökce, B.; De Giacomo, A.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials 2020, 10, 2317. [Google Scholar] [CrossRef] [PubMed]
- De Giacomo, A.; Shakhatov, V.A.; Senesi, G.S.; Orlando, S. Spectroscopic investigation of the technique of plasma assisted pulsed laser deposition of titanium dioxide. Spectrochim. Acta B 2001, 56, 1459–1472. [Google Scholar] [CrossRef]
- De Giacomo, A.; Shakhatov, V.A.; Senesi, G.S.; De Pascale, O.; Prudenzano, F. Plasma-assisted pulsed laser deposition for the improvement of the film growth process. Appl. Surf. Sci. 2002, 186, 533–537. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. 2002, R37, 129–281. [Google Scholar]
- Robertson, J. Diamond-like carbon. Pure Appl. Chem. 1994, 66, 1789–1796. [Google Scholar] [CrossRef]
- Niemczyk, A.; Moszynski, D.; Goszczyńska, A.; Kwiatkowska, M.; Jedrzejczak, A.; Nowak, D.; Sośnicki, J.G.; El Fray, M.; Baranowska, J. Understanding the DLC film—Polyamide 12 substrate interrelation during pulsed laser deposition. Appl. Surf. Sci. 2022, 576 Part B, 151872. [Google Scholar] [CrossRef]
- Yoshida, S.; Okoshi, M.; Inoue, N. Femtosecond-pulsed laser deposition of diamond-like carbon films onto silicone rubber. J. Phys. Conf. Ser. 2007, 59, 368. [Google Scholar] [CrossRef]
- Lackner, J.M.; Waldhauser, W.; Major, R.; Major, B.; Czarnowska, E.; Bruckert, F. Industrially scaled pulsed laser deposition based coating techniques for the realization of hemocompatible surfaces for blood contact applications. In High-Power Laser Ablation VII; SPIE: Cergy, France, 2008; Volume 7005, p. 70050Q. [Google Scholar]
- Cesaria, M.; Serra, A.; Manno, D.; Rizwan Aziz, M.; Rella, S.; Malitesta, C.; Martino, M.; Verwilligen, P.; Caricato, A.P. Tailoring sheet resistance through laser fluence and study of the critical impact of a V-shaped plasma plume on the properties of PLD-deposited DLC films for micro-pattern gaseous detector applications. Diam. Relat. Mater. 2022, 124, 108909. [Google Scholar] [CrossRef]
- Ursu, C.; Nica, P.; Rusu, B.G.; Focsa, C. V-shape plasma generated by excimer laser ablation of graphite in argon: Spectroscopic investigations. Spectrochim. Acta B 2020, 163, 105743. [Google Scholar] [CrossRef]
- Patsalas, P.; Kaziannis, S.; Kosmidis, C.; Papadimitriou, D.; Abadias, G.; Evangelakis, G.A. Optimized pulsed laser deposition by wavelength and static electric field control: The case of tetrahedral amorphous carbon films. J. Appl. Phys. 2007, 101, 15–18. [Google Scholar] [CrossRef]
- Xu, W.; Lin, S.; Dai, M.; Shi, Q.; Wei, C.; Zhang, X.; Zhou, K. Effects of bias voltage on the microstructure and properties of Al-doped hydrogenated amorphous carbon films synthesized by a hybrid deposition technique. Vacuum 2018, 154, 159–166. [Google Scholar] [CrossRef]
- Zemek, J.; Houdkova, J.; Jiricek, P.; Jelinek, M. Surface and in-depth distribution of sp2 and sp3 coordinated carbon atoms in diamond-like carbon films modified by argon ion beam bombardment during growth. Carbon 2018, 134, 71–79. [Google Scholar] [CrossRef]
- Jelinek, M.; Voss, A.; Kocourek, T.; Mozafari, M.; Vymetalova, V.; Zezulova, M.; Pisaik, P.; Kotzianova, A.; Popov, C.; Miksovsky, J. Comparison of the surface properties of DLC and ultrananocrystalline diamond films with respect to their bio-applications. Phys. Status Solidi A 2013, 210, 2106–2110. [Google Scholar] [CrossRef]
- Jelínek, M.; Písařík, P.; Kocourek, T.; Zemek, J.; Lukeš, J. Influence of ion bombardment on growth and properties of PLD created DLC films. Appl. Phys. A 2013, 110, 943–947. [Google Scholar] [CrossRef]
- Kocourek, T.; Jelínek, M.; Písařík, P.; Remsa, J.; Janovská, M.; Landa, M.; Zemek, J.; Havránek, V. Diamond-like carbon layers modified by ion bombardment during growth and researched by Resonant Ultrasound Spectroscopy. Appl. Surf. Sci. 2017, 417, 213–217. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, Y.; Huang, G.; Xi, L.; Wang, S.; Wei, S.; Mi, C. Effects of external magnetic field on the micro-structure of diamondlike carbon film prepared by pulsed laser deposition. Mater. Res. Express 2019, 6, 116433. [Google Scholar] [CrossRef]
- Modabberasl, A.; Kameli, P.; Ranjbar, M.; Salamati, H.; Ashiri, R. Fabrication of DLC thin films with improved diamond-like carbon character by the application of external magnetic field. Carbon 2015, 94, 485–493. [Google Scholar] [CrossRef]
- Garrelie, F.; Bourquard, F.; Loir, A.-S.; Donnet, C.; Colombier, J.-P. Control of femtosecond pulsed laser ablation and deposition by temporal pulse shaping. Opt. Laser Technol. 2016, 78, 42–51. [Google Scholar] [CrossRef]
- Jegenyes, N.; Toth, Z.; Hopp, B.; Klebniczki, J.; Bor, Z.; Fotakis, C. Femtosecond pulsed laser deposition of diamond-like carbon films: The effect of double laser pulses. Appl. Surf. Sci. 2006, 252, 4667–4671. [Google Scholar] [CrossRef]
- Jelinek, M.; Zemek, J.; Remsa, J.; Miksovsky, J.; Kocourek, T.; Pisarik, P.; Travnickova, M.; Filova, E.; Bacakova, L. Hybrid laser technology and doped biomaterials. Appl. Surf. Sci. 2017, 417, 73–83. [Google Scholar] [CrossRef]
- Constantinou, M.; Pervolaraki, M.; Nikolaou, P.; Prouskas, C.; Patsalas, P.; Kelires, P.; Giapintzakis, J.; Constantinides, G. Microstructure and nanomechanical properties of pulsed excimer laser deposited DLC: Ag films: Enhanced nanotribological response. Surf. Coat. Technol. 2017, 309, 320–330. [Google Scholar] [CrossRef]
- Pisarik, P.; Jelinek, M.; Remsa, J.; Mikšovsky, J.; Zemek, J.; Jurek, K.; Kubinova, S.; Lukes, J.; Sepitka, J. Antibacterial, mechanical and surface properties of Ag-DLC films prepared by dual PLD for medical applications. Mater. Sci. Eng. C 2017, 77, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Foong, Y.M.; Koh, A.T.T.; Chua, D.H.C. Experimental and theoretical study on the energy-dependent surface evolution and microstructure changes in copper nanostructured composites. J. Phys. D Appl. Phys. 2011, 44, 385401. [Google Scholar] [CrossRef]
- Constantinou, M.; Pervolaraki, M.; Koutsokeras, L.; Prouskas, C.; Patsalas, P.; Kelires, P.; Giapintzakis, J.; Constantinides, G. Enhancing the nanoscratch resistance of pulsed laser deposited DLC films through molybdenum-doping. Surf. Coat. Technol. 2017, 330, 185–195. [Google Scholar] [CrossRef]
- Jelinek, M.; Kocourek, T.; Zemek, J.; Miksovsky, J.; Kubinova, S.; Remsa, J.; Kopecek, J.; Jurek, K. Chromium-doped DLC for implants prepared by laser-magnetron deposition. Mater. Sci. Eng. C 2015, 46, 381–386. [Google Scholar] [CrossRef]
- Jelinek, M.; Zemek, J.; Vandrovcova, M.; Bacakova, L.; Kocourek, T.; Remsa, J.; Pisarik, P. Bonding and bio-properties of hybrid laser/magnetron Cr-enriched DLC layers. Mater. Sci. Eng. C 2016, 58, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, I.; Khare, A. Optical nonlinearity in nanostructured carbon thin films fabricated by pulsed laser deposition technique. Thin Solid Films 2016, 611, 56–61. [Google Scholar] [CrossRef]
- Rau, J.V.; Teghil, R.; De Bonis, A.; Generosi, A.; Paci, B.; Generosi, R.; Fosca, M.; Ferro, D.; Rossi Albertini, V.; Chilingarov, N.S. Pulsed laser deposition of hard and superhard carbon thin films from C60 targets. Diam. Relat. Mater. 2010, 19, 7–14. [Google Scholar] [CrossRef]
- Modabber Asl, A.; Kameli, P.; Ranjbar, M.; Salamati, H.; Jannesari, M. Correlations between microstructure and hydrophobicity properties of pulsed laser deposited diamond-like carbon films. Superlatt. Microstruct. 2015, 81, 64–79. [Google Scholar] [CrossRef]
- Salah, N.; Alshahrie, A.; Iqbal, J.; Hasan, P.M.Z.; Abdel-Wahab, M.S. Tribological behavior of diamond-like carbon thin films deposited by the pulse laser technique at different substrate temperatures. Tribol. Int. 2016, 103, 274–280. [Google Scholar] [CrossRef]
- De Bonis, A.; Rau, J.V.; Santagata, A.; Teghil, R. Diamond-like carbon thin films produced by femtosecond pulsed laser deposition of fullerite. Surf. Coat. Technol. 2011, 205, 3747–3753. [Google Scholar] [CrossRef]
- Alawajji, R.A.; Kannarpady, G.K.; Nima, Z.A.; Kelly, N.; Watanabe, F.; Biris, A.S. Electrical properties of multilayer (DLC-TiC) films produced by pulsed laser deposition. Appl. Surf. Sci. 2018, 437, 429–440. [Google Scholar] [CrossRef]
- Hara, T.; Yoshitake, T.; Fukugawa, T.; yun Zhu, L.; Itakura, M.; Kuwano, N.; Tomokiyo, Y.; Nagayama, K. Nanocrystalline diamond film prepared by pulsed laser deposition in a hydrogen atmosphere. Diam. Relat. Mater. 2004, 13, 679–683. [Google Scholar] [CrossRef]
- Dementjev, A.P.; Petukhov, M.N. The roles of H and O atoms in diamond growth. Diam. Relat. Mater. 1997, 6, 486. [Google Scholar] [CrossRef]
- Budai, J.; Toth, S.; Toth, Z.; Koos, M. Diamond-like carbon films prepared by reactive pulsed laser deposition in hydrogen and methane ambient. Appl. Surf. Sci. 2007, 253, 8220–8225. [Google Scholar] [CrossRef]
- Ohmagari, S.; Yoshitake, T.; Nagano, A.; Al-Riyami, S.; Ohtani, R.; Setoyama, H.; Kobayashi, E.; Nagayama, K. Near-edge X-ray absorption fine structure of ultrananocrystalline diamond/hydrogenated amorphous carbon films prepared by pulsed laser deposition. J. Nanomater. 2009, 2009, 876561. [Google Scholar] [CrossRef] [Green Version]
- Nagano, A.; Yoshitake, T.; Hara, T.; Nagayama, K. Optical properties of ultrananocrystalline diamond/amorphous carbon composite films prepared by pulsed laser deposition. Diam. Relat. Mater. 2008, 17, 1199–1202. [Google Scholar] [CrossRef]
- Yoshitake, T.; Nagano, A.; Itakura, M.; Kuwano, N.; Hara, T.; Nagayama, K. Spectral absorption properties of ultrananocrystalline diamond/amorphous carbon composite thin films prepared by pulsed laser deposition. Jpn. J. Appl. Phys. 2007, 46, L936–L938. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Yoshida, K.; Maruta, H.; Hishitani, Y.; Koinuma, H.; Nishio, S.; Kakihana, M.; Tachibana, T. Epitaxial diamond growth on sapphire in an oxidizing environment. Nature 1999, 399, 340–342. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Furusawa, M.; Nakajima, K.; Takakura, M.; Hishitani, Y. Diamond film growth in an oxygen atmosphere. Diam. Relat. Mater. 2001, 10, 295–299. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Zhao, J.P.; Yano, T.; Ooie, T.; Yoneda, M.; Sakakibara, J. Growth of nano-crystalline diamond by pulsed laser deposition in oxygen atmosphere. J. Cryst. Growth 2001, 226, 62–66. [Google Scholar] [CrossRef]
- Yoshitake, T.; Hara, T.; Nagayama, K. The influence of the repetition rate of laser pulses on the growth of diamond thin films by pulsed laser ablation of graphite. Diam. Relat. Mater. 2003, 12, 306–309. [Google Scholar] [CrossRef]
- Yoshitake, T.; Nishiyama, T.; Nagayama, K. The role of hydrogen and oxygen gas in the growth of carbon thin films by pulsed laser deposition. Diam. Relat. Mater. 2000, 9, 689–692. [Google Scholar] [CrossRef]
- Hara, T.; Yoshitake, T.; Fukugawa, T.; yun Zhu, L.; Itakura, M.; Kuwano, N.; Tomokiyo, Y.; Nagayama, K. Consideration of diamond film growth on various orientation substrates of diamond in oxygen and hydrogen atmospheres by reactive pulsed laser deposition. Diam. Relat. Mater. 2004, 13, 622–626. [Google Scholar] [CrossRef]
- Hara, T.; Yoshitake, T.; Fukugawa, T.; Kubo, H.; Itakura, M.; Kuwano, N.; Tomokiyo, Y.; Nagayama, K. Ultrananocrystalline diamond prepared by pulsed laser deposition. Diam. Relat. Mater. 2006, 15, 649–653. [Google Scholar] [CrossRef]
- Lackner, J.M.; Stotter, C.; Waldhauser, W.; Ebner, R.; Lenz, W.; Beutl, M. Pulsed laser deposition of diamond-like carbon coatings for industrial tribological applications. Surf. Coat. Technol. 2003, 174–175, 402–407. [Google Scholar] [CrossRef]
- Popescu, A.C.; Stan, G.E.; Duta, L.; Nita, C.; Popescu, C.; Surdu, V.-A.; Husanu, M.-A.; Bita, B.; Ghisleni, R.; Himcinschi, C.; et al. The Role of Ambient Gas and Pressure on the Structuring of Hard Diamond-Like Carbon Films Synthesized by Pulsed Laser Deposition. Materials 2015, 8, 3284–3305. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, H.; Osozawa, R.; Mohnai, Y.; Nara, Y. Synthesis of boron/nitrogen-incorporated diamond-like carbon films by pulsed laser deposition using nitrogen gas and a boron-containing graphite target. Jpn. J. Appl. Phys. 2017, 56, 105501. [Google Scholar] [CrossRef]
- Al-Riyami, S.; Gima, H.; Akamine, H.; Yoshitake, T. Chemical bonding of nitrogenated ultrananocrystalline diamond films deposited on titanium substrates by pulsed laser deposition. ECS J. Solid State Sci. Technol. 2013, 2, M33–M38. [Google Scholar] [CrossRef]
- Ray, S.C.; Mbiombi, W.; Papakonstantinou, P. Electrical and electronic properties of nitrogen doped amorphous carbon (a-CNx) thin films. Curr. Appl. Phys. 2014, 14, 1845–1848. [Google Scholar] [CrossRef]
- Ray, S.C.; Pong, W.F.; Papakonstantinou, P. Iron, nitrogen and silicon doped diamond like carbon (DLC) thin films: A comparative study. Thin Solid Films 2016, 610, 42–47. [Google Scholar] [CrossRef]
- Menegazzo, N.; Kahn, M.; Berghauser, R.; Waldhauser, W.; Mizaikoff, B. Nitrogen-doped diamond-like carbon as optically transparent electrode for infrared attenuated total reflection spectroelectrochemistry. Analyst 2011, 136, 1831–1839. [Google Scholar] [CrossRef]
- Guzman, F.; Favre, M.; Ruiz, H.M.; Hevia, S.; Caballero, L.S.; Wyndham, E.S.; Bhuyan, H.; Flores, M.; Mandl, S. Pulsed laser deposition of thin carbon films in a neutral gas background. J. Phys. D Appl. Phys. 2013, 46, 215202. [Google Scholar] [CrossRef]
- Ossi, P.M.; Bottani, C.E.; Miotello, A. Pulsed-laser deposition of carbon: From DLC to cluster-assembled films. Thin Solid Films 2005, 482, 2–8. [Google Scholar] [CrossRef]
- Gaudiuso, R. Experimental Techniques for the Study of Laser Induced Plasmas and Their Applications to Chemical Analysis and Materials Production. Ph.D. Thesis, University of Bari “A. Moro”, Bari, Italy, 2010. [Google Scholar]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urade, A.R.; Lahiri, I.; Suresh, K.S. Graphene Properties, Synthesis and Applications: A Review. JOM 2023, 75, 614–630. [Google Scholar] [CrossRef] [PubMed]
- Ghany, N.A.A.; Elsherif, S.A.; Handal, H.T. Revolution of Graphene for different applications: State-of-the-art. Surf. Interfaces 2017, 9, 93–106. [Google Scholar] [CrossRef]
- Cappelli, E.; Iacobucci, S.; Scilletta, C.; Flammini, R.; Orlando, S.; Mattei, G.; Ascarelli, P.; Borgatti, F.; Giglia, A.; Mahn, N.; et al. Orientation tendency of PLD carbon films as a function of substrate temperature: A NEXAFS. study. Diam. Relat. Mater. 2005, 14, 959–964. [Google Scholar] [CrossRef]
- Cappelli, E.; Orlando, S.; Servidori, M.; Scilletta, C. Nano-graphene structures deposited by N-IR pulsed laser ablation of graphite on Si. Appl. Surf. Sci. 2007, 254, 1273–1278. [Google Scholar] [CrossRef]
- Benhagouga, R.H.; Abdelli-Messaci, S.; Abdesselam, M.; Blondeau-Patissier, V.; Yahiaoui, R.; Siad, M.; Rahal, A. Temperature effect on hydrogenated amorphous carbon leading to hydrogenated graphene by pulsed laser deposition. Appl. Surf. Sci. 2017, 426, 874–880. [Google Scholar] [CrossRef]
- Ren, P.; Pu, E.; Liu, D.; Wang, Y.; Xiang, B.; Ren, X. Fabrication of nitrogen-doped graphenes by pulsed laser deposition and improved chemical enhancement for Raman spectroscopy. Mater. Lett. 2017, 204, 65–68. [Google Scholar] [CrossRef]
- Lopez, D.; Castellanos, M.A.; Riascos, H. Influence of substrate temperature on graphene oxide thin films synthesis by laser ablation technique. J. Vac. Sci. Technol. A 2022, 40, 013402. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Gartiser, V.; Loir, A.-S.; Caja-Munoz, B.; Avila, J.; Barnier, V.; Garrelie, F.; Donnet, C. Graphene synthesis on SiO2 using pulsed laser deposition with bilayer predominance. Mater. Chem. Phys. 2019, 238, 121905. [Google Scholar] [CrossRef]
- Kumar, S.R.S.; Alshareef, H.N. Ultraviolet laser deposition of graphene thin films without catalytic layers. Appl. Phys. Lett. 2013, 102, 012110. [Google Scholar] [CrossRef] [Green Version]
- Kumar, I.; Khare, A. Multi- and few-layer graphene on insulating substrate via pulsed laser deposition technique. Appl. Surf. Sci. 2014, 317, 1004–1009. [Google Scholar] [CrossRef]
- Milenov, T.; Dikovska, A.; Avdeev, G.; Avramova, I.; Kirilov, K.; Karashanova, D.; Terziyska, P.; Georgieva, B.; Arnaudov, B.; Kolev, S.; et al. Pulsed laser deposition of thin carbon films on SiO2/Si substrates. Appl. Surf. Sci. 2019, 480, 323–329. [Google Scholar] [CrossRef]
- Xu, S.C.; Man, B.Y.; Jiang, S.; Liu, A.H.; Hu, G.; Chen, C.S.; Liu, M.; Yang, C.; Feng, D.J.; Zhang, C. Direct synthesis of graphene on any nonmetallic substrate based on KrF laser ablation of ordered pyrolytic graphite. Laser Phys. Lett 2014, 11, 096001. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Yu, J.; Xiao, T.; Peng, L.; Fan, L.; Wang, C.; Shen, Q.; Wu, W. Tailoring the Grain Size of Bi-Layer Graphene by Pulsed Laser Deposition. Nanomaterials 2018, 8, 885. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Fan, L.; Wang, X.; Xiao, T.; Peng, L.; Wang, X.; Yu, J.; Cao, L.; Xiong, Z.; Fu, Y.; et al. Pulsed laser deposition of monolayer and bilayer graphene. Appl. Surf. Sci. 2019, 494, 651–658. [Google Scholar] [CrossRef]
- Juvaid, M.M.; Sarkar, S.; Gogoi, P.K.; Ghosh, S.; Annamalai, M.; Lin, Y.-C.; Prakash, S.; Goswami, S.; Li, C.; Hooda, S.; et al. Direct Growth of Waferscale, Transparent, p-Type Reduced Graphene Oxide Like Thin Films by Pulsed Laser Deposition. ACS Nano 2020, 14, 3290–3298. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Lahiri, I.I.; Mitra, A. Nickel mediated few-layer graphene growth on glass substrates by pulsed laser deposition. Results Phys. 2019, 14, 102350. [Google Scholar] [CrossRef]
- Kumar, P.; Lahiri, I.; Mitra, A. Influence of the buffer layers on growth and quality of graphene films grown by pulsed laser deposition. Mater. Res. Express 2019, 6, 125625. [Google Scholar] [CrossRef]
- Wang, K.; Tai, G.; Wong, K.H.; Lau, S.P.; Guo, W. Ni induced few-layer graphene growth at low temperature by pulsed laser deposition. AIP Adv. 2011, 1, 022141. [Google Scholar] [CrossRef]
- Bourquard, F.; Bleu, Y.; Loir, A.-S.; Caja-Munoz, B.; Avila, J.; Asensio, M.-C.; Raimondi, G.; Shokouhi, M.; Rassas, I.; Farre, C.; et al. Electroanalytical Performance of Nitrogen-Doped Graphene Films Processed in One Step by Pulsed Laser Deposition Directly Coupled with Thermal Annealing. Materials 2019, 12, 666. [Google Scholar] [CrossRef] [Green Version]
- Larki, F.; Kameli, P.; Nikmanesh, H.; Jafari, M.; Salamati, H. The influence of external magnetic field on the pulsed laser deposition growth of graphene on nickel substrate at room temperature. Diam. Relat. Mater. 2019, 93, 233–240. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Loir, A.-S.; Barnier, V.; Garrelie, F.; Donnet, C. Raman study of the substrate influence on graphene synthesis using a solid carbon source via rapid thermal annealing. J. Raman Spectrosc. 2019, 50, 1630–1641. [Google Scholar] [CrossRef]
- Maddi, C.; Bourquard, F.; Barnier, V.; Avila, J.; Asensio, M.C.; Tite, T.; Donnet, C.; Garrelie, F. Nano-architecture of nitrogen-doped graphene films synthesized from a solid CN source. Sci. Rep. 2018, 8, 3247. [Google Scholar] [CrossRef] [Green Version]
- Tite, T.; Donnet, C.; Loir, A.-S.; Reynaud, S.; Michalon, J.-Y.; Vocanson, F.; Garrelie, F. Graphene-based textured surface by pulsed laser deposition as a robust platform for surface enhanced Raman scattering applications. Appl. Phys. Lett. 2014, 104, 041912. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Liu, S.; Song, H.; Gu, P. Growth of large-area, few-layer graphene by femtosecond pulsed laser deposition with double-layer Ni catalyst. J. Mater. Sci. 2017, 52, 2060–2065. [Google Scholar] [CrossRef]
- Tite, T.; Barnier, V.; Donnet, C.; Loir, A. –S.; Reynaud, S.; Michalon, J.–Y.; Vocanson, F.; Garrelie, F.; Surface enhanced Raman spectroscopy platform based on graphene with one-year stability. Thin Solid Films 2016, 604, 74–80. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Colombo, L.; Ruoff, R.S. Evolution of Graphene Growth on Ni and Cu by Carbon Isotope Labeling. Nano Lett. 2009, 9, 4268–4272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishwakarma, R.; Rosmi, M.S.; Takahashi, K.; Wakamatsu, Y.; Yaakob, Y.; Araby, M.I.; Kalita, G.; Kitazawa, M.; Tanemurab, M. Transfer free graphene growth on SiO2 substrate at 250 °C. Sci. Rep. 2017, 7, 43756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd Elhamid, A.M.; Aboulfotouh, A.M.; Hafez, M.A.; Azzouz, I.M. Room temperature graphene growth on complex metal matrix by PLD. Diam. Relat. Mater. 2017, 80, 162–167. [Google Scholar] [CrossRef]
- Koh, A.T.T.; Foong, Y.M.; Chua, D.H.C. Comparison of the mechanism of low defect few-layer graphene fabricated on different metals by pulsed laser deposition. Diam. Relat. Mater. 2012, 25, 98–102. [Google Scholar] [CrossRef]
- Maddi, C.; Bourquard, F.; Tite, T.; Loir, A.S.; Donnet, C.; Garrelie, F.; Barnier, V.; Wolski, K.; Fortgang, P.; Zehani, N.; et al. Structure, electrochemical properties and functionalization of amorphous CN films deposited by femtosecond pulsed laser ablation. Diam. Relat. Mater. 2016, 65, 17–25. [Google Scholar] [CrossRef]
- Scilletta, C.; Servidori, M.; Orlando, S.; Cappelli, E.; Barba, L.; Ascarelli, P. Influence of substrate temperature and atmosphere on nano-graphene formation and texturing of pulsed Nd:YAG laser-deposited carbon films. Appl. Surf. Sci. 2006, 252, 4877–4881. [Google Scholar] [CrossRef]
- Qian, M.; Zhou, Y.S.; Gao, Y.; Park, J.B.; Feng, T.; Huang, S.M.; Sun, Z.; Jiang, L.; Lu, Y.F. Formation of graphene sheets through laser exfoliation of highly ordered pyrolytic graphite. Appl. Phys. Lett. 2011, 98, 173108. [Google Scholar] [CrossRef]
- Ershov, I.V.; Prutsakova, N.V.; Holodova, O.M.; Lavrentyev, A.A.; Mardasova, I.V.; Zhdanova, T.P. Structural Properties and Composition of Graphite-Like Carbon Films Obtained by Pulsed Laser Deposition. Tech. Phys. 2021, 66, 580–587. [Google Scholar] [CrossRef]
- Ershov, I.V.; Lavrentyev, A.A.; Prutsakova, N.V.; Holodova, O.M.; Mardasova, I.V.; Zhdanova, T.P.; Kozakov, A.T. Characterization of Graphenic Carbon Produced by Pulsed Laser Ablation of Sacrificial Carbon Tapes. Appl. Sci. 2021, 11, 11972. [Google Scholar] [CrossRef]
- Kumar, S.R.S.; Nayak, P.K.; Hedhili, M.N.; Khan, M.A.; Alshareef, H.N. In situ growth of p and n-type graphene thin films and diodes by pulsed laser deposition. Appl. Phys. Lett. 2013, 103, 192109. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleu, Y.; Bourquard, F.; Barnier, V.; Lefkir, Y.; Reynaud, S.; Loir, A.-S.; Garrelie, F.; Donnet, C. Boron-doped graphene synthesis by pulsed laser co-deposition of carbon and boron. Appl. Surf. Sci. 2020, 513, 145843. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Plšek, J.; Drogowska-Horná, K.A.; Guerra, V.L.P.; Mikšátko, J.; Valeš, V.; Kalbáč, M. Towards Catalytically Active Porous Graphene Membranes with Pulsed Laser Deposited Ceria Nanoparticles. Chem. Eur. J. 2021, 27, 4150–4158. [Google Scholar] [CrossRef]
- Casari, C.S.; Milani, A. Carbyne: From the elusive allotrope to stable carbon atom wires. MRS Commun. 2018, 8, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Hearth, J.R.; Zhang, Q.; O’Brien, S.C.; Curl, R.F.; Kroto, H.W.; Smalley, R.E. The formation of long carbon chain molecules during laser vaporization of graphite. J. Am. Chem. Soc. 1987, 109, 359–363. [Google Scholar] [CrossRef]
- Compagnini, G.; Battiato, S.; Puglisi, O.; Baratta, G.A.; Strazzulla, G. Ion irradiation of sp rich amorphous carbon thin films: A vibrational spectroscopy investigation. Carbon 2005, 43, 3025. [Google Scholar] [CrossRef]
- Hu, A.; Rybachuk, M.; Lu, Q.-B.; Duley, W.W. Direct synthesis of sp-bonded carbon chains on graphite surface by femtosecond laser irradiation. Appl. Phys. Lett. 2007, 91, 131906. [Google Scholar] [CrossRef] [Green Version]
- Hu, A.; Lu, Q.-B.; Duley, W.W.; Rybachuk, M. Spectroscopic characterization of carbon chains in nanostructured tetrahedral carbon films synthesized by femtosecond pulsed laser deposition. J. Chem. Phys. 2007, 126, 154705. [Google Scholar] [CrossRef]
- Casari, C.S.; Giannuzzi, C.S.; Russo, V. Carbon-atom wires produced by nanosecond pulsed laser deposition in a background gas. Carbon 2016, 104, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.E.; Shin, S.K.; Park, S.M. The physical effects on the formation of polyynes by laser ablation. Chem. Phys. Lett. 2013, 568–569, 112–116. [Google Scholar] [CrossRef]
- Pan, B.; Xiao, J.; Li, J.; Liu, P.; Wang, C.; Yang, G. Carbyne with finite length: The one-dimensional sp carbon. Sci. Adv. 2015, 1, e1500857. [Google Scholar] [CrossRef] [Green Version]
- Tabata, H.; Fujii, M.; Hayashi, S. Laser ablation of diamond nanoparticles suspended in solvent: Synthesis of polyynes. Chem. Phys. Lett. 2004, 395, 138–142. [Google Scholar] [CrossRef]
- Tabata, H.; Fujii, M.; Hayashi, S. Laser ablation of diamond particles suspended in ethanol: Effective formation of long polyynes. Carbon 2006, 44, 522–529. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Nagayama, H.; Daigoku, K.; Kiyooka, Y.; Hashimoto, K. Laser induced emission spectra of polyyne molecules C2nH2 (n = 5–8). Chem. Phys. Lett. 2007, 446, 65–70. [Google Scholar] [CrossRef]
- Kucherik, A.O.; Osipov, A.V.; Arakelian, S.M.; Garnov, S.V.; Povolotckaya, A.V.; Kutrovskaya, S.V. The laser-assisted synthesis of linear carbon chains stabilized by noble metal particle. J. Phys. Conf. Ser. 2019, 1164, 012006. [Google Scholar] [CrossRef]
- Baughman, R.H. Dangerously Seeking Linear Carbon. Science 2006, 312, 1009–1110. [Google Scholar] [CrossRef]
- Dave, H.A.B.; Matthijn, D.; Guus, R. Pulsed laser deposition in Twente: From research tool towards industrial deposition. J. Phys. D Appl. Phys. 2014, 47, 034006. [Google Scholar]
- Zanoni, K.P.S.; Pérez-del-Rey, D.; Dreessen, C.; Rodkey, N.; Sessolo, M.; Soltanpoor, W.; Morales-Masis, M.; Bolink, H.J. Tin(IV) Oxide Electron Transport Layer via Industrial-Scale Pulsed Laser Deposition for Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2023, 15, 32621–32628. [Google Scholar] [CrossRef]
- Lackner, J.M. Industrially-scaled large-area and high-rate tribological coating by Pulsed Laser Deposition. Surf. Coat. Technol. 2005, 200, 1439–1444. [Google Scholar] [CrossRef]
- Yao, J.D.; Zheng, Z.Q.; Yang, G.W. Production of large-area 2D materials for high-performance photodetectors by pulsed-laser deposition. Prog. Mater. Sci. 2019, 106, 100573. [Google Scholar] [CrossRef]



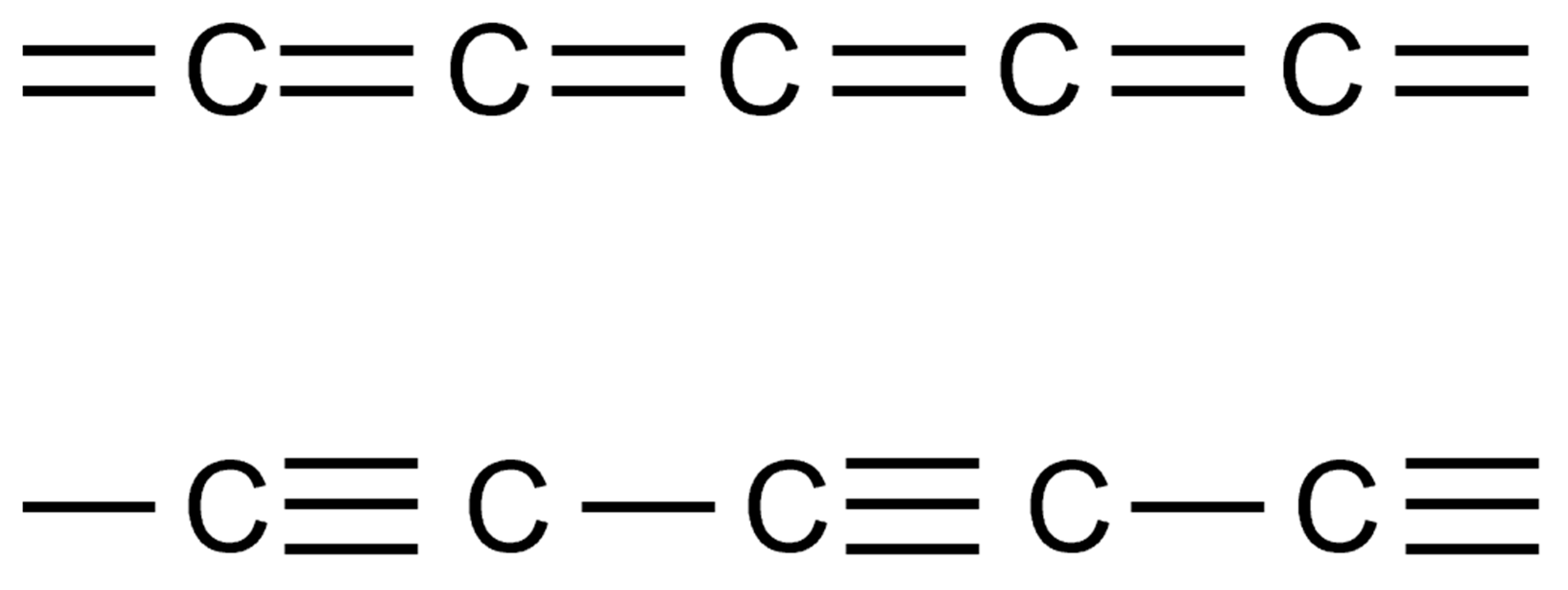
| Properties of Natural Diamond | Applications of DLC Films |
|---|---|
| Mechanical hardness (~90 GPa) Highest known bulk modulus (1.2 × 1012 N/m2) Lowest known compressibility (8.3 × 10−13 m2/N) |
|
| Highest known thermal conductivity at room temperature (2 × 103 W m−1 K−1) |
|
| Optical transparency from deep UV to far IR |
|
| Good electrical insulator (resistivity at room temperature ~1016 Ω cm) Possibility of doping to obtain wide band-gap semiconductor (5.4 eV) |
|
| High chemical inertness and resistance to corrosion |
|
| Biological compatibility (high inertness, low friction) |
| Properties of Single-Layer Graphene | Applications |
|---|---|
| High mechanical strength (42 N/m) |
|
| High elastic modulus (1 TPa) |
|
| High specific surface area (2630 m2/g) |
|
| High carrier mobility (2 × 105 cm2/V*s) |
|
| High transparency (97.7%) |
|
| High thermal conductivity (5000 W/m*K) |
|
| Chemical stability Biocompatibility Resistance to oxidation and corrosion |
|
| Hydrophobicity |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaudiuso, R. Pulsed Laser Deposition of Carbon-Based Materials: A Focused Review of Methods and Results. Processes 2023, 11, 2373. https://doi.org/10.3390/pr11082373
Gaudiuso R. Pulsed Laser Deposition of Carbon-Based Materials: A Focused Review of Methods and Results. Processes. 2023; 11(8):2373. https://doi.org/10.3390/pr11082373
Chicago/Turabian StyleGaudiuso, Rosalba. 2023. "Pulsed Laser Deposition of Carbon-Based Materials: A Focused Review of Methods and Results" Processes 11, no. 8: 2373. https://doi.org/10.3390/pr11082373
APA StyleGaudiuso, R. (2023). Pulsed Laser Deposition of Carbon-Based Materials: A Focused Review of Methods and Results. Processes, 11(8), 2373. https://doi.org/10.3390/pr11082373





