Nanofluids as a Waste Heat Recovery Medium: A Critical Review and Guidelines for Future Research and Use
Abstract
:1. Introduction
2. Waste Heat Sources
3. Nanofluids
3.1. Preparation Methods
3.1.1. One-Step Method
3.1.2. Two-Step Method
3.2. Thermophysical Properties
3.2.1. Thermal Conductivity
3.2.2. Specific Heat
3.2.3. Dynamic Viscosity
3.2.4. Density
3.3. Thermal Conductivity Influencing Factors
3.3.1. Base Fluid
3.3.2. Type of Nanoparticles
3.3.3. Concentration of the Nanoparticles
3.3.4. Size of the Nanoparticles
3.3.5. Shape of the Nanoparticles
3.3.6. Operating Temperature
3.3.7. Addition of Surfactants
3.4. Nusselt Number
3.5. Rheological Behavior
4. Heat-to-Heat Waste Heat Recovery with Nanofluids
4.1. Waste Heat Recovery Using Heat Exchangers
4.2. Waste Heat Recovery Using Heat Pipes
4.2.1. Pulsating Heat Pipes
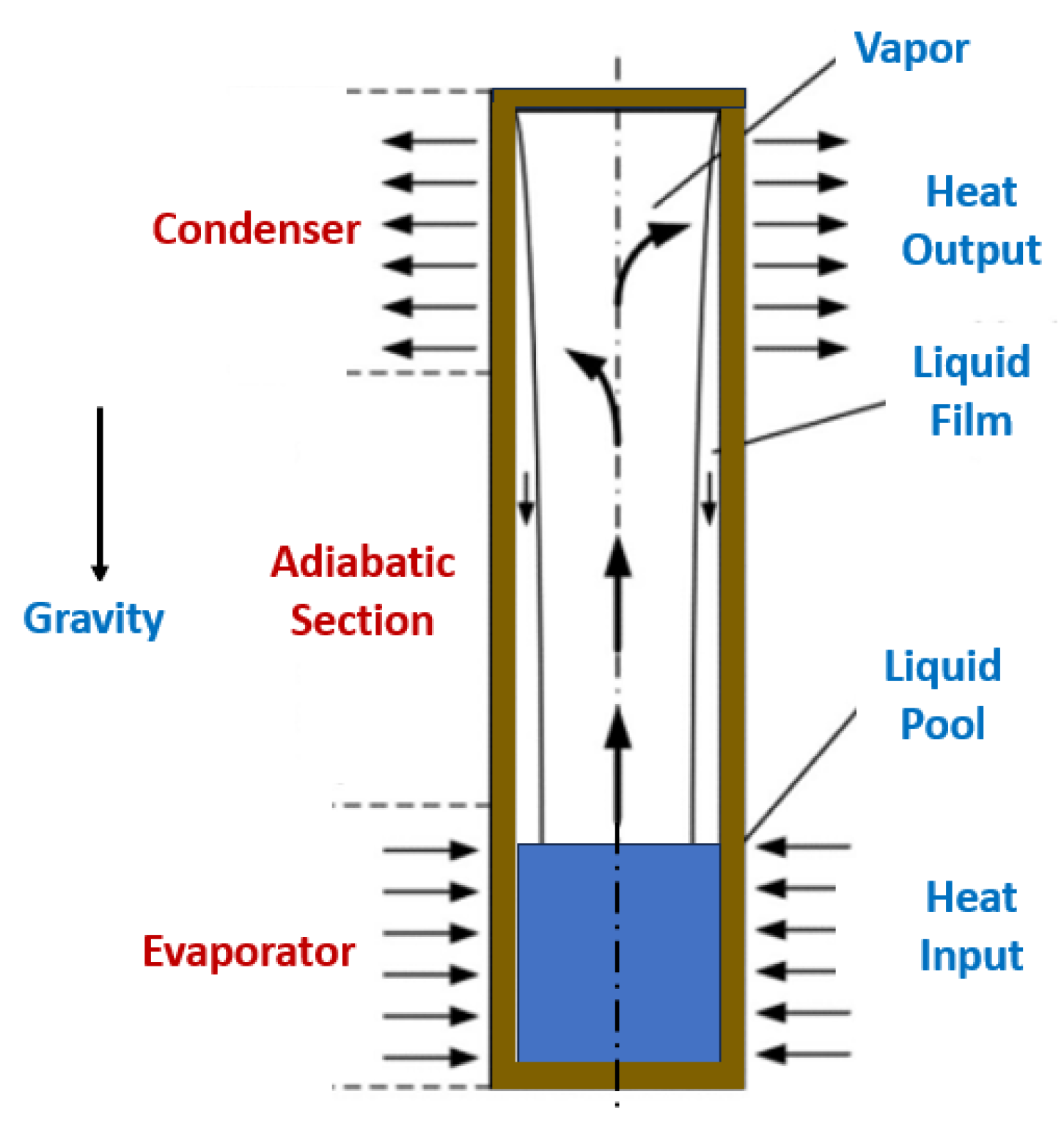
4.2.2. Gravity Heat Pipes
4.2.3. Heat Pipes Heat Exchangers
4.3. Pool Boiling Waste Heat Recovery
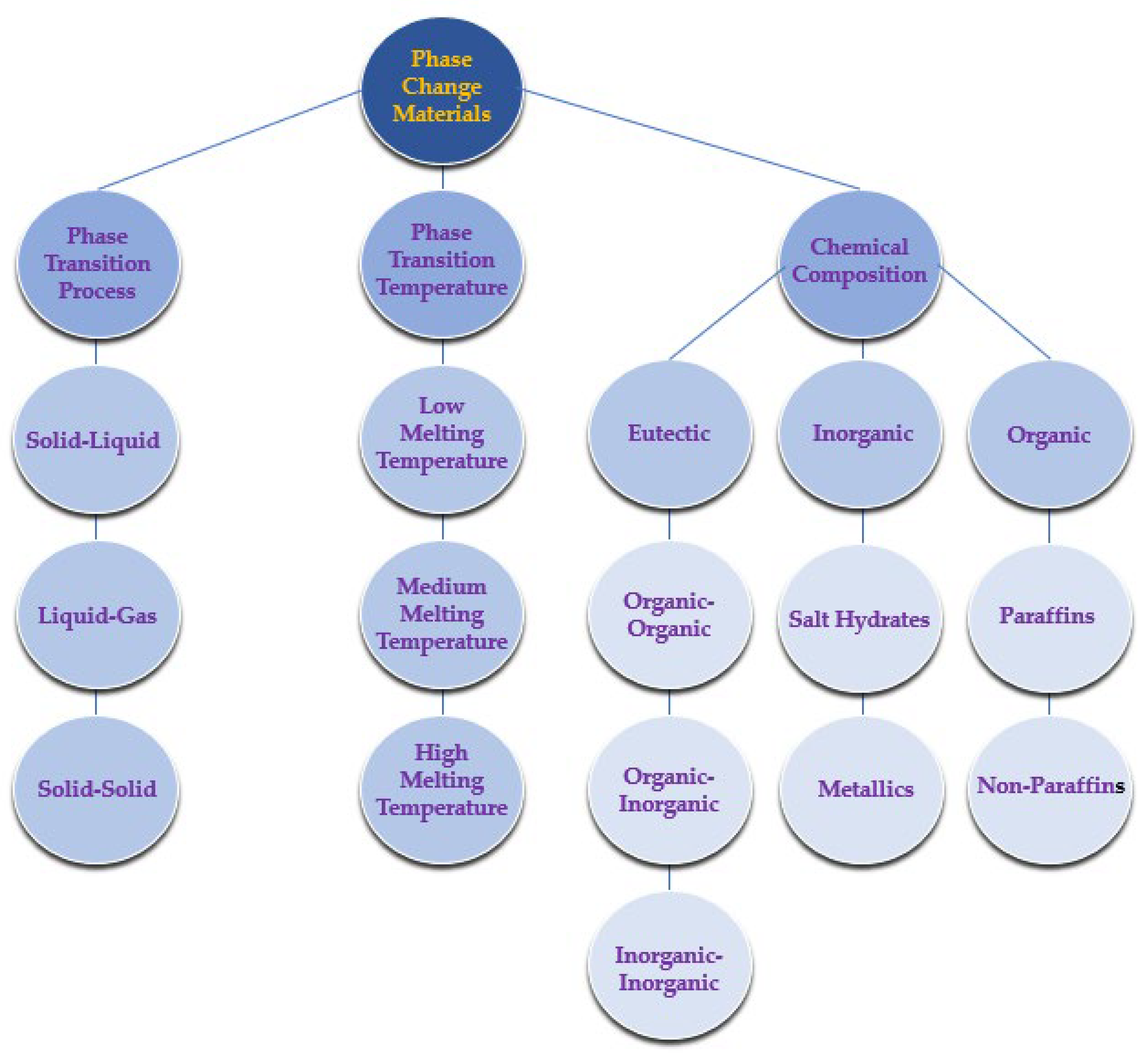
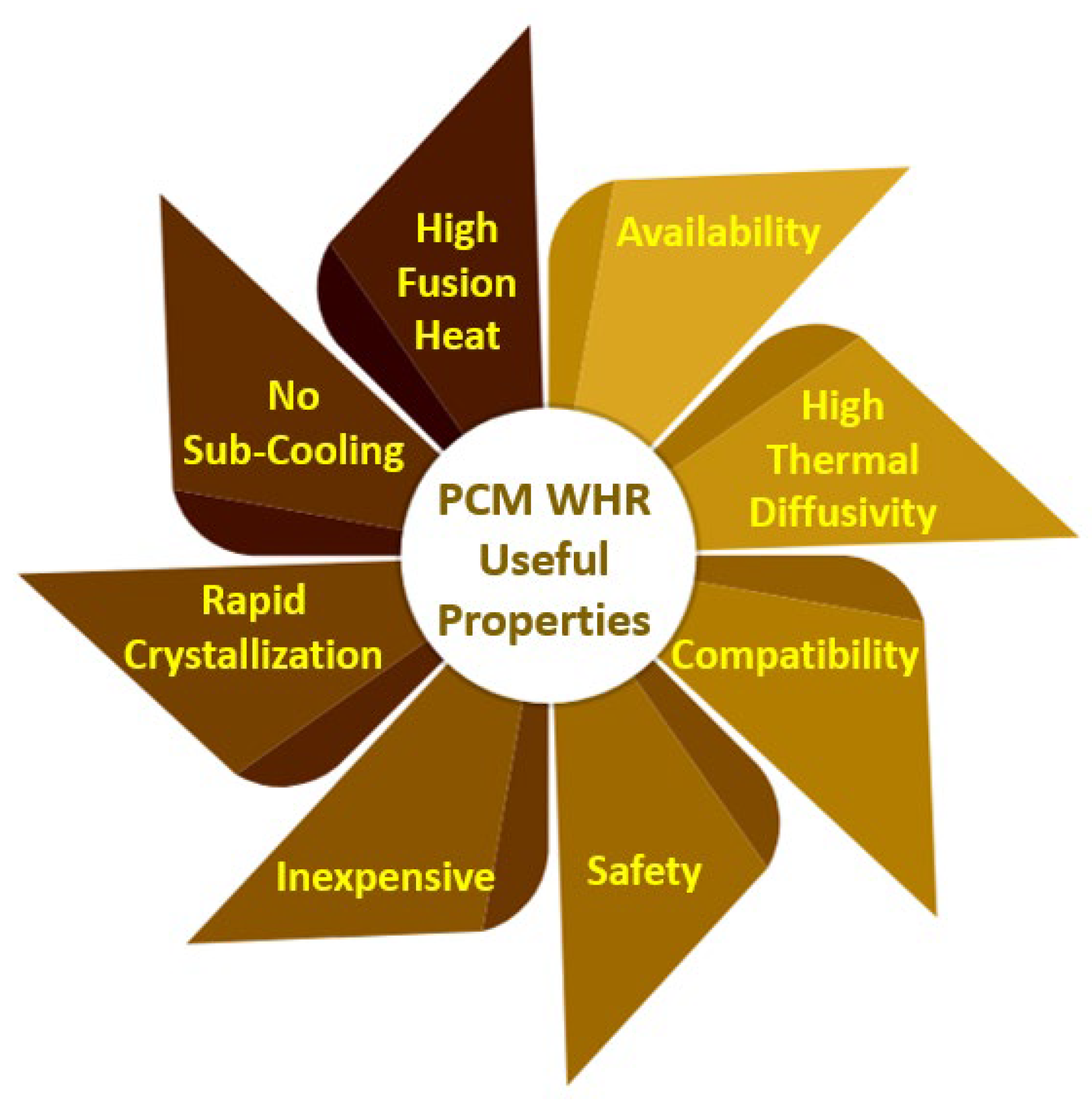
4.4. Waste Heat Recovery Using Phase-Change Materials
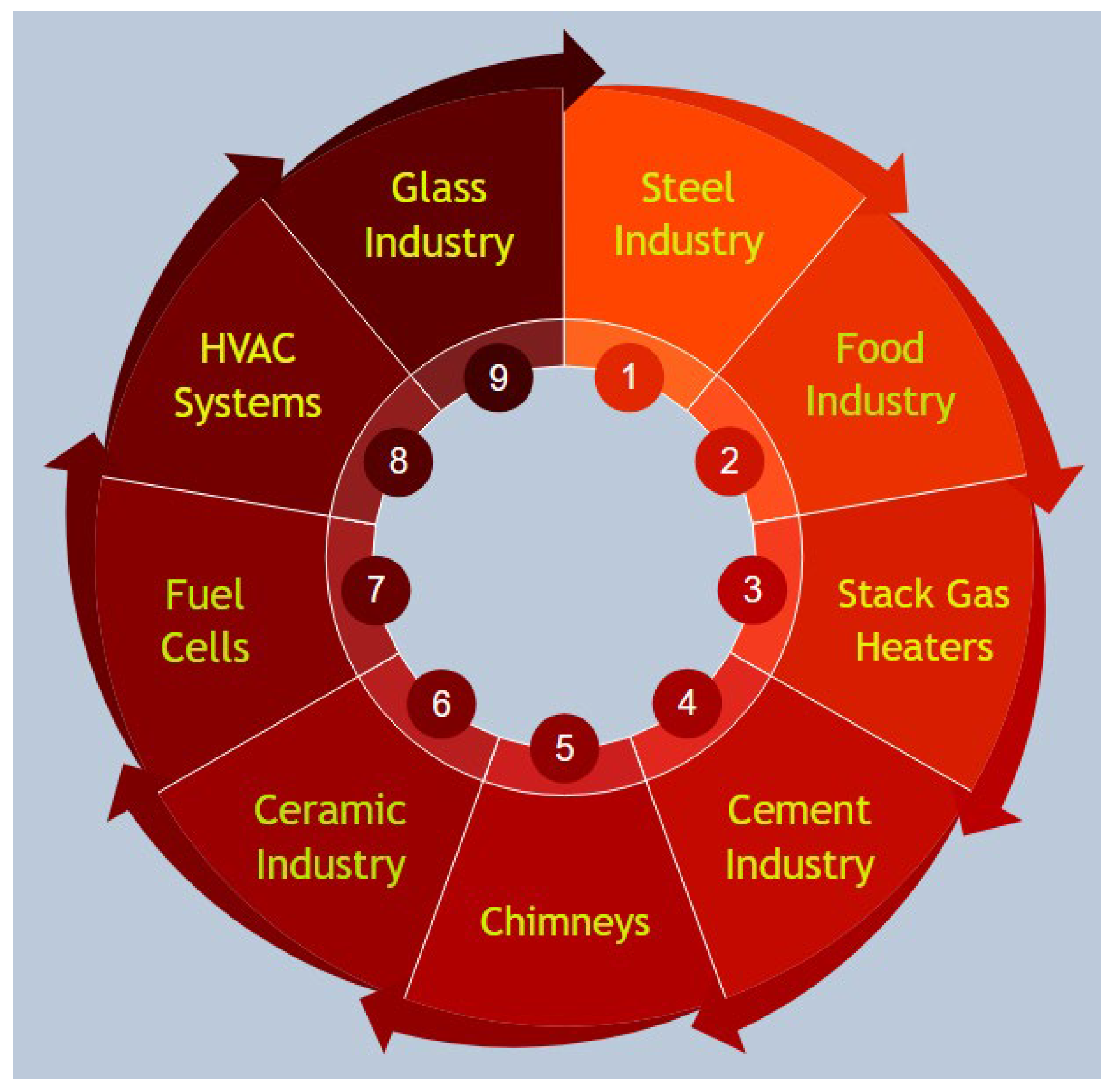
4.5. Main Areas of Actuation of the Heat-to-Heat Waste Heat Recovery
4.5.1. HVAC Systems
4.5.2. Cement Industry Waste Heat Recovery
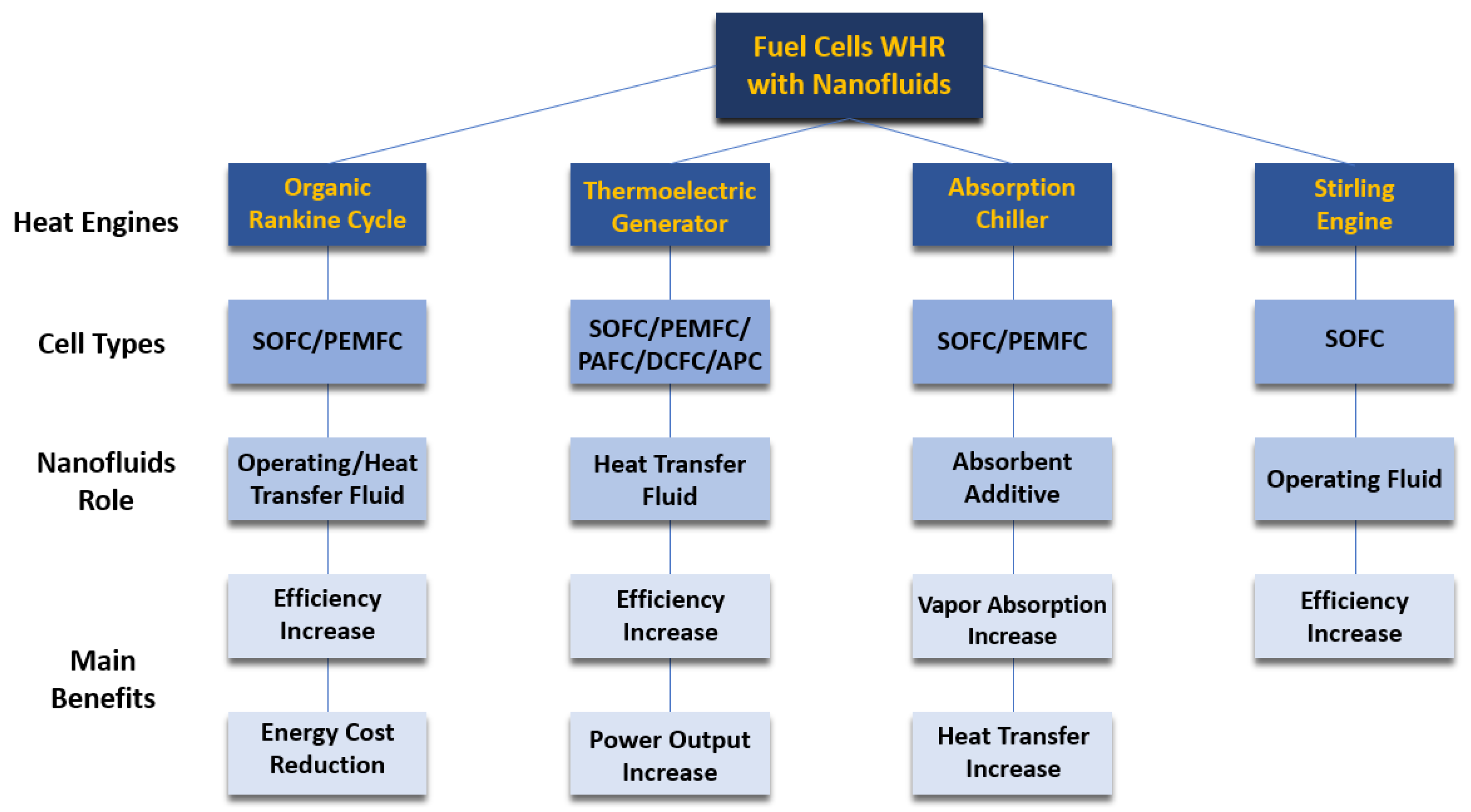
4.5.3. Fuel Cells Waste Heat Recovery
4.5.4. Chimneys Waste Heat Recovery
4.5.5. Stack Gas Heater Waste Heat Recovery
5. Heat-to-Work Waste Heat Recovery with Nanofluids
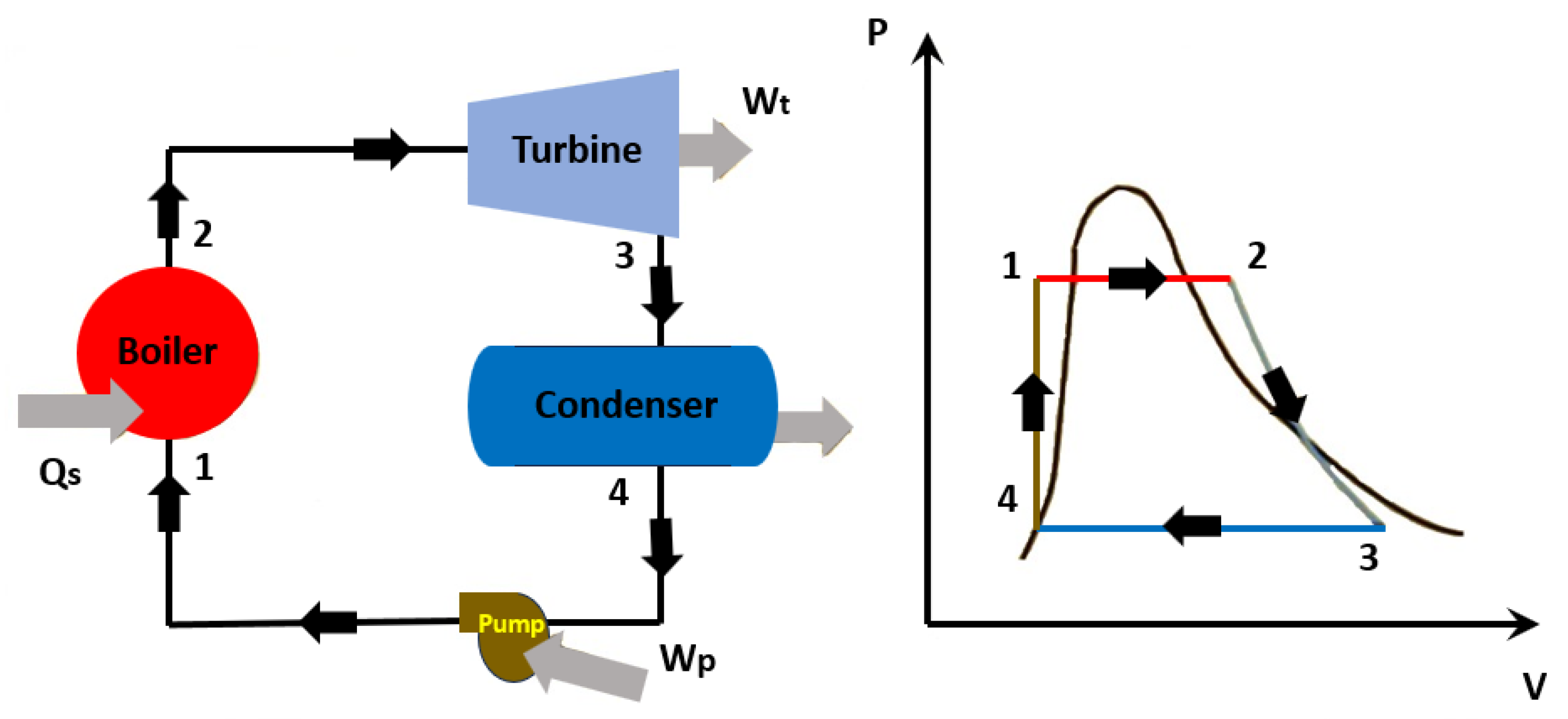
6. Heat-to-Power Waste Heat Recovery with Nanofluids
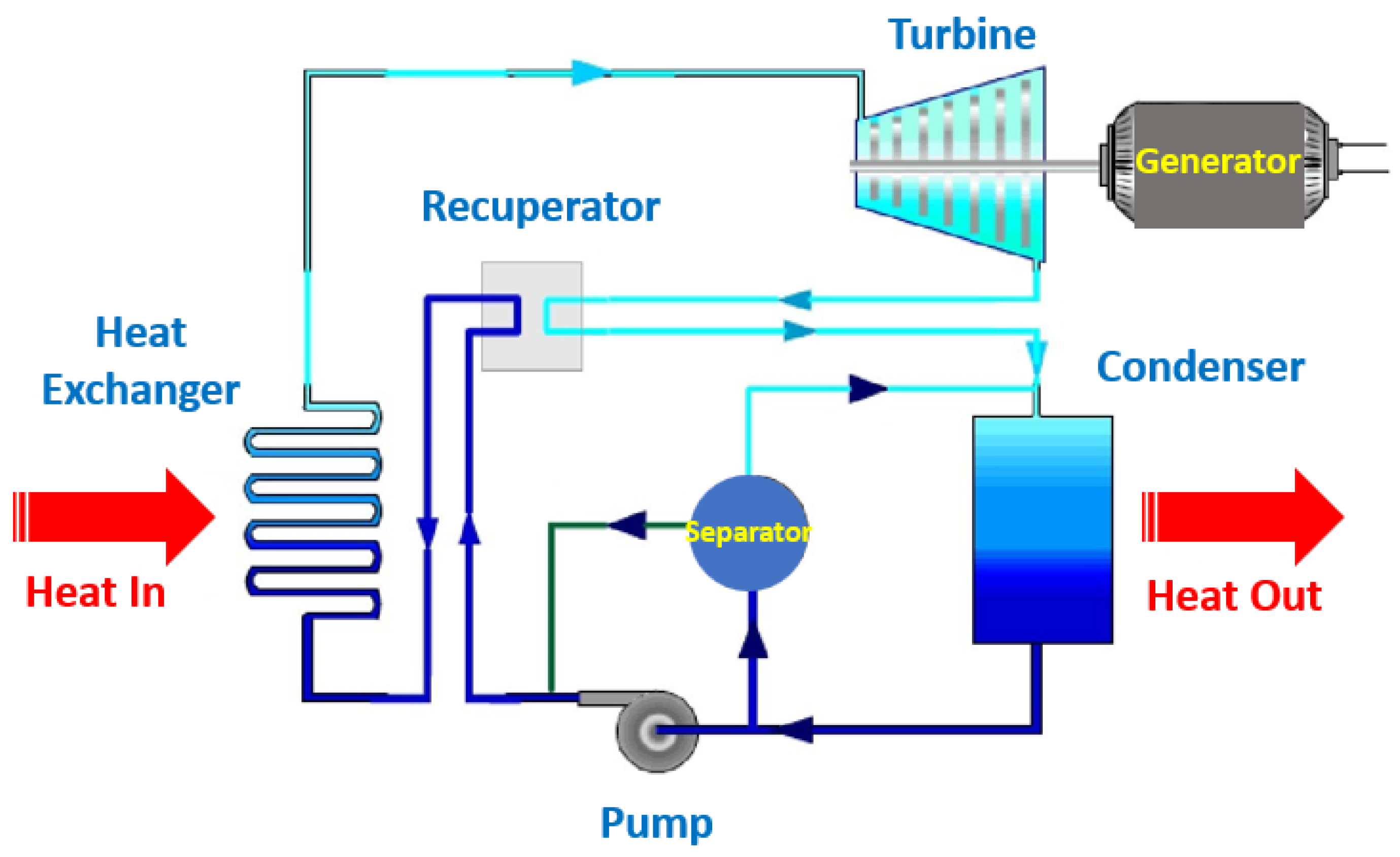
6.1. Solar Thermal Energy Waste Heat Recovery
6.2. Automotive Waste Heat Recovery
7. Recommendations for Future Research Works
- The weight and overall cost of the thermoelectric generators should be further diminished. Another challenge related to these waste heat recovery devices is the thermal resistance between the hot reservoir and the hot side of the thermoelectric generators. Additionally, the thermal resistance between their cold sides and the heat exchanger will reduce the efficiency. Such effects can be mitigated by reducing the number of thermoelectric legs, employing high thermally conductive interface materials such as graphene and carbon nanotubes, or by alternatively augmenting the contact area;
- The innovative material and construction methods of the thermoelectric generators, including, for instance, the flexible thermoelectric generator approach, should be further studied and implemented;
- Further studies are required to obtain guidelines and alternative improvements to overcome the main limitations of the use of thermoelectric generators using nanofluids, namely, the high investment cost and poor energy conversion efficiency;
- It should be seriously considered the global energy recovery in techno-economic feasible degrees. Considering this, waste heat grade and potential waste heat recovery routes using nanofluids should be in-depth technically and economically assessed to be urgently implemented;
- There is a pressing demand to further analyze the friction factors and pressure drops associated with the utilization of nanofluids in waste heat recovery approaches since these factors will determine the required pumping power demand;
- It is suggested to perform further studies on the clustering and sedimentation effects of the nanoparticles included in nanofluids applied in waste heat recovery. The accumulation effect of the suspended nanoparticles is more evident in the circuit stagnation sections in which the fluid velocity is very low. This will deteriorate the heat transfer performance of the system in extended periods of utilization;
- We welcome studies that elaborate on the evaluation of the corrosive character of some of the added nanoparticles in the nanofluid operating in waste heat recovery processes. The nanoparticles collide with the circuit surfaces and may induce the corrosion of the equipment. This problem should be mitigated in a dual mode, which implies the search for less corrosion-sensitive constitutive materials of the channels and equipment where nanofluids flow and profound studies on the potentially corrosive nature of the included nanoparticles;
- Waste heat recovery processes with nanofluids, rather than only the heat-to-heat applications, should be further expanded and evaluated. Namely, the ones concerning waste heat recovery involve the use of heat exchangers or heat pipes in which nanofluids act effectively, but they are not employed in further processing;
- The theoretical and simulation work on the waste’s significant recovery with nanofluids and the associated experimental validation should be further developed;
- Despite the large number of published techno-economic analyses for nanofluids and waste heat recovery separately, there are only a few studies presenting such assessments for the application of nanofluids in the waste heat recovery technological area. The qualitative evaluation of the technical and economic viability and the environmentally benevolent features inherent to the use of nanofluids in waste heat recovery are well-known by the scientific community. Nonetheless, further quantitative studies are needed instead of qualitative evaluations. Such a route should be accompanied by in-depth studies of waste heat recovery devices and equipment, together with the design and implementation of the process and stream from which the heat will be recovered;
- More detailed research works are needed to comprehensively evaluate the environmental impacts of the use of nanofluids in waste heat recovery areas comprehensively. Through the life cycle assessment tool, there should be revealed the impacts inherent to the addition of nanoparticles derived from their mining, extraction, purification, and synthesis. All these operations require a considerable amount of energy and fossil fuel global depletion that should be strongly diminished. Also, the disposal of nanofluids after use involves additional toxicity impacting the surrounding ecosystems mainly because of the presence of nanoparticles, which in some cases may hold, for instance, heavy metals;
- There should be further addressed the applicability and advantageous features of using heat pipe heat exchangers operating with nanofluids for dedicated ventilation and air-conditioning systems. This route will increase the coefficient of performance of the systems and prevent the extra energy demand required for the re-heating and de-humidification processes;
- It is highly recommended to elaborate further studies on the use of heat pipes working with nanofluids for recovering the sensible heat in systems where the inlet air and return air should always be separated, including surgery rooms and laboratory facilities in which the air must be changed up to 40 times per hour.
8. Conclusions
- There are currently three different approaches in the waste heat recovery area, namely the heat-to-heat, heat-to-work, and heat-to-power. The heat-to-heat is a straightforward and effective methodology using heat exchangers, heat pipes, thermosyphons, waste heat boilers, and phase-change materials. Nonetheless, the recovered energy is still in the form of heat, which can be considered an efficiency-limited form of energy since it is constrained by the temperature difference;
- The heat-to-power is a waste heat recovery approach that has great potential since it converts heat into electricity, having broad applicability. It can be performed indirectly by extending the heat-to-work through the connection to an electrical generator. Alternatively, it can also be done via thermoelectric generators. Waste heat recovery through thermoelectric generators could be a rated part for several applications because of its installation easiness and facile operation. The utilization of nanofluids could make the thermoelectric generators yield higher power output;
- Nanofluids have been demonstrated to be an enhanced technological solution for waste heat recovery processes. Their superior thermophysical properties induced by the incorporation of nanoparticles into base fluids, together with the enhanced thermal conductivity and reduced thermal resistance, have been demonstrated to ameliorate the heat transfer performance and recovery rate;
- Nanofluids used in the heat-to-work waste heat recovery approaches are still limited and fundamentally utilized indirectly to increase heat transport from the primary waste heat source, like in the organic Rankine cycle. The direct utilization of nanofluids as operating fluids in the organic Rankine cycle has been investigated theoretically by numeric simulations with no experimental validation;
- Nanofluids have been applied so far for waste heat recovery in heat-to-power approaches only to increase the heat transport capability of the primary waste heat source and as refrigerants for the thermoelectric generators’ cold and hot sides;
- Most studies concerning waste heat recovery in the cement industry have only been conducted on waste gases from the pre-heater and cooler of the clinker. It requires to be further examined intensively to decrease the losses derived from the radiation and convection effects in the cooler. Such losses should be recuperated to improve the efficiency of the clinker cooler.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Nomenclature: | |
| cpbf | Specific Heat of the Base Fluid [J/kg·K] |
| cpnf | Specific Heat of the Nanofluid [J/kg·K] |
| cpnp | Specific Heat of the Nanoparticles [J/kg·K] |
| cnf | Specific Heat Capacity of the Nanofluid per Unit Volume [J/m3·K] |
| D | Inner Diameter of the Tube [m] |
| dnp | Average Diameter of the Nanoparticles [m] |
| h | Heat Transfer Coefficient [W/m2·K] |
| Keff | Effective Thermal Conductivity [W/m·K] |
| kbf | Thermal Conductivity of the Base Fluid [W/m·K] |
| knf | Thermal Conductivity of the Nanofluid [W/m·K] |
| knp | Thermal Conductivity of the Nanoparticles [W/m·K] |
| KB | Boltzmann’s constant [J/K] |
| Nu | Nusselt Number |
| n | Empirical Shape Factor |
| Pe | Peclet Number |
| Pr | Prandtl Number |
| Q | Volumetric Flow Rate [m3/s] |
| rC | Radius of the Clusters [m] |
| Ra | Rayleigh Number |
| Re | Reynolds number |
| t | Time [s] |
| T | Temperature [°C] or [K] |
| um | Average Flow Velocity of the Nanofluid [m/s] |
| up | Brownian Velocity of the Nanoparticles [m/s] |
| Greek Symbols: | |
| αnf | Thermal Diffusivity of the Nanofluid [m2/s] |
| µ | Dynamic Viscosity [Pa·s] |
| μbf | Dynamic Viscosity of the Base Fluid [Pa·s] |
| μnf | Dynamic Viscosity of the Nanofluid [Pa·s] |
| νnf | Kinematic Viscosity of the Nanofluid [m2/s] |
| ρbf | Density of the Base Fluid [kg/m3] |
| ρnf | Density of the Nanofluid [kg/m3] |
| ρnp | Density of the Nanoparticles [kg/m3] |
| τ | Particle Relaxation Time [s] |
| ϕ | Volumetric Fraction of the Nanoparticles [% vol.] |
| ψ | Sphericity Factor |
| ω | Empirical Constant |
| Abbreviations: | |
| ASHRAE | American Society of Heating, Refrigerating, and Air-Conditioning Engineers |
| CHF | Critical Heat Flux |
| MWCNT | Multi-Walled Carbon Nanotubes |
| HTC | Heat Transfer Coefficient |
| HVAC | Heating, Ventilation, and Air-Conditioning |
| FTIR | Fourier Transform Infrared Spectroscopy |
| ORC | Organic Rankine Cycle |
| PSD | Particle Size Distribution |
| SEM | Scanning Electron Microscopy |
| TEG | Thermoelectric Generator |
| WHR | Waste Heat Recovery |
References
- Brough, D.; Jouhara, H. The aluminium industry: A review on state-of-the-art technologies, environmental impacts and possibilities for waste heat recovery. Int. J. Thermofluids 2020, 1–2, 100007. [Google Scholar] [CrossRef]
- Delpech, B.; Milani, M.; Montorsi, L.; Boscardin, D.; Chauhan, A.; Almahmoud, S.; Axcell, B.; Jouhara, H. Energy efficiency enhancement and waste heat recovery in industrial processes by means of the heat pipe technology: Case of the ceramic industry. Energy 2018, 158, 656–665. [Google Scholar] [CrossRef]
- Elsaid, J.K.; Sayed, E.T.; Yousef, B.A.; Rabaia, M.K.H.; Abdelkareem, M.A.; Olabi, A. Recent progress on the utilization of waste heat for desalination: A review. Energy Convers. Manag. 2020, 221, 113105. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Ming, J.; Chen, L.; Shu, C.; Qu, T.; Tan, Q.; Liu, Y.; Asefa, T. Harvesting waste heat energy by promoting H+-ion concentration difference with a fuel cell structure. Nano Energy 2019, 57, 101–107. [Google Scholar] [CrossRef]
- Egilegor, B.; Jouhara, H.; Zuazua, J.; Al-Mansour, F.; Plesnik, K.; Montorsi, L.; Manzini, L. ETEKINA: Analysis of the potential for waste heat recovery in three sectors: Aluminium low pressure die casting, steel sector and ceramic tiles manufacturing sector. Int. J. Thermofluids 2020, 1–2, 100002. [Google Scholar] [CrossRef]
- Erguvan, M.; MacPhee, D.W. Second law optimization of heat exchangers in waste heat recovery. Int. J. Energy Res. 2019, 43, 5714–5734. [Google Scholar] [CrossRef]
- Garcia, S.I.; Garcia, R.F.; Carril, J.C.; Garcia, D.I. A review of thermodynamic cycles used in low temperature recovery systems over the last two years. Renew. Sustain. Energy Rev. 2018, 81, 760–767. [Google Scholar] [CrossRef]
- Pan, Q.; Zhao, R.; Jiang, Q.; Gosselin, L. Technological and economic analyses on power generation from the waste heat in a modified aluminum smeltingpot. Int. J. Energy Res. 2020, 44, 1735–1750. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.H.; Kim, T.S. Thermo-economic analysis of using an organic Rankine cycle for heat recovery from both the cell stack and reformer in a PEMFC for power generation. Int. J. Hydrogen Energy 2019, 44, 3876–3890. [Google Scholar] [CrossRef]
- Mirhosseini, M.; Rezania, A.; Rosendahl, L. Power optimization and economic evaluation of thermoelectric waste heat recovery system around a rotary cement kiln. J. Clean. Prod. 2019, 232, 1321–1334. [Google Scholar] [CrossRef]
- Gao, C.; Lee, S.W.; Yang, Y. Thermally regenerative electrochemical cycle for low-grade heat harvesting. ACS Energy Lett. 2017, 2, 2326–2334. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, P. High Seebeck Coefficient Electrochemical Thermocells for Efficient Waste Heat Recovery. CS Appl. Energy Mater. 2018, 1, 1424–1428. [Google Scholar] [CrossRef]
- Mai, V.-P.; Haung, W.-H.; Yang, R.-J. Charge Regulation and pH Effects on Thermo-Osmotic Conversion. Nanomaterials 2022, 12, 2774. [Google Scholar] [CrossRef]
- Straub, A.P.; Yip, N.Y.; Lin, S.; Lee, J.; Elimelech, M. Harvesting low-grade heat energy using thermo-osmotic vapour transport through nanoporous membranes. Nat. Energy 2016, 1, 16090. [Google Scholar] [CrossRef]
- Pandya, S.; Velarde, G.; Zhang, L.; Wilbur, J.D.; Smith, A.; Hanrahan, B.; Dames, C.; Martin, L.W. New approach to waste-heat energy harvesting: Pyroelectric energy conversion. NPG Asia Mater. 2019, 11, 26. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A. A review on nanofluids: Fabrication, stability, and thermophysical properties. J. Nanomater. 2018, 2018, 6978130. [Google Scholar] [CrossRef] [Green Version]
- Prajapati, P.P.; Patel, V.K. Comparative analysis of nanofluid-based Organic Rankine Cycle through thermoeconomic optimization. Heat Transf.—Asian Res. 2019, 48, 3013–3038. [Google Scholar] [CrossRef]
- Aljaghtham, M.; Celik, E. Design optimization of oil pan thermoelectric generator to recover waste heat from internal combustion engines. Energy 2020, 200, 117547. [Google Scholar] [CrossRef]
- US-EPA. Greenhouse Gas Equivalence Calculator. 2023. Available online: https://www.epa.gov/energy/greenhouse-gas-equivalencies-calculator (accessed on 11 July 2023).
- Dal Magro, F.; Savino, S.; Meneghetti, A.; Nardin, G. Coupling waste heat extraction by phase change materials with superheated steam generation in the steel industry. Energy 2017, 137, 1107–1118. [Google Scholar] [CrossRef]
- Zaric, M.; Stijepovic, M.Z.; Linke, P.; Stajic-Trosic, J.; Bugarski, B.; Kijevcanin, M. Targeting heat recovery and reuse in industrial zone. Chem. Ind. Chem. Eng. Q. 2017, 23, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Forman, C.; Muritala, I.K.; Pardemman, R.; Meyer, B. Estimating the global waste heat potential. Renew. Sustain. Energy Rev. 2016, 57, 1568–1579. [Google Scholar] [CrossRef]
- Vance, D.; Nimbalkar, S.; Thekdi, A.; Armstrong, K.; Wenning, T.; Cresko, J.; Jin, M. Estimation of and barriers to waste heat recovery from harsh environments in industrial processes. J. Clean. Prod. 2019, 222, 539–549. [Google Scholar] [CrossRef]
- Cheng, Z.; Guo, Z.; Tan, Z.; Yang, J.; Wang, Q. Waste heat recovery from high-temperature solid granular materials: Energy challenges and opportunities. Renew. Sustain. Energy Rev. 2019, 116, 109428. [Google Scholar] [CrossRef]
- Lokare, O.R.; Tavakkoli, S.; Rodriguez, G.; Khanna, V.; Vidic, R.D. Integrating membrane distillation with waste heat from natural gas compressor stations for produced water treatment in Pennsylvania. Desalination 2017, 413, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Clarendon Press: Clarendon, UK, 1973. [Google Scholar]
- Hamilton, R.L.; Crosser, O.K. Thermal conductivity of heterogeneous two-component systems. IEC Fundam 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. Int. J. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q.; Hu, W. Aggregation structure and thermal conductivity of nanofluids. AIChE J. 2003, 49, 1038–1043. [Google Scholar] [CrossRef]
- Yang, B. Thermal conductivity equations based on Brownian motion in suspensions of nanoparticles (nanofluids). J. Heat Transf. 2008, 130, 042408. [Google Scholar] [CrossRef]
- Xuan, Y.; Roetzel, W. Conceptions for heat transfer correlation of nanofluids. Int. J. Heat Mass Transf. 2002, 43, 3701–3707. [Google Scholar] [CrossRef]
- Zhou, L.P.; Wang, B.X.; Peng, X.F.; Du, X.Z.; Yang, Y.P. On the specific heat capacity of CuO nanofluid. Adv. Mech. Eng. 2010, 2, 172085. [Google Scholar] [CrossRef] [Green Version]
- Einstein, A. A new determination of molecular dimensions. Ann. Phys. 1906, 19, 2. [Google Scholar]
- Brinkman, H.C. The viscosity of concentrated suspensions and solution. J. Chem. Phys. 1952, 20, 571–581. [Google Scholar] [CrossRef]
- Abu-Nada, E. Effects of variable viscosity and thermal conductivity of Al2O3–water nanofluid on heat transfer enhancement in natural convection. Int. J. Heat Fluid Flow 2009, 30, 679–690. [Google Scholar] [CrossRef]
- Namburu, P.K.; Das, D.K.; Tanguturi, K.M.; Vajjha, R.S. Numerical study of turbulent flow and heat transfer characteristics of nanofluids considering variable properties. Int. J. Therm. Sci. 2009, 48, 290–302. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Desgranges, F.; Roy, G.; Galanis, N.; Maré, T.; Boucher, S.; Mintsa, H.A. Temperature and particle-size dependent viscosity data for water-based nanofluids–hysteresis phenomenon. Int. J. Heat Fluid Flow 2007, 28, 1492–1506. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Choi, S.U.S. Thermal conductivity of nanoparticle-fluid mixture. J. Thermophys. Heat Transf. 1999, 13, 474–480. [Google Scholar] [CrossRef]
- Naddaf, A.; Heris, S.Z. Density and rheological properties of different nanofluids based on diesel oil at different mass concentrations: An experimental study. J. Therm. Anal. Calorim. 2019, 135, 1229–1242. [Google Scholar] [CrossRef]
- Barbés, B.; Páramo, R.; Blanco, E.; Casanova, C. Thermal conductivity and specific heat capacity measurements of CuO nanofluids. J. Therm. Anal. Calorim. 2014, 115, 1883–1891. [Google Scholar] [CrossRef]
- Xie, H.; Chen, L. Adjustable thermal conductivity in carbon nanotube nanofluids. Phys. Lett. A 2009, 373, 1861–1864. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Routbort, J.L.; Singh, D. Particle shape effects on thermophysical properties of alumina nanofluids. J. Appl. Phys. 2009, 106, 014304. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.H.; Lee, J.H.; Jang, S.P. Effect of particle shape on suspension stability and thermal conductivities of water-based bohemite alumina nanofluids. Energy 2015, 90, 1290–1297. [Google Scholar] [CrossRef]
- Duangthongsuk, W.; Wongwises, S. An experimental study on the heat transfer performance and pressure drop of TiO2-water nanofluids flowing under a turbulent flow regime. Int. J. Heat Mass Transf. 2010, 53, 334–344. [Google Scholar] [CrossRef]
- Khairul, M.A.; Shah, K.; Doroodchi, E.; Azizian, R.; Moghtaderi, B. Effects of surfactant on stability and thermo-physical properties of metal oxide nanofluids. Int. J. Heat Mass Transf. 2016, 98, 778–787. [Google Scholar] [CrossRef]
- Heris, S.Z.; Noie, S.H.; Talaii, E.; Sargolzaei, J. Numerical investigation of Al2O3/water nanofluid laminar convective heat transfer through triangular ducts. Nanoscale Res. Lett. 2011, 6, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, Y.; Li, Q. Investigation on convective heat transfer and flow features of nanofluids. J. Heat Transf. 2003, 125, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Maiga, S.B.; Nguyen, C.T.; Galanis, N.; Roy, G.; Maré, T.; Coqueux, M. Heat transfer enhancement in turbulent tube flow using Al2O3 nanoparticle suspension. Int. J. Numer. Methods Heat Fluid Flow 2006, 16, 275–292. [Google Scholar] [CrossRef]
- Hatami, M.; Ganji, D.; Gorji-Bandpy, M. A review of different heat exchangers designs for increasing the diesel exhaust waste heat recovery. Renew. Sustain. Energy Rev. 2014, 37, 168–181. [Google Scholar] [CrossRef]
- Fakheri, A. Heat Exchanger Efficiency. J. Heat Transfer. 2007, 129, 1268–1276. [Google Scholar] [CrossRef]
- Pordanjani, A.H.; Aghakhani, S.; Afrand, M.; Mahmoudi, B.; Mahian, O.; Wongwises, S. An updated review on application of nanofluids in heat exchangers for saving energy. Energy Convers. Manag. 2019, 198, 111886. [Google Scholar] [CrossRef]
- Zamzamian, A.; Oskouie, S.N.; Doosthoseini, A.; Joneidi, A.; Pazouki, M. Experimental investigation of forced convective heat transfer coefficient in nanofluids of Al2O3/EG and CuO/EG in a double pipe and plate heat exchangers under turbulent flow. Exp. Therm. Fluid Sci. 2011, 35, 495–502. [Google Scholar] [CrossRef]
- Attalla, M.; Maghrabie, H.M. An experimental study on heat transfer and fluid flow of rough plate heat exchanger using Al2O3/water nanofluid. Exp. Heat Transf. 2020, 33, 261–281. [Google Scholar] [CrossRef]
- Leong, K.; Saidur, R.; Mahlia, T.; Yau, Y. Modeling of shell and tube heat recovery exchanger operated with nanofluid based coolants. Int. J. Heat Mass Transf. 2012, 55, 808–816. [Google Scholar] [CrossRef]
- Kong, R.; Asanakham, A.; Deethayat, T.; Kiatsiriroat, T. Heat transfer characteristics of deionized water-based graphene nanofluids in helical coiled heat exchanger for waste heat recovery of combustion stack gas. Heat Mass Transf. 2019, 55, 385–396. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Akhoundi, M. A feasibility study and economic analysis for application of nanofluids in waste heat recovery. Energy Equip. Syst. 2016, 4, 205–214. [Google Scholar] [CrossRef]
- Shafieian, A.; Khiadani, M.; Nosrati, A. A review of latest developments, progress, and applications of heat pipe solar collectors. Renew. Sustain. Energy Rev. 2018, 95, 273–304. [Google Scholar] [CrossRef]
- Noie-Baghban, S.H.; Majideian, G. Waste heat recovery using heat pipe heat exchanger (HPHE) for surgery rooms in hospitals. Appl. Therm. Eng. 2000, 20, 1271–1282. [Google Scholar] [CrossRef]
- Martınez, F.J.R.; Plasencia, M.A.A.-G.; Gomez, E.V.; Diez, F.V.; Martin, R.H. Design and experimental study of a mixed energy recovery system, heat pipes and indirect evaporative equipment for air conditioning. Energy Build. 2003, 35, 1021–1030. [Google Scholar] [CrossRef]
- Lukitobudi, A.; Akbarzadeh, A.; Johnson, P.; Hendy, P. Design, construction and testing of a thermosyphon heat exchanger for medium temperature heat recovery in bakeries. Heat Recovery Syst. CHP 1995, 15, 481–491. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, X.; Lin, G. Waste heat recovery using heat pipe heat exchanger for heating automobile using exhaust gas. Appl. Therm. Eng. 2003, 23, 367–372. [Google Scholar] [CrossRef]
- Riffat, S.; Gan, G. Determination of effectiveness of heat-pipe heat recovery for naturally-ventilated buildings. Appl. Therm. Eng. 1998, 18, 121–130. [Google Scholar] [CrossRef]
- Wu, X.P.; Johnson, P.; Akbarzadeh, A. Application of heat pipe heat exchangers to humidity control in air-conditioning systems. Appl. Therm. Eng. 1997, 17, 561–568. [Google Scholar] [CrossRef]
- Ghosayeshi, H.R.; Safaei, M.R.; Goodarzi, M.; Dahari, M. Particle size and type effects on heat transfer enhancement of Ferro-nanofluids in a pulsating heat pipe. Powder Technol. 2016, 301, 1218–1226. [Google Scholar] [CrossRef]
- Kang, S.-W.; Wang, Y.-C.; Liu, Y.-C.; Lo, H.-M. Visualization and thermal resistance measurements for a magnetic nanofluid pulsating heat pipe. Appl. Therm. Eng. 2017, 126, 1044–1150. [Google Scholar] [CrossRef]
- Ramachandran, R.; Ganesan, K.; Rajkumar, M.R.; Asirvatham, L.G.; Wongwises, S. Comparative study of the effect of hybrid nanoparticle on the thermal performance of cylindrical screen mesh heat pipe. Int. Commun. Heat Mass Transf. 2016, 76, 294–300. [Google Scholar] [CrossRef]
- Zufar, M.; Gunnasegaran, P.; Kumar, H.M.; Ng, K.C. Numerical and experimental investigations of hybrid nanofluids on pulsating heat pipe performance. Int. J. Heat Mass Transf. 2020, 146, 118887. [Google Scholar] [CrossRef]
- Qian, N.; Jiang, F.; Chen, J.; Fu, Y.; Zhang, J.; Xu, J. Heat transfer enhancement by diamond nanofluid in gravity heat pipe for waste heat recovery. Funct. Diam. 2022, 2, 236–244. [Google Scholar] [CrossRef]
- Chaudhari, V.; Dharmadhikari, M.; Kolhe, V. Performance Evaluation of Heat Pipe Heat Exchanger with Nanofluid: An Experimental Study. Lett. Appl. NanoBioScience 2023, 12, 121. [Google Scholar] [CrossRef]
- Shinde, S.M.; Arakerimath, R.R.; Bansod, P.J. Performance Study of Heat Pipe Heat Exchanger Using Nanofluid (BN) Used in Boiler Plant- Experimental Study. Int. J. Innov. Res. Sci. Eng. Technol. 2017, 6, 20941–20949. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, N.; Sonawane, S.S. Enhancement of pool boiling performance using MWCNT based nanofluids: A sustainable method for the wastewater and incinerator heat recovery. Sustain. Energy Technol. Assess. 2021, 45, 101115. [Google Scholar] [CrossRef]
- Tao, Y.; He, Y.-L. A review of phase change material and performance enhancement method for latent heat storage system. Renew. Sustain. Energy Rev. 2018, 93, 245–259. [Google Scholar] [CrossRef]
- Rathod, M.K.; Banerjee, J. Thermal stability of phase change materials used in latent heat energy storage systems: A review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- Nomura, T.; Okinaka, N.; Akiyama, T. Waste heat transportation system, using phase change material (PCM) from steelworks to chemical plant. Resour. Conserv. Recycl. 2010, 54, 1000–1006. [Google Scholar] [CrossRef]
- Begum, L.; Hasan, M.; Vatistas, G.H. Energy storage in a complex heat storage unit using commercial grade phase change materials: Effect of convective heat transfer boundary conditions. Appl. Therm. Eng. 2018, 131, 621–641. [Google Scholar] [CrossRef]
- Milian, Y.E.; Gutierrez, A.; Grageda, M.; Ushak, S. A review on encapsulation techniques for inorganic phase change materials and the influence on their thermophysical properties. Renew. Sustain. Energy Rev. 2017, 73, 983–999. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, S.; Zhou, D.; Tian, Y. Experimental study on thermal conductivity of paraffin-based shape-stabilized phase change material with hybrid carbon nano-additives. Renew. Energy 2020, 146, 2637–2645. [Google Scholar] [CrossRef]
- Wickramaratne, C.; Dhau, J.S.; Kamal, R.; Myers, P.; Goswami, D.Y.; Stefanakos, E. Macro-encapsulation and characterization of chloride based inorganic Phase change materials for high temperature thermal energy storage systems. Appl. Energy 2018, 221, 587–596. [Google Scholar] [CrossRef]
- Jeyaseelan, T.R.; Azhagesan, N.; Pethurajan, V. Thermal characterization of NaNO3/KNO3 with different concentrations of Al2O3 and TiO2 nanoparticles. J. Therm. Anal. Calorim. 2019, 136, 235–242. [Google Scholar] [CrossRef]
- Firouzfar, E.; Soltanieh, M.; Noie, S.H.; Saidi, S.H. Energy saving in HVAC systems using nanofluid. Appl. Therm. Eng. 2011, 31, 1543–1545. [Google Scholar] [CrossRef]
- Byrne, P.; Miriel, J.; Lénat, Y. Experimental study of an air-source heat pump for simultaneous heating and cooling–part 2: Dynamic behavior and two-phase thermosiphon defrosting technique. Appl. Therm. Eng. 2011, 88, 3072–3078. [Google Scholar] [CrossRef] [Green Version]
- Jouhara, H.; Merchant, H. Experimental investigation of a thermosyphon based heat exchanger used in energy efficient air handling units. Energy 2012, 39, 82–89. [Google Scholar] [CrossRef]
- Danielewicz, J.; Sayegh, M.A.; Sniechowska, B.; Szulgowska-Zgrzywa, M.; Jouhara, H. Experimental and analytical performance investigation of air to air two phase closed thermosyphon based heat exchangers. Energy 2014, 77, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Meena, P.; Tammasaeng, P.; Kanphirom, J.; Ponkho, A.; Setwong, S. Enhancement of the performance heat transfer of a thermosyphon with fin and without fin heat exchangers using Cu-nanofluid as working fluids. J. Eng. Thermophys. 2014, 23, 331–340. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.-F. Research on Energy Saving Potential for Dedicated Ventilation Systems Based on Heat Recovery Technology. Energies 2014, 7, 4261–4280. [Google Scholar] [CrossRef] [Green Version]
- Ahmadzadehtalatapeh, M. Improving the Energy Performance of HVAC Systems in Operating Theatres by Using Heat Recovery Devices. Int. J. Renew. Energy Res. 2014, 4, 586–592. [Google Scholar] [CrossRef]
- Karamarkovic, V.; Marasevic, M.; Karamarkovic, R.; Karamarkovic, M. Recuperator for waste heat recovery from rotary kilns. Appl. Therm. Eng. 2013, 54, 470–480. [Google Scholar] [CrossRef]
- Heinz, A.; Streicher, W. Application of Phase Change Materials and PCM Slurries for Thermal Energy Storage. In Proceedings of the 10th International Conference on Thermal Energy Storage, Pomona, CA, USA, 31 May 2006. [Google Scholar]
- Abedin, A.H.; Marc, A.R. A Critical Review of Thermo-Chemical Energy Storage Systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on Thermal Energy Storage with Phase Change Materials and Applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Khurana, S.; Banerjee, R.; Gaitonde, U. Energy balance and cogeneration for cement plants. Appl. Therm. Eng. 2002, 22, 485–494. [Google Scholar] [CrossRef]
- Madlool, N.A.; Saidur, R.; Hossain, M.S.; Rahim, N.A. A critical review on energy use and savings in the cement industries. Renew. Sustain. Energy Rev. 2011, 15, 2042–2060. [Google Scholar] [CrossRef]
- Madlool, N.A.; Hadi, A.A.A. Enhancement of waste heat recovery by using Cu nanoparticles in cement industries. Int. Sci. J.-J. Environ. Sci. 2015. [Google Scholar]
- Sulaiman, M.S.; Mohamed, W.; Singh, B.; Ghazali, M.F. Validation of a waste heat recovery model for a 1kW PEM fuel cell using thermoelectric generator. IOP Conf. Ser. Mater. Sci. Eng. 2017, 226, 012148. [Google Scholar] [CrossRef]
- Sehgal, S.; Alvarado, J.L.; Hassan, I.G.; Kadam, S.T. A comprehensive review of recent developments in falling-film, spray, bubble and microchannel absorbers for absorption systems. Renew. Sustain. Energy Rev. 2021, 142, 110807. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Q.; Zeng, M.; Xu, R.; Xie, G.; Chu, W. Investigation on mass transfer characteristics of the falling film absorption of LiBr aqueous solution added with nanoparticles. Int. J. Refrig 2018, 89, 149–158. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, Z.; Liu, Y.; Jin, L.; Zhang, Q.; Hu, W. Experimental study on enhancement of falling film absorption process by adding various nanoparticles. Int. Commun. Heat Mass Transf. 2018, 92, 100–106. [Google Scholar] [CrossRef]
- Pourfayaz, F.; Imani, M.; Mehrpooya, M.; Shirmohammadi, R. Process development and exergy analysis of a novel hybrid fuel cell-absorption refrigeration system utilizing nanofluid as the absorbent liquid. Int. J. Refrig. 2019, 97, 31–41. [Google Scholar] [CrossRef]
- Beigzadeh, M.; Pourfayaz, F.; Ahmadi, M.H. Modeling and improvement of solid oxide fuel cell-single effect absorption chiller hybrid system by using nanofluids as heat transporters. Appl. Therm. Eng. 2020, 166, 114707. [Google Scholar] [CrossRef]
- Baroutaji, A.; Arjunan, A.; Ramadan, M.; Robinson, J.; Alaswad, A.; Abdelkareem, M.A.; Olabi, A.-G. Advancements and prospects of thermal management and waste heat recovery of PEMFC. Int. J. Thermofluids 2021, 9, 100064. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, H.; Hu, Z.; Zhang, Z.; Zhang, J. Performance characteristics of a direct carbon fuel cell/thermoelectric generator hybrid system. Energy Convers. Manag. 2015, 89, 683–689. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Y.; Zhang, P.; Zhang, H.; Hu, Z.; Zhang, J. Performance evaluation of an alkaline fuel cell/thermoelectric generator hybrid system. Int. J. Hydrogen Energy 2014, 39, 11756–11762. [Google Scholar] [CrossRef]
- Jouhara, H.; Zabnienska-Gora, A.; Khordehgah, N.; Doraghi, Q.; Ahmad, L.; Norman, L.; Axcell, B.; Wrobel, L.; Dai, S. Thermoelectric generator (TEG) technologies and applications. Int. J. Thermofluids 2021, 9, 100063. [Google Scholar] [CrossRef]
- Selimefendigil, F.; Okulu, D.; Mamur, H. Numerical analysis for performance enhancement of thermoelectric generator modules by using CNT–water and hybrid Ag/MgO–water nanofluids. J. Therm. Anal. Calorim. 2020, 143, 1611–1621. [Google Scholar] [CrossRef]
- Xing, J.-J.; Wu, Z.-H.; Xie, H.-Q.; Wang, Y.-Y.; Li, Y.-H.; Mao, J.-H. Performance of thermoelectric generator with graphene nanofluid cooling. Chin. Phys. B 2017, 26, 104401. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Xie, H.; Xing, J.; Mao, J.; Wang, Y.; Li, Z. Study on the performance of TEG with heat transfer enhancement using graphene-water nanofluid for a TEG cooling system. Sci. China Technol. Sci. 2017, 60, 1168–1174. [Google Scholar] [CrossRef]
- Li, Z.; Li, W.; Chen, Z. Performance analysis of thermoelectric based automotive waste heat recovery system with nanofluid coolant. Energies 2017, 10, 1489. [Google Scholar] [CrossRef] [Green Version]
- Eldesoukey, A.; Hassan, H. Study of the performance of thermoelectric generator for waste heat recovery from chimney: Impact of nanofluid-microchannel cooling system. Environ. Sci. Pollut. Res. 2022, 29, 74242–74263. [Google Scholar] [CrossRef]
- Dalvand, H.M.; Moghadam, A.J. Experimental investigation of a water/nanofluid jacket performance in stack heat recovery. J. Therm. Anal. Calorim. 2019, 135, 657–669. [Google Scholar] [CrossRef]
- Jouhara, H.; Khordehgah, N.; Almahmoud, S.; Delpech, B.; Chauhan, A.; Tassou, S.A. Waste heat recovery technologies and applications. Therm. Sci. Eng. Prog. 2018, 6, 268–289. [Google Scholar] [CrossRef]
- Júnior, E.P.B.; Arrieta, M.D.P.; Arrieta, F.R.P.; Silva, C.H.F. Assessment of a Kalina cycle for waste heat recovery in the cement industry. Appl. Therm. Eng. 2019, 147, 421–437. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Yang, B.; Ge, Y.; Sun, F. Thermodynamic analysis and optimization of an air Brayton cycle for recovering waste heat of blast furnace slag. Appl. Therm. Eng. 2015, 90, 742–748. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C. Parametric analysis and optimization of an Organic Rankine Cycle with nanofluid based solar parabolic trough collectors. Renew. Energy 2017, 114, 1376–1393. [Google Scholar] [CrossRef]
- Saadatfar, B.; Fakhrai, R.; Fransson, T. Conceptual modeling of nano fluid ORC for solar thermal polygeneration. Energy Procedia 2014, 57, 2696–2705. [Google Scholar] [CrossRef] [Green Version]
- Mondejar, M.E.; Andreasen, J.G.; Regidor, M.; Riva, S.; Kontogeorgis, G.; Persico, G.; Haglind, F. Prospects of the use of nanofluids as working fluids for organic Rankine cycle power systems. Energy Procedia 2017, 129, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Cavazzini, G.; Bari, S.; McGrail, P.; Benedetti, V.; Pavesi, G.; Ardizzon, G. Contribution of Metal-Organic-Heat Carrier nanoparticles in a R245fa low-grade heat recovery Organic Rankine Cycle. Energy Convers. Manag. 2019, 199, 111960. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, Z.; Yan, B. Comparison of the performance of two different Dual-loop organic Rankine cycles (DORC) with nanofluid for engine waste heat recovery. Energy Convers. Manag. 2016, 126, 99–109. [Google Scholar] [CrossRef]
- Sami, S. Analysis of Nanofluids Behavior in a PV-Thermal-Driven Organic Rankine Cycle with Cooling Capability. Appl. Syst. Innov. 2020, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Refiei, A.; Loni, R.; Najafi, G.; Sahin, A.; Bellos, E. Effect of use of MWCNT/oil nanofluid on the performance of solar organic Rankine cycle. Energy Rep. 2020, 6, 782–794. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Dehqani, M.; Mousavi, S.A. Heat transfer and economic analysis of using various nanofluids in shell and tube heat exchangers for the cogeneration and solar-driven organic Rankine cycle systems. Int. J. Low-Carbon Technol. 2021, 17, 11–22. [Google Scholar] [CrossRef]
- Prajapati, P.P.; Patel, V.K. Thermo-economic optimization of a nanofluid based organic Rankine cycle: A multi-objective study and analysis. Therm. Sci. Eng. Prog. 2020, 17, 100381. [Google Scholar] [CrossRef]
- Soltani, S.; Kasaeian, A.; Sarrafha, H.; Wen, D. An experimental investigation of a hybrid photovoltaic/thermoelectric system with nanofluid application. Sol. Energy 2017, 155, 1033–1043. [Google Scholar] [CrossRef]
- Rajaee, F.; Rad, M.A.V.; Kasaeian, A.; Mahian, O.; Yan, W.-M. Experimental analysis of a photovoltaic/thermoelectric generator using cobalt oxide nanofluid and phase change material heat sink. Energy Convers. Manag. 2020, 212, 112780. [Google Scholar] [CrossRef]
- Sami, S.; Marin, E. Modelling and Simulation of PV Solar-Thermoelectric Generators using Nano fluids. Int. J. Sustain. Energy Environ. Res. 2019, 8, 70–99. [Google Scholar] [CrossRef]
- Ramamurthi, P.V.; Nadar, E.R.S. An Integrated SiGe Based Thermoelectric Generator with Parabolic Trough Collector Using Nano HTF for Effective Harvesting of Solar Radiant Energy. J. Electron. Mater. 2019, 48, 7780–7791. [Google Scholar] [CrossRef]
- Mohammadnia, A.; Ziapour, B.M.; Sedaghati, F.; Rosendahl, L.; Rezania, A. Utilizing thermoelectric generator as cavity temperature controller for temperature management in dish-Stirling engine. Appl. Therm. Eng. 2020, 165, 114568. [Google Scholar] [CrossRef]
- Smith, K.; Thornton, M. Feasibility of Thermoelectrics for Waste Heat Recovery in Conventional Vehicles; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2009. [Google Scholar]
- Karana, D.R.; Sahoo, R.R. Effect on TEG performance for waste heat recovery of automobiles using MgO and ZnO nanofluid coolants. Case Stud. Therm. Eng. 2018, 12, 358–364. [Google Scholar] [CrossRef]
- Karana, D.R.; Sahoo, R.R. Performance effect on the TEG system for waste heat recovery in automobiles using ZnO and SiO2 nanofluid coolants. Heat Transf.—Asian Res. 2019, 48, 216–232. [Google Scholar] [CrossRef] [Green Version]
- Hilmin, M.N.H.M.; Remeli, M.F.; Singh, B.; Affandi, N.D.N. Thermoelectric Power Generations from Vehicle Exhaust Gas with TiO2 Nanofluid Cooling. Therm. Sci. Eng. Prog. 2020, 18, 100558. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.; Moita, A.; Moreira, A. Nanofluids as a Waste Heat Recovery Medium: A Critical Review and Guidelines for Future Research and Use. Processes 2023, 11, 2443. https://doi.org/10.3390/pr11082443
Pereira J, Moita A, Moreira A. Nanofluids as a Waste Heat Recovery Medium: A Critical Review and Guidelines for Future Research and Use. Processes. 2023; 11(8):2443. https://doi.org/10.3390/pr11082443
Chicago/Turabian StylePereira, José, Ana Moita, and António Moreira. 2023. "Nanofluids as a Waste Heat Recovery Medium: A Critical Review and Guidelines for Future Research and Use" Processes 11, no. 8: 2443. https://doi.org/10.3390/pr11082443
APA StylePereira, J., Moita, A., & Moreira, A. (2023). Nanofluids as a Waste Heat Recovery Medium: A Critical Review and Guidelines for Future Research and Use. Processes, 11(8), 2443. https://doi.org/10.3390/pr11082443








