Extraction and Microencapsulation of Phytochemical Compounds from Mango Peel (Mangifera indica L.) var. “Kent” and Assessment of Bioaccessibility through In Vitro Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. Mango Samples
2.3. Sample Conditioning
2.4. Extraction of Phytochemicals
2.5. Optimization of Extraction Conditions
2.5.1. Number of Consecutive Extractions
2.5.2. Box–Behnken Design
2.6. Analytical Methods
2.6.1. Phenolic Compounds (PC)
2.6.2. Flavonoid Content (FC)
2.6.3. Mangiferin Content (MC)
2.6.4. Anthocyanin Content (AC)
2.6.5. Carotenoid Content (CC)
2.6.6. Ascorbic Acid Content (AA)
2.6.7. Antioxidant Capacity
2.7. Microencapsulation
2.7.1. Microencapsulation by Spray Drying (SD)
2.7.2. Microencapsulation by Spout-Fluid Bed Dryer (SFB)
2.8. Characterization of the Microcapsules
2.8.1. Total Phenolic Compounds on the Surface of the Microcapsules
2.8.2. Retention Efficiency (RE) and Encapsulation Efficiency (EE)
2.8.3. Moisture Content and aw (Water Activity)
2.8.4. Particle Size Distribution
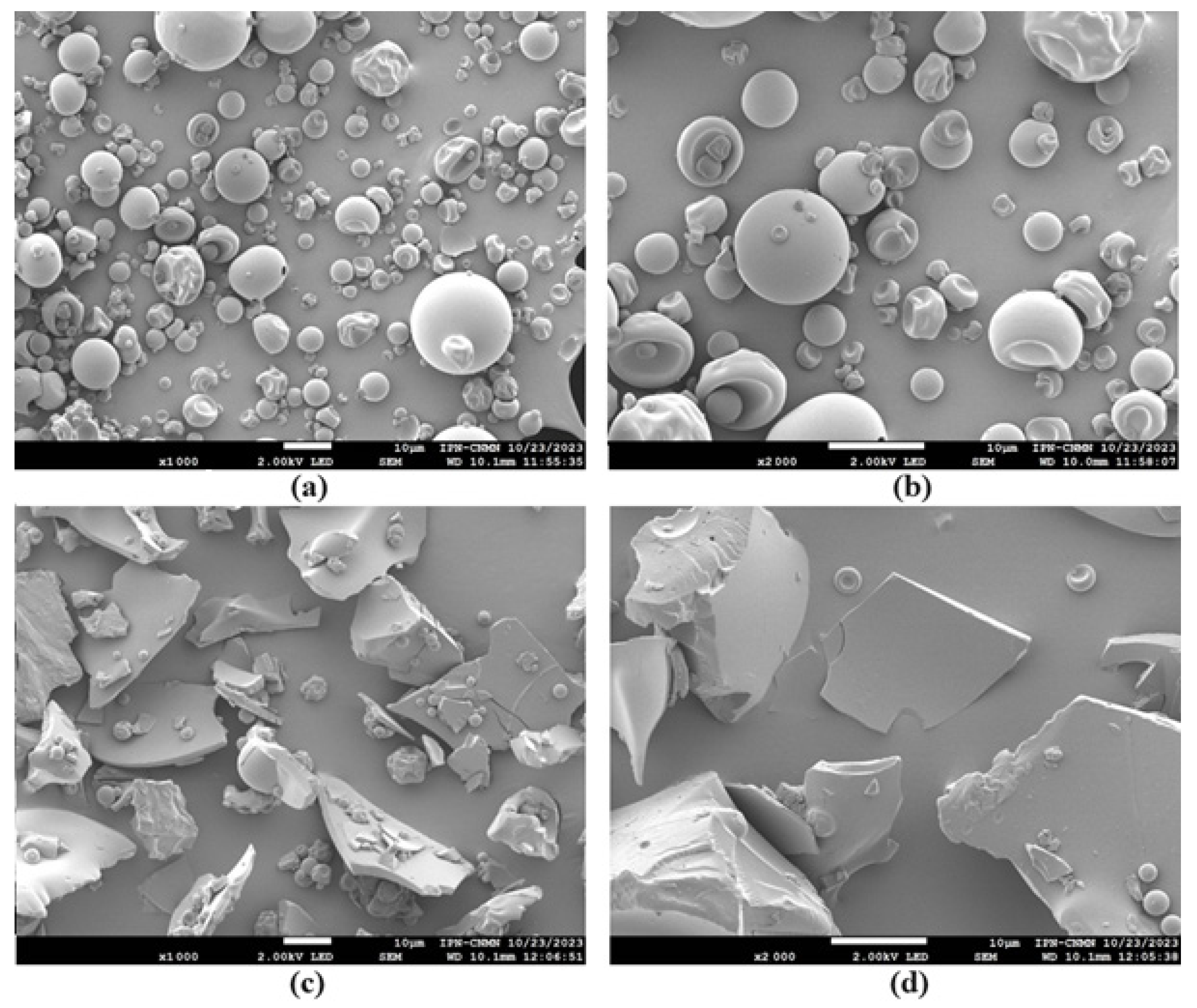
2.8.5. Morphology of Microcapsules
2.9. In Vitro Digestion Procedure
2.10. Statistical Analysis
3. Results
3.1. Yield of “Kent” Mango
3.2. Bioactive Compounds (BC) and Antioxidant Capacity (AC) of Mango Peel
3.3. Optimization of the Extraction of PC and FC of Mango Peel Powder
3.3.1. Effect of Sequential Extractions
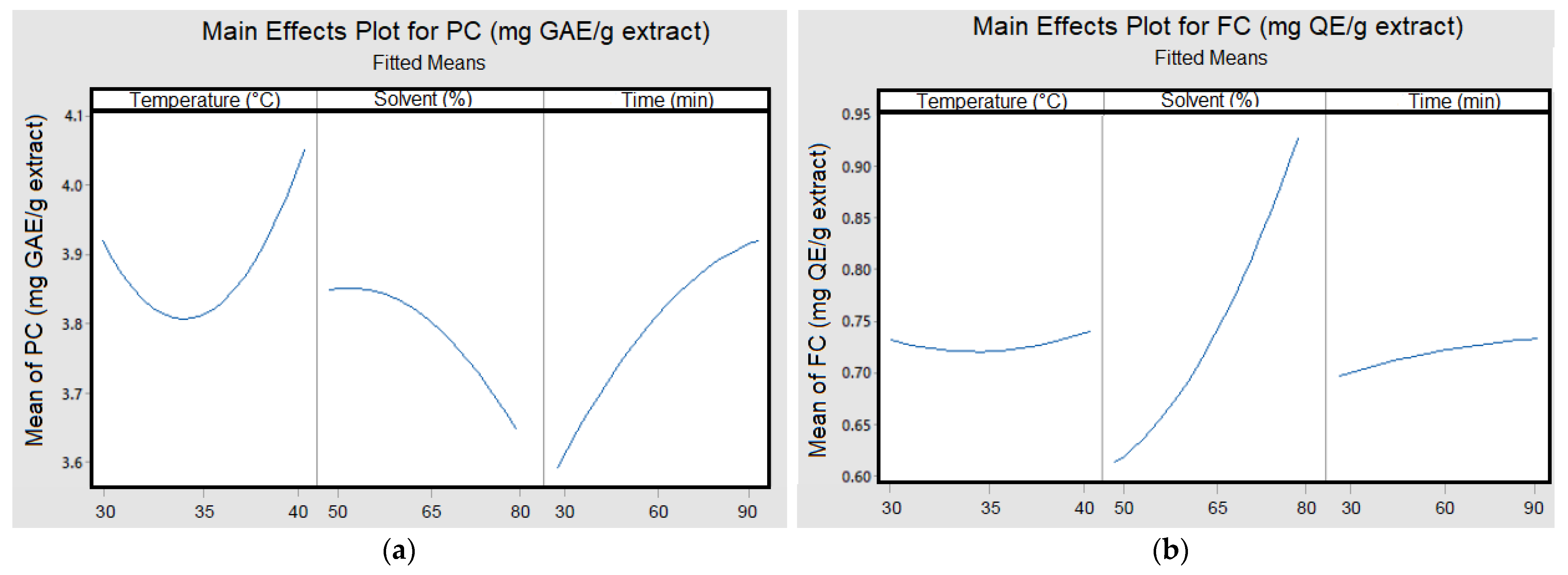
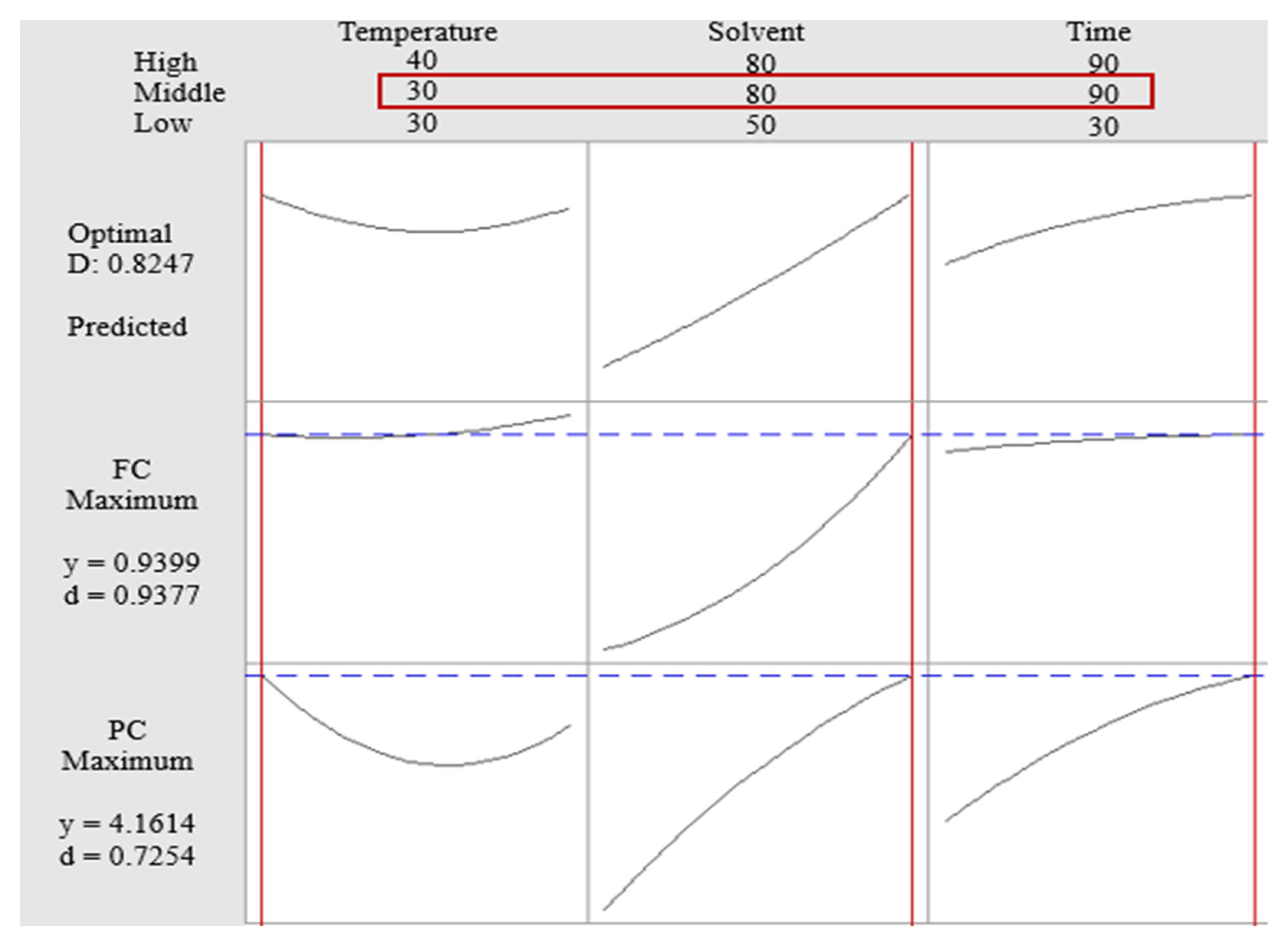
3.3.2. Box–Behnken Design
3.4. Microencapsulation through SD and SFB
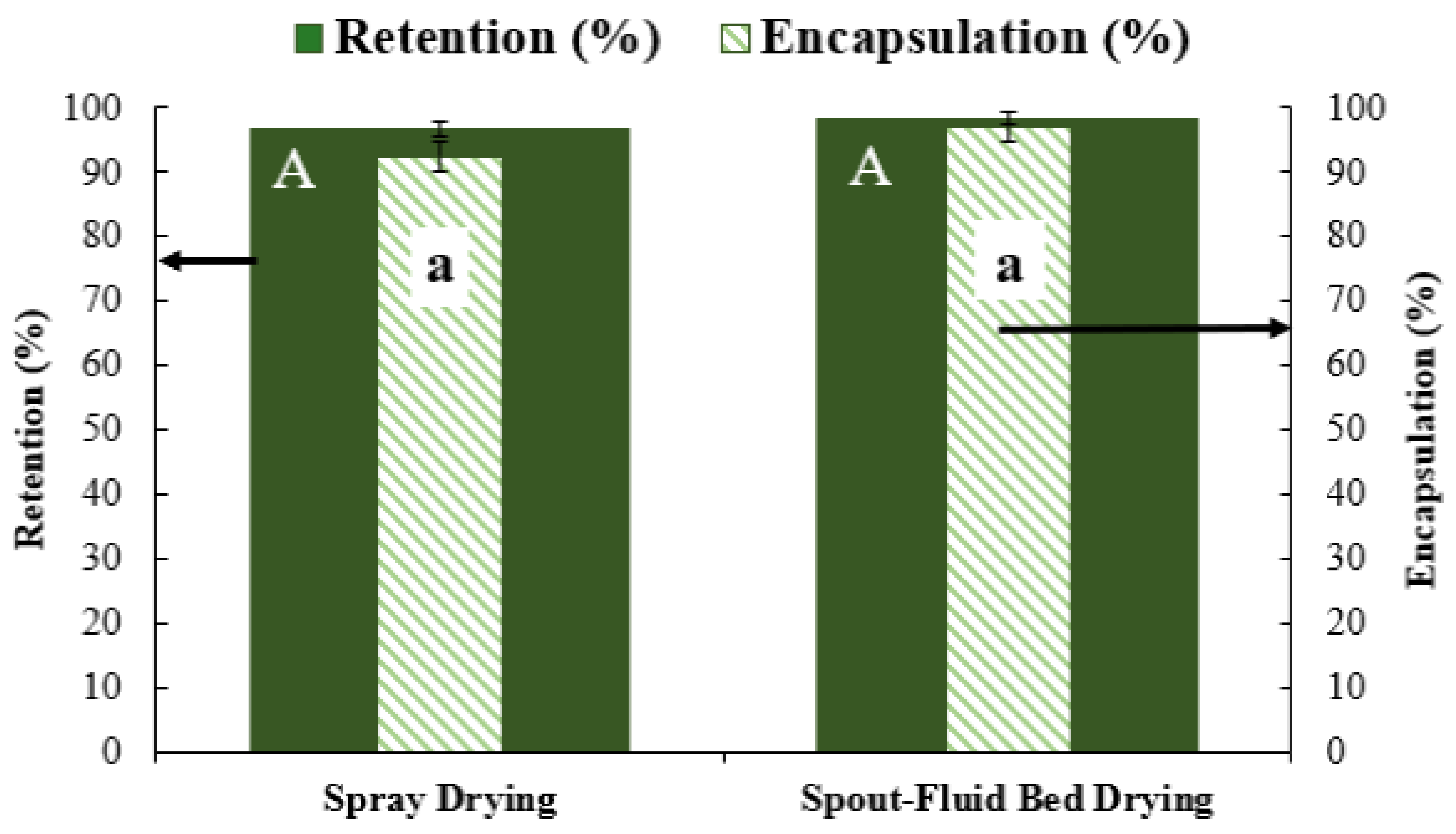
3.4.1. Retention and Encapsulation Efficiency
3.4.2. Contents of BC and AC in Microcapsules
3.4.3. Moisture Content and aw
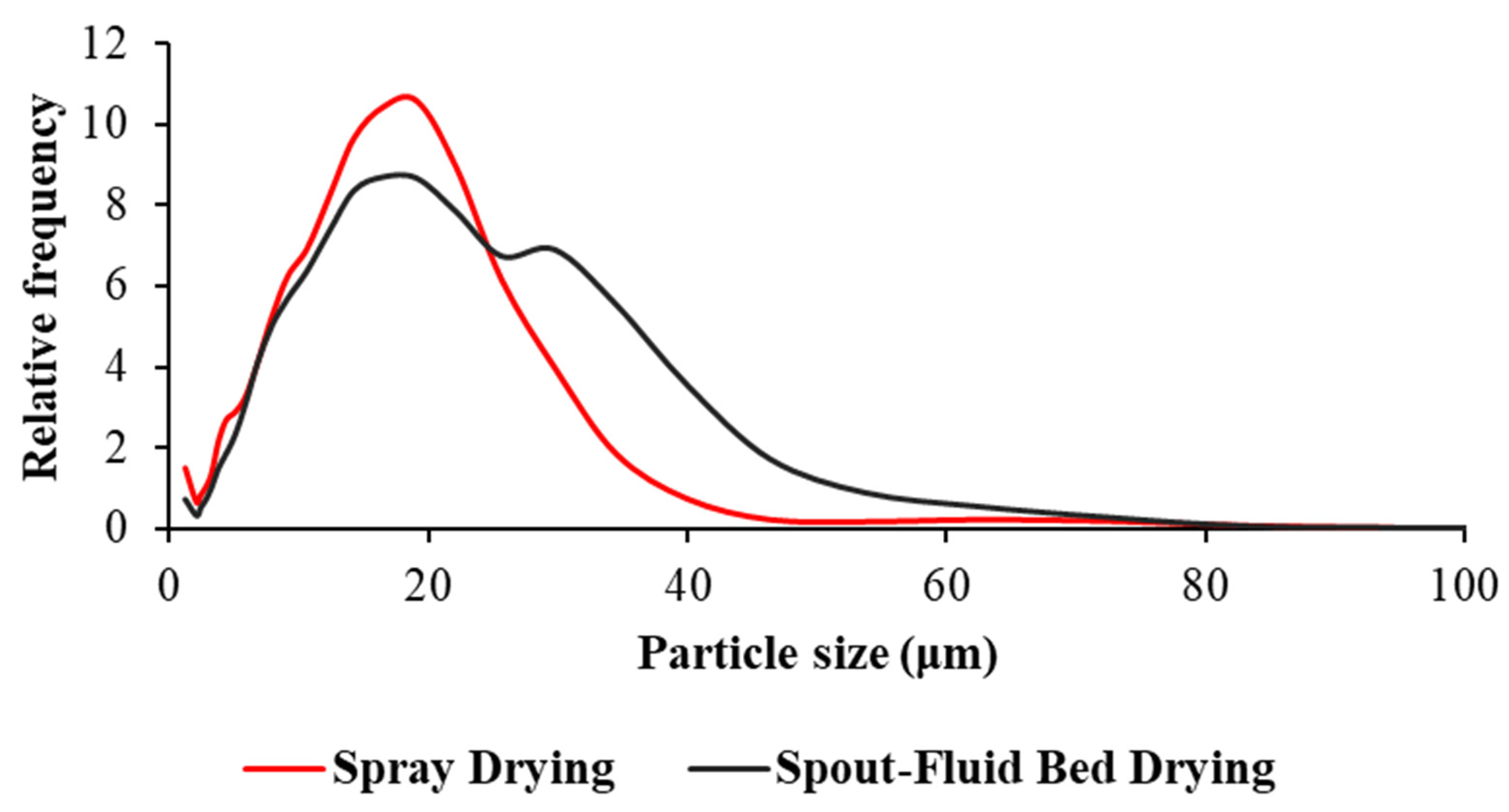
3.4.4. Particle Size Distribution
3.4.5. Morphology of Microcapsules
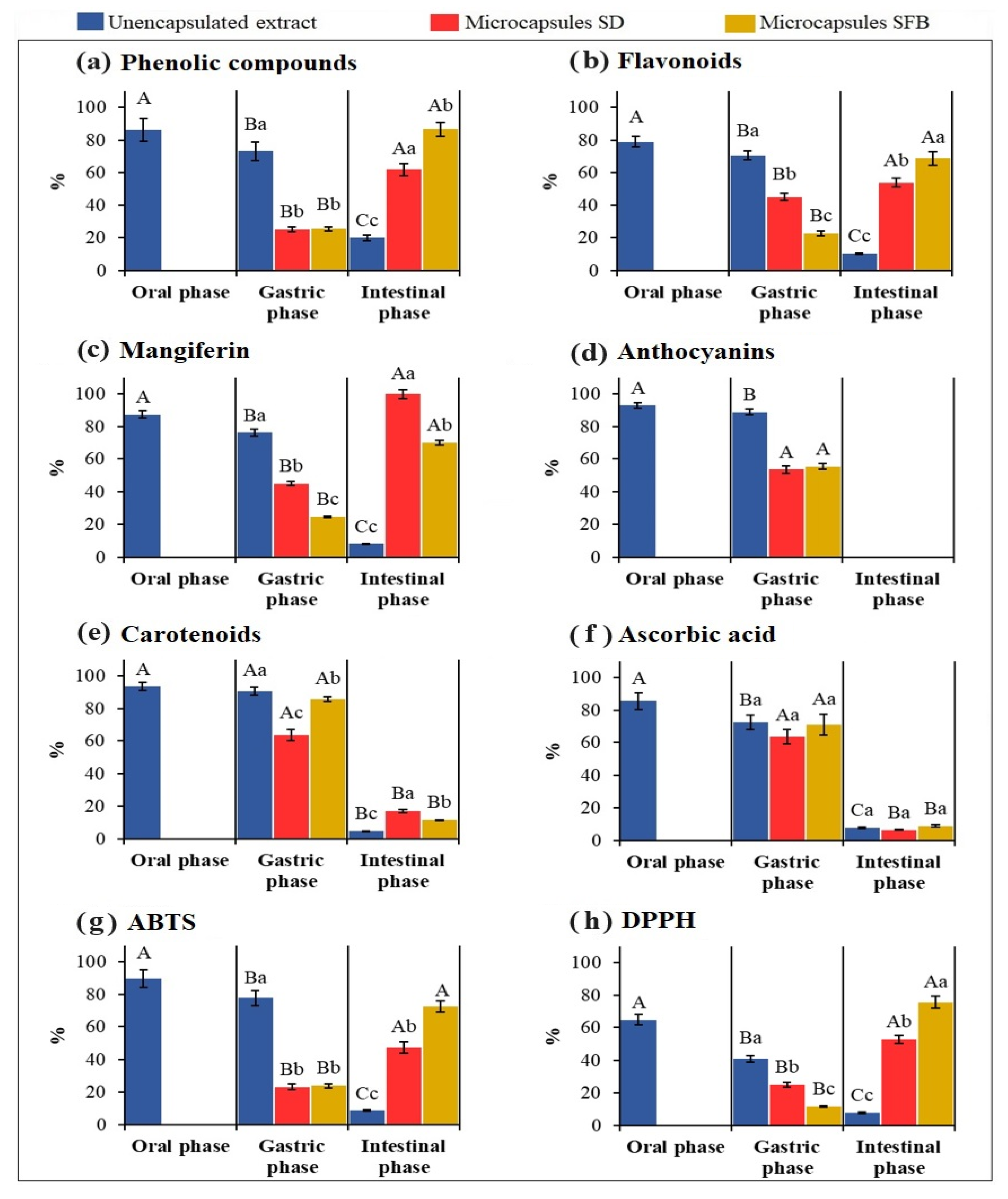
3.5. In Vitro Gastrointestinal Digestion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larios-Medrano, I.; Campos-Serrano, M.K.; Padilla-Sahagún, M.; Villanueva-Rodríguez, S.J. Materia prima. In Introducción a la Tecnología del Mango; Villanueva-Rodríguez, S.J., Ed.; CIATEJ: Jalisco, Mexico, 2016; pp. 1–13. [Google Scholar]
- Bezerra de Sousa, A.S.; Alves da Silva, M.C.; Pereira-Lima, R.; Lins de Albuquerque-Meireles, B.R.; Magalhaes-Cordeiro, A.T.; da Silva Santos, E.F.; Amaro, A.L.; Estevez-Pintado, M.M.; de Melo-Silva, S. Phenolic compounds and antioxidant activity as discriminating markers and adding value of mango varieties. Sci. Hortic. 2021, 287, 110259–110268. [Google Scholar] [CrossRef]
- Gálvez-López, D.; Salvador-Figueroa, M.; Becerra-León, E.N.; González-Paz, M.; Hernández-Delgado, S.; Mayek-Pérez, N. Diversidad molecular y relaciones genéticas de germoplasma de mango de Chiapas, México. Agrociencia 2010, 44, 907–915. [Google Scholar]
- Marcillo-Parra, V.; Anaguano, M.; Molina, M.; Tupuna-Yerovi, D.S.; Ruales, J. Characterization and quantification of bioactive compounds and antioxidant activity in three different varieties of mango (Mangifera indica L.) peel from the Ecuadorian region using HPLC-UV/VIS and UPLC-PDA. NFS J. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Spray drying, freeze drying, and related processes for food ingredient and nutraceutical encapsulation. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Garti, N., McClements, D.J., Eds.; WP Woodhead Publishing: Oxford, UK, 2012; pp. 73–109. [Google Scholar]
- Ajila, C.M.; Jaganmohan-Rao, L.; Prasada-Rao, U.J.S. Characterization of bioactive compounds from raw and ripe Mangifera indica L. peel extracts. Food Chem. Toxicol. 2010, 45, 1406–3411. [Google Scholar] [CrossRef]
- Singh-Sogi, D.; Siddiq, M.; Greiby, I.; Dolan, K.D. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef]
- López-Cobo, A.; Verardo, V.; Diaz-de-Cerio, E.; Segura-Carretero, A.; Fernández- Gutiérrez, A.; Gómez-Caravaca, A.M. Use of HPLC- and GC-QTOF to determine hydrophilic and lipophilic phenols in mango fruit (Mangifera indica L.) and its by-products. Food Res. Int. 2017, 100, 423–434. [Google Scholar] [CrossRef]
- De Ancos, B.; Sánchez-Moreno, C.; Zacarías, L.; Rodrigo, M.J.; Sáyago-Ayerdi, S.; Blancas-Benítez, F.J.; Domínguez-Avila, J.A.; González-Aguilar, G.A. Effects of two different drying methods (freeze-drying and hot airdrying) on the phenolic and carotenoid profile of ‘Ataulfo’ mango byproducts. J. Food Meas. Charact. 2018, 12, 2145–2157. [Google Scholar] [CrossRef]
- Pacheco-Ordaz, R.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; González-Aguilar, G.A. Intestinal permeability and cellular antioxidant activity of phenolic compounds from mango (Mangifera indica cv. Ataulfo) peels. Int. J. Mol. Sci. 2018, 19, 514. [Google Scholar] [CrossRef]
- Castro-Vargas, H.I.; Ballesteros-Vivas, D.; Ortega-Barbosa, J.; Morantes-Medina, S.J.; Aristizabal-Gutiérrez, F.; Parada-Alfonso, F. Bioactive phenolic compounds from the agroindustrial waste of Colombian mango cultivars ‘Sugar Mango’ and ‘Tommy Atkins’—An alternative for their use and valorization. Antioxidants 2019, 8, 41. [Google Scholar] [CrossRef]
- Espinosa-Espinosa, L.; Garduño-Siciliano, L.; Rodríguez-Canales, M.; Hernández-Portilla, L.B.; Canales-Martinez, M.M.; Rodriguez-Monroy, M.A. The wound-healing effect of mango peel extract on incision wounds in a murine model. Molecules 2022, 27, 259. [Google Scholar] [CrossRef] [PubMed]
- Mugwagwa, L.R.; Chimphango, A.F.A. Box-Behnken design based multi-objective optimisation of sequential extraction of pectin and anthocyanins from mango peels. Carbohydr. Polym. 2019, 219, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sumaya-Martínez, M.T.; Medina-Carrillo, R.E.; González-Ocegueda, E.; Jiménez-Ruiz, E.I.; Balois-Morales, R.; Sánchez-Herrera, L.M.; López-Nahuatt, G. Mango (Mangifera indica L.) pulping byproducts: Antioxidant activity and bioactive compounds of three mango cultivars. BioCiencias 2019, 6, 1–20. [Google Scholar]
- Alañón, M.E.; Pimentel-Moral, S.; Arráez-Román, D.; Segura-Carretero, A. Profiling phenolic compounds in underutilized mango peel by-products from cultivars grown in Spanish subtropical climate over maturation course. Food Res. Int. 2021, 140, 109852. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramos, T.; Benedito-Fort, J.; Watson, N.J.; Ruiz-López, I.I.; Che-Galicia, G.; Corona-Jiménez, E. Effect of solvent composition and its interaction with ultrasonic energy on the ultrasound-assisted extraction of phenolic compounds from Mango peels (Mangifera indica L.). Food Bioprod. Process. 2020, 122, 41–54. [Google Scholar] [CrossRef]
- González-Montelongo, R.; Gloria, L.M.; González, M. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chem. 2010, 119, 1030–1039. [Google Scholar] [CrossRef]
- Yin-Thoo, Y.; Kheng-Hoa, D.; Yun-Liangb, J.; Wai-Hob, C.; Ping-Tan, C. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia). Food Chem. 2010, 120, 290–295. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Anthocyanins. Characterization and measurement of anthocyanins by UV Visible Spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R., Ed.; Wiley: New York, NY, USA, 2001; pp. 21–30. [Google Scholar]
- Dürüst, N.; Sümengen, D.; Dürüst, Y. Ascorbic acid and element contents of foods of Trabzon (Turkey). J. Agric. Food Chem. 1997, 45, 2085–2087. [Google Scholar] [CrossRef]
- Re, R.; Nicoletta, P.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical methods to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rigon, R.T.; Zapata, N.C.P. Microencapsulation by spray-drying of bioactive compounds extracted from blackberry (Rubus fruticosus). J. Food Sci. Technol. 2016, 53, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenolns and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Pinazo, A.; Heredia, A.; Andrés, A. Evaluation studies of persimmon plant (Diospyros kaki) for physiological benefits and bioaccessibility of antioxidants by in vitro simulated gastrointestinal digestion. Food Chem. 2017, 214, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Marçal, S.; Pintado, M. Mango peels as food ingredient/additive: Nutritional value, processing, safety and applications. Trends Food Sci. Technol. 2021, 114, 472–489. [Google Scholar] [CrossRef]
- Thakare, V.B.; Jadeja, G.C.; Desai, M.A. Extraction of mangiferin and pectin from mango peels using process intensified tactic: A step towards waste valorization. Chem. Eng. Res. Design 2023, 192, 280–288. [Google Scholar] [CrossRef]
- Chongchatuporn, U.; Ketsa, S.; van Doorn, W.G. Chilling injury in mango (Mangifera indica) fruit peel: Relationship with ascorbic acid concentrations and antioxidant enzyme activities. Postharvest Biol. Technol. 2013, 86, 409–417. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.; Ballesteros-Vivas, D.; Buelvas-Puello, L.M.; Martínez-Correa, H.A.; Parada-Alfonso, F.; Cifuentes, A.; Ferreira, S.R.S.; Gutiérrez, L.F. Microwave-assisted extraction of phenolic compounds with antioxidant and anti-proliferative activities from supercritical CO2 pre-extracted mango peel as valorization strategy. LWT 2021, 137, 110414. [Google Scholar] [CrossRef]
- Pajaro-Castro, N.P.; Granados-Conde, C.; Torrenegra-Alarcón, M.E.; Osorio-Fortich, M.; Pajaro-Castro, E.J.; Leon-Mendez, G. Microencapsulation of pulp of Mangifera indica L. by spray drying and antioxidant activity. Int. J. Pharm. Pharm. Sci. 2017, 9, 181–185. [Google Scholar] [CrossRef]
- Lim, K.J.A.; Cabajar, A.A.; Migallos, M.K.V.; Lobarbio, C.F.Y.; Taboada, E.B. Microencapsulation of Phenolic Compounds from Waste Mango Seed Kernel Extract by Spray Drying Technology. Nat. Environ. Pollut. Technol. 2019, 18, 765–775. [Google Scholar]
- Nikolić, N.C.; Stanisavljević, N.; Šavikin, K.; Kaluševic, A.; Nedović, V.; Bigović, D.; Janković, A.T. Application of gum arabic in the production of spray-dried chokeberry polyphenols, microparticles characterisation and in vitro digestion method. Lek. Sirovine 2018, 38, 9–16. [Google Scholar] [CrossRef]
- Ketnawa, S.; Suwannachot, J.; Ogawa, Y. In vitro gastrointestinal digestion of crisphead lettuce: Changes in bioactive compounds and antioxidant potential. Food Chem. 2020, 311, 125885. [Google Scholar] [CrossRef] [PubMed]
- Blancas-Benitez, F.J.; Mercado-Mercado, G.; Quirós-Sauceda, A.E.; Montalvo-González, E.; González-Aguilar, G.A.; Sáyago-Ayerdi, S.G. Bioaccessibility of polyphenols associated with dietary fiber and in vitro kinetics release of polyphenols in Mexican ‘Ataulfo’ mango (Mangifera indica L.) by-products. Food Funct. 2015, 6, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Deng, J.; Wen, L.; You, L.; Zhao, Z.; Zhou, L. Release of phenolic compounds and antioxidant capacity of Chinese hawthorn “Crataegus pinnatifida” during in vitro digestion. J. Funct. Foods 2018, 40, 76–85. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Cilla, A.; Bosch, L.; Barberá, R.; Alegría, A. Effect of processing on the bioaccessibility of bioactive compounds—A review focusing on carotenoids, minerals, ascorbic acid, tocopherols and polyphenols. J. Food Comp. Anal. 2018, 68, 3–15. [Google Scholar] [CrossRef]
- Cabezas-Terán, K.; Gootaert, C.; Ortiz, J.; Donoso, S.; Ruales, J.; Van Bockstaele, F.; Van Camp, J.; Van de Wiele, T. In vitro bioaccessibility and uptake of β-carotene from encapsulated carotenoids from mango by-products in a coupled gastrointestinal digestion/Caco-2 cell model. Food Res. Int. 2023, 164, 11201. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]

| Parameter | Fresh Mango Peel | Mango Peel Dried Powder |
|---|---|---|
| PC (mg GAE/gdw) | 58.33 ± 2.97 a | 57.57 ± 2.53 a |
| FC (mg QE/gdw) | 11.35 ± 0.21 a | 11.04 ± 0.16 a |
| MC (mg ME/gdw) | 3.20 ± 0.23 a | 3.01 ± 0.10 a |
| AC (mg cya-3-glu/gdw) | 0.15 ± 0.01 a | 0.14 ± 0.02 a |
| CC (mg β-carotene/gdw) | 2.65 ± 0.08 a | 2.38 ± 0.33 a |
| AA (mg AA/gdw) | 12.66 ± 0.04 a | 11.63 ± 0.12 b |
| Antioxidant capacity | ||
| ABTS (μmol TE/gdw) | 144.86 ± 2.07 a,A | 142.69 ± 0.31 a,A |
| DPPH (μmol TE/gdw) | 185.07 ± 1.15 a,B | 181.74 ± 0.22 b,B |
| Parameter | SD | SFB |
|---|---|---|
| PC (mg GAE/gdw) | 102.35 ± 6.83 a | 105.54 ± 4.34 a |
| FC (mg QE/gdw) | 58.77 ± 0.42 b | 67.56 ± 1.84 a |
| MC (mg ME/gdw) | 7.26 ± 1.29 b | 10.97 ± 0.45 a |
| AC (mg cya-3-glu/gdw) | 0.064 ± 0.007 a | 0.064 ± 0.005 a |
| CC (mg β-carotene/gdw) | 0.11 ± 0.004 b | 0.16 ± 0.002 a |
| AAC (mg AA/gdw) | 0.95 ± 0.17 b | 1.47 ± 0.02 a |
| Antioxidant capacity | ||
| ABTS (μmol TE/gdw) | 141.34 ± 9.96 b,B | 170.54 ± 4.36 a,B |
| DPPH (μmol TE/gdw) | 268.15 ± 2.98 b,A | 297.92 ± 6.03 a,A |
| Microcapsules | Moisture Content (%) | Water Activity (aw) |
|---|---|---|
| Spray Drying | 3.70 ± 0.33 a | 0.24 ± 0.01 a |
| Spout-Fluid Bed Drying | 2.70 ± 0.26 b | 0.16 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roa-Tort, A.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Gallardo-Velázquez, T.; Ramos-Monroy, O.A. Extraction and Microencapsulation of Phytochemical Compounds from Mango Peel (Mangifera indica L.) var. “Kent” and Assessment of Bioaccessibility through In Vitro Digestion. Processes 2024, 12, 154. https://doi.org/10.3390/pr12010154
Roa-Tort A, Meza-Márquez OG, Osorio-Revilla G, Gallardo-Velázquez T, Ramos-Monroy OA. Extraction and Microencapsulation of Phytochemical Compounds from Mango Peel (Mangifera indica L.) var. “Kent” and Assessment of Bioaccessibility through In Vitro Digestion. Processes. 2024; 12(1):154. https://doi.org/10.3390/pr12010154
Chicago/Turabian StyleRoa-Tort, Arantxa, Ofelia Gabriela Meza-Márquez, Guillermo Osorio-Revilla, Tzayhri Gallardo-Velázquez, and Oswaldo Arturo Ramos-Monroy. 2024. "Extraction and Microencapsulation of Phytochemical Compounds from Mango Peel (Mangifera indica L.) var. “Kent” and Assessment of Bioaccessibility through In Vitro Digestion" Processes 12, no. 1: 154. https://doi.org/10.3390/pr12010154
APA StyleRoa-Tort, A., Meza-Márquez, O. G., Osorio-Revilla, G., Gallardo-Velázquez, T., & Ramos-Monroy, O. A. (2024). Extraction and Microencapsulation of Phytochemical Compounds from Mango Peel (Mangifera indica L.) var. “Kent” and Assessment of Bioaccessibility through In Vitro Digestion. Processes, 12(1), 154. https://doi.org/10.3390/pr12010154






