1. Introduction
The constant-temperature operation of a freezer is one of the most important aspects to ensure the freshness of refrigerated and frozen foods. Large temperature variations in the internal volume of a refrigerator lead to moisture loss of food, which, in turn, causes degradation in the food’s freshness. Generally, the expiration dates of refrigerated and frozen foods are affected primarily by their storage temperatures and are determined by microorganism activities [
1,
2,
3,
4,
5].
The constant-temperature operation of a refrigerator for keeping food fresh requires a reduction in the refrigerator’s heat loss and an efficient temperature management system. One way of reducing heat loss is to improve the insulation of sections where heat loss takes place, such as cabinets and doors. An insulator, including a vacuum insulation panel (VIP) inside the cabinet, can result in energy savings of approximately 21% on average [
6]. However, this approach can only be applied to limited parts [
7].
Another way is to develop high-efficiency freezing cycles for refrigerators, which enable large volumes to quickly reach desired temperatures. Refrigerants in compressors must be examined to achieve the high efficiency of freezing cycles for refrigerators. R-600a, R-436a, etc., were developed as refrigerants for refrigerators that can replace R-134a, which is the refrigerant that was used worldwide. These refrigerants are eco-friendly and can save about 10% of energy [
8]. However, they present combustion risks because they are made from isobutene, resulting in an increase in price, as suitable compressors also need to be designed and developed [
9].
In addition, in order to effectively improve the constant temperature operation of the refrigerator, a method of storing thermal energy using a phase change material can be considered. Heat storage techniques that use PCMs are eco-friendly without causing toxicity or pollution in the process of storing and reusing energy and are therefore used in different ways for application in food transport, building insulation, and textiles [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19]. Recently, PCMs have also been used as refrigerator parts to achieve improved efficiencies with respect to storage temperature and energy reduction. Adolmaleki et al. [
10] produced a PCM with a phase change temperature between −20 and −10 °C by mixing PEG200 and PEG300. When the aluminum pack containing the produced PCM was mounted on a refrigerator shelf, changes in energy consumption were studied in a freezer. They reported that energy consumption in the freezer was reduced by up to 8.37%, and temperature fluctuations were improved by up to 40.59%. Ghodrati et al. [
11] employed water as a PCM in a freezer to evaluate the degree of internal heat energy loss following a 100-minute power outage. They found that the energy loss was only 63% in comparison to the existing freezer. Lee and Park [
12] developed a refrigerator component containing PCM on the outlet of the duct passage of a home refrigerator that is divided into an upper portion for freezing and a lower portion for refrigeration. They claimed that a modified component efficiently kept the internal temperature of the refrigerator from rising during power outages and defrost operations.
PCM selection is particularly important for improving efficient constant temperature operation inside the refrigerator. Hydrate-based inorganics with high moisture content, widely used as low-temperature PCMs, are accompanied by supercooling. Because supercooling is not an intrinsic property of substances, it can be influenced by a variety of parameters, such as liquid volume, concentration, cooling rate, and container condition. To prevent the supercooling phenomenon of PCMs, a nucleating agent is often used to promote the crystallization phase by forming crystalline nuclei in the coagulation process or by increasing the viscosity of the liquid [
13,
14,
15]. Lee et al. [
16] attempted to improve supercooling by using sodium acetate trihydrate (CH
3COONa·3H
2O, SAT). They reported that the thermal properties of a PCM were optimal when 5 wt% to 6.5 wt% of Na
3P
2O
7·10H
2O was used as a nucleating agent, and 3 wt% of CMC–Na was used as a thickener. Yoon et al. [
17] used SAT as a PCM for solar energy storage and added gelatin and sodium pyrophosphate decahydrate (Na
4P
2O
7·10H
2O) to improve supercooling and phase separation. Liu et al. [
18] examined the thermal properties, such as the supercooling temperature and latent heat, to select the best hydrate salt-based PCM for low-temperature food transportation that has a melting point between −30.6 °C and −26.7 °C. Park and Kim [
15] used 0.5 wt% ethanol as a nucleating agent in a TMA 25 wt% inclusion compound when developing air-conditioning solutions. They reported that the supercooling temperature decreased by 5.7 °C compared with the case of not adding the nucleating agent. Furthermore, Cui [
19] argued that the supercooling temperature could be maintained at approximately 0.5 °C by using 0.5% nano-Cu particles as a nucleating agent in an SAT with a melting point of 58 °C.
The degree of supercooling of PCM is directly affected by crystal growth, nucleation, and material properties. Wada et al. [
20,
21,
22] investigated the thermal stability of SAT (acetic acid trihydrate). As a solution to supercooling and phase separation that occurs during cooling, a method of adding some water-soluble polymers was proposed. However, care should be taken since changes in the properties of the solution, such as latent heat, density, and thermal conductivity, may occur as a result of the addition of such substances. Given the variety of PCMs, research on PCM supercooling at the required phase change temperature level must be customized for the system.
Methods for improving the supercooling of salt hydrate-based PCMs generally include an active method of applying external force, such as mechanical stirring, high-pressure air injection, or sonication, and a passive method of adding a nucleating agent [
23]. The nucleating agent serves as a support for the crystallization of the PCM once the PCM reaches the solidification temperature. Since the salt hydrate PCM applied to refrigerators is filled with the liquid of the component and attached to a shelf or duct or buried as an insulating material, it is difficult to use an active method that applies external force. Research has shown that the passive method reduces the supercooling of the PCM by up to 90%, using a 1 wt% nucleating agent [
24].
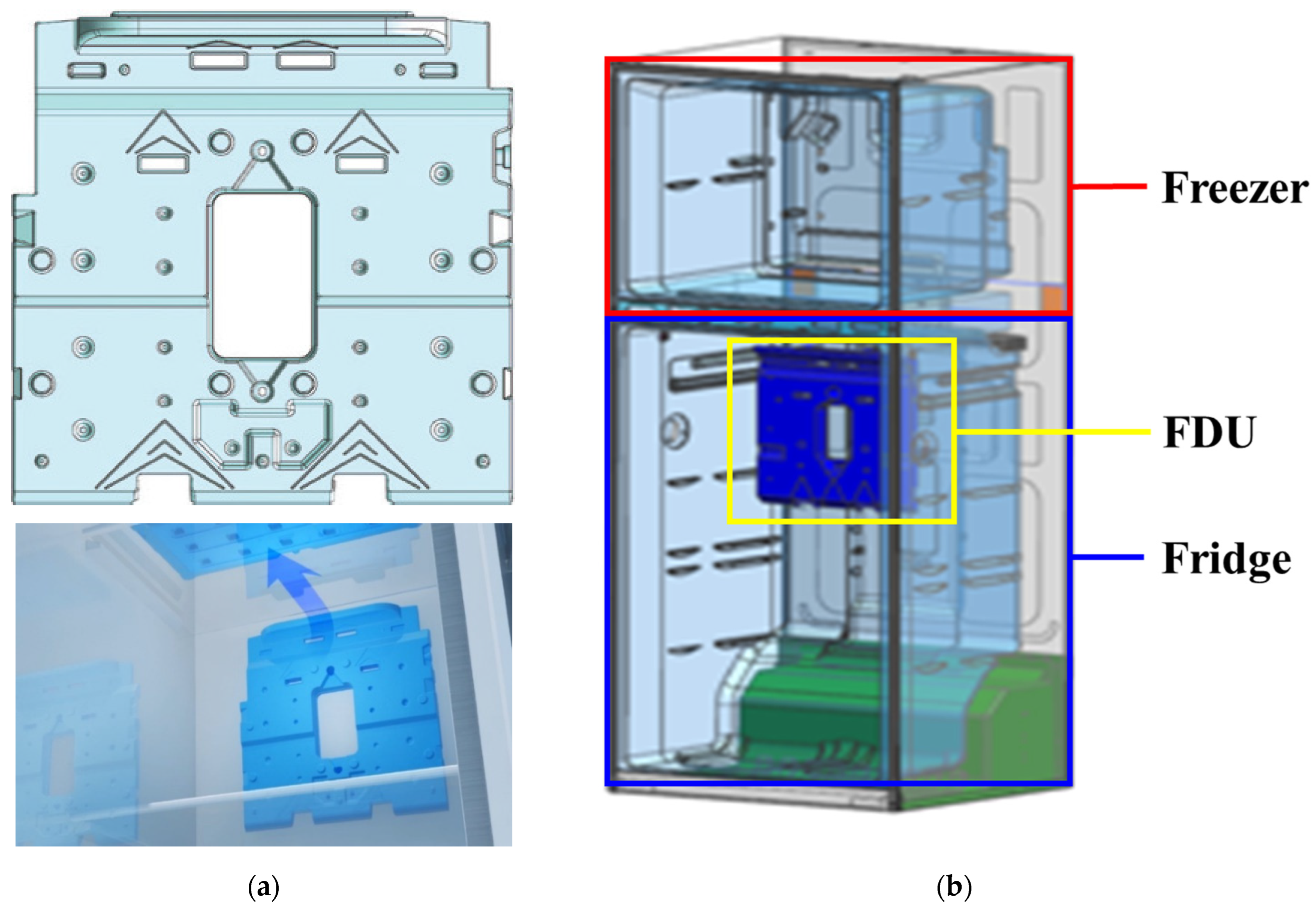
In this study, we examined the thermal properties of the PCM charged in a functional duct unit (FDU) to improve the constant-temperature operation effect of a refrigerator with a top-mounted freezer (TMF), which is shown in
Figure 1. The air temperature measured at the outlet of the FDU (which connects the freezer to the refrigerator) ranges from −3 °C to −5 °C depending on the refrigerator temperature setting [
25]. Therefore, pure water, which is most widely used as a low-temperature PCM, is not a good option for our application because it has a supercooling temperature of around −7 °C.
While seeking for the PCM whose phase change temperature ranges from −3 °C to −5 °C and has a lower supercooling temperature than around −7 °C, we also examined the feasibility of a superabsorbent polymer (SAP) addition to PCM as a thickener and as a nucleating agent to prevent the leakage of the PCM from the FDU and to elucidate its attendant effects on changes in thermal properties of PCM.
2. Experimental Setup
The PCMs used in this study were produced by diluting eutectic molten salt compounds with water to adjust and optimize the thermal properties, such as the supercooling and phase change temperatures. It is important to note that the compositions of the eutectic molten salt compounds listed in
Table 1 were always kept constant while diluting with water.
To observe the phase change characteristics relative to the concentrations of eutectic molten salt compounds diluted with water, the PCM weight percent was defined using Equation (1) and adjusted to range from 1 wt% to 5 wt%.
In the above equation, mTotal is the total mass of the aqueous PCM solution, and meutectic is the mass of the eutectic molten salt compounds. After the eutectic molten salt compound PCMs were produced, SAP was used as an additive to investigate their effects on the latent heat, while acting as a nucleating agent, as well as a thickener, in accordance with the purpose of this study. We prepared samples with SAP concentrations in PCMs from 0.1 to 1.0 wt%, with an increment of 0.1 wt%.
In order to determine the added concentration of the SAP in a PCM solution and whether it can act as a thickener, a series of viscosity measurements were performed. Distilled water and the PCM without the SAP were also measured to objectively compare the viscosity experiment results.
Figure 2 shows a schematic of a viscometer used for the viscosity measurements. The viscometer uses a tuning fork vibration method, and its measurement range is 0.3–10,000 cP. The amount of sample used for one viscosity measurement was about 45 mL, and the sample temperature was maintained at 20 °C during the measurements. The viscosity experiment was repeated five times, and the average of five measurements was taken.
To investigate the thermal properties of the PCM in relation to the addition of SAP, DSC analyses were conducted. For the DSC analyses, samples were prepared with weights of 4.0 mg to 8.0 mg. The temperature was calibrated using indium, and the mean was taken after three or more measurements. The entire calibration process and measurements were carried out in an inert gas (N2) environment, and the DSC operation conditions are summarized as follows:
- i.
40.0~−40.0 °C, cooling, −2.00 K/min.
- ii.
−40.0 °C, 3.00 min, isothermal.
- iii.
−40.0~40.0 °C, heating, 2.00 K/min.
A brine refrigeration apparatus, shown in
Figure 3, and DSC were simultaneously used to investigate the thermal characteristics of the SAP-added PCM samples. Unlike DSC measurements, which require a small sample, the brine refrigeration apparatus uses a relatively appropriate quantity of around 100 g, enabling us to effectively investigate thermal properties, such as the supercooling temperature and phase change temperature, and the degradation and phase separation characteristics through repeated experiments. The apparatus consists of a test chamber filled with brine and a copper tube through which a refrigerant flows and cools the brine through heat exchange. To minimize temperature stratification inside the test chamber, the cooled brine was designed to circulate by force, using a centrifugal circulation pump, and a test tube filled with a PCM sample was inserted into the test chamber to allow for a heat exchange to take place. During the experiment, the temperatures of the PCM sample and brine were recorded on a PC, using a K-type thermocouple and DAQ. The hole on the test tube container cover was finished with epoxy to prevent sample leakage, and a PETE tube was used to support the K-type thermocouple.
3. Experimental Results and Discussion
We performed experiments to check the viscous characteristics that change according to the added SAP concentration in the PCM solution, and the experimental results are shown in
Figure 4. The viscosity of the samples without the SAP was about 1 cP. The added SAP concentrations from 0.1 wt% to 0.6 wt% did not result in a large increase in viscosity. In addition, regardless of the type of sample, the viscosity of the sample according to the addition of SAP showed a similar trend. The experimental results showed that the viscosity of the cases with the added SAP concentration of up to 0.6 wt% was increased about twofold compared to the case without the SAP. As shown in
Figure 4, the viscosity of the samples increased rapidly from the added SAP concentration of 0.7 wt%.
The SAP concentration of 1.0–1.2 wt% resulted in a viscosity of 6000 cP or higher. The viscosity of commercially available ice packs manufactured in gel form for the purpose of preventing water leakage was tested through an experiment and found to be over 6000 cP on average, as shown in
Table 2. Therefore, the optimal viscosity was determined to be SAP 1.0 wt%, which is similar to the viscosity value of an ice pack manufactured for the same purpose, and was applied to PCM for FDU.
DSC measurements were conducted to qualitatively verify how the addition of SAP affects the thermal properties of the PCM.
Figure 5a shows a graph of the caloric changes for distilled water, which is the reference material and the PCM solutions without SAP addition. The PCM weight percent of samples used for caloric measurements was 1 wt%, 3 wt%, and 5 wt%, respectively. In order to normalize the DSC data, the measured heat flow value of each sample was divided by the measured weight and is shown in
Figure 5a,b. As seen in
Figure 5a, a caloric change occurred at around −11 °C and 0 °C for all samples without regard to the PCM weight percent. As the PCM weight increased, the caloric change at around −11 °C increased. These results confirm that the eutectic molten salt compound starts to melt at around −11 °C.
The thermal properties confirmed through the above figures are summarized in
Table 3. The study defined the maximum melting temperature confirmed in the DSC experiment as the peak temperature (
Tpeak), the temperature where melting begins as the onset temperature (
Ton), the temperature where melting ends as the end set temperature (
Tend), and the endothermic capacity as latent heat, respectively, and interpreted the results. Regardless of the weight percent of the eutectic molten salt compound, the melting onset temperature (
Ton) was constant at around −11.5 °C. The results demonstrated that the heat capacity around −11 °C increased as the weight percent of the PCM increased, and the concentration of the PCM affected the heat capacity around −11 °C. Since the mass of water decreased as the weight percent of the PCM increased, the heat capacity at 0 °C decreased and the heat capacity of the entire sample also decreased. Furthermore, density inversion occurred at around 4 °C in water, which is the reference material, on the caloric change curve, which indicates that the fluid was moved by the difference in density according to the sample temperature [
28].
Figure 5 shows the results of the DSC analysis, which was conducted to proactively observe the thermal property changes that occurred owing to the SAP addition. The
Ton and
Tend of water were 1.0 °C and 4.5 °C, respectively, and the water melting temperature (
Tpeak) was 2.7 °C, which was confirmed to be similar to the value reported in the literature [
28]. The
Ton value of the water with SAP added was −0.4 °C, which was lower than that of pure water, and the
Tend was 3.0 °C, which was lower than that of pure water. The latent heat was 330 J/g, which is 3 J/g lower than that of pure water. These results confirm that the SAP addition decreased the
Ton and slightly decreased the latent heat. Based on the experiment shown in
Figure 5, the samples were prepared by mixing 1 wt% of SAP into samples of eutectic molten salt compound PCM with concentrations of 1 wt%, 3 wt%, and 5 wt%, and a DSC analysis was performed with these samples.
Figure 5b shows the caloric change graph of the PCM with the addition of SAP and pure water. The latent heat of the 1 wt% sample with the addition of SAP was similar to that of the sample with no SAP, and the caloric change at around −11 °C decreased. The 3 wt% and 5 wt% samples with the addition of SAP showed a similar caloric change graph compared with that of the sample with no SAP, and no significant change in thermal properties was observed. The thermal properties shown in
Figure 5b are outlined in
Table 4. The
Tpeak value was observed at around −11.0 °C and 0 °C for the 3 wt% and 5 wt% samples, respectively, but it was observed only at 0.5 °C for the 1 wt% sample. The PCMs with added SAP showed lower values of
compared with the PCM with no SAP, and this indicates that the addition of SAP in the PCM makes it begin to melt faster than the sample with no SAP. The
Ton values of the 3 wt% and 5 wt% samples with the addition of SAP decreased by approximately 2 °C. This result showed that the addition of SAP affected the melting temperature of the PCM. Furthermore, the latent heat of the PCMs with added SAP decreased at a lower rate than that of the PCM with no SAP, indicating that SAP did not have a significant effect on the latent heat of the samples.
The results of the DSC experiment confirmed that the addition of SAP in eutectic molten salt compound PCMs could accelerate the crystallization of the samples as the melting temperature (Ton) decreased. Furthermore, the latent heat decreased by 3 J/g to 24.4 J/g compared with the sample with no SAP.
In addition to the DSC analysis, the brine refrigeration apparatus was used to verify the improvement of supercooling and the thermal properties of the PCM. Before the PCM experiment, SAP was added to the water, which is widely used for food transportation, and it was confirmed that the supercooling was improved.
As shown in
Figure 6, the supercooling temperature of water (pure) was about −7.7 °C, but when SAP was added, the supercooling was improved by 2.5 °C. In addition, the density inversion confirmed at around 4 °C was not observed when SAP was added, and it was confirmed that SAP also acted as a thickener.
Figure 7a shows schematics with a comparison of thermal properties after the addition of SAP in the PCM with a 1 wt% of the eutectic molten salt compound in the cooling process. The experiment results revealed that the PCM with a 1 wt% of the eutectic molten salt compound had a supercooling temperature of −4.6 °C and a phase change temperature of 0.1 °C. In contrast, the 1 wt% sample with the addition of SAP showed a supercooling temperature of −2.9 °C and a phase change temperature of −0.5 °C. The experiment results shown in
Figure 7a clearly confirmed that the addition of SAP improved supercooling, exhibiting the same tendency as the DSC experiment results. As the supercooling temperature decreased, the liquid retention time shortened, which indicates that the energy consumption during the phase change in the PCM can be reduced. Based on the results of this experiment, the thermal properties of the 3 wt% PCM (
Figure 7b) and 5 wt% PCM (
Figure 7c) in the cooling process were also verified.
The thermal properties of the PCM derived using the brine refrigeration test equipment are summarized in
Table 5. It was observed that the phase change temperature decreased as the weight percent increased when the eutectic molten salt compound was added compared to the reference material, water. In addition, as the weight percent of the eutectic molten salt compound increased, the phase change temperature gradually decreased. It is believed that the eutectic molten salt compound particles are dissolved in water, a pure substance, and the phase change temperature is reduced by the freezing point depression [
29,
30]. The supercooling temperature was improved when SAP was added to water and 1 wt%, 3 wt%, and 5 wt% PCM, and the supercooling degree of water and the PCM was believed to be improved, as SAP particles played the role of condensation nuclei during phase change [
30,
31]. In particular, 1 wt% + SAP PCM showed the best degree of improvement with a supercooling degree of 2.4, which is believed to be due to a decrease in the phase change temperature due to a freezing point depression due to the addition of eutectic molten salt compound and an improvement in the supercooling phenomenon due to the addition of SAP. In addition, even when SAP was added to each PCM, there was no significant difference in the change in phase change temperature.
The requirements for PCM that can be applied to the duct part of the refrigerator presented in this study are a supercooling temperature at about −5 °C and an excellent latent heat amount. As a result of examining the thermal properties of PCM through DSC and brine refrigeration test equipment, the most appropriate PCM is 1 wt% + SAP with a supercooling temperature of −2.9 °C and a latent heat amount of 293 J/g.
This study observed the operation characteristics of the refrigerator by applying the 1 wt% + SAP PCM optimized in this paper to the refrigerator’s duct part and analyzed the refrigerator containing operation characteristics of the general refrigerator (
Figure 8a) and PCM (
Figure 8b) [
12]. Care must be taken to determine whether changes in the functional range of the system to which the previously optimized PCM is applied and changes in thermal characteristics such as phase change temperature are observed. As mentioned in the Introduction, among the factors that cause supercooling, changes are possible due to various parameters, such as the PCM volume, cooling rate, container characteristics, and thermal history, so this verification procedure must be carried out [
32]. The experiment result demonstrated that the chamber of a general refrigerator operates at a temperature between −0.8 °C and 6.76 °C, and the maximum/minimum temperature deviation (ΔT) was 7.56 °C. The chamber of the refrigerator applying PCM operates at a temperature between −1.6 °C and 5.4 °C, and the maximum/minimum temperature deviation (ΔT) was found to be 7.0 °C. In addition, when the defrosting of the refrigerator was operated, the maximum temperature at the duct outlet rose rapidly to 8.1 °C due to the heater being turned for defrosting, resulting in a temperature increase inside the refrigerator chamber. On the other hand, in the defrosting of the refrigerator with the PCM, the outlet temperature of the duct was at the maximum level of −0.9 °C, indicating that the heat capacity of the PCM prevented the high-temperature thermal resistance entry/exit of the heater. The overall analysis confirmed that refrigerators containing PCM were generally superior in temperature uniformity to general refrigerators and that they can especially contribute significantly to defending against the heater thermal resistance that occurs during defrosting.
4. Conclusions
In this study, SAP was added to eutectic molten salt compound PCM, which is used as a low temperature heat storage material. Adding thickeners and water-soluble polymers to PCM can help improve supercooling, but caution must be taken because it causes changes in solution properties, such as the latent heat and phase change temperature. Then, the improvements in supercooling, phase separation, and thermal properties such as latent heat were analyzed. By adding 1.0 wt% of SAP to the PCM, this study confirmed that a viscosity of 6000 cP or more can be secured, which is the level of the existing product, and that SAP can be used as a thickener to prevent product leakage. The results of the experiment using DSC confirmed that when SAP was added to PCM, it accelerated the rate of sample melting as the melting temperature (Ton) decreased, and that SAP could improve the viscosity and act as a nucleating agent. However, when SAP was added, the latent heat decreased slightly from 3 J/g to 24.4 J/g compared to the previous product, but adding SAP was determined to be more effective to prevent leakage and enhance the safety of the product. When SAP was added to the PCM, the sample’s density inversion was not observed, and the phase separation was improved as the viscosity of PCM improved. In the experiment using the brine refrigeration device, the result confirmed that the supercooling temperature improved when SAP was added to the PCM, and the phase change temperature did not change significantly. It appears that the supercooling phenomenon of PCM, which occurs due to a lack of condensation nuclei, acted as condensation nuclei as SAP was added, thereby contributing to phase change. As a result of comparing the thermal properties of pure water and eutectic molten salt compound PCM, the phase change temperature appears to gradually decrease as the concentration of the eutectic molten salt compound PCM increases. The phase change temperature appears to have decreased due to the freezing point depression phenomenon due to the dissolution of eutectic molten salt compound particles in pure water. The PCM satisfying the required temperature level of −3 °C~−5 °C for the refrigerator duct was 1.0 wt% + SAP, and the DSC test showed it was 293.0 J/g, which is a 10% decrease in latent heat from water. The supercooling temperature of 1.0 wt% + SAP was −2.9 °C, which showed the best performance. Although it is recommended to use water for the optimal efficiency of thermal energy storage, water cannot be used due to the temperature requirements of the functional duct unit. The study results showed that 1 wt% + SAP of PCM would be appropriate to use. Moreover, when an experiment was conducted to identify the effect of PCM in this paper on the operation performance of the refrigerator, the maximum/minimum temperature deviation improved by 0.56 °C from the existing refrigerator. Due to the thermal resistance of the heater generated during the refrigerator defrosting, the outlet temperature of the EPS duct of the existing refrigerator rose sharply to 8.1 °C. However, the duct outlet temperature for the refrigerator applied with PCM was at around −0.9 °C, preventing temperature loss by PCM’s heat capacity. Based on the results that the supercooling of PCM improved, this research confirmed that SAP, which was mainly used only as a thickener, could also be considered for PCM to be used for cold distribution systems and freezers.