One-Dimensional Numerical Simulation on Removal of CO2 Hydrate Blockage around Wellbore by N2 Injection
Abstract
1. Introduction
2. Numerical Code and Simulation Method
2.1. Simulation Code
2.2. Geometry and System Description
2.3. Initial Conditions and Simulation Cases
3. Results and Discussions
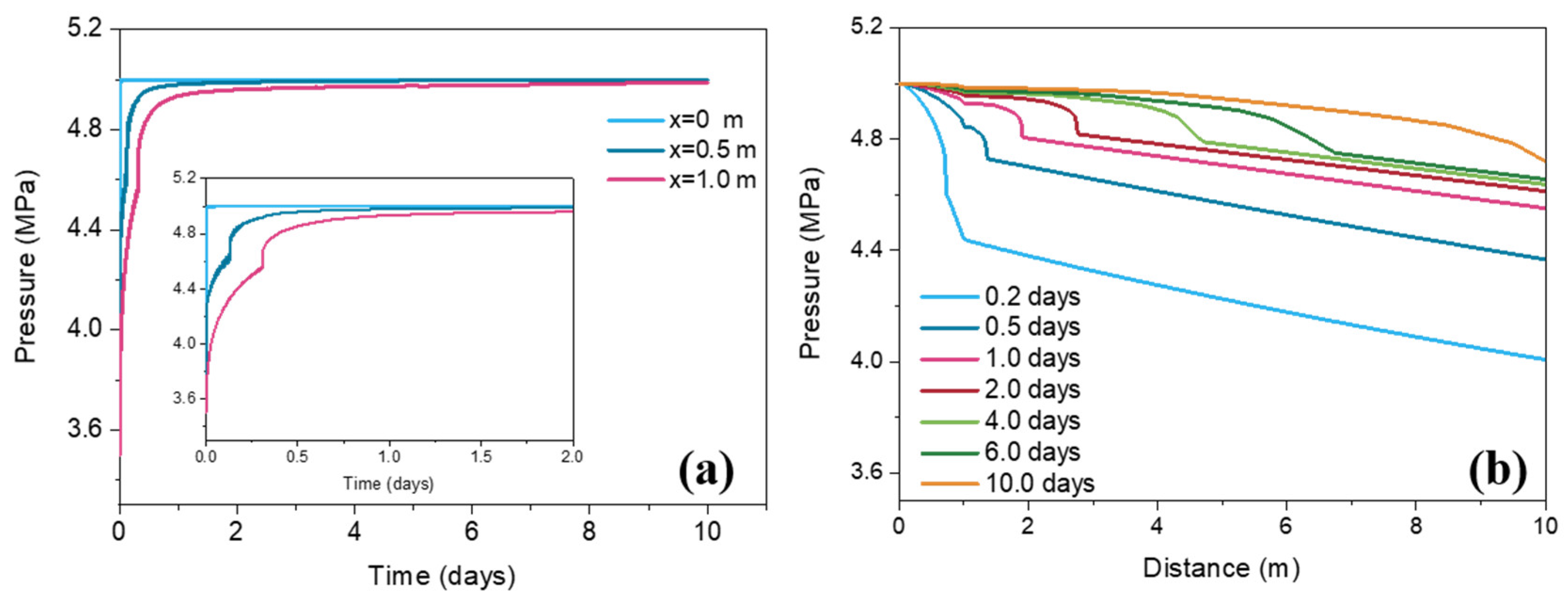
3.1. Pressure Change
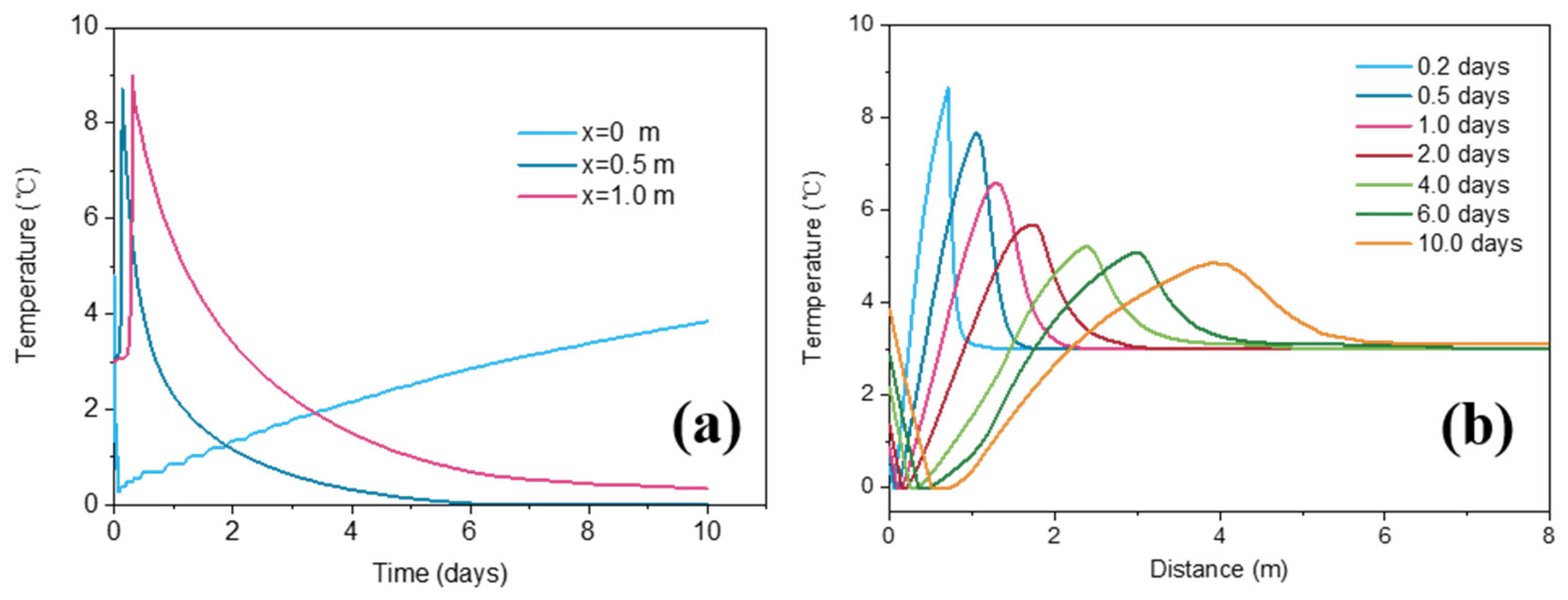
3.2. Temperature Change
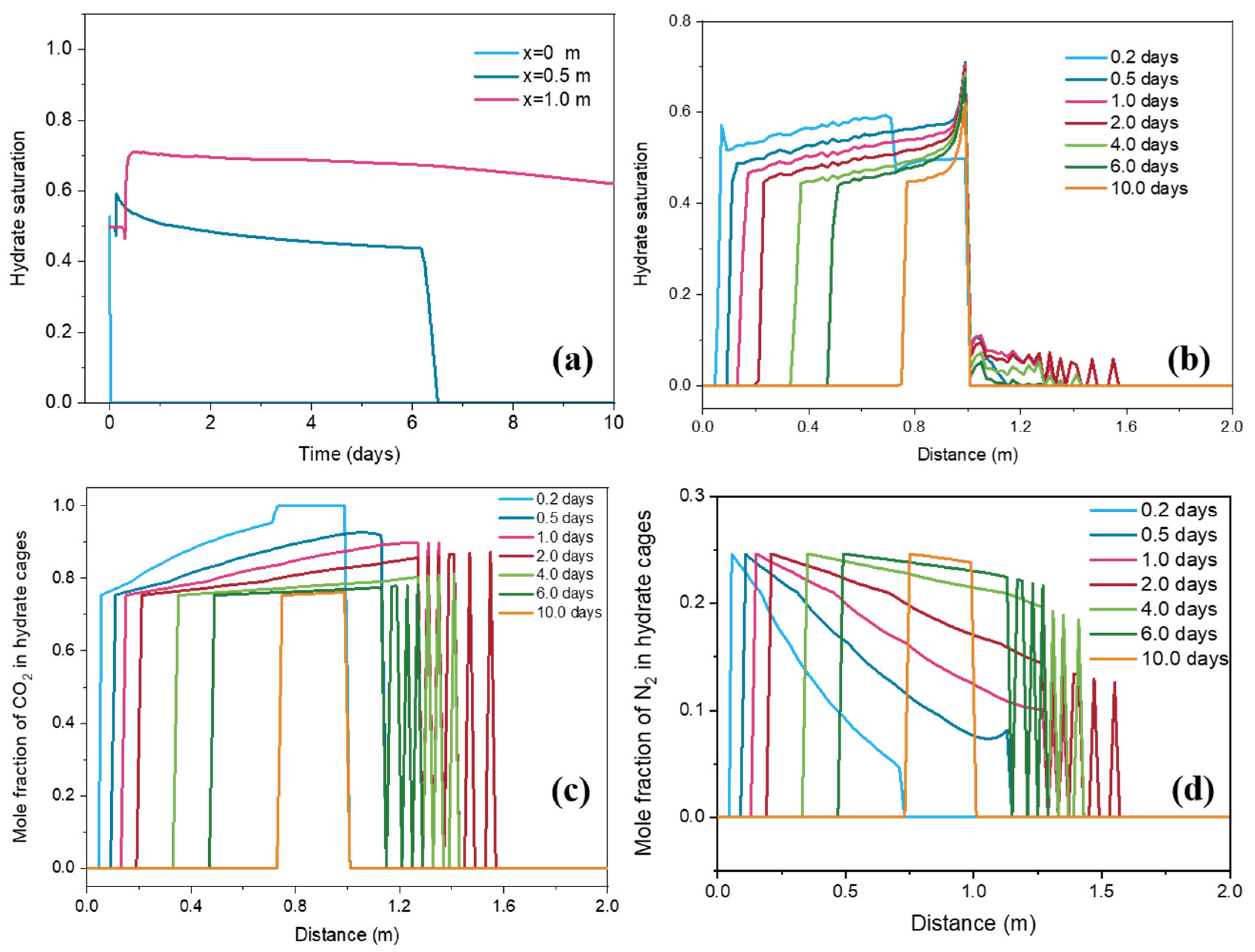
3.3. Hydrate Saturation Change
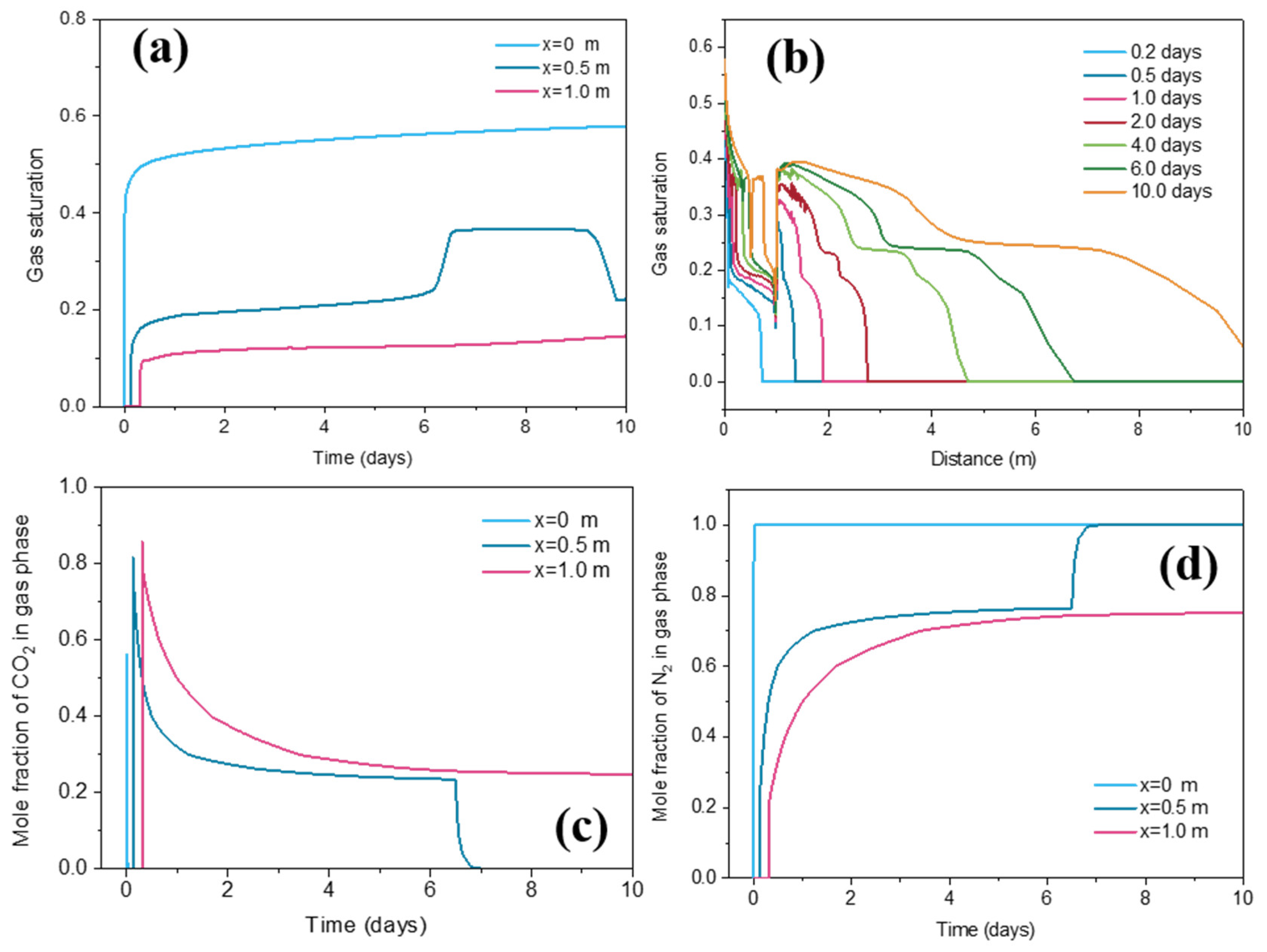
3.4. Gas Saturation Change
3.5. Comparison of N2 Injection and Depressurization
4. Conclusions
- (1)
- Compared with the direct depressurization method, N2 injection can quickly remove the blocked CO2 hydrate. The CO2 hydrate dissociation rate can be improved by increasing the N2 injection pressure. N2 injection temperature has a relatively weak effect on the removal of CO2 hydrate blockage;
- (2)
- The injection of N2 can lead to the rise of sediment pressure. With the influence of injected N2, rapid CO2 hydrate dissociation can decrease the temperature of sediments. However, with the combined effect of temperature, pressure, and gas composition, secondary CO2-N2 hydrate can be formed far away from the injection point and lead to the rise of local temperature and hydrate saturation. The continuous injection of N2 can eliminate the secondary hydrate formation;
- (3)
- Our work provides a new method for eliminating the problem of hydrate blockage, which has prominent significance for the development of hydrate-based CO2 geological storage technology.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| CR | heat capacity of the dry rock (J/kg/K) |
| g | gravitational acceleration (m/s2) |
| h | enthalpy of phase β or component κ in phase β (J/kg) |
| k | intrinsic permeability (m2) |
| kr | relative permeability of phase β |
| m | advective mass flux vector of phase β (kg/m2/s) |
| n | the inward unit normal vector |
| P | pressure (Pa) |
| P1 | entry capillary pressure (Pa) |
| q | source/sink term of mass component κ (kg/m3/s) |
| Qdiss | hydrate dissociation heat (J/m3) |
| s | source/sink term of heat (J/m3/s) |
| S | phase saturation |
| T | temperature (°C) |
| U | the internal energy of phase β (J/kg) |
| V | volume (m3) |
| ϕ | porosity |
| λR | composite thermal conductivity (W/m/K) |
| λRD | dry thermal conductivity (W/m/K) |
| λRW | wet thermal conductivity (W/m/K) |
| λI | thermal conductivity of ice (W/m/K) |
| μ | viscosity of phase β (Pa s) |
| ρ | density of phase β (kg/m3) |
| ρR | rock density (kg/m3) |
| Subscripts and superscripts | |
| b | van Genuchten exponent |
| cap | capillary |
| irA | irreducible aqueous phase |
| irG | irreducible gas phase |
| nA | permeability reduction exponent for the aqueous phase |
| nG | permeability reduction exponent for gas phase |
| β | phase (G = gas, A = aqueous, H = hydrate, I = ice) |
| κ | mass component (n = nitrogen, c = carbon dioxide, w = water) |
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrate of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Boswell, R.; Collett, T.S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 2011, 4, 1206–1215. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Z.; Song, Y.; Liu, W.; Zhang, Y.; Wang, D. Analyzing the process of gas production for natural gas hydrate using depressurization. Appl. Energy 2015, 142, 125–134. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.S.; Li, G.; Zhang, Y.; Li, B.; Feng, J. A three-dimensional study on methane hydrate decomposition with different methods using five-spot well. Appl. Energy 2013, 112, 83–92. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, J.; Li, F.; Yang, M. Gas permeability characteristics of marine sediments with and without methane hydrates in a core holder. J. Nat. Gas. Sci. Eng. 2020, 76, 103215. [Google Scholar] [CrossRef]
- Okwananke, A.; Hassanpouryouzband, A.; Farahani, M.V.; Yang, J.; Tohidi, B.; Chuvilin, E.; Istomin, V.; Bukhanov, B. Methane recovery from gas hydrate-bearing sediments: An experimental study on the gas permeation characteristics under varying pressure. J. Petrol. Sci. Eng. 2019, 180, 435–444. [Google Scholar] [CrossRef]
- Le, Q.D.; Rodriguez, C.T.; Legoix, L.N.; Pirim, C.; Chazallon, B. Influence of the initial CH4-hydrate system properties on CO2 capture kinetics. Appl. Energy 2020, 280, 115843. [Google Scholar] [CrossRef]
- Ohgaki, K.; Takano, K.; Sangawa, H.; Matsubara, T.; Nakano, S. Methane exploitation by CO2 from gas hydrates—Phase equilibria for CO2-CH4 mixed hydrate system. J. Chem. Eng. JPN 1996, 29, 478–483. [Google Scholar] [CrossRef]
- Yuan, Q.; Sun, C.Y.; Liu, B.; Yang, X.; Ma, P.C.; Ma, Z.W.; Ma, Q.L.; Yang, L.Y.; Chen, G.J. Recovery of methane from hydrate reservoir with gaseous CO2 using a three-dimensional middle-size reactor. Energy 2012, 40, 47–58. [Google Scholar] [CrossRef]
- Yuan, Q.; Sun, C.Y.; Yang, X.; Wang, X.H.; Ma, Z.W.; Ma, Q.L.; Yang, L.Y.; Chen, G.J.; Li, Q.P.; Li, S. Methane recovery from natural gas hydrate in porous sediment using pressurized liquid CO2. Energ. Convers. Manag. 2013, 67, 257–264. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, X.H.; Dandekar, A.; Sun, C.Y.; Li, Q.P.; Ma, Z.W.; Liu, B.; Chen, G.J. Replacement of methane from hydrates in porous sediments with CO2-in-water emulsions. Ind. Eng. Chem. Res. 2014, 53, 12476–12484. [Google Scholar] [CrossRef]
- Park, Y.; Kim, D.Y.; Lee, J.W.; Huh, D.G.; Park, K.P.; Lee, J.; Lee, H. Sequestering CO2 into complex structures of naturally occurring gas hydrates. Proc. Natl. Acad. Sci. USA 2006, 103, 12690–12694. [Google Scholar] [CrossRef]
- Kang, H.; Koh, D.Y.; Lee, H. Nondestructive natural gas hydrate recovery driven by air and CO2. Sci. Rep. 2014, 4, 6616. [Google Scholar] [CrossRef]
- Li, B.; Xu, T.; Zhang, G.; Guo, W.; Liu, H.; Wang, Q.; Qu, L.; Sun, Y. An experimental study on gas production from fracture-filled hydrate by CO2 and CO2/N2 replacement. Energ. Convers. Manag. 2018, 165, 738–747. [Google Scholar] [CrossRef]
- Yang, J.; Okwananke, A.; Tohidi, B.; Chuvilin, E.; Maerle, K.; Istomin, V.; Bukhanov, B.; Cheremisin, A. Flue gas injection into gas hydrate reservoirs for methane recovery and CO2 sequestration. Energ. Convers. Manag. 2017, 136, 431–438. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Yang, J.; Okwananke, A.; Burgass, R.; Thohidi, B.; Chuvilin, E.; Istomin, V.; Bukhanov, B. An experimental investigation on the kinetics of integrated methane recovery and CO2 sequestration by injection of flue gas into permafrost methane hydrate reservoirs. Sci. Rep. 2019, 9, 16206. [Google Scholar] [CrossRef] [PubMed]
- Schoderbek, D.; Martin, K.L.; Howard, J.; Silpngarmlert, S.; Hester, K. North slope hydrate fieldtrial: CO2/CH4 exchange. In Proceedings of the Arctic Technology Conference, Houston, TX, USA, 3–5 December 2012. [Google Scholar]
- Wang, X.H.; Sun, Y.F.; Wang, Y.F.; Li, N.; Sun, C.Y. Gas production from hydrates by CH4-CO2/H2 replacement. Appl. Energy 2017, 188, 305–314. [Google Scholar] [CrossRef]
- Cao, L.Y.; Qian, W.M.; Gong, P.; Guan, S.; Duan, Y.; He, Y. Application Practices and Evaluation of Gas Injection Technology for CO2 Flooding in Subei Oilfield. Xinjiang Oil Gas 2022, 18, 46–50. [Google Scholar]
- Xiong, X.Q.; Liao, T.; Xing, X.K.; Zhang, Z.; Dong, Z.; Bi, Y. Study Progress on Characteristics and Separation of Produced Fluid of CO2 Flooding. Xinjiang Oil Gas 2022, 18, 33–39. [Google Scholar]
- Zhao, Z.G.; Zhao, B.; Yang, H.J.; He, C.Z. Methods to prevent blockage of natural gas hydrates. Xinjiang Oil Gas 2009, 5, 81–83. [Google Scholar]
- Liang, S.; Gao, F.X.; Yue, G.; Liu, Z.Q.; Sun, Q.; Wang, Y.W.; Liu, A.X.; Guo, X.Q. Research on the Preparation of Civil Natural Gas from Oil Shale Retorting Gas by the Hydration Process. Xinjiang Oil Gas 2021, 17, 56–61. [Google Scholar]
- Wang, X.H.; Sun, C.Y.; Chen, G.J.; He, Y.N.; Sun, Y.F.; Wang, Y.F.; Li, N.; Zhang, X.X.; Liu, B.; Yang, L.Y. Influence of gas sweep on methane recovery from hydrate-bearing sediments. Chem. Eng. Sci. 2015, 134, 727–736. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Joonaki, E.; Vasheghani Farahani, M.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H.; et al. Gas hydrates in sustainable chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar] [CrossRef] [PubMed]
- Moridis, G.; Kowalsky, M.; Pruess, K. TOUGH+ HYDRATE v1. 0 User’s Manual: A Code for the Simulation of System Behaviour in Hydrate-Bearing Porous Media; Lawrence Berkeley National Laboratory Report LBNL-149E; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2008. [Google Scholar]
- Konno, Y.; Masuda, Y.; Hariguchi, Y.; Masuda, Y.; Hariguch, Y.; Kurihara, M.; Ouchi, H. Key factors for depressurization-induced gas production from oceanic methane hydrates. Energy Fuels 2010, 24, 1736–1744. [Google Scholar] [CrossRef]
- Anderson, B.J.; Kurihara, M.; White, M.D.; Moridis, G.J.; Wilson, S.J.; Darvish, M.P.; Gaddipati, M.; Masuda, Y.; Collett, T.; Hunter, R.B.; et al. Regional long-term production modeling from a single well test, Mount Elbert Gas Hydrate Stratigraphic Test Well, Alaska North Slope. Mar. Petrol. Geol. 2011, 28, 493–501. [Google Scholar] [CrossRef]
- Yonkofski, C.M.; Horner, J.A.; White, M.D. Experimental and numerical investigation of hydrate-guest molecule exchange kinetics. J. Nat. Gas. Sci. Eng. 2016, 35, 1480–1489. [Google Scholar] [CrossRef]
- Kan, J.Y.; Sun, Y.; Dong, B.C.; Yuan, Q.; Liu, B.; Sun, C.Y.; Chen, G.J. Numerical simulation of gas production from permafrost hydrate deposits enhanced with CO2/N2 injection. Energy 2021, 221, 119919. [Google Scholar] [CrossRef]
- Chen, G.J.; Guo, T.M. A new approach to gas hydrate modelling. Chem. Eng. J. 1998, 71, 145–151. [Google Scholar] [CrossRef]
- van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil. Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]


| Parameter | Value & Unit |
|---|---|
| Temperature | 3 °C |
| Pressure | 3.5 MPa |
| Hydrate saturation | 0.50 |
| Intrinsic permeability | 5.0 × 10−14 m2 |
| Porosity | 0.30 |
| Composite thermal conductivity model [25] | λRD = 1.0 W/m/K λRW = 3.1 W/m/K |
| Relative permeability model [31] | ; nA = 3.5, nG = 3.5, SirA = 0.3, SirG = 0.05 |
| Capillary pressure model [25] | b = 0.45, P1 = 105 Pa |
| Simulation ID | Pinj (MPa) | Tinj (°C) |
|---|---|---|
| A1 | 4.5 | 25 |
| A2 | 4.5 | 50 |
| A3 | 5 | 10 |
| A4 | 5 | 25 |
| A5 | 5 | 50 |
| A6 | 5.5 | 25 |
| A7 | 5.5 | 50 |
| B1 | 1.5(Pdep) | – |
| B2 | 2.5(Pdep) | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, T.; Yuan, L.; Li, W.; Kan, J.; Luo, W.; Xiong, X.; Li, N. One-Dimensional Numerical Simulation on Removal of CO2 Hydrate Blockage around Wellbore by N2 Injection. Processes 2024, 12, 204. https://doi.org/10.3390/pr12010204
Liao T, Yuan L, Li W, Kan J, Luo W, Xiong X, Li N. One-Dimensional Numerical Simulation on Removal of CO2 Hydrate Blockage around Wellbore by N2 Injection. Processes. 2024; 12(1):204. https://doi.org/10.3390/pr12010204
Chicago/Turabian StyleLiao, Tao, Liang Yuan, Wei Li, Jingyu Kan, Wei Luo, Xiaoqin Xiong, and Nan Li. 2024. "One-Dimensional Numerical Simulation on Removal of CO2 Hydrate Blockage around Wellbore by N2 Injection" Processes 12, no. 1: 204. https://doi.org/10.3390/pr12010204
APA StyleLiao, T., Yuan, L., Li, W., Kan, J., Luo, W., Xiong, X., & Li, N. (2024). One-Dimensional Numerical Simulation on Removal of CO2 Hydrate Blockage around Wellbore by N2 Injection. Processes, 12(1), 204. https://doi.org/10.3390/pr12010204










