The Contribution of Cornelian Cherry (Cornus mas L.) Alcoholic Beverages on the Sensory, Nutritional and Anti-Nutritional Characteristics—In Vitro and In Silico Approaches
Abstract
1. Introduction
2. Material and Methods
2.1. Cornelian Cherry Liquors
2.2. Analytical Methods
2.2.1. Basic Physicochemical Parameters
2.2.2. Amygdalin Concentration
2.2.3. In Silico Calculation of Amygdalin Properties and UV-VIS Spectra
2.2.4. Determination of Polyphenol Concentrations
2.2.5. Chlorophyll and Total Carotenoid Fractions
2.2.6. Antiradical Capacity
2.3. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coldea, T.E.; Socaciu, C.; Mudura, E.; Socaci, S.A.; Ranga, F.; Pop, C.R.; Vriesekoop, F.; Pasqualone, A. Volatile and Phenolic Profiles of Traditional Romanian Apple Brandy after Rapid Ageing with Different Wood Chips. Food Chem. 2020, 320, 126643. [Google Scholar] [CrossRef]
- López, F.; Rodríguez-Bencomo, J.J.; Orriols, I.; Pérez-Correa, J.R. Fruit Brandies. In Science and Technology of Fruit Wine Production; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 531–556. ISBN 9780128010341. [Google Scholar]
- Alamprese, C.; Pompei, C. Influence of Processing Variables on Some Characteristics of Nocino Liqueur. Food Chem. 2005, 92, 203–209. [Google Scholar] [CrossRef]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, Flavones, Flavanones, and Human Health: Epidemiological Evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, P.; Pietta, P.; Testolin, G. Polyphenol Content and Total Antioxidant Potential of Selected Italian Wines. J. Agric. Food Chem. 1997, 45, 1152–1155. [Google Scholar] [CrossRef]
- Lugasi, A. Polyphenol Content and Antioxidant Properties of Beer. Acta Aliment. 2003, 32, 181–192. [Google Scholar] [CrossRef]
- Gupta, A.; Sanwal, N.; Bareen, M.A.; Barua, S.; Sharma, N.; Joshua Olatunji, O.; Prakash Nirmal, N.; Sahu, J.K. Trends in Functional Beverages: Functional Ingredients, Processing Technologies, Stability, Health Benefits, and Consumer Perspective. Food Res. Int. 2023, 170, 113046. [Google Scholar] [CrossRef]
- Finassi, C.M.; Calixto, L.A.; Segura, W.; Bocato, M.Z.; Barbosa Júnior, F.; Fonseca, F.L.A.; Lamy, E.; Castelo, P.M. Effect of Sweetened Beverages Intake on Salivary Aspartame, Insulin and Alpha-Amylase Levels: A Single-Blind Study. Food Res. Int. 2023, 173, 113406. [Google Scholar] [CrossRef]
- Tamayo-Sánchez, J.C.; Meza-González, D.A.; Warren-Vega, W.M.; Zárate-Guzmán, A.I.; Romero-Cano, L.A. Advances in the Development of Tailor-Made Color Alcoholic Beverages Based on an Accelerated Maturation Process. Food Res. Int. 2023, 169, 112859. [Google Scholar] [CrossRef]
- Toomey, V.M.; Nickum, E.A.; Flurer, C.L. Cyanide and Amygdalin as Indicators of the Presence of Bitter Almonds in Imported Raw Almonds. J. Forensic Sci. 2012, 57, 1313–1317. [Google Scholar] [CrossRef]
- Hamel, J. A Review of Acute Cyanide Poisoning with a Treatment Update. Crit. Care Nurse 2011, 31, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.M.; Kwon, H. Metabolites of Amygdalin under Simulated Human Digestive Fluids. Int. J. Food Sci. Nutr. 2010, 61, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Alshimali, S.I.; Abdellatif, A.A.H. Amygdalin: A Review on Its Characteristics, Antioxidant Potential, Gastrointestinal Microbiota Intervention, Anticancer Therapeutic and Mechanisms, Toxicity, and Encapsulation. Biomolecules 2022, 12, 1514. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xu, X. Advanced Research on Anti-Tumor Effects of Amygdalin. J. Cancer Res. Ther. 2014, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L. (Ron); Leblanc, J.C.; Nebbia, C.S.; et al. Evaluation of the Health Risks Related to the Presence of Cyanogenic Glycosides in Foods Other than Raw Apricot Kernels. EFSA J. 2019, 17, e05662. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, O.M.; Ligaj, M.; Kobus-Cisowska, J.; Maciejewska, P.; Tichoniuk, M.; Szulc, P. Application for Novel Electrochemical Screening of Antioxidant Potential and Phytochemicals in Cornus mas Extracts. CYTA-J. Food 2019, 17, 781–789. [Google Scholar] [CrossRef]
- He, X.; Gaca, A.; Jeleń, H.H. Determination of Volatile Compounds in Baijiu Using Simultaneous Chromatographic Analysis on Two Columns. J. Inst. Brew. 2020, 126, 206–212. [Google Scholar] [CrossRef]
- (EC) No 110/2008; Regulation (EC) No 110/2008 of the European Parliament and of the Council of 15 January 2008 on the Definition, Description, Presentation, Labelling and the Protection of Geographical Indications of Spirit Drinks and Repealing Council Regulation (EEC) No 1576/89. Official Journal of the European Union: Luxembourg, 2008.
- Polish Standard PN-A-79523: 2002; Raw Spirits. Polish Committee for Standardisation: Warsaw, Poland, 2002.
- Miao, X.; Zhao, Z.; Zhu, H.; Li, M.; Zhao, Q. Comparison of Second-Derivative Spectrophotometry and HPLC for Determination of Amygdalin in Wild Apricot Kernels. ScienceAsia 2013, 39, 444–447. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Kurasiak-Popowska, D.; Nawracała, J.; Perkowski, J. Response of Non-Enzymatic Antioxidative Mechanisms to Stress Caused by Infection with Fusarium Fungi and Chemical Protection in Different Wheat Genotypes. Chem. Ecol. 2017, 33, 949–962. [Google Scholar] [CrossRef]
- Abou-Arab, A.E.; Abou-Arab, A.A.; Abu-Salem, M.F. Physico-Chemical Assessment of Natural Sweeteners Steviosides Produced from Stevia Rebaudiana Bertoni Plant. Afr. J. Food Sci. 2010, 4, 269–281. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Connor, T.P.; O’Brien, N.M. Comparison of the Antioxidant Activity of Commercial Honeys, before and after in-Vitro Digestion. Pol. J. Food Nutr. Sci. 2013, 63, 167–171. [Google Scholar] [CrossRef]
- Szczepaniak, O.M.; Kobus-Cisowska, J.; Kusek, W.; Przeor, M. Functional Properties of Cornelian Cherry (Cornus mas L.): A Comprehensive Review. Eur. Food Res. Technol. 2019, 245, 2071–2087. [Google Scholar] [CrossRef]
- Li, J.; Peng, S.; Huo, M.; Cao, Y.; Chen, Z.; Zhang, Y.; Qiao, Y. Discovery of the Relationship between Bitter Taste and Bitter Flavor (Efficacy) Based on HTAS2Rs. J. Tradit. Chin. Med. Sci. 2023, 10, 170–178. [Google Scholar] [CrossRef]
- Lu, Z.; Xie, G.; Wu, D.; Yang, L.; Jin, Z.; Hu, Z.; Xu, X.; Lu, J. Isolation and Identification of the Bitter Compound from Huangjiu. Food Chem. 2021, 349, 129133. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Dhua, S.; Pratiksha; Kumar, V.; Naik, B.; Magwaza, L.S.; Ncama, K.; Opara, U.L.; McClements, D.J.; Mishra, P. Current and Emerging Applications in Detection and Removal of Bitter Compounds in Citrus Fruit Juice: A Critical Review. Food Biosci. 2023, 55, 102995. [Google Scholar] [CrossRef]
- Tirado-Kulieva, V.A.; Hernández-Martínez, E.; Minchán-Velayarce, H.H.; Pasapera-Campos, S.E.; Luque-Vilca, O.M. A Comprehensive Review of the Benefits of Drinking Craft Beer: Role of Phenolic Content in Health and Possible Potential of the Alcoholic Fraction. Curr. Res. Food Sci. 2023, 6, 100477. [Google Scholar] [CrossRef]
- Luo, Y.; Kong, L.; Xue, R.; Wang, W.; Xia, X. Bitterness in Alcoholic Beverages: The Profiles of Perception, Constituents, and Contributors. Trends Food Sci. Technol. 2020, 96, 222–232. [Google Scholar] [CrossRef]
- Tonutti, I.; Liddle, P. Aromatic Plants in Alcoholic Beverages. A Review. Flavour. Fragr. J. 2010, 25, 341–350. [Google Scholar] [CrossRef]
- Aharon, T.; Caricato, M. Compact Basis Sets for Optical Rotation Calculations. J. Chem. Theory Comput. 2020, 16, 4408–4415. [Google Scholar] [CrossRef]
- Suendo, V.; Viridi, S. Ab Initio Calculation of UV-Vis Absorption Spectra of a Single Chlorophyll a Molecule: Comparison Study between RHF/CIS, TDDFT, and Semi-Empirical Methods. ITB J. Sci. 2012, 44A, 93–112. [Google Scholar] [CrossRef]
- Szczepaniak, O.; Kobus-Cisowska, J.; Kmiecik, D. Freezing Enhances the Phytocompound Content in Cornelian Cherry (Cornus mas L.) Liqueur. Czech J. Food Sci. 2020, 38, 259–263. [Google Scholar] [CrossRef]
- Tešević, V.; Nikićević, N.; Milosavljević, S.; Bajić, D.; Vajs, V.; Vučković, I.; Vujisić, L.; Dordević, I.; Stanković, M.; Veličković, M. Characterization of Volatile Compounds of “Drenja”, an Alcoholic Beverage Obtained from the Fruits of Cornelian Cherry. J. Serbian Chem. Soc. 2009, 74, 117–128. [Google Scholar] [CrossRef]
- Alonso, E.; Torrado, A.; Guerra, N.P. Major Volatile Composition of Four Distilled Alcoholic Beverages Obtained from Fruits of the Forest. Chem. Eng. Trans. 2015, 44, 253–258. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid.-Based Complement. Altern. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef] [PubMed]
- Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A.; Piórecki, N. Fruit Low-Alcoholic Beverages with High Contents of Iridoids and Phenolics from Apple and Cornelian Cherry (Cornus mas L.) Fermented with Saccharomyces Bayanus. Pol. J. Food Nutr. Sci. 2019, 69, 307–317. [Google Scholar] [CrossRef]
- Polanowska, K.; Varghese, R.; Kuligowski, M.; Majcher, M. Carob Kibbles as an Alternative Raw Material for Production of Kvass with Probiotic Potential. J. Sci. Food Agric. 2021, 101, 5487–5497. [Google Scholar] [CrossRef]
- Siddeeg, A.; AlKehayez, N.M.; Abu-Hiamed, H.A.; Al-Sanea, E.A.; AL-Farga, A.M. Mode of Action and Determination of Antioxidant Activity in the Dietary Sources: An Overview. Saudi J. Biol. Sci. 2021, 28, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin-Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Katsube, T.; Imawaka, N.; Kawano, Y.; Yamazaki, Y.; Shiwaku, K.; Yamane, Y. Antioxidant Flavonol Glycosides in Mulberry (Morus Alba L.) Leaves Isolated Based on LDL Antioxidant Activity. Food Chem. 2006, 97, 25–31. [Google Scholar] [CrossRef]
- Peng, K.; Lv, X.; Zhao, H.; Chen, B.; Chen, X.; Huang, W. Antioxidant and Intestinal Recovery Function of Condensed Tannins in Lateolabrax Maculatus Responded to in Vivo and in Vitro Oxidative Stress. Aquaculture 2022, 547, 737399. [Google Scholar] [CrossRef]
- Gao, N.; Cui, H.; Lang, Y.; Zhang, W.; Shu, C.; Wang, Y.; Bian, Y.; Li, D.; Li, B. Conversion of Condensed Tannin from Chokeberry to Cyanidin: Evaluation of Antioxidant Activity and Gut Microbiota Regulation. Food Res. Int. 2022, 158, 111456. [Google Scholar] [CrossRef] [PubMed]
- Magalhães dos Santos, D.; Sanches, M.P.; Poffo, C.M.; Parize, A.L.; Sagrera Darelli, G.J.; Rodrigues de Lima, V. Syringic and Cinnamic Acids Antiradical/Antioxidant Activities as R. Ferruginea Extract Components and Membrane Physico-Chemical Influence. J. Mol. Struct. 2020, 1220, 128749. [Google Scholar] [CrossRef]
- Bayrakçeken Güven, Z.; Saracoglu, I.; Nagatsu, A.; Yilmaz, M.A.; Basaran, A.A. Anti-Tyrosinase and Antimelanogenic Effect of Cinnamic Acid Derivatives from Prunus Mahaleb L.: Phenolic Composition, Isolation, Identification and Inhibitory Activity. J. Ethnopharmacol. 2023, 310, 116378. [Google Scholar] [CrossRef] [PubMed]
| Sample | Liquor | Brandy |
|---|---|---|
| Organoleptic parameters: | ||
| Color | Straw | Dark reddish |
| Clarity | Transparent | Transparent |
| Aroma | Specific, sweet and fruity (cornelian cherry fruit noticeable), delicate, pleasant | Specific, sweet and fruity (cornelian cherry fruit slightly noticeable), pleasant |
| Taste | Sweet, slightly fruity (cornelian cherry fruit noticeable), slightly sour, harmonious | Slightly sweet, slightly fruity (cornelian cherry fruit noticeable), tart, harmonious |
| Physicochemical parameters | ||
| Alcohol content at 20 °C (%v/v) | 22.2 ± 0.5 a | 34.8 ± 0.5 b |
| Extract after alcohol distillation (Bx) | 39.0 ± 0.5 b | 17.0 ± 0.5 a |
| pH | 3.7 ± 0.2 a | 3.8 ± 0.2 a |
| Centrifugation residue (g/L) | <0.1 | <0.1 |
| Relative viscosity † | 12.7 ± 0.2 b | 4.83 ± 0.23 a |
| Color-instrumental measurement CIE Lab system | L* = 21.5 ± 1.2 a a* = 3.2 ± 0.2 b b* = 0.05 ± 0.02 b | L* = 21.2 ± 0.2 a a* = 2.4 ± 0.2 a b* = −0.19 ± 0.02 a |
| ΔE = 0.89 | ||
| Basis Set/Solvent | Gas State | Water | Ethanol |
|---|---|---|---|
| single point energy (kJ/mol) | |||
| b3lyp/6–31+g(2d,2p) | −4,255,888.40 | −4,259,820.71 | −4,259,816.57 |
| b3lyp/6–31++g(2d,2p) | −4,256,234.30 | −4,256,323.02 | −4,256,318.61 |
| b3lyp/3–21+g* | −4,233,830.95 | −4,233,945.76 | −4,233,939.93 |
| b3lyp/3–21+g | −4,233,319.52 | −4,233,435.80 | −4,233,429.99 |
| optical rotation angle | |||
| b3lyp/6–31+g(2d,2p) | 100.41 | 96.36 | 97.49 |
| b3lyp/6–31++g(2d,2p) | 100.01 | 95.41 | 96.12 |
| b3lyp/3–21+g* | 111.10 | 105.86 | 106.73 |
| b3lyp/3–21+g | 111.10 | 105.86 | 106.73 |
| UV spectra: wavelength (nm); ε | |||
| b3lyp/6–31+g(2d,2p) | 239.77 | 240.19 | 240.21 |
| 0.0040 | 0.0073 | 0.0073 | |
| 235.90 | 228.40 | 228.60 | |
| 0.0144 | 0.1103 | 0.1095 | |
| 232.75 | 219.90 | 220.66 | |
| 0.0253 | 0.0057 | 0.0065 | |
| b3lyp/6–31++g(2d,2p) | 239.00 | 239.46 | 239.48 |
| 0.0040 | 0.0074 | 0.0075 | |
| 235.28 | 227.61 | 227.80 | |
| 0.0138 | 0.1158 | 0.1150 | |
| 232.13 | 218.82 | 219.61 | |
| 0.0268 | 0.0054 | 0.0063 | |
| b3lyp/3–21+g* | 233.91 | 234.58 | 234.59 |
| 0.0038 | 0.0089 | 0.0089 | |
| 232.62 | 223.70 | 223.88 | |
| 0.0103 | 0.1294 | 0.1284 | |
| 229.26 | 214.61 | 215.47 | |
| 0.0305 | 0.0053 | 0.0061 | |
| b3lyp/3–21+g | 233.91 | 234.58 | 234.58 |
| 0.0038 | 0.0089 | 0.0089 | |
| 232.62 | 233.70 | 223.88 | |
| 0.0103 | 0.1294 | 0.1284 | |
| 229.26 | 214.61 | 215.47 | |
| 0.0305 | 0.0053 | 0.0061 | |
| UV-VIS spectra | liquor | brandy | |
| Λmax (nm); | 233 | 234 | |
| Absorbance | 0.31397 | 0.46112 | |
| Sample | Gallic Acid | 2,5-DHBA | 4-HBA | PCA | Caffeic Acid | Syringic Acid | p-Coumaric Acid | Ferulic Acid | CGA | Sinapic Acid | t-Cinnamic Acid | CA | CB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| brandy | 14.22 ± 0.22 a | 1.30 ± 0.10 a | 22.35 ± 0.67 a | 13.50 ± 0.19 b | 33.10 ± 2.10 a | 12.60 ± 0.98 a | 3.31 ± 0.15 b | 28.52 ± 2.12 b | 6.10 ± 0.57 a | 1.14 ± 0.17 b | 5.32 ± 1.21 a | 1.66 ± 0.00 b | 4.08 ± 0.01 b |
| liquor | 16.10 ± 0.31 b | 1.60 ± 0.12 a | 21.20 ± 0.54 a | 10.40 ± 0.09 a | 36.50 ± 2.22 a | 10.40 ± 1.02 a | 2.80 ± 0.62 a | 16.31 ± 1.15 a | 5.21 ± 0.38 a | 0.70 ± 0.02 a | 8.93 ± 1.98 b | 0.53 ± 0.00 a | 1.24 ± 0.00 a |
| sample | vanillic acid | salicyl acid | Naringenin | vitexin | rutin | quercetin | apigenin | kaempferol | luteolin | catechin | amygdalin | TC | |
| brandy | 15.60 ± 2.15 | 2.25 ± 0.77 a | 378.31 ± 9.17 b | 34.52 ± 2.46 b | 169.13 ± 7.35 b | 201.33 ± 9.36 b | 79.11 ± 5.38 b | 17.42 ± 2.16 b | 10.70 ± 0.39 b | 345.23 a ± 10.52 | 10.64 ± 12.10 a | 6.57 ± 0.00 b | |
| liquor | 14.90 ± 1.98 | 8.64 ± 0.94 b | 281.56 ± 10.02 a | 12.61 ± 1.07 a | 106.3 ± 4.13 a | 115.46 ± 5.72 a | 51.20 ± 3.16 a | 6.56 ± 1.52 a | 2.12 ± 0.91 a | 300.71 a ± 11.43 | 1.45 ± 2.05 a | 1.58 ± 0.00 a |
| Compound (PubChem CID) | Description of the Odor | Cornelian Cherry Liqueur | Cornelian Cherry Brandy | |
|---|---|---|---|---|
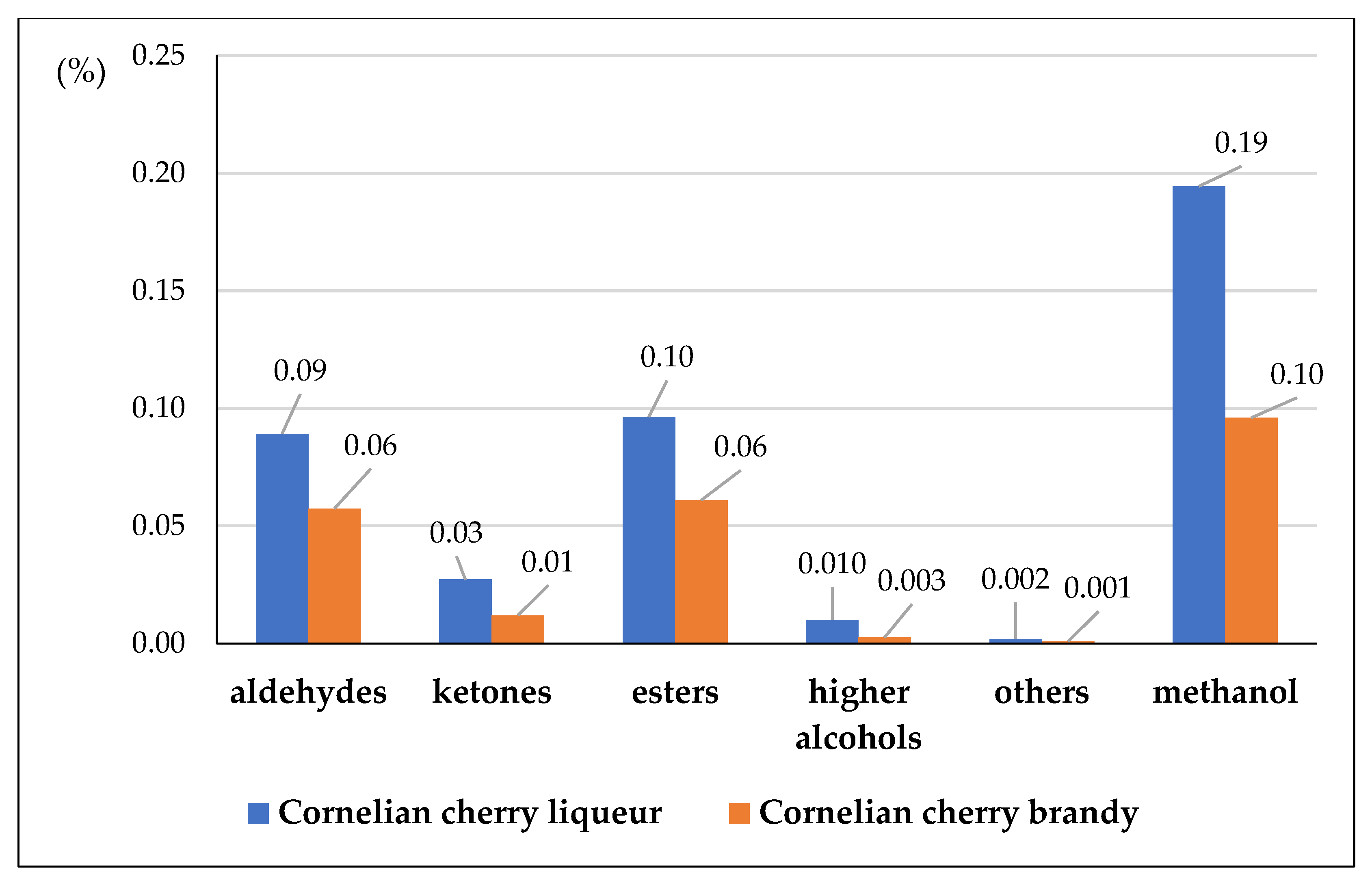
| Aldehydes Ketones Esters Higher alcohols Others Methanol (887) Total volatiles | acetaldehyde (177) 1.1-diethoxyethane (92620) propan-2-one (180) ethyl acetate (8857) ethyl pentanoate (10882) propyl acetate (7997) butan-1-ol (263) 3-methylbutan-1-ol (31260) hexan-1-ol (8103) heptan-1-ol (8129) 2-phenylethanol (6054) 2.3.5-trimethylpyrazine (26808) | fruity fruity solvent-like, pungent solvent-like fruity petrol-like medicinal, fusel fusel oil fatty, fruity, woody not found floral, honey-like earthy pungent, aromatic - | 146.57 ± 0.82 b 15.11 ± 1.76 a 47.12 ± 0.44 b 149.68 ± 3.06 b 3.60 ± 0.33 15.81 ± 0.05 a 3.67 ± 0.18 b 2.11 ± 0.16 b 2.21 ± 0.50 3.81 ± 0.33 b 4.69 ± 1.10 3.38 ± 0.03 b 342.15 ± 1.46 b 737.36 ± 11.39 b | 127.87 ± 3.77 a 29.42 ± 2.25 b 33.01 ± 1.02 a 133.26 ± 5.25 a 2.95 ± 0.48 31.61 ± 2.70 b 2.62 ± 0.03 a 1.19 ± 0.01 a 1.66 ± 0.04 2.28 ± 0.10 a nd 1.85 ± 0.07 a 264.31 ± 9.88 a 632.84 ± 26.61 a |
| Sample | ABTS (mM Trolox) * | DPPH (mM Trolox) † |
|---|---|---|
| liquor | 1.3 a ± 0.4 | 163.6 a ± 24.9 |
| brandy | 2.6 b ± 0.5 | 52.9 b ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepaniak, O.; Stachowiak, B.; Jeleń, H.; Stuper-Szablewska, K.; Szambelan, K.; Kobus-Cisowska, J. The Contribution of Cornelian Cherry (Cornus mas L.) Alcoholic Beverages on the Sensory, Nutritional and Anti-Nutritional Characteristics—In Vitro and In Silico Approaches. Processes 2024, 12, 237. https://doi.org/10.3390/pr12010237
Szczepaniak O, Stachowiak B, Jeleń H, Stuper-Szablewska K, Szambelan K, Kobus-Cisowska J. The Contribution of Cornelian Cherry (Cornus mas L.) Alcoholic Beverages on the Sensory, Nutritional and Anti-Nutritional Characteristics—In Vitro and In Silico Approaches. Processes. 2024; 12(1):237. https://doi.org/10.3390/pr12010237
Chicago/Turabian StyleSzczepaniak, Oskar, Barbara Stachowiak, Henryk Jeleń, Kinga Stuper-Szablewska, Katarzyna Szambelan, and Joanna Kobus-Cisowska. 2024. "The Contribution of Cornelian Cherry (Cornus mas L.) Alcoholic Beverages on the Sensory, Nutritional and Anti-Nutritional Characteristics—In Vitro and In Silico Approaches" Processes 12, no. 1: 237. https://doi.org/10.3390/pr12010237
APA StyleSzczepaniak, O., Stachowiak, B., Jeleń, H., Stuper-Szablewska, K., Szambelan, K., & Kobus-Cisowska, J. (2024). The Contribution of Cornelian Cherry (Cornus mas L.) Alcoholic Beverages on the Sensory, Nutritional and Anti-Nutritional Characteristics—In Vitro and In Silico Approaches. Processes, 12(1), 237. https://doi.org/10.3390/pr12010237










