Influence of Cold Plasma Processing on the Stability of Phenolic Compounds of Araça-Boi (Eugenia stipitata) Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
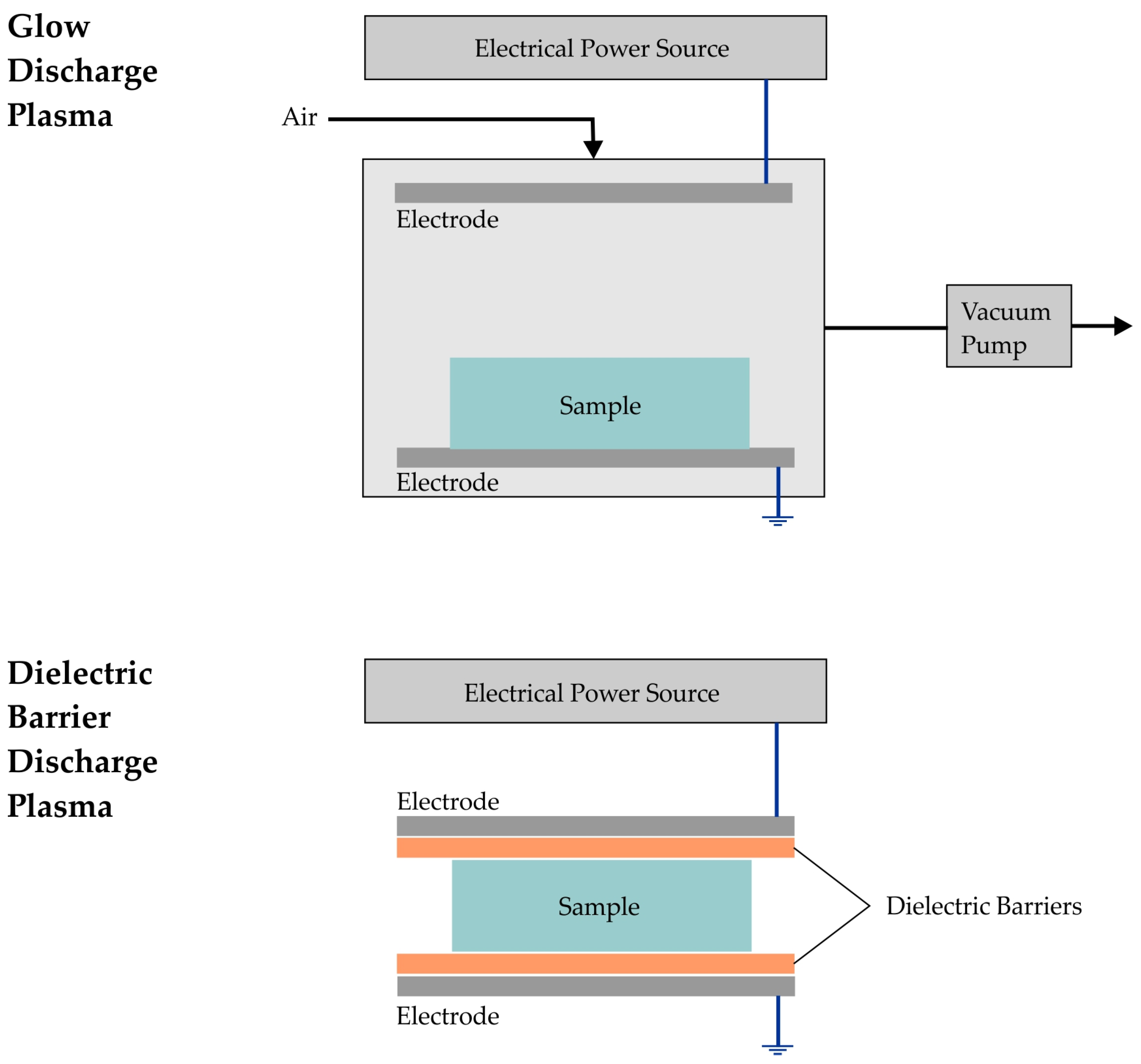
2.2. Plasma Processing
2.3. Extraction and Concentration of Phenolic Compounds
2.4. Chromatographic Analysis
2.5. Statistical Analysis
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Misra, N.N.; Tiwari, B.K.; Raghavarao, K.S.M.S.; Cullen, P.J. Nonthermal Plasma Inactivation of Food-Borne Pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljevic, V.; O’Donnell, C.P.; Bourke, P.; Cullen, P.J. Applications of Cold Plasma Technology in Food Packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Joshi, I.; Salvi, D.; Schaffner, D.W.; Karwe, M.V. Characterization of Microbial Inactivation Using Plasma-Activated Water and Plasma-Activated Acidified Buffer. J. Food Prot. 2018, 81, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, C.; Jiang, A.; Guan, Q.; Sun, X.; Liu, S.; Hao, K.; Hu, W. The Effects of Cold Plasma-Activated Water Treatment on the Microbial Growth and Antioxidant Properties of Fresh-Cut Pears. Food Bioprocess Technol. 2019, 12, 1842–1851. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, D.U.; Min, S.C. Microbial Decontamination of Red Pepper Powder by Cold Plasma. Food Microbiol. 2014, 38, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Jabbar, S.; Ayub, Z.; Muhammad Aadil, R.; Abid, M.; Zhang, J.; Liqing, Z. Recent Advances in Plasma Technology: Influence of Atmospheric Cold Plasma on Spore Inactivation. Food Rev. Int. 2022, 38, 789–811. [Google Scholar] [CrossRef]
- Bie, P.; Pu, H.; Zhang, B.; Su, J.; Chen, L.; Li, X. Structural Characteristics and Rheological Properties of Plasma-Treated Starch. Innov. Food Sci. Emerg. Technol. 2016, 34, 196–204. [Google Scholar] [CrossRef]
- Carvalho, A.P.M.G.; Barros, D.R.; da Silva, L.S.; Sanches, E.A.; da Costa Pinto, C.; de Souza, S.M.; Clerici, M.T.P.S.; Rodrigues, S.; Fernandes, F.A.N.; Campelo, P.H. Dielectric Barrier Atmospheric Cold Plasma Applied to the Modification of Ariá (Goeppertia allouia) Starch: Effect of Plasma Generation Voltage. Int. J. Biol. Macromol. 2021, 182, 1618–1627. [Google Scholar] [CrossRef]
- Lee, H.J.; Jung, H.; Choe, W.; Ham, J.S.; Lee, J.H.; Jo, C. Inactivation of Listeria Monocytogenes on Agar and Processed Meat Surfaces by Atmospheric Pressure Plasma Jets. Food Microbiol. 2011, 28, 1468–1471. [Google Scholar] [CrossRef]
- Ulbin-Figlewicz, N.; Brychcy, E.; Jarmoluk, A. Effect of Low-Pressure Cold Plasma on Surface Microflora of Meat and Quality Attributes. J. Food Sci. Technol. 2015, 52, 1228–1232. [Google Scholar] [CrossRef]
- Dorraki, N.; Mahdavi, V.; Ghomi, H.; Ghasempour, A. Elimination of Diazinon Insecticide from Cucumber Surface by Atmospheric Pressure Air-Dielectric Barrier Discharge Plasma. Biointerphases 2016, 11, 041007. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-W.; Niu, D.; Zhou, Y.-X.; Cheng, J.-H.; El-Din Bekhit, A.; Aadil, R.M. Oxidation Induced by Dielectric-Barrier Discharge (DBD) Plasma Treatment Reduces Soybean Agglutinin Activity. Food Chem. 2021, 340, 128198. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.A.N.; Rodrigues, S. Cold Plasma Processing on Fruits and Fruit Juices: A Review on the Effects of Plasma on Nutritional Quality. Processes 2021, 9, 2098. [Google Scholar] [CrossRef]
- Kungsuwan, K.; Sawangrat, C.; Ounjaijean, S.; Chaipoot, S.; Phongphisutthinant, R.; Wiriyacharee, P. Enhancing Bioactivity and Conjugation in Green Coffee Bean (Coffea arabica) Extract through Cold Plasma Treatment: Insights into Antioxidant Activity and Phenolic–Protein Conjugates. Molecules 2023, 28, 7066. [Google Scholar] [CrossRef] [PubMed]
- Mazine, F.F.; Bünger, M.; Faria, J.E.Q.; Fernandes, T.; Giaretta, A.; Valdemarin, K.S.; Santana, K.C.; Souza, M.A.D.; Sobral, M. Eugenia. In Flora e Funga do Brasil; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2023. [Google Scholar]
- Abouelenein, D.; Mustafa, A.M.; Nzekoue, F.K.; Caprioli, G.; Angeloni, S.; Tappi, S.; Castagnini, J.M.; Dalla Rosa, M.; Vittori, S. The Impact of Plasma Activated Water Treatment on the Phenolic Profile, Vitamins Content, Antioxidant and Enzymatic Activities of Rocket-Salad Leaves. Antioxidants 2022, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Fernandes, F.A.N. Changing Ready-to-Drink Coffee Aroma Using Dielectric Barrier Discharge Plasma. Processes 2022, 10, 2056. [Google Scholar] [CrossRef]
- Farias, T.R.B.; Alves Filho, E.G.; Campelo, P.H.; Rodrigues, S.; Fernandes, F.A.N. Influence of Atmospheric and Vacuum Plasma Processing on the Organic Composition of Araça-Boi (Eugenia stipitata) Juice. Food Chem. Adv. 2023, 3, 100345. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, C.; Zhan, J. Separation, Characterization, and Quantitation of Benzoic and Phenolic Antioxidants in American Cranberry Fruit by GC−MS. J. Agric. Food Chem. 2002, 50, 3789–3794. [Google Scholar] [CrossRef]
- Guimarães, A.C.G.; de Souza Gomes, M.; Zacaroni Lima, L.M.; Sales, P.F.; da Cunha, M.C.; Rodrigues, L.J.; de Barros, H.E.A.; Pires, C.R.F.; dos Santos, V.F.; Lima Natarelli, C.V.; et al. Application of Chemometric Techniques in the Evaluation of Bioactive Compounds and Antioxidant Activity of Fruit From Brazilian Cerrado. J. Food Meas. Charact. 2023, 17, 2095–2106. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; de Souza, F.G.; Catharino, R.R.; do Sacramento, C.K.; Pastore, G.M. Influence of High-Intensity Ultrasound on Color, Chemical Composition and Antioxidant Properties of Araçá-Boi Pulp. Food Chem. 2021, 338, 127747. [Google Scholar] [CrossRef]
- Chagas Barros, R.G.; Santos de Oliveira, C.; Santos Oliveira, L.T.; Pereira, U.C.; Matos Silva, T.O.; Denadai, M.; Narain, N. Enhancement of Phenolic Antioxidants Production in Submerged Cultures of Endophytic Microorganisms Isolated from Achachairu (Garcinia humilis), Araçá-Boi (Eugenia stipitata) and Bacaba (Oenocarpus bacaba) Fruits. LWT 2019, 111, 370–377. [Google Scholar] [CrossRef]
- Latza, S.; Ganßer, D.; Berger, R.G. Carbohydrate Esters of Cinnamic Acid from Fruits of Physalis peruviana, Psidium guajava and Vaccinium vitis-idaea. Phytochemistry 1996, 43, 481–485. [Google Scholar] [CrossRef]
- Cheel, J.; Theoduloz, C.; Rodríguez, J.; Saud, G.; Caligari, P.D.S.; Schmeda-Hirschmann, G. E-Cinnamic Acid Derivatives and Phenolics from Chilean Strawberry Fruits, Fragaria chiloensis ssp. Chiloensis. J. Agric. Food Chem. 2005, 53, 8512–8518. [Google Scholar] [CrossRef]
- Nair, A.; Preetha Rani, M.R.; Salin Raj, P.; Ranjit, S.; Rajankutty, K.; Raghu, K.G. Cinnamic Acid Is Beneficial to Diabetic Cardiomyopathy via Its Cardioprotective, Anti-inflammatory, Anti-dyslipidemia, and Antidiabetic Properties. J. Biochem. Mol. Toxicol. 2022, 36, e23215. [Google Scholar] [CrossRef] [PubMed]
- Chavarria, D.; Silva, T.; Martins, D.; Bravo, J.; Summavielle, T.; Garrido, J.; Borges, F. Exploring Cinnamic Acid Scaffold: Development of Promising Neuroprotective Lipophilic Antioxidants. Medchemcomm 2015, 6, 1043–1053. [Google Scholar] [CrossRef]
- Sousa, M.; Afonso, A.C.; Teixeira, L.S.; Borges, A.; Saavedra, M.J.; Simões, L.C.; Simões, M. Hydrocinnamic Acid and Perillyl Alcohol Potentiate the Action of Antibiotics against Escherichia coli. Antibiotics 2023, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Porto, E.C.M.; de Brito, E.S.; Rodrigues, S.; Fernandes, F.A.N. Effect of Atmospheric Cold Plasma on the Aroma of Pineapple Juice: Improving Fresh and Fruity Notes and Reducing Undesired Pungent and Sulfurous Aromas. Processes 2023, 11, 2303. [Google Scholar] [CrossRef]
- Lee, M.; Rho, H.S.; Choi, K. Anti-Inflammatory Effects of a P-Coumaric Acid and Kojic Acid Derivative in LPS-Stimulated RAW264.7 Macrophage Cells. Biotechnol. Bioprocess Eng. 2019, 24, 653–657. [Google Scholar] [CrossRef]
- Venkatesan, A.; Samy, J.V.R.A.; Balakrishnan, K.; Natesan, V.; Kim, S.-J. In Vitro Antioxidant, Anti-Inflammatory, Antimicrobial, and Antidiabetic Activities of Synthesized Chitosan-Loaded p-Coumaric Acid Nanoparticles. Curr. Pharm. Biotechnol. 2023, 24, 1178–1194. [Google Scholar] [CrossRef]
- Yoon, S.-A.; Kang, S.-I.; Shin, H.-S.; Kang, S.-W.; Kim, J.-H.; Ko, H.-C.; Kim, S.-J. P-Coumaric Acid Modulates Glucose and Lipid Metabolism via AMP-Activated Protein Kinase in L6 Skeletal Muscle Cells. Biochem. Biophys. Res. Commun. 2013, 432, 553–557. [Google Scholar] [CrossRef]
- Cui, K.; Wu, H.; Fan, J.; Zhang, L.; Li, H.; Guo, H.; Yang, R.; Li, Z. The Mixture of Ferulic Acid and P-Coumaric Acid Suppresses Colorectal Cancer through LncRNA 495810/PKM2 Mediated Aerobic Glycolysis. Int. J. Mol. Sci. 2022, 23, 12106. [Google Scholar] [CrossRef] [PubMed]
- Sefidi-Heris, Y.; Zarei, E.; Saadat, I. Metformin and P-Coumaric Acid Downregulate the Expression of HTERT in Gastric Cancer Cell Line AGS. Gene Rep. 2023, 32, 101795. [Google Scholar] [CrossRef]
- Wijerathna, T.M.; Mohamed, F.; Dissanayaka, D.; Gawarammana, I.; Palangasinghe, C.; Shihana, F.; Endre, Z.; Shahmy, S.; Buckley, N.A. Albuminuria and Other Renal Damage Biomarkers Detect Acute Kidney Injury Soon after Acute Ingestion of Oxalic Acid and Potassium Permanganate. Toxicol. Lett. 2018, 299, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Otsubo, S.; Komai, M.; Takada, H.; Maemoto, M.; Kobayashi, A.; Otsubo, N. The Design, Synthesis and Evaluation of 2-Aminobenzoxazole Analogues as Potent and Orally Efficacious ChemR23 Inhibitors. Bioorg. Med. Chem. 2020, 28, 115622. [Google Scholar] [CrossRef]
- Zhou, D.; Sun, R.; Zhu, W.; Shi, Y.; Ni, S.; Wu, C.; Li, T. Impact of Dielectric Barrier Discharge Cold Plasma on the Quality and Phenolic Metabolism in Blueberries Based on Metabonomic Analysis. Postharvest Biol. Technol. 2023, 197, 112208. [Google Scholar] [CrossRef]


| Phenolic Compound | Control | Frequency | ||
|---|---|---|---|---|
| 50 Hz | 500 Hz | 1000 Hz | ||
| 2-Aminobenzoxazole | 0.71 ± 0.04 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
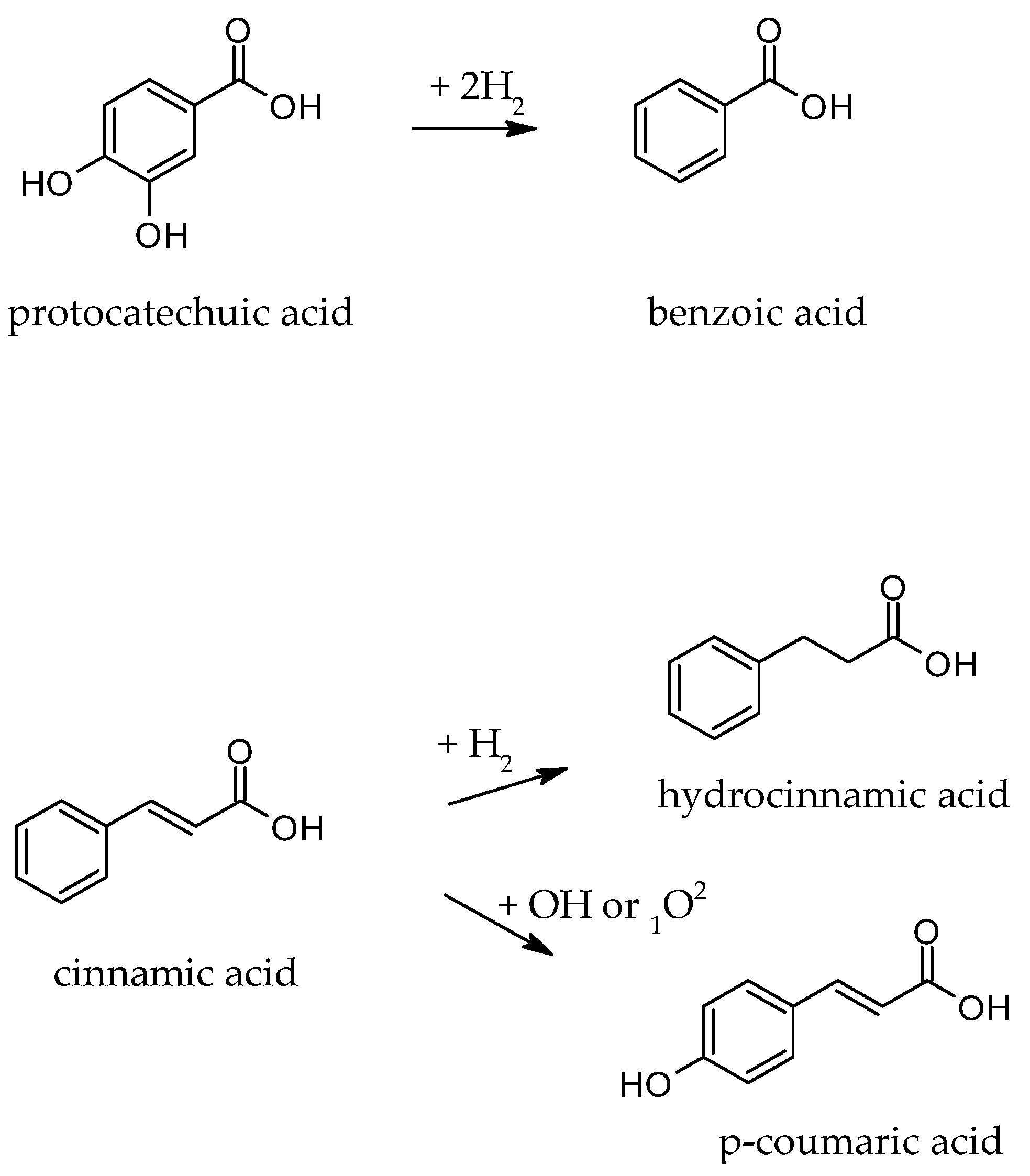
| Benzoic acid | 0.84 ± 0.04 c | 1.03 ± 0.05 b | 1.58 ± 0.08 a | 0.69 ± 0.03 d |
| Cinnamic acid | 90.34 ± 1.81 b | 96.70 ± 1.93 a | 96.63 ± 1.93 a | 95.40 ± 1.91 a |
| Hydrocinnamic acid | 0.63 ± 0.03 c | 1.61 ± 0.08 a | 1.78 ± 0.09 a | 1.05 ± 0.05 b |
| Oxanilic acid | 0.47 ± 0.02 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| p-Coumaric acid | 0.00 ± 0.00 d | 1.26 ± 0.06 b | 1.97 ± 0.10 a | 0.93 ± 0.05 c |
| Phenylacetic acid | 0.29 ± 0.01 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| Protocatechuic acid | 5.49 ± 0.27 a | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 2.63 ± 0.13 b |
| Phenolic Compound | Control | Plasma Flow Rate | ||
|---|---|---|---|---|
| 10 mL/min | 20 mL/min | 30 mL/min | ||
| 2-Aminobenzoxazole | 0.71 ± 0.04 a | 0.75 ± 0.04 a | 0.29 ± 0.01 b | 0.13 ± 0.01 c |
| Benzoic acid | 0.84 ± 0.04 c | 1.21 ± 0.06 a | 0.97 ± 0.05 b | 0.76 ± 0.04 c |
| Cinnamic acid | 90.34 ± 1.81 c | 96.38 ± 1.93 a | 93.40 ± 1.87 bc | 93.15 ± 1.86 bc |
| Hydrocinnamic acid | 0.63 ± 0.03 c | 0.00 ± 0.00 d | 1.22 ± 0.06 b | 2.20 ± 0.11 a |
| Oxanilic acid | 0.47 ± 0.02 a | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.38 ± 0.02 b |
| p-Coumaric acid | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.91 ± 0.05 a | 0.44 ± 0.02 b |
| Phenylacetic acid | 0.29 ± 0.01 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| Protocatechuic acid | 5.49 ± 0.27 a | 0.60 ± 0.03 c | 3.48 ± 0.17 b | 3.38 ± 0.17 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porto, E.C.; Maia, D.L.H.; Rodrigues, S.; Fernandes, F.A.N.; Campelo, P.H. Influence of Cold Plasma Processing on the Stability of Phenolic Compounds of Araça-Boi (Eugenia stipitata) Juice. Processes 2024, 12, 73. https://doi.org/10.3390/pr12010073
Porto EC, Maia DLH, Rodrigues S, Fernandes FAN, Campelo PH. Influence of Cold Plasma Processing on the Stability of Phenolic Compounds of Araça-Boi (Eugenia stipitata) Juice. Processes. 2024; 12(1):73. https://doi.org/10.3390/pr12010073
Chicago/Turabian StylePorto, Elaine C., Dayanne L. H. Maia, Sueli Rodrigues, Fabiano A. N. Fernandes, and Pedro H. Campelo. 2024. "Influence of Cold Plasma Processing on the Stability of Phenolic Compounds of Araça-Boi (Eugenia stipitata) Juice" Processes 12, no. 1: 73. https://doi.org/10.3390/pr12010073
APA StylePorto, E. C., Maia, D. L. H., Rodrigues, S., Fernandes, F. A. N., & Campelo, P. H. (2024). Influence of Cold Plasma Processing on the Stability of Phenolic Compounds of Araça-Boi (Eugenia stipitata) Juice. Processes, 12(1), 73. https://doi.org/10.3390/pr12010073









