Integrating Suspended Sludge and Fixed Film into a Biological Wastewater Treatment System to Enhance Nitrogen Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Description
2.2. The Carrier Materials
2.3. Seed Sludge and Domestic Wastewater
2.4. Factors Affecting Treatment Effectiveness
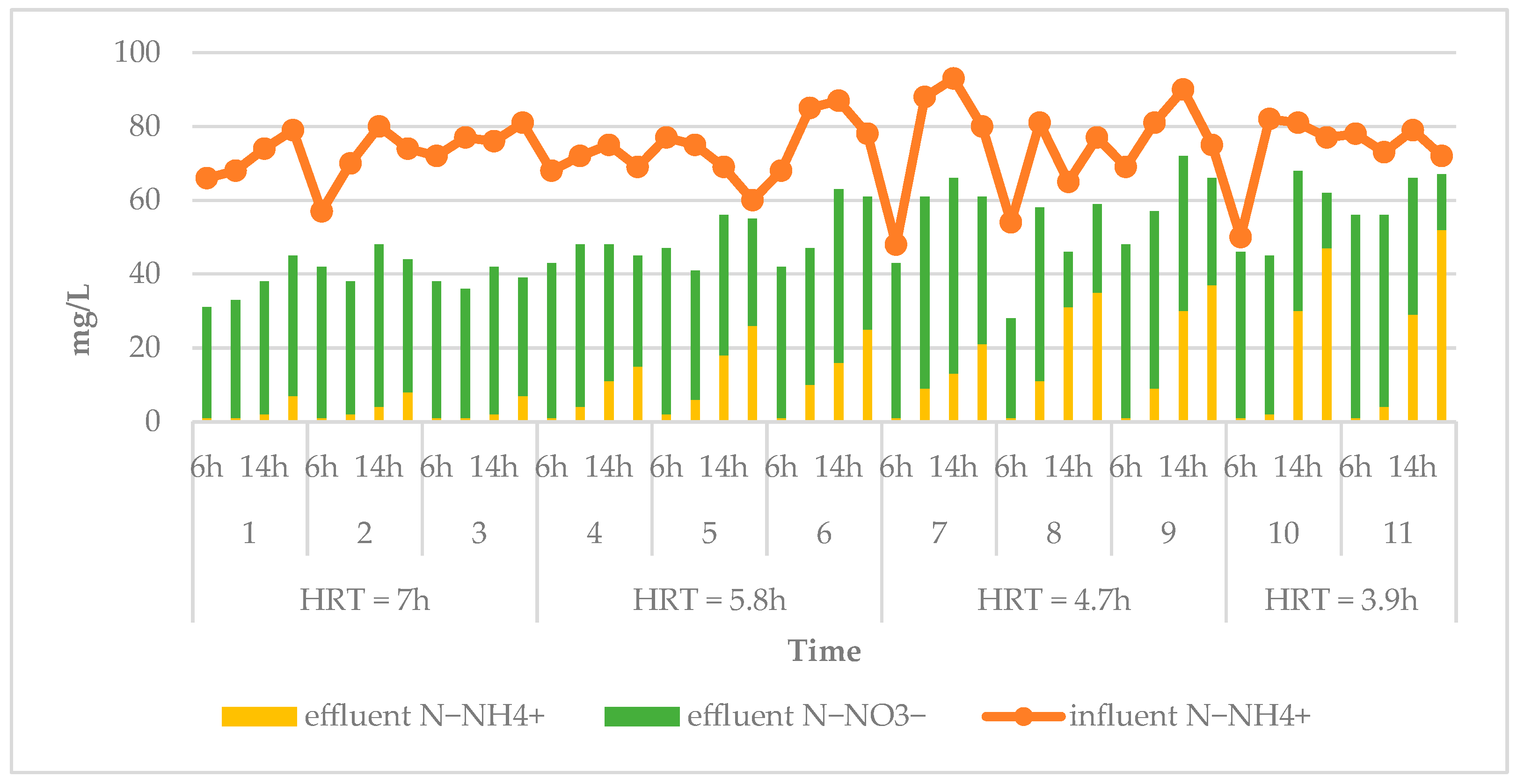
2.4.1. Hydraulic Retention Time (HRT)
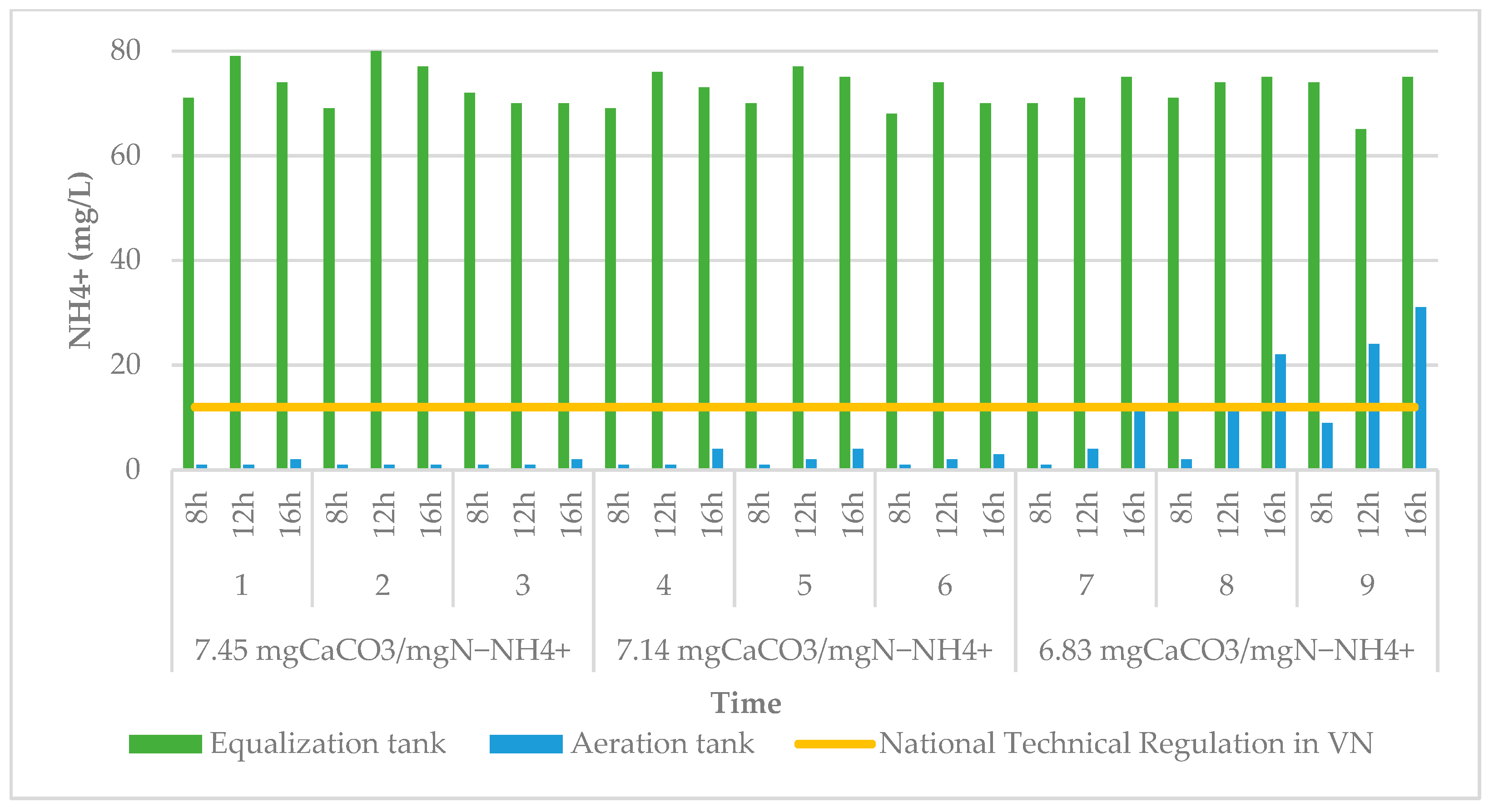
2.4.2. Alkalinity
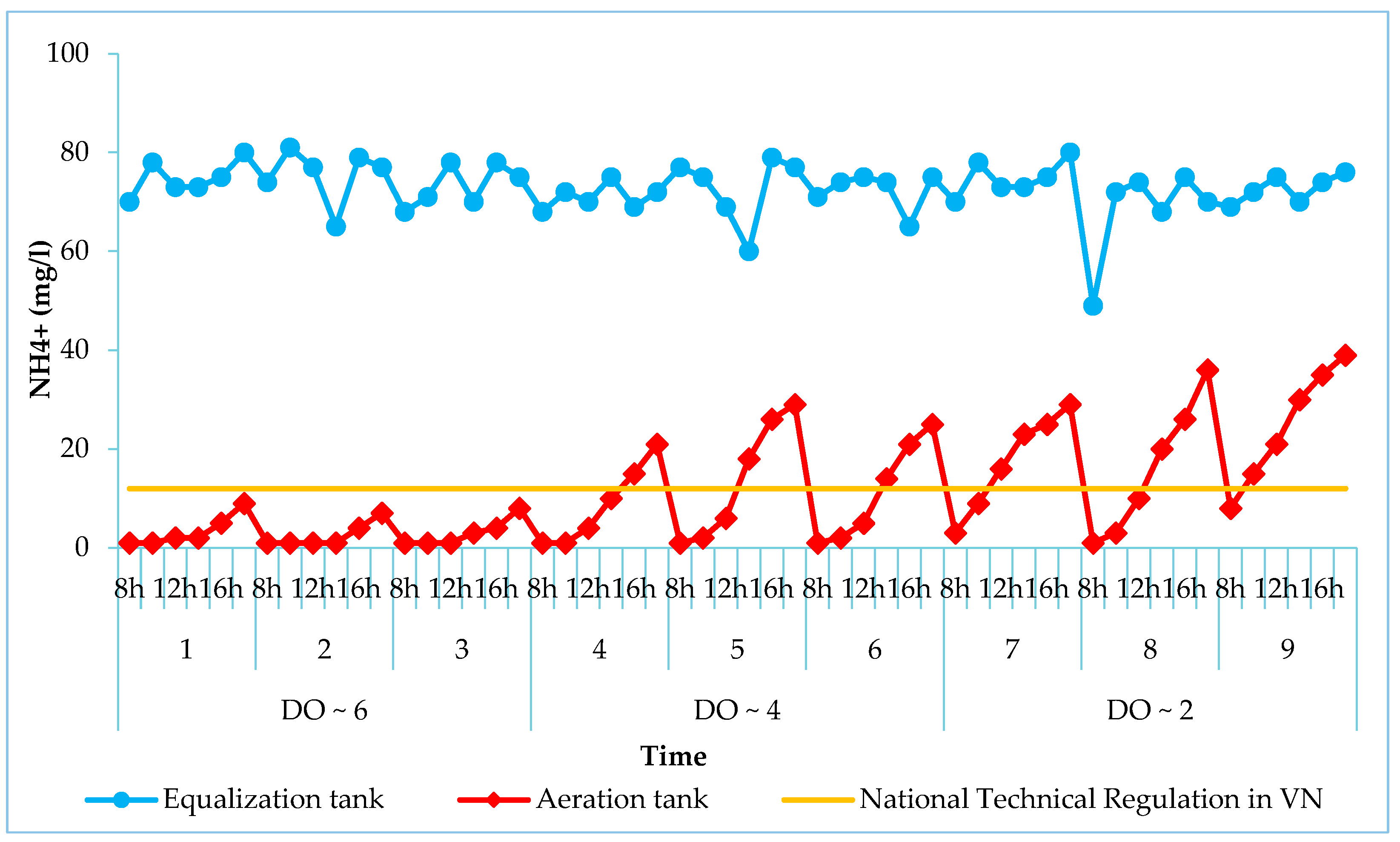
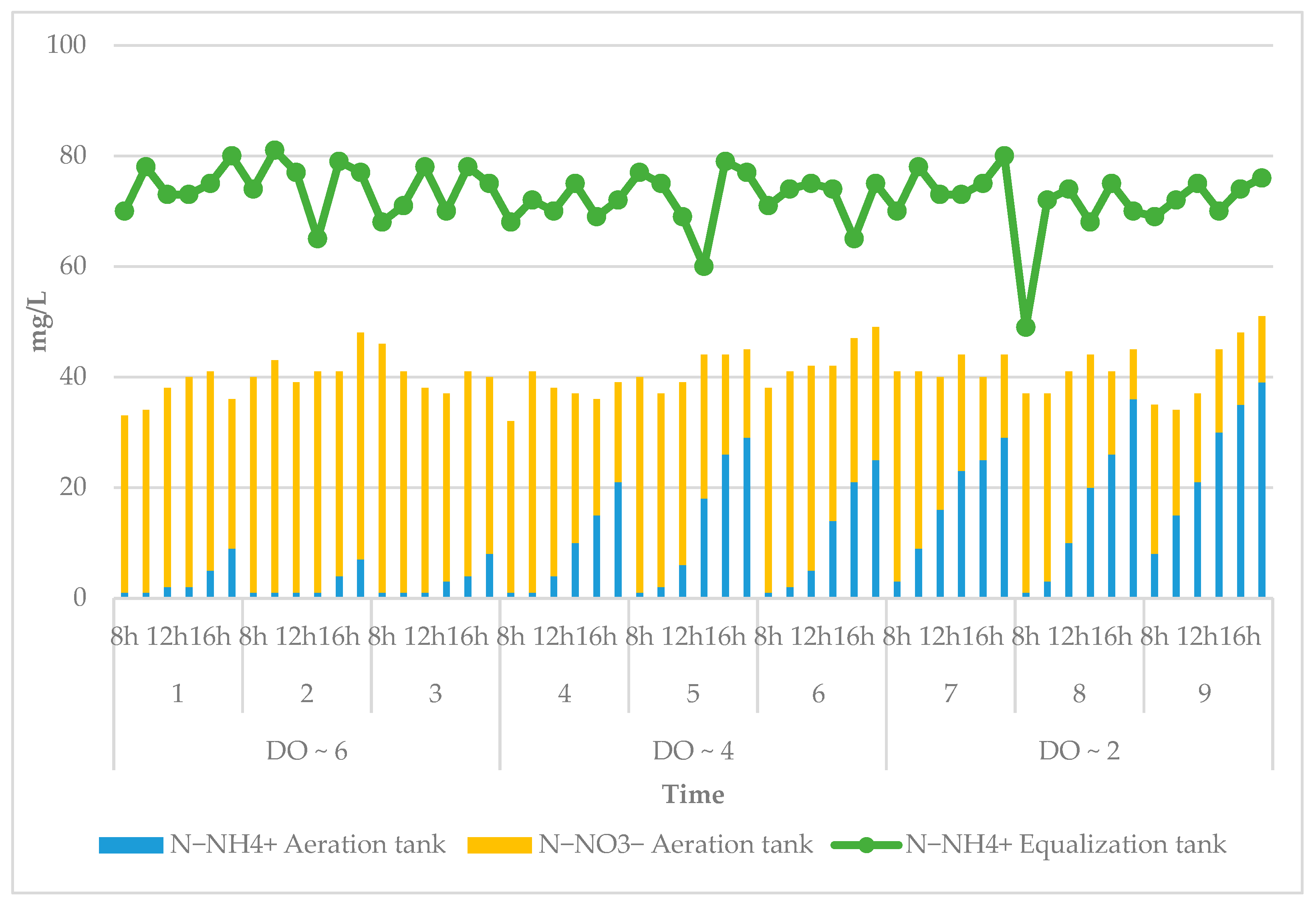
2.4.3. Dissolved Oxygen (DO)
2.5. Sampling and Analysis
3. Results
3.1. Effects of HRT on Treatment Efficiency
3.2. Effect of Alkalinity on Treatment Efficiency
3.3. Effect of DO on Treatment Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rashidi, H.; GhaffarianHoseini, A.; Sulaiman, N.M.N.; Tookey, J.; Hashim, N.A. Application of wastewater treatment in sustainable design of green built environments: A review. Renew. Sustain. Energy Rev. 2015, 49, 845–856. [Google Scholar] [CrossRef]
- He, X. Does the rapid development of China’s urban residential buildings matter for the environment? Build. Environ. 2013, 64, 130–137. [Google Scholar] [CrossRef]
- Va, V.; Setiyawan, A.S.; Soewondo, P.; Putri, D.W. The Characteristics of Domestic Wastewater from Office Buildings in Bandung, West Java, Indonesia. Indones. J. Urban Environ. Technol. 2018, 1, 199–214. [Google Scholar] [CrossRef]
- Va, V.; Setiyawan, A.S.; Soewondo, P.; Putri, D.W. Effect of recirculation ratio on the performance of modified septic tank in treating office building wastewater. In E3S Web Conference; EDP Sciences: Les Ulis, France, 2020; Volume 148, p. 01001. [Google Scholar]
- Mester, T.; Balla, D.; Karancsi, G.; Bessenyei, É.; Szabó, G. Effects of nitrogen loading from domestic wastewater on groundwater quality. Water SA 2019, 45, 349–358. [Google Scholar] [CrossRef]
- Li, Y.-H.; Li, H.-B.; Xu, X.-Y.; Xiao, S.-Y.; Wang, S.-Q.; Xu, S.-C. Fate of nitrogen in subsurface infiltration system for treating secondary effluent. Water Sci. Eng. 2017, 10, 217–224. [Google Scholar] [CrossRef]
- Hewawasam, C.; Matsuura, N.; Maharjan, N.; Hatamoto, M.; Yamaguchi, T. Oxygen transfer dynamics and nitrification in a novel rotational sponge reactor. Biochem. Eng. J. 2017, 128, 162–167. [Google Scholar] [CrossRef]
- Li, F.; An, X.; Feng, C.; Kang, J.; Wang, J.; Yu, H. Research on Operation Efficiency and Membrane Fouling of A2/O-MBR in Reclaimed Water Treatment. Membranes 2019, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Pedrouso, A.; Tocco, G.; del Río, A.V.; Carucci, A.; Morales, N.; Campos, J.L.; Milia, S.; Mosquera-Corral, A. Digested blackwater treatment in a partial nitritation-anammox reactor under repeated starvation and reactivation periods. J. Clean. Prod. 2020, 244, 118733. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, Y.; Zhang, L.; Li, X.; Zhang, Q. Simultaneous partial nitritation and denitritation coupled with polished anammox for advanced nitrogen removal from low C/N domestic wastewater at low dissolved oxygen conditions. Bioresour. Technol. 2020, 305, 123045. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Peng, Y.; Huang, D.; Fan, J.; Du, R. Enhanced Nitrogen Removal from Domestic Wastewater by Partial-Denitrification/Anammox in an Anoxic/Oxic Biofilm Reactor. Processes 2022, 10, 109. [Google Scholar] [CrossRef]
- Halicki, W.; Halicki, M. Effective Removal of Biogenic Substances Using Natural Treatment Systems for Wastewater for Safer Water Reuse. Water 2022, 14, 3977. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R.; Man, Z.; Wibisono, Y.; Jaafar, J.; Mahlia, T.M.I.; Khan, A.L.; Aslam, M. Recent progress in integrated fixed-film activated sludge process for wastewater treatment: A review. J. Environ. Manag. 2020, 268, 110718. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Quan, X.; Zhao, H.; Zhang, Y.; Chen, S.; Liu, T. Enhancing nitrogen removal efficiency in a dyestuff wastewater treatment plant with the IFFAS process: The pilot-scale and full-scale studies. Water Sci. Technol. 2018, 77, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, L.; Cheng, J.; Zhang, S.; Li, B.; Peng, Y. Achieve efficient nitrogen removal from real sewage in a plug-flow integrated fixed-film activated sludge (IFAS) reactor via partial nitritation/anammox pathway. Bioresour. Technol. 2017, 239, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Zhang, Y.; Liang, R.; Wang, Z.; Sun, J.; Lu, X.; He, Y.; Wang, Y. Comprehensive comparison of integrated fixed-film activated sludge (IFAS) and AAO activated sludge methods: Influence of different operational parameters. Chemosphere 2024, 357, 142068. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Irvin, S. The comparison of alkalinity and ORP as indicators for nitrification and denitrification in a sequencing batch reactor (SBR). Biochem. Eng. J. 2007, 34, 248–255. [Google Scholar] [CrossRef]
- Zeng, W.; Peng, Y.; Wang, S. Process evaluation of an alternating aerobic-anoxic process applied in a sequencing batch reactor for nitrogen removal. Front. Environ. Sci. Eng. China 2007, 1, 28–32. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Hassan, G.K.; Maktabifard, M.; Grubba, D.; Majtacz, J.; Mąkinia, J. Integrating conventional nitrogen removal with anammox in wastewater treatment systems: Microbial metabolism, sustainability and challenges. Environ. Res. 2022, 215, 114432. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Janyasupab, P.; Jampeetong, A. Performance of Porous Substrates for Domestic Wastewater Treatment under Prolonged Hydraulic Retention Time. Appl. Environ. Res. 2022, 44, 45–58. [Google Scholar] [CrossRef]
- Ren, L.; Xu, L.; Zhang, Y.; Pan, W.; Yin, S.; Zhou, Y.; Yu, L.; Chen, Y.; An, S. Effects of Connection Mode and Hydraulic Retention Time on Wastewater Pollutants Removal in Constructed Wetland Microcosms. CLEAN Soil Air Water 2015, 43, 1574–1581. [Google Scholar] [CrossRef]
- Singh, N.K.; Bhatia, A.; Kazmi, A.A. Effect of intermittent aeration strategies on treatment performance and microbial community of an IFAS reactor treating municipal wastewater. Environ. Technol. 2017, 38, 2866–2876. [Google Scholar] [CrossRef]
- Paśmionka, I.B.; Bulski, K.; Herbut, P.; Boligłowa, E.; Vieira, F.M.C.; Bonassa, G.; Bortoli, M.; de Prá, M.C. Toxic Effect of Ammonium Nitrogen on the Nitrification Process and Acclimatisation of Nitrifying Bacteria to High Concentrations of NH4-N in Wastewater. Energies 2021, 14, 5329. [Google Scholar] [CrossRef]
- Spataru, P. Influence of organic ammonium derivatives on the equilibria between NH4+, NO2− and NO3− ions in the Nistru River water. Sci. Rep. 2022, 12, 13505. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, H.; Zhang, F.; Zhou, Y.; Wang, J.; Liu, Z.; Su, Y. Analysis of rapid culture of high-efficiency nitrifying bacteria and immobilized filler application for the treatment of municipal wastewater. RSC Adv. 2020, 10, 19240–19246. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yan, Q.; Wang, C.; Luo, C.; Zhou, N.; Jian, C. Treatment of Ammonia Nitrogen Wastewater in Low Concentration by Two-Stage Ozonization. Int. J. Environ. Res. Public Health 2015, 12, 11975–11987. [Google Scholar] [CrossRef]
- Naghipour, D.; Rouhbakhsh, E.; Jaafari, J. Application of the biological reactor with fixed media (IFAS) for removal of organic matter and nutrients in small communities. Int. J. Environ. Anal. Chem. 2022, 102, 5811–5821. [Google Scholar] [CrossRef]
- Martin, C.L.; Ii, C.J.C. Traditional Nitrogen Removal Coupled with SND to Meet Advanced WWTP Standards at a Full Scale SBR Wastewater Treatment Facility. J. Water Resour. Prot. 2017, 9, 1169–1183. [Google Scholar] [CrossRef]
- Jimenez, J.; Dursun, D.; Dold, P.; Bratby, J.; Keller, J.; Parker, D. Simultaneous Nitrification-Denitrification to Meet Low Effluent Nitrogen Limits: Modeling, Performance and Reliability. Proc. Water Environ. Fed. 2010, 2010, 2404–2421. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Xu, Y.; Shutes, B.; Yan, B.; Zhou, Q. Effect of Aeration Modes and COD/N Ratios on Organic Matter and Nitrogen Removal in Horizontal Subsurface Flow Constructed Wetland Mesocosms. Water 2018, 10, 1530. [Google Scholar] [CrossRef]
- Paredes, D.; Kuschk, P.; Mbwette, T.S.A.; Stange, F.; Müller, R.A.; Köser, H. New Aspects of Microbial Nitrogen Transformations in the Context of Wastewater Treatment—A Review. Eng. Life Sci. 2007, 7, 13–25. [Google Scholar] [CrossRef]
- Jensen, V.B.; Darby, J.L.; Seidel, C.; Gorman, C. Nitrate in Potable Water Supplies: Alternative Management Strategies. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2203–2286. [Google Scholar] [CrossRef]
- Raboni, M.; Torretta, V.; Viotti, P.; Urbini, G. Pilot Experimentation with Complete Mixing Anoxic Reactors to Improve Sewage Denitrification in Treatment Plants in Small Communities. Sustainability 2013, 6, 112–122. [Google Scholar] [CrossRef]
- Waqas, S.; Harun, N.Y.; Sambudi, N.S.; Abioye, K.J.; Zeeshan, M.H.; Ali, A.; Abdulrahman, A.; Alkhattabi, L.; Alsaadi, A.S. Effect of Operating Parameters on the Performance of Integrated Fixed-Film Activated Sludge for Wastewater Treatment. Membranes 2023, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Guo, Z.; Ma, B.; Wang, F.; You, H.; Mei, J.; Hou, X.; Liang, Z.; Li, Z.; Jin, C. The Influence of Different Operation Conditions on the Treatment of Mariculture Wastewater by the Combined System of Anoxic Filter and Membrane Bioreactor. Membranes 2021, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- Gieseke, A.; Tarre, S.; Green, M.; de Beer, D.; Gieseke, A.; Tarre, S.; Green, M.; de Beer, D. Nitrification in a Biofilm at Low pH Values: Role of In Situ Microenvironments and Acid Tolerance. Appl. Environ. Microbiol. 2006, 72, 4283–4292. [Google Scholar] [CrossRef]
- Saijai, S.; Ando, A.; Inukai, R.; Shinohara, M.; Ogawa, J. Analysis of microbial community and nitrogen transition with enriched nitrifying soil microbes for organic hydroponics. Biosci. Biotechnol. Biochem. 2016, 80, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-Y.; Sheng, G.-P.; Li, X.-Y.; Yu, H.-Q. Operation of a sequencing batch reactor for cultivating autotrophic nitrifying granules. Bioresour. Technol. 2010, 101, 2960–2964. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, L.; Fukuzaki, Y.; Hira, D.; Furukawa, K. High-rate nitrogen removal by the Anammox process with a sufficient inorganic carbon source. Bioresour. Technol. 2010, 101, 9471–9478. [Google Scholar] [CrossRef]
- Hu, D.; Zhou, Z.; Shen, X.; Wei, H.; Jiang, L.-M.; Lv, Y. Effects of alkalinity on membrane bioreactors for reject water treatment: Performance improvement, fouling mitigation and microbial structures. Bioresour. Technol. 2015, 197, 217–226. [Google Scholar] [CrossRef]
- Ji, J.; Peng, Y.; Wang, B.; Li, X.; Zhang, Q. Synergistic Partial-Denitrification, Anammox, and in-situ Fermentation (SPDAF) Process for Advanced Nitrogen Removal from Domestic and Nitrate-Containing Wastewater. Environ. Sci. Technol. 2020, 54, 3702–3713. [Google Scholar] [CrossRef]
- Kampschreur, M.J.; Temmink, H.; Kleerebezem, R.; Jetten, M.S.; van Loosdrecht, M.C. Nitrous oxide emission during wastewater treatment. Water Res. 2009, 43, 4093–4103. [Google Scholar] [CrossRef]
- Blackburne, R.; Yuan, Z.; Keller, J. Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation 2008, 19, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Kim, J.H.; Behera, S.K.; Park, H.S. Influence of dissolved oxygen concentration and aeration time on nitrite accumulation in partial nitrification process. Int. J. Environ. Sci. Technol. 2008, 5, 527–534. [Google Scholar] [CrossRef]
- Park, S.; Bae, W. Modeling kinetics of ammonium oxidation and nitrite oxidation under simultaneous inhibition by free ammonia and free nitrous acid. Process. Biochem. 2009, 44, 631–640. [Google Scholar] [CrossRef]
- Hartop, K.; Sullivan, M.; Giannopoulos, G.; Gates, A.; Bond, P.; Yuan, Z.; Clarke, T.; Rowley, G.; Richardson, D. The metabolic impact of extracellular nitrite on aerobic metabolism of Paracoccus denitrificans. Water Res. 2017, 113, 207–214. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, C.; Rong, H.; Zheng, G.; Zhao, L. The effect of dissolved oxygen concentration (DO) on oxygen diffusion and bacterial community structure in moving bed sequencing batch reactor (MBSBR). Water Res. 2017, 108, 86–94. [Google Scholar] [CrossRef]
- Waqas, S.; Harun, N.Y.; Bilad, M.R.; Samsuri, T.; Nordin, N.A.H.M.; Shamsuddin, N.; Nandiyanto, A.B.D.; Huda, N.; Roslan, J. Response Surface Methodology for Optimization of Rotating Biological Contactor Combined with External Membrane Filtration for Wastewater Treatment. Membranes 2022, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, X.; Han, Y.; Liu, J.; Ren, J.; Wang, Y.; Guo, Y. Enhancing nitrogen removal in an Orbal oxidation ditch by optimization of oxygen supply: Practice in a full-scale municipal wastewater treatment plant. Bioprocess Biosyst. Eng. 2012, 35, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Q.; Huang, G.; Zhang, L.; Liu, Y. Effect of dissolved oxygen concentration on nitrogen removal and electricity generation in self pH-buffer microbial fuel cell. Int. J. Hydrogen Energy 2020, 45, 34099–34109. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, L.; Wang, J.; Yang, S.; Hou, Z.; Wang, X.C.; Chen, R. Partial-nitritation of low-strength anaerobic effluent: A moderate-high dissolved oxygen concentration facilitates ammonia-oxidizing bacteria disinhibition and nitrite-oxidizing bacteria suppression. Sci. Total. Environ. 2021, 770, 145337. [Google Scholar] [CrossRef]
- Yang, S.; Yang, F.; Fu, Z.; Lei, R. Comparison between a moving bed membrane bioreactor and a conventional membrane bioreactor on organic carbon and nitrogen removal. Bioresour. Technol. 2009, 100, 2369–2374. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, R.; Guo, B.; Zhang, L.; Zou, X.; Xia, S.; Liu, Y. Greywater treatment using an oxygen-based membrane biofilm reactor: Formation of dynamic multifunctional biofilm for organics and nitrogen removal. Chem. Eng. J. 2020, 386, 123989. [Google Scholar] [CrossRef]
- Fu, B.; Liao, X.; Ding, L.; Ren, H. Characterization of microbial community in an aerobic moving bed biofilm reactor applied for simultaneous nitrification and denitrification. World J. Microbiol. Biotechnol. 2010, 26, 1981–1990. [Google Scholar] [CrossRef]
- Meng, F.; Wang, Y.; Huang, L.; Li, J.; Jiang, F.; Li, S.; Chen, G. A novel nonwoven hybrid bioreactor (NWHBR) for enhancing simultaneous nitrification and denitrification. Biotechnol. Bioeng. 2013, 110, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Almstrand, R.; Persson, F.; Daims, H.; Ekenberg, M.; Christensson, M.; Wilén, B.-M.; Sörensson, F.; Hermansson, M. Three-Dimensional Stratification of Bacterial Biofilm Populations in a Moving Bed Biofilm Reactor for Nitritation-Anammox. Int. J. Mol. Sci. 2014, 15, 2191–2206. [Google Scholar] [CrossRef] [PubMed]
- Machat, H.; Boudokhane, C.; Roche, N.; Dhaouadi, H. Effects of C/N Ratio and DO concentration on Carbon and Nitrogen removals in a Hybrid Biological Reactor. Biochem. Eng. J. 2019, 151, 107313. [Google Scholar] [CrossRef]
- Layer, M.; Villodres, M.G.; Hernandez, A.; Reynaert, E.; Morgenroth, E.; Derlon, N. Limited simultaneous nitrification-denitrification (SND) in aerobic granular sludge systems treating municipal wastewater: Mechanisms and practical implications. Water Res. X 2020, 7, 100048. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Pi, S.; Zhu, W.; Wang, X.; Xu, X. Nitrification and aerobic denitrification in solid phase denitrification systems with various biodegradable carriers for ammonium-contaminated water purification. J. Chem. Technol. Biotechnol. 2019, 94, 3569–3577. [Google Scholar] [CrossRef]
- Gu, S.; Liu, L.; Zhuang, X.; Qiu, J.; Zhou, Z. Enhanced Nitrogen Removal in a Pilot-Scale Anoxic/Aerobic (A/O) Process Coupling PE Carrier and Nitrifying Bacteria PE Carrier: Performance and Microbial Shift. Sustainability 2022, 14, 7193. [Google Scholar] [CrossRef]
- Le, T.; Peng, B.; Su, C.; Massoudieh, A.; Torrents, A.; Al-Omari, A.; Murthy, S.; Wett, B.; Chandran, K.; DeBarbadillo, C.; et al. Impact of carbon source and COD/N on the concurrent operation of partial denitrification and anammox. Water Environ. Res. 2019, 91, 185–197. [Google Scholar] [CrossRef]
- Raboni, M.; Torretta, V.; Viotti, P.; Urbini, G. Calculating specific denitrification rates in pre-denitrification by assessing the influence of dissolved oxygen, sludge loading and mixed-liquor recycle. Environ. Technol. 2014, 35, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Aqeel, H.; Liss, S.N. Fate of sloughed biomass in integrated fixed-film systems. PLoS ONE 2022, 17, e0262603. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Tseng, S.; Chang, Y. Simultaneous nitrification and denitrification using an autotrophic membrane-immobilized biofilm reactor. Lett. Appl. Microbiol. 2002, 35, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Han, D.-W.; Yun, H.-J.; Kim, D.-J. Autotrophic nitrification and denitrification characteristics of an upflow biological aerated filter. J. Chem. Technol. Biotechnol. 2001, 76, 1112–1116. [Google Scholar] [CrossRef]
- Bao, R.; Yu, S.; Shi, W.; Zhang, X.; Wang, Y. Aerobic granules formation and nutrients removal characteristics in sequencing batch airlift reactor (SBAR) at low temperature. J. Hazard. Mater. 2009, 168, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Li, J.; Wang, R. Nitrogen removal performance and microbial community structure dynamics response to carbon nitrogen ratio in a compact suspended carrier biofilm reactor. Ecol. Eng. 2008, 32, 256–262. [Google Scholar] [CrossRef]
- Sriwiriyarat, T.; Ungkurarate, W.; Fongsatitkul, P.; Chinwetkitvanich, S. Effects of dissolved oxygen on biological nitrogen removal in integrated fixed film activated sludge (IFAS) wastewater treatment process. J. Environ. Sci. Health Part A 2008, 43, 518–527. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Unit | Values |
|---|---|---|
| pH | - | 6.6–7.7 |
| SS | mg/L | 34–80 |
| COD | mgO2/L | 144–432 |
| N-organic | mg/L | 9–15 |
| N-NH4+ | mg/L | 49–90 |
| N-NO2− | mg/L | 0.2–8.0 |
| Alkalinity | mgCaCO3/L | 160–480 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, Q.C.; Phan, N.N.M.; Nguyen, T.V.; Yang, C.-C.; Chen, K.-F.; Tsai, Y.-P. Integrating Suspended Sludge and Fixed Film into a Biological Wastewater Treatment System to Enhance Nitrogen Removal. Processes 2024, 12, 2131. https://doi.org/10.3390/pr12102131
Bui QC, Phan NNM, Nguyen TV, Yang C-C, Chen K-F, Tsai Y-P. Integrating Suspended Sludge and Fixed Film into a Biological Wastewater Treatment System to Enhance Nitrogen Removal. Processes. 2024; 12(10):2131. https://doi.org/10.3390/pr12102131
Chicago/Turabian StyleBui, Quang Chi, Nguyen Nguyet Minh Phan, Trung Viet Nguyen, Chih-Chi Yang, Ku-Fan Chen, and Yung-Pin Tsai. 2024. "Integrating Suspended Sludge and Fixed Film into a Biological Wastewater Treatment System to Enhance Nitrogen Removal" Processes 12, no. 10: 2131. https://doi.org/10.3390/pr12102131






