Aerated Concrete, Based on the Ash of Thermal Power Plants, Nanostructured with Water-Soluble Fullerenols
Abstract
:1. Introduction
2. Materials and Methods of Material Synthesis
2.1. Concrete M400
2.2. Ash and Slag Waste—ASW
2.3. Fullerenol-m
2.4. Aerated Concrete
- -
- cement/slag waste/lime 1.0/1.0/0.0 (in grams 2200/2200/0);
- -
- cement/slag waste/lime 1.0/0.9/0.1 (in grams 2200/1980/220);
- -
- cement/slag waste/lime 1.0/0.8/0.2 (in grams 2200/1760/440).
2.5. Testing and Research Methods
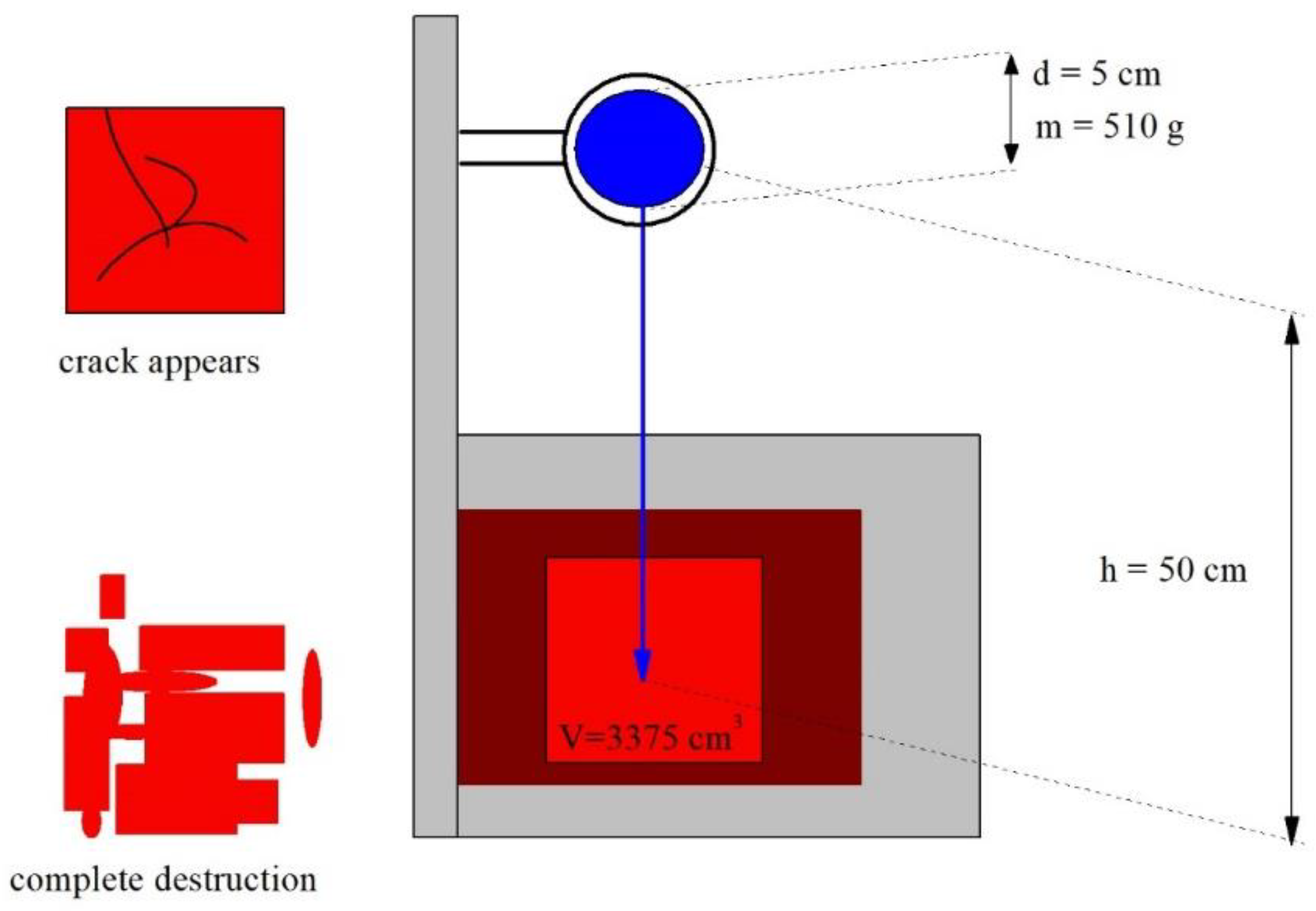
2.5.1. Specific Impact Strength
2.5.2. Compressive Strength
2.5.3. Density
2.5.4. Humidity
2.5.5. Thermal Conductivity
3. Results
4. Discussion
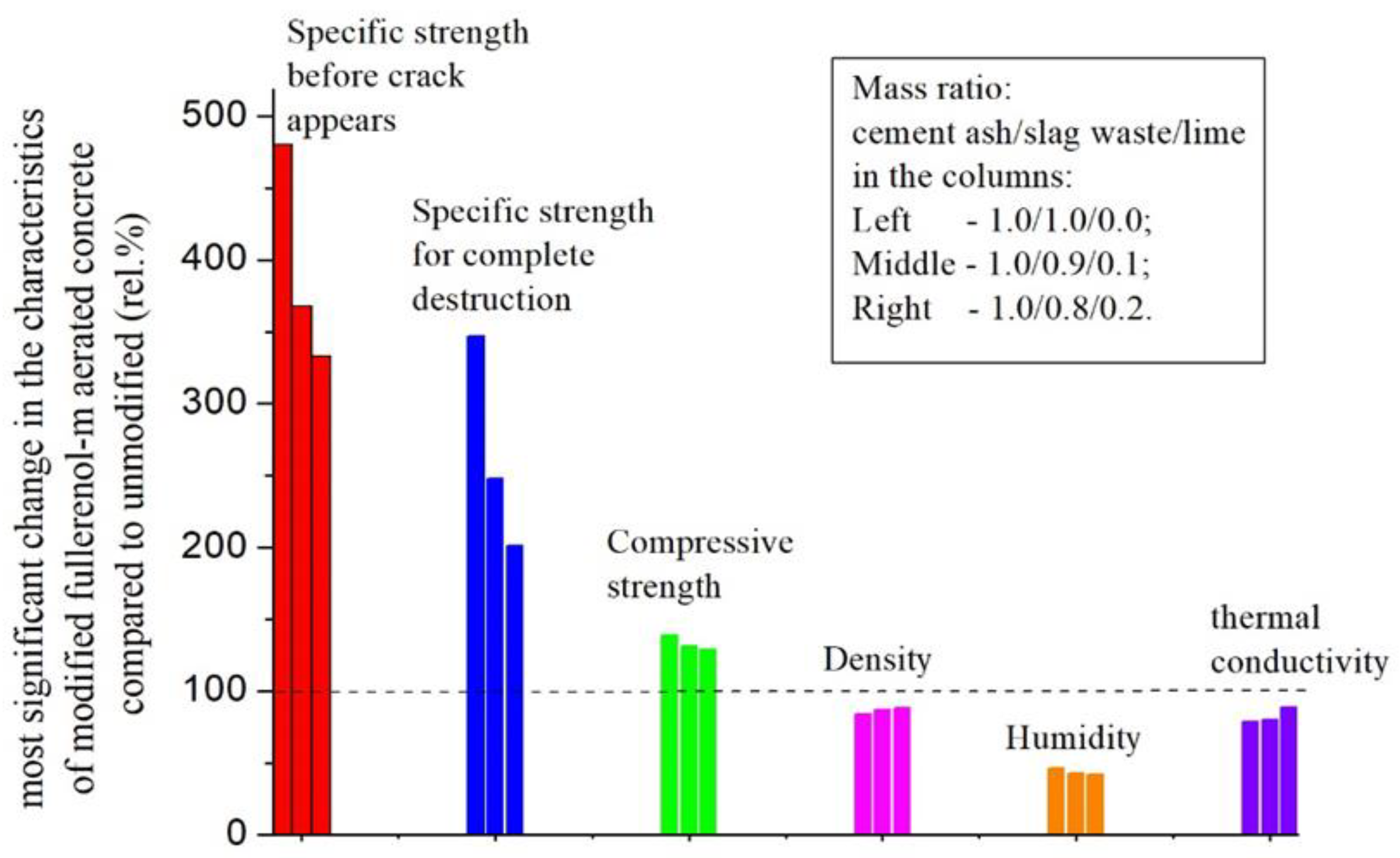
- The specific impact strength increases ≈3 ÷ 5 times (crack appears, maximum effect);
- The specific impact strength increases ≈2 ÷ 3 times (complete destruction);
- The compressive strength increases by ≈20 ÷ 30 rel.%;
- The density decreases by ≈12 ÷ 16 rel.%;
- The humidity decreases by ≈55 ÷ 60 rel.%;
- The thermal conductivity decreases by ≈10 ÷ 20 rel.%.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sabitov, Y.Y.; Dyussembinov, D.S.; Zhumagulova, A.A.; Bazarbayev, D.O.; Lukpanov, R.E. Composite non-autoclaved aerated concrete based on an emulsion. Mag. Civ. Eng. 2021, 106, 10605. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Ou, J. Abrasion resistance of concrete containing nano-particles for pavement. Wear 2006, 260, 1262–1266. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J.; Alnuaimi, N.A. Recent Advancements in the Nanomaterial Application in Concrete and Its Ecological Impact. Materials 2021, 14, 6387. [Google Scholar] [CrossRef]
- Yadav, S.; Saleem, H.; Ibrar, I.; Naji, O.; Hawari, A.A.; Alanezi, A.A.; Zaidi, S.J.; Altaee, A.; Zhou, J. Recent developments in forward osmosis membranes using carbon-based nanomaterials. Desalination 2020, 482, 114375. [Google Scholar] [CrossRef]
- Huseien, G.F. A Review on Concrete Composites Modified with Nanoparticles. J. Compos. Sci. 2023, 7, 67. [Google Scholar] [CrossRef]
- Alhassan, M.; Alkhawaldeh, A.; Betoush, N.; Alkhawaldeh, M.; Huseien, G.F.; Amaireh, L.; Elrefae, A. Life Cycle Assessment of the Sustainability of Alkali-Activated Binders. Biomimetics 2023, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Li, J.; Lu, Z.; Niu, Y. Influence of foaming agent on cement and foam concrete. Constr. Build. Mater. 2021, 280, 122399. [Google Scholar] [CrossRef]
- Gołaszewski, J.; Klemczak, B.; Smolana, A.; Gołaszewska, M.; Cygan, G.; Mankel, C.; Peralta, I.; Röser, F.; Koenders, E.A.B. Effect of Foaming Agent, Binder and Density on the Compressive Strength and Thermal Conductivity of Ultra-Light Foam Concrete. Buildings 2022, 12, 1176. [Google Scholar] [CrossRef]
- Al-Saffar, F.; Wong, L.; Paul, S. An Elucidative Review of the Nanomaterial Effect on the Durability and Calcium-Silicate-Hydrate (C-S-H) Gel Development of Concrete. Gels 2023, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, G.; Kerien, J.; Plechanova, T.; Krutikov, V. Nanobewehrung von Schaumbeton. Beton- und Stahl. Стрoительные Материалы 2007, 102, 120–124. [Google Scholar]
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Urkhanova , L.A.; Rosina, V.E. High-Strength Concrete with the Use of Fly Ash and Microsilica. Proceedings of Irkutsk State Technical University. 2011. № 10. С. 97–100. Available online: https://journals.istu.edu/vestnik_irgtu/journals/2011/10 (accessed on 8 July 2024).
- Fediuk, R.S.; Yushin, A.M. The use of fly ash the thermal power plants in the construction. IOP Conf. Ser. Mater. Sci. Eng. 2015, 93, 012070. [Google Scholar] [CrossRef]
- Zolotarev, А.; Lushin, A.; Charykov, A.; Semenov, K.; Namazbaev, V.; Keskinov, V.; Kritchenkov, A. Impact Resistance of Cement and Gypsum Plaster Nanomodified by Water-Soluble Fullerenols. Ind. Eng. Chem. Res. 2013, 52, 14583–14591. [Google Scholar] [CrossRef]
- Podolsky, N.E.; Lelet, M.I.; Ageev, S.V.; Petrov, A.V.; Mazur, A.S.; Iamalova, N.R.; Zakusilo, D.N.; Charykov, N.A.; Vasina, L.V.; Semenov, K.N.; et al. Thermodynamic properties of the C70(OH)12 fullerenol in the temperature range T = 9.2 K to 304.5 K. J. Chem. Thermodyn. 2020, 144, 106029. [Google Scholar] [CrossRef]
- Sharoyko, V.V.; Ageev, S.V.; Meshcheriakov, A.A.; Akentiev, A.V.; Noskov, B.A.; Rakipov, I.T.; Charykov, N.A.; Kulenova, N.A.; Shaimardanova, B.K.; Podolsky, N.E.; et al. Physicochemical study of water-soluble C60(OH)24 fullerenol. J. Mol. Liq. 2020, 311, 113360–113411. [Google Scholar] [CrossRef]
- Semenov, K.N.; Charykov, N.A.; Keskinov, V.A.; Letenko, D.G.; Nikitin, V.A.; Namazbaev, V.I. Synthesis and identification of mixed fullerenol, obtained by the method of the direct oxidation of fullerene soot. Russ. J. Phys.Chem. 2011, 65, 1108–1115. [Google Scholar]
- Kratschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A New Form of Carbon; Nature: London, UK, 1990; Volume 347, pp. 354–358. [Google Scholar]
- Smalley, R.E.; Haufler, R.E. Electric arc Process for Making Fullerenes. United. States Patent № 5227038, 13 July 1993. [Google Scholar]
- Gruzinskaya, E.A.; Keskinov, V.A.; Keskinova, M.V.; Semenov, K.N.; Charykov, N.A. Fullerenovaya Sazha Elektrodugovogo Sinteza. Nanosistemy Fiz. Khimiya Mat. 2012, 3, 83–90. (In Russia) [Google Scholar]
- Hasanshin, I.Y.; Popkova, O.S. Synthesis of fullerene soot by plasma enhanced chemical vapour deposition in impulsing discharge at atmospheric pressure from liquid hydrocarbon. Sci. J. Kuban State Agrar. Univ. 2012, 80, 1–13. [Google Scholar]
- Abduguev, R.M.; Alekhin, O.S.; Gerasimov, V.I.; Losev, G.M.; Nekrasov, K.V.; Nikonov, Y.A.; Charykov, N.A. Device for Producing a Fullerene-Containing Black // WO2005087662 (A1), МПК C01B31/02, № PCT/RU2005/000119; published 22.09.2005.
- Abduguevб, R.M.; Alekhin, O.S.; Gerasimov, V.I.; Losev, G.M.; Nekrasov, K.V.; Nikonov, Y.A.; Soroka, A.I.; Charykov, N.A. Method for Production a Fullerene-Containing Black // WO2005070826 (A1), МПК C01B31/02, C09C1/48, № PCT/RU2005/000025; published 04.08.2005.
- Federal Agency for Technical Regulation and Metrology of the Russian Federation Certificate of Approval of the Type of Measuring Instruments. Federal Agency for Technical Regulation of Metrology. Russia. Chelyabinsk. 2017.RU.C.28.059.A. N 45609. 49 P.
- GOST 25485-89; Cellular Concretes. Technical Conditions. State Construction Committee of the USSR: Moscow, Russia, 1990; 36p.
- Limited Liability Company "Special Design Bureau Stroypribor". Humidity Meter Electronic Moisture Meter-MG4-U; Federal Agency for Metrology: Chelyabinsk, Russia, 2015; 36p. (In Russia) [Google Scholar]
- GOST 30515; The Interstate Council for Standardization, Metrology and Certification. CEMENTS General Technical Conditions: Moscow, Russia, 2013; 37p. (In Russia)
- Limited Liability Company "Special Design Bureau Stroypribor". Thermal Conductivity Meter ITP-MG4; Federal Agency for Metrology: Chelyabinsk, Russia, 2020; 39p. (In Russia) [Google Scholar]
- Brooks, J. Elasticity, shrinkage, creep and thermal movement. In Advanced Concrete Technology; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
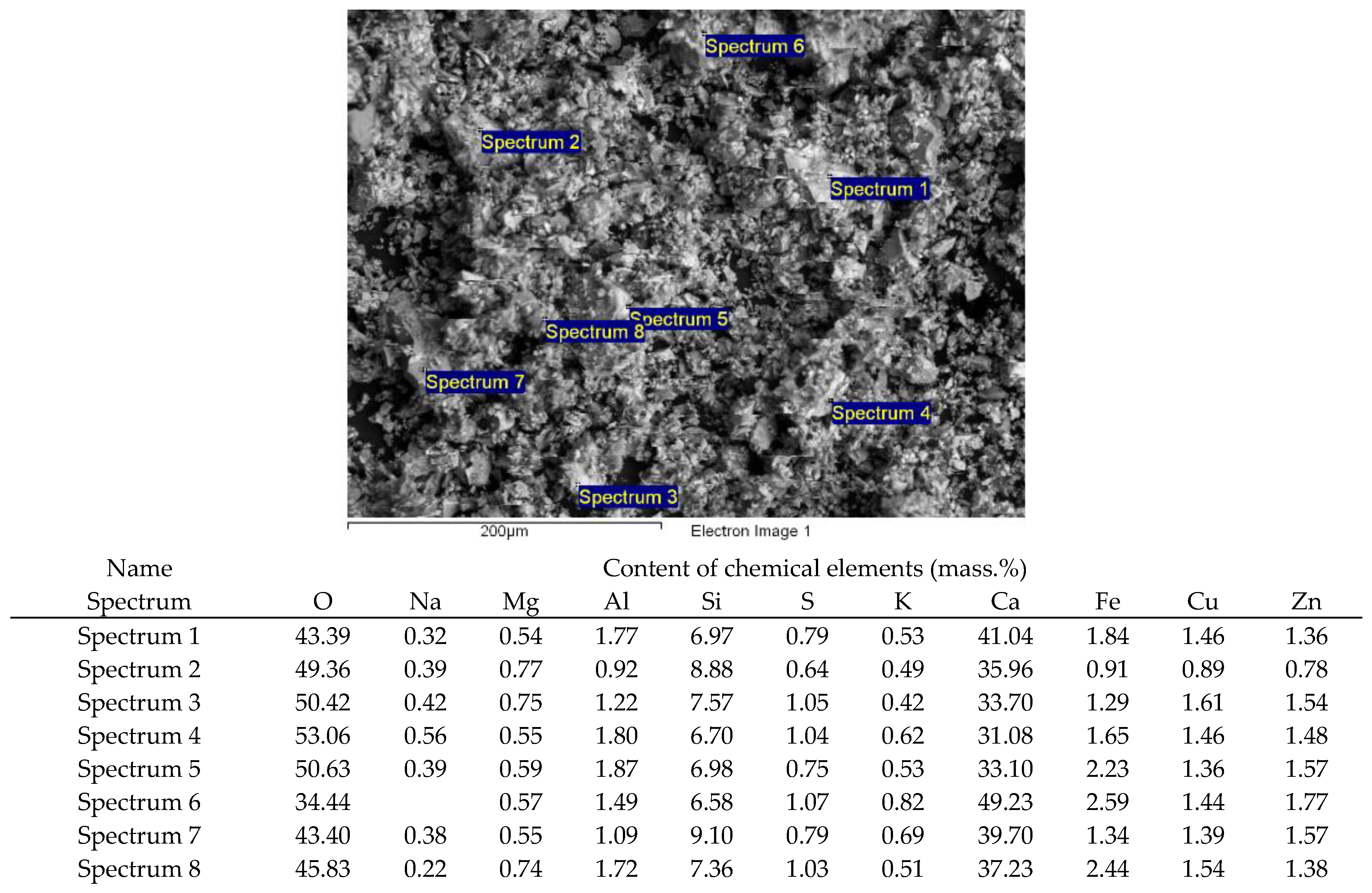

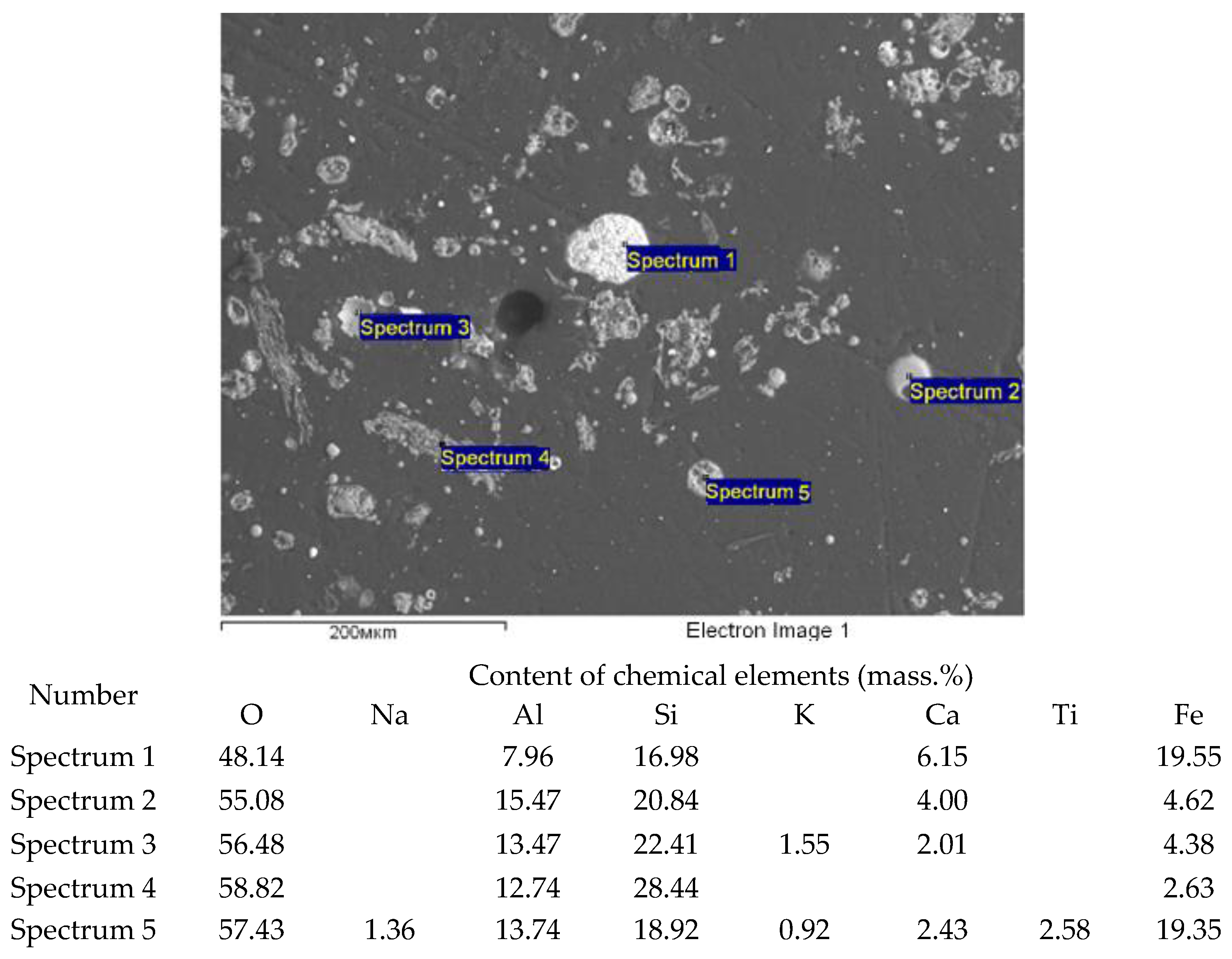








| Name of Product | Content of Oxides (Mass. %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O + K2O | Others | ||
| Cement M400 | 23.74 | 4.67 | 3.68 | 64.82 | 1.63 | 0.21 | 0.87 | 0.38 | |
| Name of Product | Content of Phases (Mass. %) | ||||||||
| 3CaO·SiO2 | 2CaO·SiO2 | 3CaO·Al2O3 | 4CaO·Al2O3·Fe2O3 | CaO | |||||
| Cement M400 | 58.4 | 22.8 | 5.8 | 11.7 | 0.6 | ||||
| Name of Product | Content of Oxides (Mass.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | Na2O + K2O | SO3 | Others | |
| ASW | 47.08 | 33.52 | 4.99 | 2.94 | 1.27 | 0.92 | 2.15 | 0.78 | 5.35 |
| Stage Number | Synthesis Stage | Stage Characteristics |
|---|---|---|
| 1. | Reaction for fullerenol-m preparation | |
| 1.1. | Fullerene soot | Plasma-arc erosion of graphite rods in He atmosphere (method W. Kratschmer [18,19,20,21,22], type of construction [23]). , C76 + C78 + C84 + … ≈ |
| 1.2. | water solution | , 99.5 mass.% |
| 1.3 | Interphase catalyst water solution | = 98 mass.% |
| 2. | Reactive mixing, conducting reaction process | = 4 Hz); time of reaction t = 7 days. |
| 3. | Filtration of reactive solution | ) |
| 4. | Evaporation of solution | |
| 5. | Acidification of solution | ; standing time = 3 h |
| 6. | Precipitation of target product with methanol | 50 cm3. Sediment extraction by filtration (blue ribbon) |
| 7. | Water–methanol recrystallization | |
| 8. | Soft drying of target product | t = 5 h |
| Method of Physical–Chemical Analysis | Results of Analysis |
|---|---|
| C/H/N Analyzer 5E-CHN2200 | |
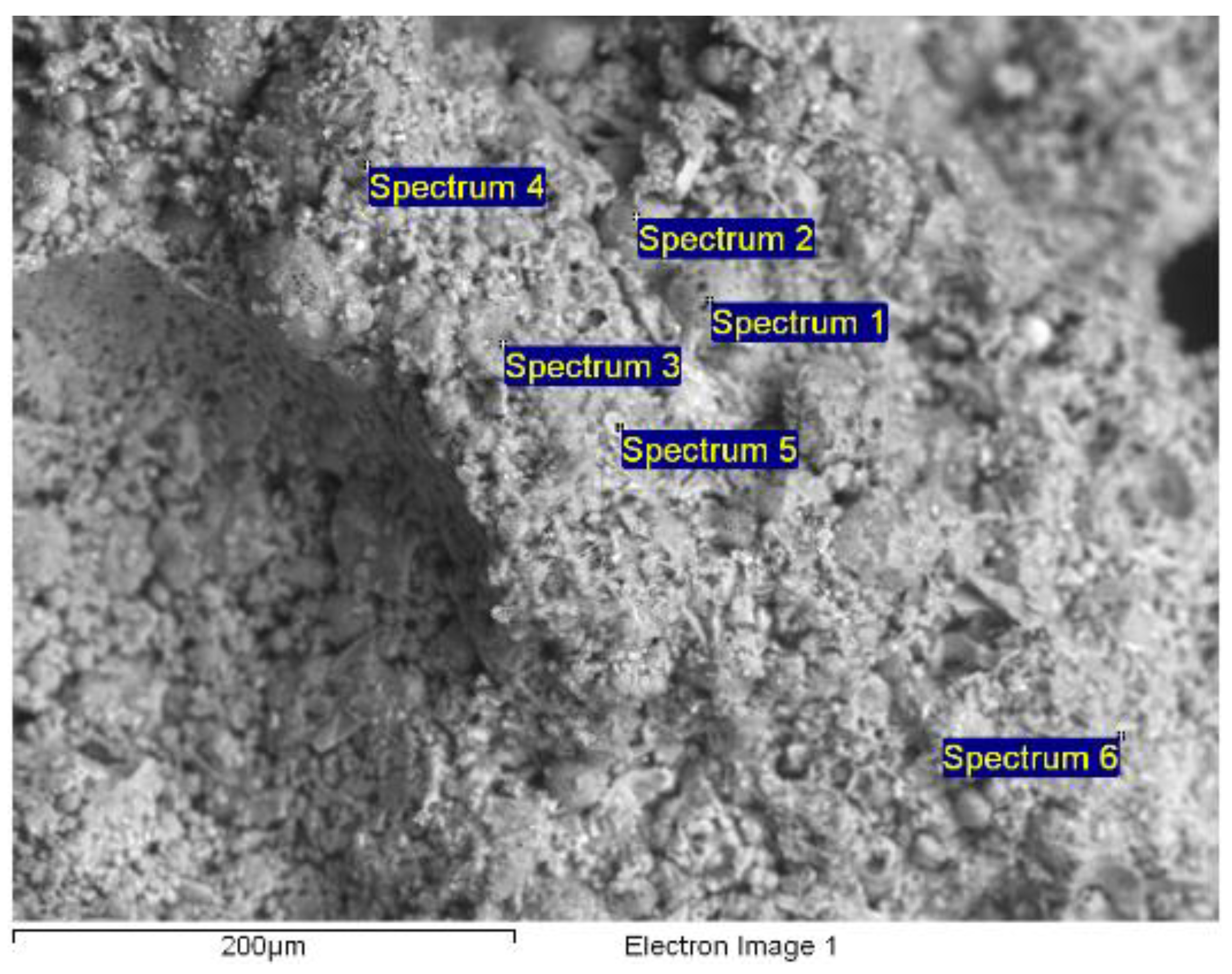
| Electronic microscopy, VEGA-3 TESCAN (XRF Analyzer) | Content in mass % (C = 65, H = 2 (difference), O = 31, Na = 2). |
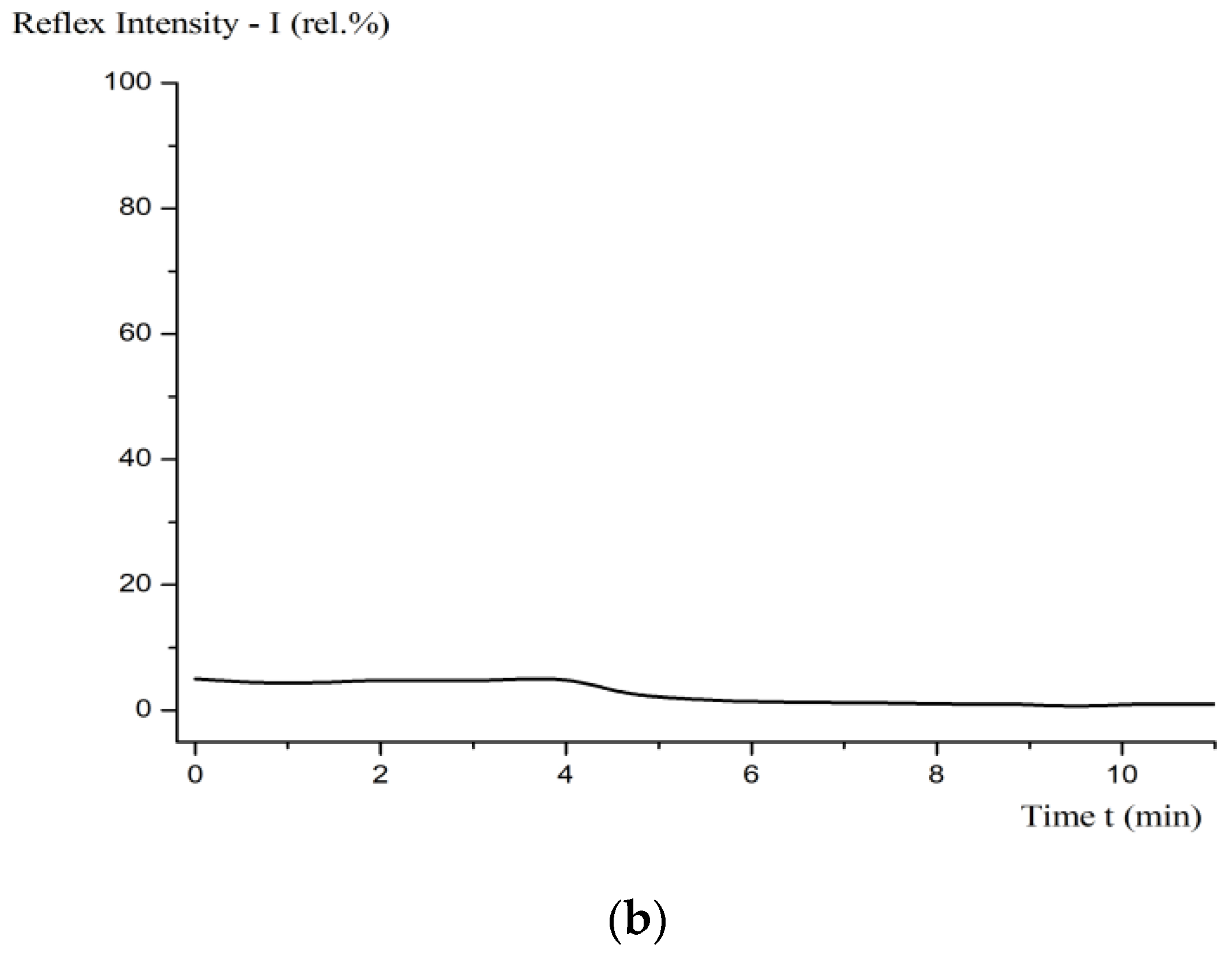
| Optical microscopy, Min-5 | (Figure 5) |
| Mass spectrometry, Mibcrotof (Bruker), ionization–electronic impact | , . Reflexes at values (M/Z = 925–1163 a.e., single-charged anions) (Figure 8) |
| ; ; 1597, 1440, 880, 714, 697 etc cm−1; v—valence oscillations, δ—deformation oscillations (Figure 6) | |
| Electronic spectrophotometry, SPECORD M-32; λ = 200–1110 nm; standard—water | Absence of adsorption peaks. Booger–Lambert–Beer light absorption law (optical way l = 1 cm; wavelength = 330 cm): (Figure 7) |
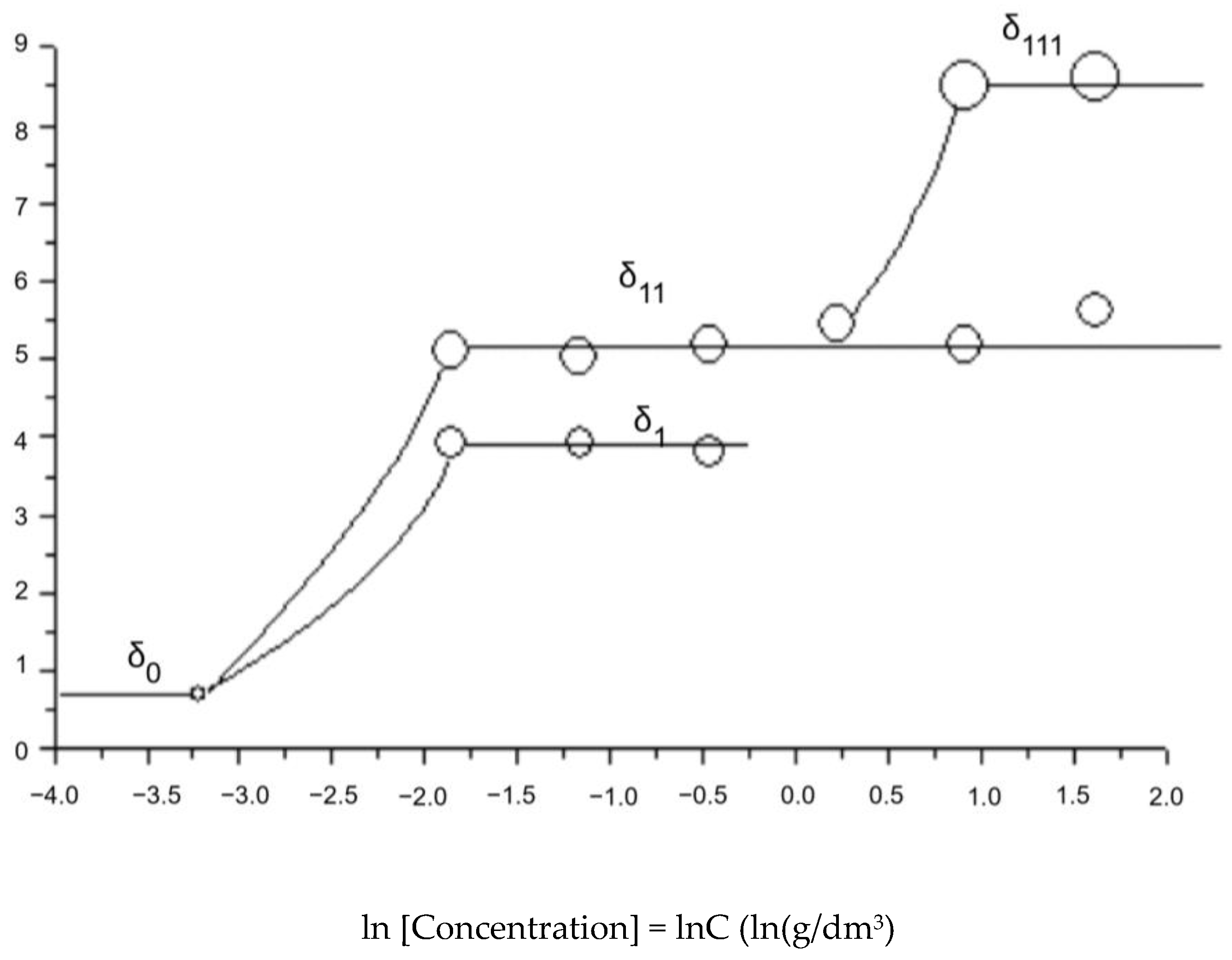
| Dynamic light scattering, Malvern Zetasizer Nano ZS90 apparatus | Diameters of different orders of spherical associates: 0-order, (Figure 11) |
| Solubility in water, method of isotherm saturation in ampoules; time of saturation = 8 h; magnetic stirrer | ; ; ; . |
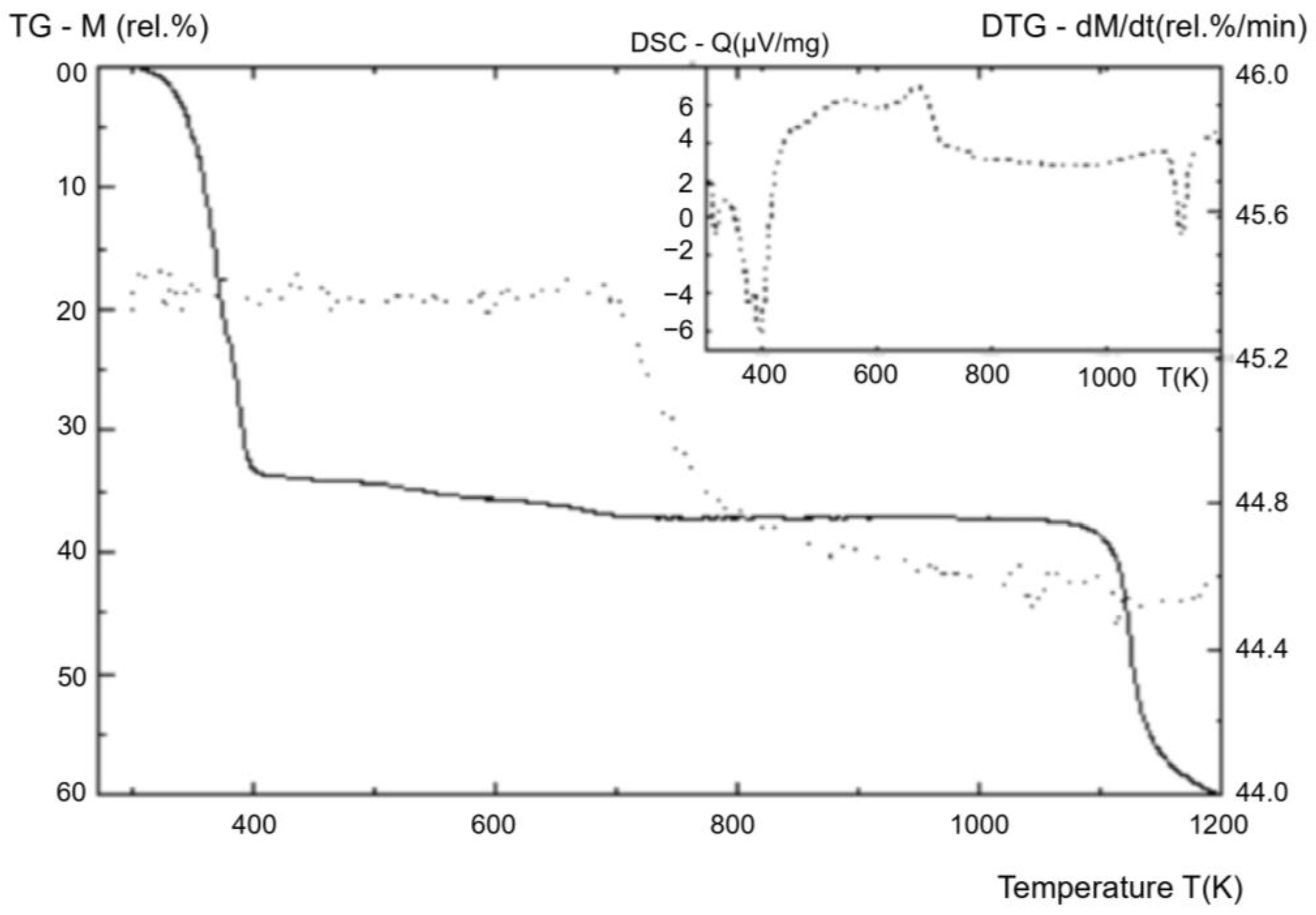
| Complex thermal analysis, Shanghai Jiahang Instruments Co., Ltd.; air atmosphere, normal pressure, temperature range | , (Figure 9) |
| ; NMR Bruker; standard—TMS | |
| ; HRK 9000 A | = 1.3388. |
| High-performance liquid-phase chromatography; HPLC system, Agilent 1200; column, Agilent Zorbax SB-C18 (4.6 mm × 150 mm, dimension of particles, 5 μm); eluent, water–acetonitrile (1/20); detection, spectrophotometry at | mass. % (Figure 10). |
| Element | Content (Mass.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Na | Mg | Al | Si | P | S | K | Ca | Ti | Fe | |
| Max | 64 | 1.5 | 1.8 | 13 | 18 | 0.6 | 0.5 | 1.2 | 28 | 0.6 | 2.3 |
| Min | 56 | 0.6 | 0.3 | 2.4 | 4.9 | 0.6 | 0.2 | 0.3 | 5.3 | 0.2 | 1.1 |
| Mass Ratio: Cement/ ASW/ Quicklime | Fullerenol Content in Sealing Water of Aerated Concrete (Mass.%) | Standard Deviation Limits (Min–Max) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0000 | 0.0075 | 0.0100 | 0.0125 | 0.0150 | 0.0200 | 0.0250 | 0.0300 | ||
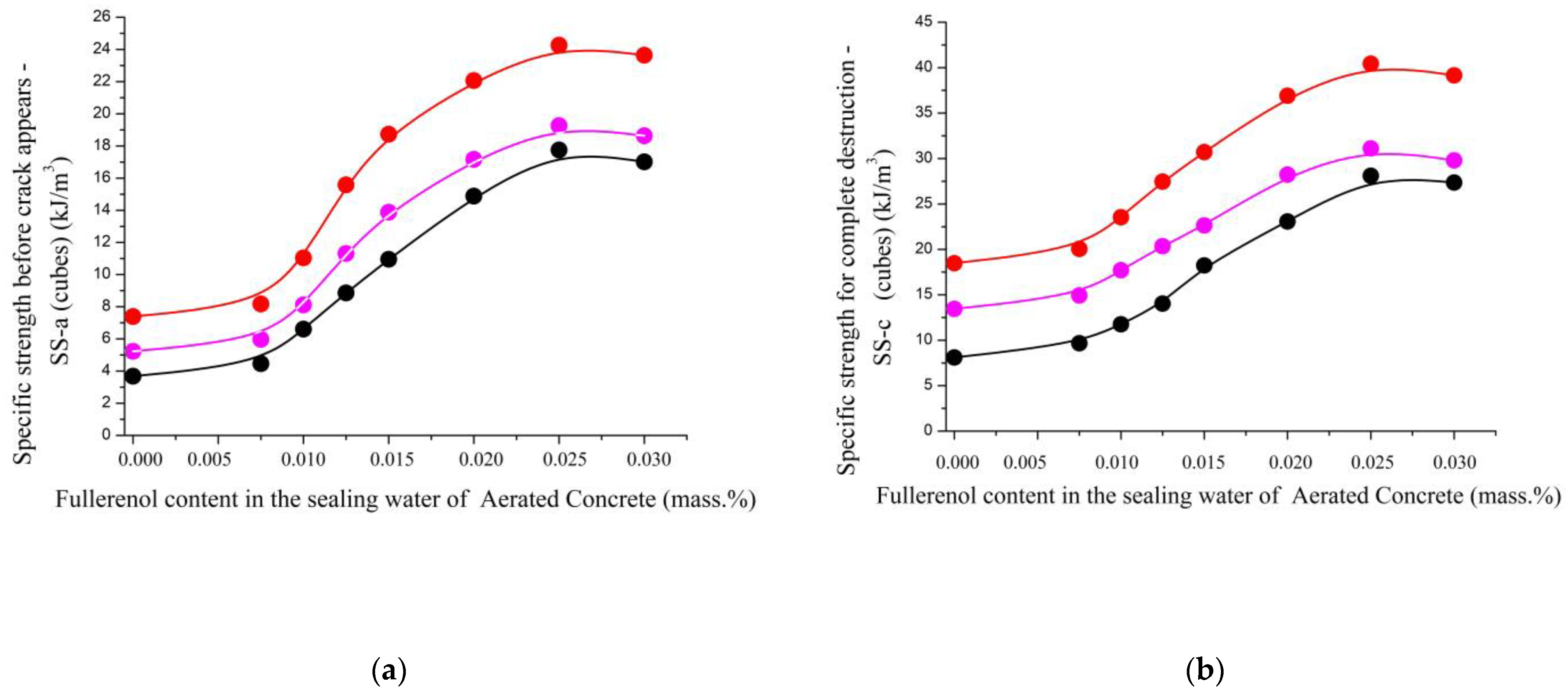
| Specific strength before crack appears—SS-a (cubes) (kJ/m3) | |||||||||
| 1.0/1.0/0.0 | 3.68 | 4.46 | 6.61 | 8.86 | 10.95 | 14.88 | 17.75 | 17.01 | 0.33–0.56 |
| 1.0/0.9/0.1 | 5.23 | 5.97 | 8.11 | 11.31 | 13.88 | 17.16 | 19.26 | 18.63 | 0.36–0.48 |
| 1.0/0.8/0.2 | 7.39 | 8.17 | 11.04 | 15.58 | 18.73 | 22.05 | 24.26 | 23.64 | 0.34–0.49 |
| Specific strength for complete destruction—SS-c (cubes) (kJ/m3) | |||||||||
| 1.0/1.0/0.0 | 8.10 | 9.67 | 11.75 | 14.03 | 18.24 | 23.07 | 28.10 | 27.36 | 0.39–0.57 |
| 1.0/0.9/0.1 | 13.45 | 14.92 | 17.70 | 20.36 | 22.64 | 28.20 | 31.11 | 29.80 | 0.38–0.61 |
| 1.0/0.8/0.2 | 18.47 | 20.05 | 23.54 | 27.46 | 30.71 | 36.90 | 40.44 | 39.15 | 0.31–0.58 |
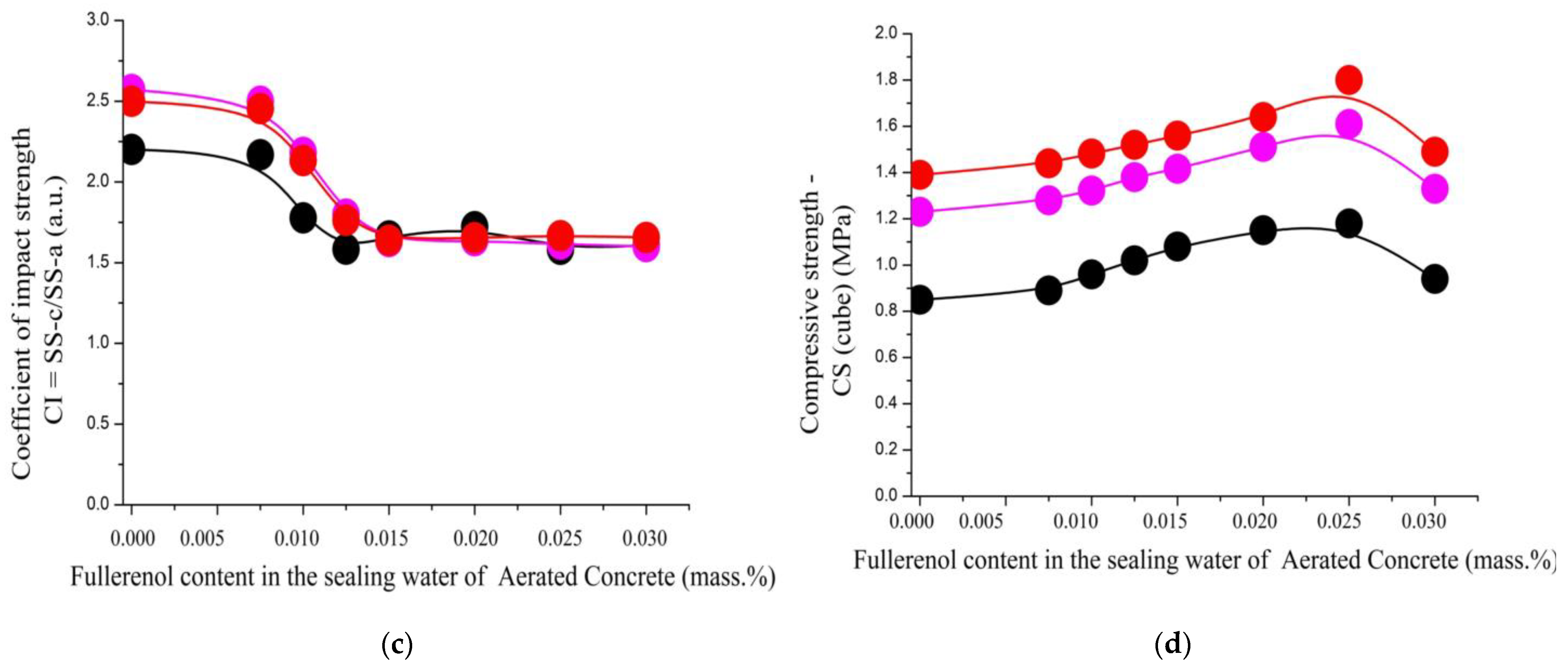
| Coefficient of impact strength CI = SS-c/SS-a (a.u.) | |||||||||
| 1.0/1.0/0.0 | 2.2 | 2.2 | 1.8 | 1.6 | 1.7 | 1.7 | 1.6 | 1.6 | 0.03–0.20 |
| 1.0/0.9/0.1 | 2.6 | 2.5 | 2.2 | 1.8 | 1.6 | 1.6 | 1.6 | 1.6 | 0.06–0.2 |
| 1.0/0.8/0.2 | 2.5 | 2.5 | 2.1 | 1.8 | 1.6 | 1.7 | 1.7 | 1.7 | 0.02–0.26 |
| Compressive strength—CS (cube) (MPa) | |||||||||
| 1.0/1.0/0.0 | 0.85 | 0.89 | 0.96 | 1.02 | 1.08 | 1.15 | 1.18 | 0.94 | 0,11–0,15 |
| 1.0/0.9/0.1 | 1.23 | 1.28 | 1.32 | 1.38 | 1.41 | 1.51 | 1.61 | 1.33 | 0.11–0.14 |
| 1.0/0.8/0.2 | 1.39 | 1.44 | 1.48 | 1.56 | 1.52 | 1.64 | 1.80 | 1.49 | 0,10–0,14 |
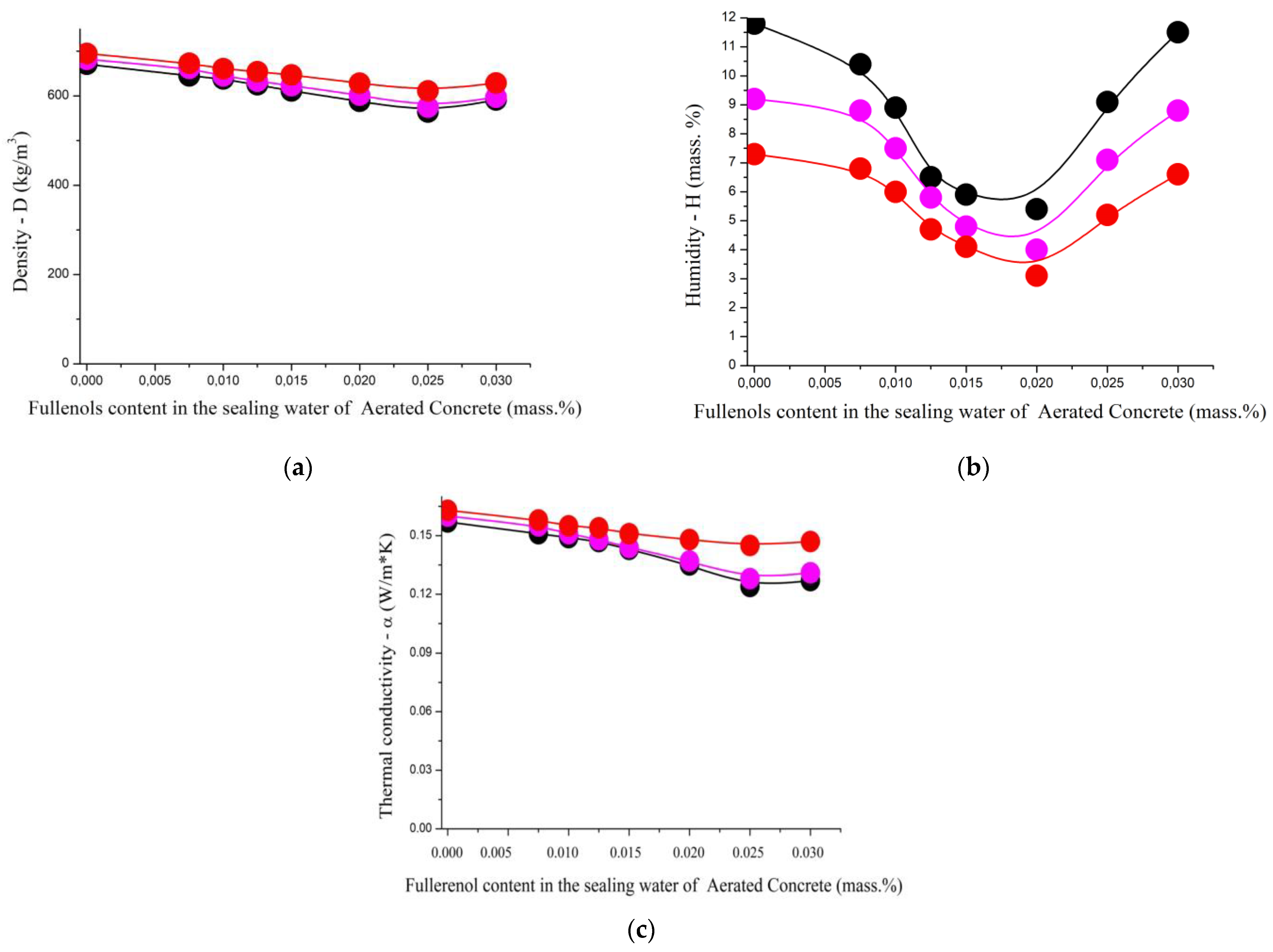
| Density—D (kg/m3) | |||||||||
| 1.0/1.0/0.0 | 671 | 645 | 638 | 625 | 611 | 588 | 564 | 591 | 3.0–6.5 |
| 1.0/0.9/0.1 | 682 | 661 | 645 | 633 | 623 | 602 | 575 | 597 | 3.1–6.0 |
| 1.0/0.8/0.2 | 695 | 673 | 661 | 654 | 647 | 628 | 611 | 629 | 2.6–6.5 |
| Humidity—H (mass. %) | |||||||||
| 1.0/1.0/0.0 | 11.8 | 10.4 | 8.9 | 6.5 | 5.9 | 5.4 | 9.1 | 11.5 | 0.14–0.41 |
| 1.0/0.9/0.1 | 9.2 | 8.8 | 7.5 | 5.8 | 4.8 | 4.0 | 7.1 | 8.8 | 0.21–0.30 |
| 1.0/0.8/0.2 | 7.3 | 6.8 | 6.0 | 4.7 | 4.1 | 3.1 | 5.2 | 6.6 | 0.32–0.47 |
| Thermal conductivity—, W/(m·K) | |||||||||
| 1.0/1.0/0.0 | 0.157 | 0.151 | 0.149 | 0.147 | 0.143 | 0.135 | 0.124 | 0.127 | 0.01–0.02 |
| 1.0/0.9/0.1 | 0.160 | 0.155 | 0.151 | 0.148 | 0.144 | 0.137 | 0.128 | 0.131 | 0.01–0.02 |
| 1.0/0.8/0.2 | 0.163 | 0.158 | 0.155 | 0.154 | 0.151 | 0.148 | 0.145 | 0.147 | 0.01–0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudenko, O.V.; Charykov, N.A.; Kulenova, N.A.; Sadenova, M.A.; Anop, D.K.; Kuldeyev, E. Aerated Concrete, Based on the Ash of Thermal Power Plants, Nanostructured with Water-Soluble Fullerenols. Processes 2024, 12, 2139. https://doi.org/10.3390/pr12102139
Rudenko OV, Charykov NA, Kulenova NA, Sadenova MA, Anop DK, Kuldeyev E. Aerated Concrete, Based on the Ash of Thermal Power Plants, Nanostructured with Water-Soluble Fullerenols. Processes. 2024; 12(10):2139. https://doi.org/10.3390/pr12102139
Chicago/Turabian StyleRudenko, Olga V., Nikolay A. Charykov, Natalya A. Kulenova, Marzhan A. Sadenova, Darya K. Anop, and Erzhan Kuldeyev. 2024. "Aerated Concrete, Based on the Ash of Thermal Power Plants, Nanostructured with Water-Soluble Fullerenols" Processes 12, no. 10: 2139. https://doi.org/10.3390/pr12102139







