Population Composition, Physiology and Ecology of Filamentous Bacteria in Activated Sludge
Abstract
:1. Introduction
2. Detection and Identification of Filamentous Bacteria
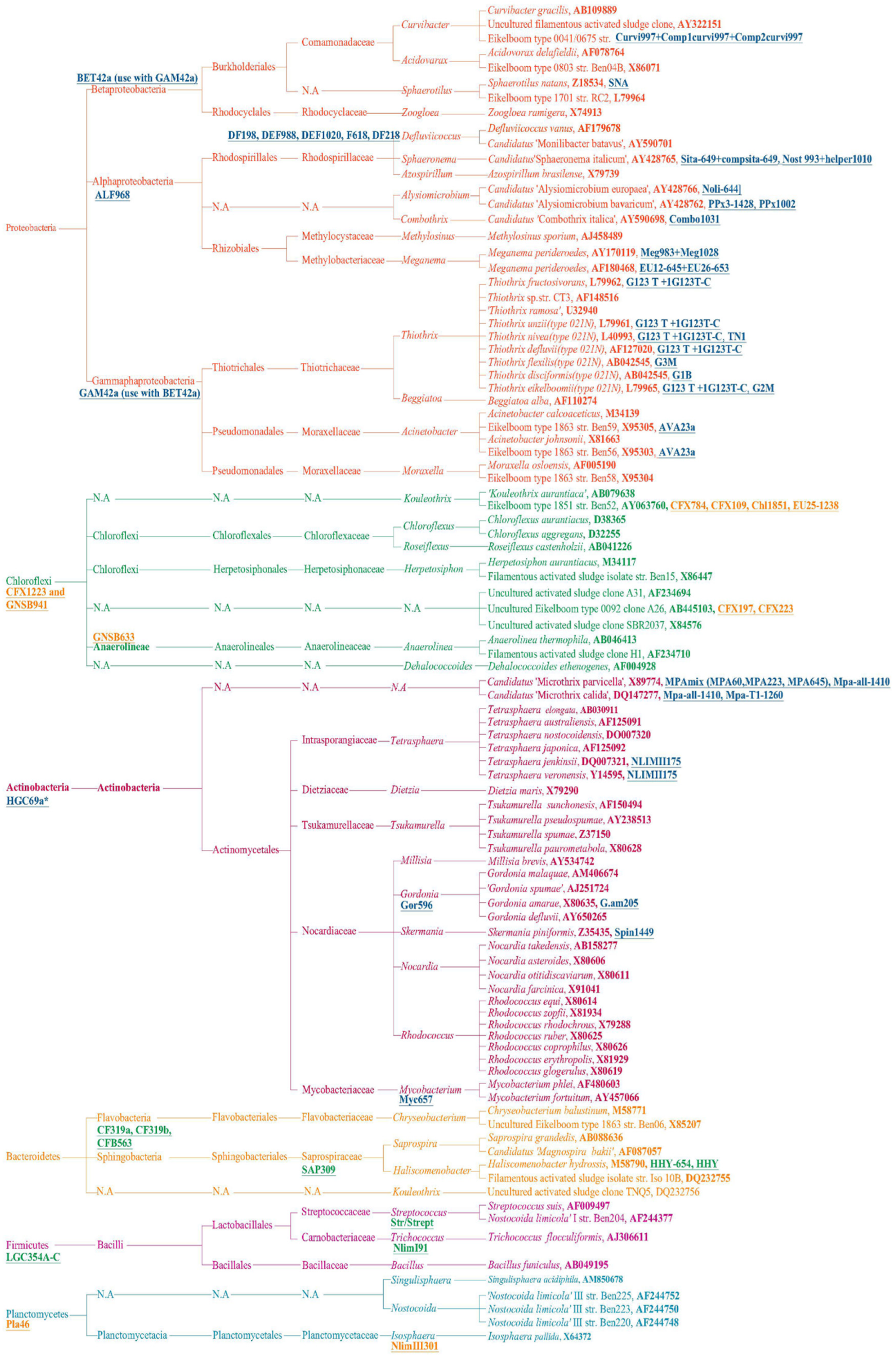
2.1. Fluorescence In Situ Hybridization (FISH) Technology
2.2. High-Throughput Sequencing (HTS) and Filamentous Bacteria Database
- N—the number of annotated filamentous bacterial sequences under different truncation values (100%, 99%, 97%, etc.);
- NT—total number of sample sequences.
3. Total Abundance and Population Structure of Filamentous Bacteria in Activated Sludge
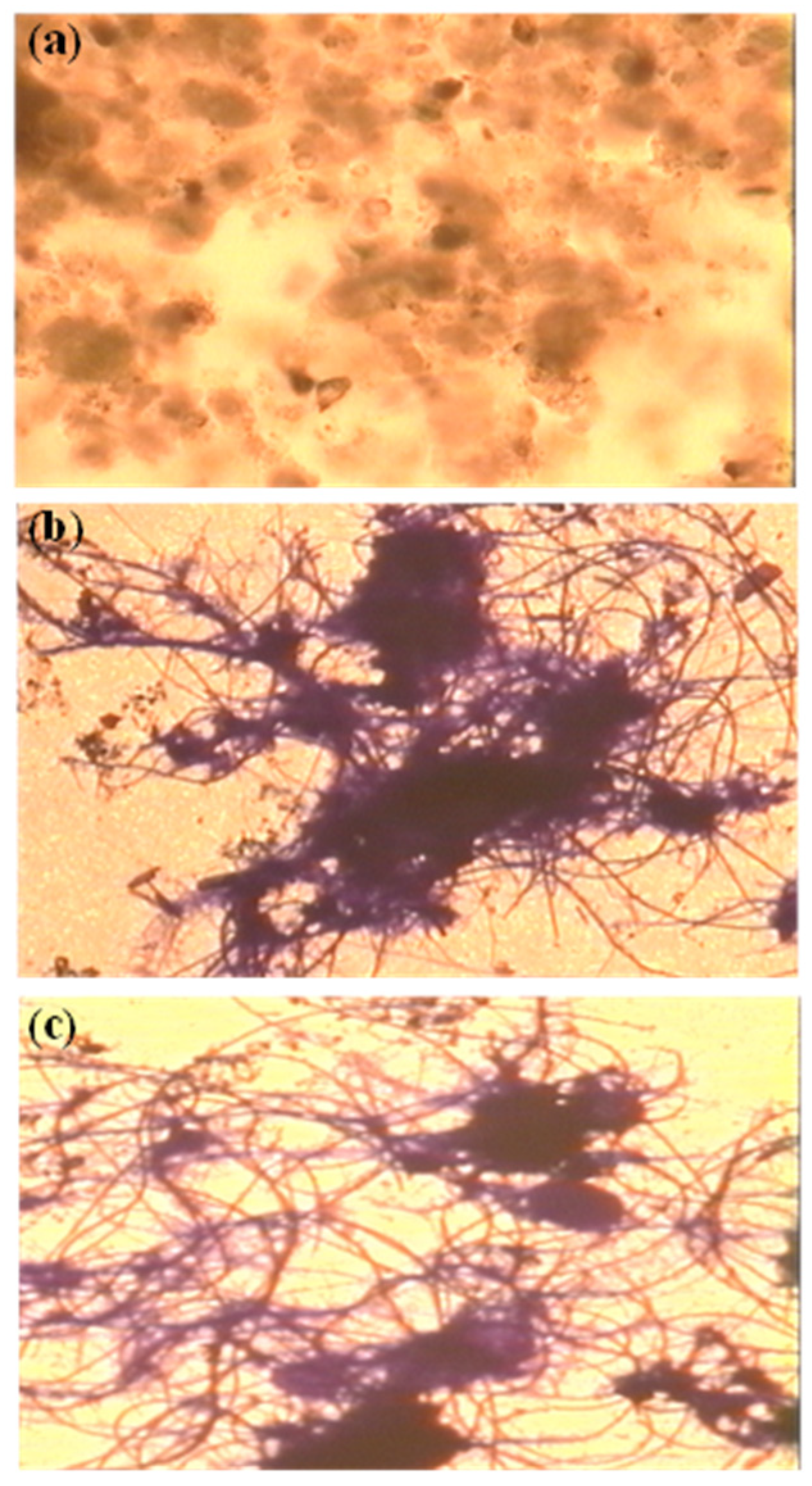
3.1. Filamentous Bacteria in Typical Activated Sludge
3.2. Filamentous Bacteria in Expanded Sludge
4. Physiological and Ecological Analysis of Core Filamentous Bacteria
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.C. The innovative direction of the 100-year activated sludge method. Water Wastewater 2014, 40, 1–3. [Google Scholar]
- Ju, F.; Zhang, T.; Ju, F.; Zhang, T. Advances in meta-omics research on activated sludge microbial Community. Microbiology 2019, 46, 2038–2052. [Google Scholar]
- Ju, F.; Zhang, T. Bacterial assembly and temporal dynamics in activated sludge of a full-scale municipal wastewater treatment plant. ISME J. 2015, 9, 683–695. [Google Scholar] [CrossRef]
- Sezgin, M.; Jenkins, D.; Parker, D.S. A Unified Theory of Filamentous Activated Sludge Bulking. Water Pollut. Control Fed. 1978, 50, 362–381. [Google Scholar]
- Parker, D.S.; Kaufman, W.J.; Jenkins, D. Physical Conditioning of Activated Sludge Floc. J. Water Pollut. Control Fed. 1971, 43, 1817–1833. [Google Scholar]
- Parker, D.S.; Kaufman, W.J.; Jenkins, D. Floc Breakup in Turbulent Flocculation Processes. J. Sanit. Eng. Div. 1972, 98, 79–99. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Y.; Wang, S.; Yang, X.; Yuan, Z. Filamentous and non-filamentous bulking of activated sludge encountered under nutrients limitation or deficiency conditions. Chem. Eng. J. 2014, 255, 453–461. [Google Scholar] [CrossRef]
- Long, T.R.; He, Q.; Lin, G. Research on the Relationships between Filamentous Organisms and Flocculate Structure in Activated Sludge. China Water Wastewater 2000, 16, 5–8. [Google Scholar]
- Bakos, V.; Gyarmati, B.; Csizmadia, P.; Till, S.; Vachoud, L.; Nagy Göde, P.; Tardy, G.M.; Szilágyi, A.; Jobbágy, A.; Wisniewski, C. Viscous and filamentous bulking in activated sludge: Rheological and hydrodynamic modelling based on experimental data. Water Res. 2022, 214, 118–155. [Google Scholar] [CrossRef]
- Gao, C.D.; Zhang, N.; Han, H.; Ren, H.; Li, Y.; Hou, C.Y.; Wang, C.D.; Peng, Y.Z. Microbial Diversity of Filamentous Sludge Bulking at Low Temperature. Environ. Sci. 2020, 41, 3373–3383. [Google Scholar]
- Wu, L.W.; Ning, D.L.; Zhang, B.; Li, Y.; Zhang, P.; Shan, X.Y.; Zhang, Q.T.; Robert Brown, M.; Li, Z.X.; Van Nostrand, J.D.; et al. Author Correction: Global diversity and biogeography of bacterial communities in wastewater tre atment plants. Nat. Microbiol. 2019, 4, 1183–1195. [Google Scholar] [CrossRef] [PubMed]
- Seviour, E.M.; Williams, C.; DeGrey, B.; Soddell, J.A.; Seviour, R.J.; Lindrea, K.C. Studies on filamentous bacteria from Australian activated sludge plants. Water Res. 1994, 28, 2335–2342. [Google Scholar] [CrossRef]
- Graveleau, L.; Cotteux, E.; Duchène, P. Bulking and foaming in France: The 1999–2001 survey. Acta Hydrochim. Et Hydrobiol. 2005, 33, 223–231. [Google Scholar] [CrossRef]
- Wanner, J.; Ruzicková, I.; Jetmarová, P.; Krhutková, O.; Paraniaková, J. A national survey of activated sludge separation problems in The Czech Republic: Filaments, floc characteristics and activated sludge metabolic properties. Water Sci. Technol. 1998, 37, 271–279. [Google Scholar] [CrossRef]
- Sun, C.X.; Zhang, B.; Ning, D.L.; Zhang, Y.; Dai, T.J.; Wu, L.W.; Li, T.L.; Liu, W.; Zhou, J.Z.; Wen, X.H. Seasonal dynamics of the microbial community in two full -scale wastewater treatment plants: Diversity, composition, phylogenetic group based assembly and co-occurrence pattern. Water Res. 2021, 200, 117295. [Google Scholar] [CrossRef]
- Du, B.; Yang, Q.; Li, X.; Yuan, W.; Wang, R. Impacts of long-term exposure to tetracycline and sulfamethoxazole on the sludge granules in an anoxic -aerobic wastewater treatment system. Sci. Total Environ. 2019, 684, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Strom, P.F.; Jenkins, D. Identification and significance of filamentous microorganisms in activated sludge. Water Pollut. Control Fed. 1984, 56, 449–459. [Google Scholar]
- Eikelboom, D.H.; Andreadakis, A.; Andreasen, K. Survey of filamentous populations in nutrient removal plants in four European countries. Water Sci. Technol. 1998, 37, 281–289. [Google Scholar] [CrossRef]
- Di Marzio, W.D. First results from a screening of filamentous organisms present in Buenos Aires’s activated sludge plants. Water Sci. Technol. 2002, 46, 119–122. [Google Scholar] [CrossRef]
- Rothman, M. Operation with biological nutrient removal with stable nitrification and control of filamentous growth. Water Sci. Technol. 1998, 37, 549–554. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, H.; Du, B. Bacteria and bacteriophage communities in bulking and non-bulking activated sludge in full-scale municipal wastewater treatment systems. Biochem. Eng. J. 2017, 119, 101–111. [Google Scholar] [CrossRef]
- Nierychlo, M.; Mcilroy, S.J.; Kucheryavskiy, S.; Jiang, C.J.; Ziegler, A.S.; Kondrotaite, Z.; Stokholm-Bjerregaard, M.A.; Halkjær Nielsen, P. Candidatus Amarolinea and Candidatus Microthrix are mainly responsible for filamentous bulking in Danish municipal wastewater treatment plants. Front. Microbio. 2020, 11, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Y.; Qiao, Y.H.; Zhang, X.X.; Ye, L. Filamentous bacteria-induced sludge bulking can alter antibiotic resistance gene profiles and increase potential risks in wastewater treatment systems. Environ. Int. 2024, 190, 108920. [Google Scholar] [CrossRef]
- Eikelboom, D.H. Filamentous Organisms Observed in Activated Sludge. Water Res. 1975, 9, 365–388. [Google Scholar] [CrossRef]
- Nierychlo, M.; Singleton, C.M.; Petriglieri, F.; Thomsen, L.; Petersen, J.F.; Peces, M.; Kondrotaite, Z.; Dueholm, M.S.; Nielsen, P.H. Low global diversity of Candidatus Microthrix, a troublesome filamentous organism in full-scale WWTPs. Front. Microbio. 2021, 12, 690251. [Google Scholar] [CrossRef] [PubMed]
- De Graaff, D.R.; Van Loosdrecht, M.C.; Pronk, M. Stable granulation of seawater -adapted aerobic granular sludge with filamentous Thiothrix bacteria. Water Res. 2020, 175, 115683. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.S.; Qi, R.; Huang, B.C.; Jin, R.C.; Yang, M. Factors influencing Candidatus Microthrix parvicella growth and specific filamentous bulking control: A review. Chemosphere 2020, 244, 125371. [Google Scholar] [CrossRef]
- Fan, N.S.; Wang, R.F.; Qi, R.; Gao, Y.X.; Rossetti, S.; Tandoi, V.; Yang, M. Control strategy for filamentous sludge bulking: Bench-scale test and full-scale application. Chemosphere 2018, 210, 709–716. [Google Scholar] [CrossRef]
- Onetto, C.A.; Grbin, P.R.; McIlroy, S.J.; Eales, K.L. Genomic insights into the metabolism of ‘Candidatus Defluviicoccus seviourii’, a member of Defluviicoccus cluster III abundant in industrial activated sludge. FEMS Microbiol. Ecol. 2019, 95, 231. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.X.; Zhang, B.; Chen, Z.; Qin, W.T.; Wen, X.H. Sludge retention time affects the microbial community structure: A large-scale sampling of aeration tanks throughout China. Environ. Pollut. 2020, 261, 114140. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.J.; Yang, J.Q.; Pan, J.Y.; Wu, S.Y.; Zou, J.T.; Li, J. Effects of enhanced biological phosphorus removal on rapid control of sludge bulking and fast formation of aerobic granular sludge. Bioresour. Technol. 2024, 402, 130820. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.T.; Wang, D.Q.; Zheng, X.; Hui, J.Y.; Cheng, W.; Zhang, Y.X. Study on specific strategies of controlling or preventing sludge bulking in S2EBPR process. J. Environ. Chem. Eng. 2023, 11, 110363. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Chen, W.K.; Zhao, Y.F.; Gong, S.; Dong, D.Y.; Wang, J.F.; Ren, H.Q. Prediction of Activated Sludge Sedimentation Performance Using Deep Transfer Learning. Environ. Sci. Technol. 2024, 4, 1367–1377. [Google Scholar] [CrossRef]
- Ma, C.Z.; Zeng, W.; Li, S.S.; Peng, X.J.; Peng, Y.Z. Metabolomic pathway regulation for prevention and control of granule sludge bulking in thiosulfate-driven denitrification. Sci. Total Environ. 2023, 893, 164657. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, D.H. Process Control of Activated Sludge Plants by Microscopic Investigation; IWA Publishing: London, UK, 2000. [Google Scholar]
- Andreasen, K.; Nielsen, P.H. Growth of Microthrix parvicella in nutrient removal activated sludge plants: Studies of in situ physiology. Water Res. 2000, 34, 1559–1569. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Asvapathanagul, P.; Huang, Z.H.; Gedalanga, P.B.; Baylor, A.; Olson, B.H. Interaction of operational and physicochemical factors leading to Gordonia amaraelike foaming in an incompletely nitrifying activated sludge plant. Appl. Environ. Microbiol. 2012, 78, 8165–8175. [Google Scholar] [CrossRef] [PubMed]
- Kaetzke, A.; Jentzsch, D.; Eschrich, K. Quantification of Microthrix parvicella in activated sludge bacterial communities by real-time PCR. Lett. Appl. Microbiol. 2005, 40, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Amann, R.; Fuchs, B.M.; Behrens, S. The Identification of Microorganisms by Fluorescence In Situ Hybridization. Curr. Opin. Biotechnol. 2001, 12, 231–236. [Google Scholar] [CrossRef]
- Wilén, B.M.; Onuki, M.; Hermansson, M.; Lumley, D.; MinoM, T. Microbial Community Structure in Activated Sludge Floc Analysed by Fluorescence In Situ Hybridization and Its Relation to Floc Stability. Water Res. 2008, 42, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.H.; Kragelund, C.; Seviour, R.J.; Nielsen, J.L. Identity and ecophysiology of filamentous bacteria in activated sludge. FEMS Microbiol. Rev. 2009, 33, 969–998. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, A.T.; Kragelund, C.; Eriksen, P.S.; Nielsen, P.H. Population dynamics of filamentous bacteria in Danish wastewater treatment plants with nutrient removal. Water Res. 2012, 46, 3781–3795. [Google Scholar] [CrossRef]
- Loy, A.; Horn, M.; Wagner, M. Probebase: An online resource for rRNA-targeted oligonucleotide probes. Nucleic Acids Res. 2003, 31, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.F.; Su, Z.B.; Tao, X.X.; Zhou, X.; Zhao, J.B.; Wang, R.Q.; Qin, J.Y. Fe controls the reproduction of zoogloeal and sludge bulking in oil-in-iron wastewater. Water 2023, 5, 1289276. [Google Scholar]
- Collins, T.J. ImageJ for microscopy. Biotechniques 2007, 43, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Zahra, S.A.; Abdullah, N.; Iwamoto, K.; Yuzir, A.; Mohamad, S.E. Alginate-like exopolysaccharides in aerobic granular sludge: A review. Mater. Today: Proc. 2022, 65, 3046–3053. [Google Scholar] [CrossRef]
- Dunkel, T.; De Leon Gallegos, E.L.; Bock, C.; Lange, A.; Hoffmann, D.; Boenigk, J.; Denecke, M. Illumina sequencing for the identification of filamentous bulking and foaming bacteria in industrial activated sludge plants. Int. J. Environ. Sci. Technol. 2018, 15, 1139–1158. [Google Scholar] [CrossRef]
- Jiang, X.T.; Guo, F.; Zhang, T. Population dynamics of bulking and foaming bacteria in a full-scale wastewater treatment plant over five years. Sci. Rep. 2016, 6, 24180. [Google Scholar] [CrossRef]
- Wang, P.; Yu, Z.; Qi, R.; Zhang, H.X. Detailed comparison of bacterial communities during seasonal sludge bulking in a municipal wastewater treatment plant. Water Res. 2016, 105, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yu, Z.; Zhao, J.; Zhang, H.X. Seasonal changes in bacterial communities cause foaming in a wastewater treatment plant. Microb. Ecol. 2016, 71, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.N.; Yue, Y.Q.; Jiao, X.M.; Chi, Y.Z.; Ding, Z.Q.; Bai, Y.Z. Effect of temperature on the relationship between quorum-sensing and sludge bulking. J. Water Process Eng. 2024, 58, 104883. [Google Scholar] [CrossRef]
- Wang, S.; Wang, G.L.; Yan, P.; Chen, Y.P.; Fang, F.; Guo, J.S. Non-filamentous sludge bulking induced by exopolysaccharide variation in structure and properties during aerobic granulation. Sci. Total Environ. 2023, 876, 162786. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Caporaso, J.G. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Ming, F.S.; Lin, Y. 454 Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J. 2012, 6, 1137–1147. [Google Scholar]
- Liu, C.; Li, J.B.; Rui, J.P.; An, J.X.; Li, X.Z. The applications of the 16S rRNA gene in microbial ecology: Current situation and problems. Acta Ecol. Sin. 2015, 35, 2769–2788. [Google Scholar]
- Nübel, U.; Engelen, B.; Felske, A.; Snaidr, J.; Wieshuber, A.; Amann, R.I.; Ludwig, W.; Backhaus, H. Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J. Bacteriol. 1996, 178, 5636–5643. [Google Scholar] [CrossRef]
- Wang, B.B.; Peng, D.C.; Hou, Y.P.; Li, H.J.; Pei, L.Y.; Yu, L.F. The Important Implications of Particulate Substrate in Determining the Physicochemical Characteristics of Extracellular Polymeric Substances (EPS) in Activated Sludge. Water Res. 2014, 58, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Qi, R.; Tandoi, V.; Yang, M. Sludge bulking impact on relevant bacterial populations in a full-scale municipal wastewater treatment plant. Process. Biochem. 2014, 49, 2258–2265. [Google Scholar] [CrossRef]
- Jenkins, D.; Richard, M.G.; Daigger, G.T. Manual on the Causes and Control of Activated Sludge Bulking, Foaming, and Other Solids Separation Problems, 3rd ed.; IWA Publishing: London, UK, 2004. [Google Scholar]
- Guo, F.; Zhang, T. Profiling bulking and foaming bacteria in activated sludge by high throughput sequencing. Water Res. 2012, 46, 2772–2782. [Google Scholar] [CrossRef]
- Li, B.B.; Peng, Z.Y.; Zhi, L.L.; Li, H.B.; Zheng, K.K.; Li, J. Distribution and diversity of filamentous bacteria in wastewater treatment plants exhibiting foaming of Taihu Lake Basin, China *. Environ. Pollut. 2020, 267, 115644. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Yin, J.; Ye, L.; Wu, X.H. Occurrence andcontrol of sludge bulking under low temperature in MUCT process. J. Harbin Inst. Technol. 2009, 41, 67–71. [Google Scholar]
- Miłobędzka, A.; Muszyn’ski, A. Population dynamics of filamentous bacteria identified in Polish full-scale wastewater treatment plants with nutrients removal. Water Sci. Technol. 2015, 71, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Stratton, H.M.; Griffiths, P.C.; Seviour, R.J. Which are the polyphosphate accumulating organisms in full scale activated sludge enhanced biological phosphate removal systems in Australia? J. Appl. Microbiol. 2006, 100, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.H.; Xia, Y.; Nielsen, J.L.; Nielsen, P.H. Structure and function of the microbial community in a full-scale enhanced biological phosphorus removal plant. Microbiology 2007, 153, 4061–4073. [Google Scholar] [CrossRef]
- Yang, K.; Lin, H.M.; Chen, T.; Yang, T.; Zhang, B. Research progress on dominant filamentous bacteria and biological control measures in bulking sludge. Appl. Chem. Ind. 2023, 52, 3365–3370. [Google Scholar]
- Kang, H.; Wang, Y.Y.; He, Y.X.; Li, J.Y.; Yu, Z.T.; Li, Q. Analysis of microbial population dynamics during the expansion of activated sludge in A2/O plant. Technol. Wind 2022, 12, 128–130. [Google Scholar]
- Zhang, M.; Yao, J.; Wang, X. The microbial community in filamentous bulking sludge with the ultra-low sludge loading and long sludge retention time in oxidation ditch. Sci. Rep 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Nittami, T.; Kasakura, R.; Kobayashi, T.; Suzuki, K.; Koshiba, Y.; Fukuda, J.; Takeda, M.; Tobino, T.; Kurisu, F.; Rice, D.; et al. Exploring the operating factors controlling Kouleothrix (type 1851), the dominant filamentous bacterial population, in a full-scale A2O plant. Sci. Rep 2020, 10, 6809. [Google Scholar] [CrossRef]
- Xu, P.; Xie, Z.; Shi, L.; Yan, X.; Fu, Z.; Ma, J.; Zhang, W.; Wang, H.; Hu, B.; He, Q. Distinct responses of aerobic granular sludge sequencing batch reactors to nitrogen and phosphorus deficient conditions. Sci. Total Environ. 2022, 834, 155369. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Amado, R.; Padrão, J.; Ferreira, V.; Dias, N.M.; Melo, L.D.R.; Santos, S.B.; Nicolau, A. The first sequenced Sphaerotilus natans bacteriophage–characterization and potential to control its filamentous bacterium host. FEMS Microbiol. Ecol. 2021, 97, 029. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Huang, D.M.; Chen, Y.P.; Yan, P.; Gao, X. New insight into filamentous sludge bulking during wastewater treatment: Surface characteristics and thermodynamics. Sci. Total Environ. 2020, 712, 135795. [Google Scholar] [CrossRef] [PubMed]
- Palm, J.C.; Jenkins, D.; Parker, D.S. Relationship Between organic loading, dissolved oxygen concentration and sludge settleability in the completely-mixed activated sludge process. J. Water Pollut. Control Fed. 1980, 52, 2482–2506. [Google Scholar]
- Cao, C.Y.; Lou, I.; Huang, C.; Lee, M.Y. Metagenomic sequencing of activated sludge filamentous bacteria community using the Ion Torrent platform. Desalination Water Treat. 2014, 57, 2175–2183. [Google Scholar] [CrossRef]
- Dunkel, T.; De León Gallegos, E.L.; Schönsee, C.D.; Hesse, T.; Jochmann, M.; Wingender, J.; Denecke, M. Evaluating the influence of wastewater composition on the growth of Microthrix parvicella by GCxGC/qMS and real-time PCR. Water Res. 2016, 88, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Kruit, J.; Hulsbeek, J.; Visser, A. Bulking sludge solved?! Water Sci. Technol. 2002, 46, 457. [Google Scholar] [CrossRef]
- Kumari, S.K.S.; Marrengane, Z.; Bux, F. Application of quantitative RT-PCR to determine the distribution of Microthrix parvicella in full-scale activated sludge treatment systems. Appl. Microbiol. Biotechnol. 2009, 83, 1135–1141. [Google Scholar] [CrossRef]
- Noutsopoulos, C.; Mamais, D.; Andreadakis, A. Effect of solids retention time on Microthrix parvicella growth. Water SA 2007, 32, 315–321. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Roslev, P.; Dueholm, T.E.; Nielsen, J.L. Microthrix parvicella, a specialized lipid consumer in anaerobic-aerobic activated sludge plants. Water Sci. Technol. 2002, 46, 73–80. [Google Scholar] [CrossRef]
- Sheik, A.R.; Muller, E.E.; Audinot, J.N.; Lebrun, L.A.; Grysan, P.; Guignard, C.; Willmes, P. In situ phenotypic heterogeneity among single cells of the filamentous bacterium Candidatus Microthrix parvicella. ISME J. 2016, 10, 1274–1279. [Google Scholar] [CrossRef]
- Rossetti, S.; Tomei, M.C.; Nielsen, P.H.; Tandoi, V. “Microthrix parvicella”, a filamentous bacterium causing bulking and foaming in activated sludge systems: A review of current knowledge. FEMS Microbiol. Rev. 2005, 29, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Seviour, E.M.; Eales, K.L.; Izzard, L.; Beer, M.; Carr, E.L.; Seviour, R.J. The in situ physiology of ‘Nostocoida limicola’ II, a filamentous bacterial morphotype in bulking activated sludge, using fluorescence in situ hybridization (FISH) and microautoradiography (MAR). Water Sci. Technol. 2006, 54, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Blackall, L.L.; Seviour, E.M.; Bradford, D.; Rossetti, S.; Tandoi, V.; Seviour, R.J. ‘Candidatus Nostocoida limicola’, a filamentous bacterium from activated sludge. Int. J. Syst. Evol. Microbiol. 2000, 50, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.R.; Seviour, R.J. Design and application of oligonucleotide probes for fluorescent in situ identification of the filamentous bacterial morphotype Nostocoida limicola in activated sludge. Environ. Microbiol. 2001, 3, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Kragelund, C.; Remesova, Z.; Nielsen, J.L.; Thomsen, T.R.; Eales, K.; Seviour, R.; Wanner, J. Ecophysiology of mycolic acid-containing Actinobacteria (Mycolata) in activated sludge foams. FEMS Microbiol. Ecol. 2007, 61, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.L.; Eales, K.L.; Seviour, R.J. Substrate uptake by Gordonia amarae in activated foams by FISH-MAR. Water Sci. Technol. 2006, 54, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kragelund, C.; Levantesi, C.; Borger, A.; Thelen, K.; Eikelboom, D.; Tandoi, V.; Kong, Y. Identity, abundance and ecophysiology of filamentous Chloroflexi species from activated sludge treatment plants. FEMS Microbiol. Ecol. 2007, 59, 671–682. [Google Scholar] [CrossRef]
- Kragelund, C.; Thomsen, T.R.; Mielczarek, A.T.; Nielsen, P.H. Eikelboom’s morphotype 0803 in activated sludge belongs to the genus Caldilinea in the phylum Chloroflexi. FEMS Microbiol. Ecol. 2011, 76, 451–462. [Google Scholar] [CrossRef]
- Xia, Y.; Kong, Y.; Thomsen, T.R.; Nielsen, P.H. Identification and ecophysiological characterization of epiphytic proteinhydrolyzing Saprospiraceae (‘Candidatus Epiflobacter’ spp.) in activated sludge. Appl Environ. Microb. 2008, 74, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, T.R.; Blackall, L.L.; De Muro, M.A.; Nielsen, J.L.; Nielsen, P.H. Meganema perideroedes gen. nov., sp. nov., a new filamentous alphaproteobacterium from activated sludge. Int. J. Syst. Evol. Microbiol. 2006, 56, 1865–1868. [Google Scholar] [CrossRef]

| No. | Type | Primer Name and Sequence (5′-3′) | Amplification Conditions | Reference |
|---|---|---|---|---|
| 1 | Bacteria | 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) 534R (5′-ATTACCGCGGCTGCTGG-3′) | 95 °C 2 min; (95 °C 20 s, 56 °C 30 s, 72 °C 60 s) × 30; 72 °C 5 min | [55] |
| 2 | Bacteria | 563f (AYTGGGYDTAAAGNG) r1 (TACCRGGGTHTCTAATCC) r2 (CAGAGTATCTAATTC) r3 (CTACDSRGGTMTC TAATC) r4 (TACNVGGGTATCTAATC) | 98 °C 2 min; (98 °C 15 s, 56 °C 20 s, 68 °C 30 s) × 28; 68 °C 10 min | [56] |
| 3 | Bacteria | 338f (ACTCCTACGGGAGGCAGCA) 806r (GGACTACHVGGGTWTCTAAT) | 95 °C 3 min; (95 °C 30 s, 55 °C 30 s, 72 °C 45 s) × 27; 72 °C 10 min | [57] |
| 4 | Bacteria | 968F (AACGCGAAGAACCTTAC) 1401r (CGGTGTGTACAAGACCC) | 95 °C 5 min; (95 °C 30 s, 63 °C 45 s, 72 °C 1 min) × 35; 72 °C 5 min | [58] |
| 5 | Universal primers | 341f (CCTACGGGNGGCWGCAGG) 785r (GACTACHVGGGTATCOTTCC) | 95 °C 5 min; (95 °C 40 s, 55 °C 120 s, 72 °C 60 s) × 25; 72 °C 7 min | [40] |
| 6 | Universal primers | 341f (CCTACGGGNGGCWGCAGG) 534R (ATTACCGCGGCTGCTGG) | 94 °C 5 min; (94 °C 30 s, 55 °C 30 s, 72 °C 30 s) × 30; 72 °C 10 min | [59] |
| 7 | Universal primers | 27f (AGA GTT TGA TCC TGG CTC AG) 1492r (TACGGYTACCTTGTTACGACTT) | 95 °C 10 min; (95 °C 60 s, 55 °C 60 s, 72 °C 90 s) × 35; 72 °C 10 min | [60] |
| 8 | Actinomycetes | 243f (GGATGAGCCCGCGGCCTA) 513r (CGGCCGCGGCTGCTGGCACGTA) | 95 °C 5 min; (95 °C 30 s, 63 °C 45 s, 72 °C 1 min) × 35; 72 °C 5 min | [61] |
| Filamentous Bacteria | Use of Acceptors | Storage Compounds | Carbon Source | Optimal Growth Temperature | Grows Rate | Characteristic |
|---|---|---|---|---|---|---|
| M. parvicella | O2, NO3−, NO2−, anaerobic | PHA | Acetate, glucose and long-chain fatty acids | 12–15 °C | 0.3~0.5 d−1 | Bulking |
| Candidatus ‘Nostocoida limicola’ | O2, NO3−, NO2− | PHA, Poly P | Hydrophilic and hydrophobic substrates, including LCFA | 15−37 °C | Not reported | Bulking and foaming |
| Gordonia | O2, NO3−, NO2− | PHA, Poly P | Phthalates, hydrocarbons | 20−37 °C | Not reported | Foaming |
| Mycobactrium fortuitoum | O2, NO3−, NO2−, anaerobic | PHA | Organic matter | 15−37 °C | grows slowly | “Skeleton” |
| Chloroflexi | O2 | PHA | Proteins, polysaccharides and dead cell debris | 15−37 °C | Not reported | Bulking |
| type 0041/0675 | O2, NO3−, NO2− | PHA | Acetate, glucose | 15−37 °C | Not reported | Bulking |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Pan, W.; Niu, L.; Lu, H.; Wang, X. Population Composition, Physiology and Ecology of Filamentous Bacteria in Activated Sludge. Processes 2024, 12, 2156. https://doi.org/10.3390/pr12102156
Gao S, Pan W, Niu L, Lu H, Wang X. Population Composition, Physiology and Ecology of Filamentous Bacteria in Activated Sludge. Processes. 2024; 12(10):2156. https://doi.org/10.3390/pr12102156
Chicago/Turabian StyleGao, Shang, Wenbo Pan, Lu Niu, Hai Lu, and Xiaoling Wang. 2024. "Population Composition, Physiology and Ecology of Filamentous Bacteria in Activated Sludge" Processes 12, no. 10: 2156. https://doi.org/10.3390/pr12102156







