Kombucha Fermentation in Coffee: Application of Constant Air Flow Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fermented Coffee Preparation
2.2.2. Change in SCOBY Mass
2.2.3. Particle Size Determination
2.2.4. pH Determination
2.2.5. TSS Analysis
2.2.6. Determination of Bioactive Compounds
Determination of Concentration of Total Phenolic Compounds in Fermented Coffee Beverage
Determination of Antioxidant Activity in Fermented Coffee Beverage
Determination of Caffeine Concentration in Fermented Coffee Beverage
2.2.7. Colorimetric Measurements
2.2.8. Microbial Analysis
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Change in SCOBY Mass
3.2. pH Determination
3.3. TSS Analysis
3.4. Determination of Bioactive Compounds
3.5. Colourimetric Measurements
3.6. Microbial Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aung, T.; Kim, M.J. A Comprehensive Review on Kombucha Biofilms: A Promising Candidate for Sustainable Food Product Development. Trends Food Sci. Technol. 2024, 144, 104325. [Google Scholar] [CrossRef]
- Jakubczyk, K.J.P.; Piotrowska, G.; Janda, K. Characteristics and Biochemical Composition of Kombucha—Fermented Tea. Med. Og. Nauk. Zdr. 2020, 26, 94–96. [Google Scholar] [CrossRef]
- Soares, M.G.; de Lima, M.; Reolon Schmidt, V.C. Technological Aspects of Kombucha, Its Applications and the Symbiotic Culture (SCOBY), and Extraction of Compounds of Interest: A Literature Review. Trends Food Sci. Technol. 2021, 110, 539–550. [Google Scholar] [CrossRef]
- Kapp, J.M.; Sumner, W. Kombucha: A Systematic Review of the Empirical Evidence of Human Health Benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha Tea—A Double Power of Bioactive Compounds from Tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical Profile and Antioxidant Activity of the Kombucha Beverage Derived from White, Green, Black and Red Tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef]
- Su, X.; Duan, J.; Jiang, Y.; Duan, X.; Chen, F. Polyphenolic Profile and Antioxidant Activities of Oolong Tea Infusion under Various Steeping Conditions. Int. J. Mol. Sci. 2007, 8, 1196–1205. [Google Scholar] [CrossRef]
- Gaggìa, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G.; et al. Kombucha Beverage from Green, Black and Rooibos Teas: A Comparative Study Looking at Microbiology, Chemistry and Antioxidant Activity. Nutrients 2019, 11, 1. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid Contents and the Effect of Fermentation Condition of Kombucha Tea Beverages on Physicochemical, Microbiological and Sensory Properties. CyTA J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef]
- Içen, H.; Corbo, M.R.; Sinigaglia, M.; Korkmaz, B.I.O.; Bevilacqua, A. Microbiology and Antimicrobial Effects of Kombucha, a Short Overview. Food Biosci. 2023, 56, 103270. [Google Scholar] [CrossRef]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Chemical Composition of Kombucha. Beverages 2022, 8, 45. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Sathishkumar, M. Kombucha Tea: Metabolites. In Fungal Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 965–978. ISBN 978-3-319-25001-4. [Google Scholar]
- Coelho, R.M.D.; de Almeida, A.L.; do Amaral, R.Q.G.; da Mota, R.N.; de Sousa, P.H.M. Kombucha: Review. Int. J. Gastron. Food Sci. 2020, 22, 100272. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.; Penas, M.R.; Pintado, M.; Oliveira-Silva, P. Kombucha: Perceptions and Future Prospects. Foods 2022, 11, 1977. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, C.; Farnworth, E. Tea, Kombucha, and Health: A Review. Food Res. Int. 2000, 33, 409–421. [Google Scholar] [CrossRef]
- Emiljanowicz, K.E.; Malinowska-Pańczyk, E. Kombucha from Alternative Raw Materials—The Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3185–3194. [Google Scholar] [CrossRef]
- Morales, D.; Gutiérrez-Pensado, R.; Bravo, F.I.; Muguerza, B. Novel Kombucha Beverages with Antioxidant Activity Based on Fruits as Alternative Substrates. LWT 2023, 189, 115482. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current Challenges, Applications and Future Perspectives of SCOBY Cellulose of Kombucha Fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Szulc, J.; Czaczyk, K.; Dobrowolska, A.; Gozdecka, G.; Błaszak, B. Elicitation as a Process of Enhancing Bioactive Compounds Concentration in Sprouts. Processes 2024, 12, 174. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Skórczewska, K.; Szulc, J.; Chmielnicki, B.; Korol, J.; Formela, K. Sustainable Upcycling of Brewers’ Spent Grain by Thermo-Mechanical Treatment in Twin-Screw Extruder. J. Clean. Prod. 2021, 285, 124839. [Google Scholar] [CrossRef]
- Vuletic, N.; Bardic, L.; Odzak, R. Spectrophotometric Determining of Caffeine Content in the Selection of Teas, Soft and Energy Drinks Available on the Croatian Market. Food Res. 2021, 5, 325–330. [Google Scholar] [CrossRef]
- Cytarska, J.; Szulc, J.; Kołodziej-Sobczak, D.; Nunes, J.A.; da Silva-Júnior, E.F.; Łączkowski, K.Z. CyreneTM as a Tyrosinase Inhibitor and Anti-Browning Agent. Food Chem. 2024, 442, 138430. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 4833-1:2013-12; Microbiology of the Food Chain Horizontal Method for the Enumeration of Microorganisms Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- Martínez-Tomé, M.; Jiménez-Monreal, A.M.; García-Jiménez, L.; Almela, L.; García-Diz, L.; Mariscal-Arcas, M.; Murcia, M.A. Assessment of Antimicrobial Activity of Coffee Brewed in Three Different Ways from Different Origins. Eur. Food Res. Technol. 2011, 233, 497–505. [Google Scholar] [CrossRef]
- Malbaša, R.; Lončar, E.; Djurić, M.; Došenović, I. Effect of Sucrose Concentration on the Products of Kombucha Fermentation on Molasses. Food Chem. 2008, 108, 926–932. [Google Scholar] [CrossRef]
- Sinamo, K.N.; Ginting, S.; Pratama, S. Effect of Sugar Concentration and Fermentation Time on Secang Kombucha Drink. IOP Conf. Ser. Earth Environ. Sci. 2022, 977, 012080. [Google Scholar] [CrossRef]
- Wang, B.; Rutherfurd-Markwick, K.; Zhang, X.-X.; Mutukumira, A.N. Isolation and Characterisation of Dominant Acetic Acid Bacteria and Yeast Isolated from Kombucha Samples at Point of Sale in New Zealand. Curr. Res. Food Sci. 2022, 5, 835–844. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, F.J.; Belo, M.P.G.; de Lima, S.L.; Silva, A.K.; Uekane, M.T.; Gonzalez, G.M.A.; Branco, N.C.V.; Pitangui, S.N.; Fernandes, F.F.; Lima, R.A. Arabic Coffee Infusion Based Kombucha: Characterization and Biological Activity during Fermentation, and in Vivo Toxicity. Food Chem. 2023, 412, 135556. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. Enhancement of the Functional Properties of Coffee Through Fermentation by “Tea Fungus” (Kombucha). J. Food Process. Preserv. 2015, 39, 2596–2603. [Google Scholar] [CrossRef]
- Laureys, D.; Britton, S.J.; De Clippeleer, J. Kombucha Tea Fermentation: A Review. J. Am. Soc. Brew. Chem. 2020, 78, 165–174. [Google Scholar] [CrossRef]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Harangozo, Ľ.; Kántor, A.; Kačániová, M. The Evaluation of Chemical, Antioxidant, Antimicrobial and Sensory Properties of Kombucha Tea Beverage. J. Food Sci. Technol. 2020, 57, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Nizioł-Łukaszewska, Z.; Ziemlewska, A.; Bujak, T.; Zagórska-Dziok, M.; Zarębska, M.; Hordyjewicz-Baran, Z.; Wasilewski, T. Effect of Fermentation Time on Antioxidant and Anti-Ageing Properties of Green Coffee Kombucha Ferments. Molecules 2020, 25, 5394. [Google Scholar] [CrossRef]
- Ahmed, R.F.; Hikal, M.S.; Abou-Taleb, K.A. Biological, Chemical and Antioxidant Activities of Different Types Kombucha. Ann. Agric. Sci. 2020, 65, 35–41. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Renard, T.; Rollan, S.; Taillandier, P. Impact of Fermentation Conditions on the Production of Bioactive Compounds with Anticancer, Anti-Inflammatory and Antioxidant Properties in Kombucha Tea Extracts. Process Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef]
- Oalđe Pavlović, M.; Stajić, M.; Gašić, U.; Duletić-Laušević, S.; Ćilerdžić, J. The Chemical Profiling and Assessment of Antioxidative, Antidiabetic and Antineurodegenerative Potential of Kombucha Fermented Camellia sinensis, Coffea arabica and Ganoderma lucidum Extracts. Food Funct. 2023, 14, 262–276. [Google Scholar] [CrossRef]
- La Torre, C.; Fazio, A.; Caputo, P.; Plastina, P.; Caroleo, M.C.; Cannataro, R.; Cione, E. Effects of Long-Term Storage on Radical Scavenging Properties and Phenolic Content of Kombucha from Black Tea. Molecules 2021, 26, 5474. [Google Scholar] [CrossRef]
- Gökcen, B.B.; Şanlier, N. Coffee Consumption and Disease Correlations. Crit. Rev. Food Sci. Nutr. 2019, 59, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Verster, J.C.; Koenig, J. Caffeine Intake and Its Sources: A Review of National Representative Studies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1250–1259. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Safety of Caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Chudy, S.; Gierałtowska, U.; Krzywdzińska-Bartkowiak, M.; Piątek, M. Pomiar Barwy Produktów Mleczarskich. In Współczesne Trendy w Kształtowaniu Jakości Zywności; Wydział Nauk o Żywności i Żywieniu Uniwersytet Przyrodniczy w Poznaniu: Poznań, Poland, 2016; pp. 85–95. ISBN 978-83-7160-834-6. [Google Scholar]
- Jarrell, J.; Cal, T.; Bennett, J.W. The Kombucha Consortia of Yeasts and Bacteria. Mycologist 2000, 14, 166–170. [Google Scholar] [CrossRef]
- Sobieszuk, P.; Strzyżewska, A.; Ulatowski, K. Investigation of the Possibility of Culturing Aerobic Yeast with Oxygen Nanobubble Addition and Evaluation of the Results of Batch and Semi-Batch Cultures of Saccharomyces cerevisiae. Chem. Eng. Process. Process Intensif. 2021, 159, 108247. [Google Scholar] [CrossRef]
- Shavyrkina, N.A.; Skiba, E.A.; Kazantseva, A.E.; Gladysheva, E.K.; Budaeva, V.V.; Bychin, N.V.; Gismatulina, Y.A.; Kashcheyeva, E.I.; Mironova, G.F.; Korchagina, A.A.; et al. Static Culture Combined with Aeration in Biosynthesis of Bacterial Cellulose. Polymers 2021, 13, 4241. [Google Scholar] [CrossRef] [PubMed]



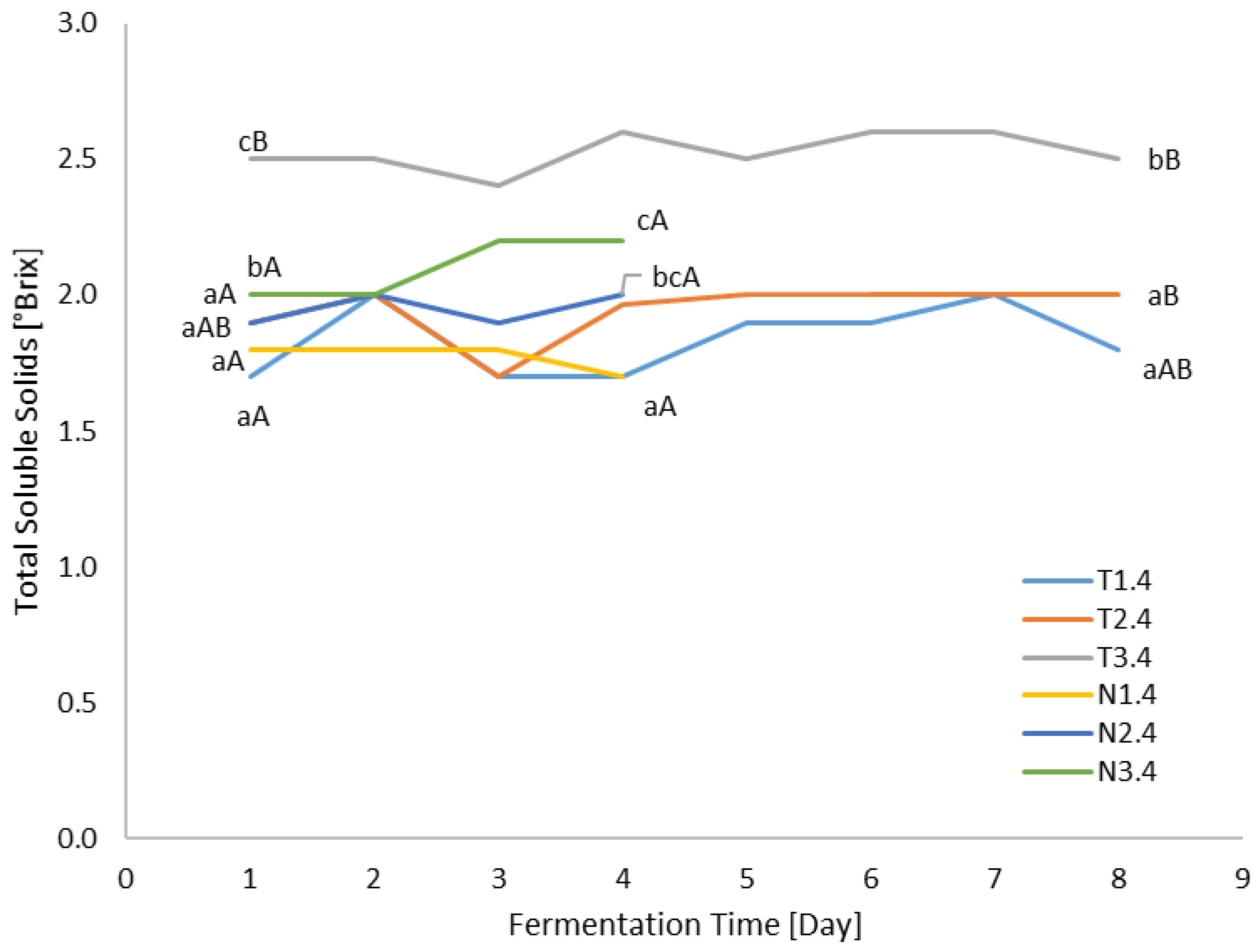
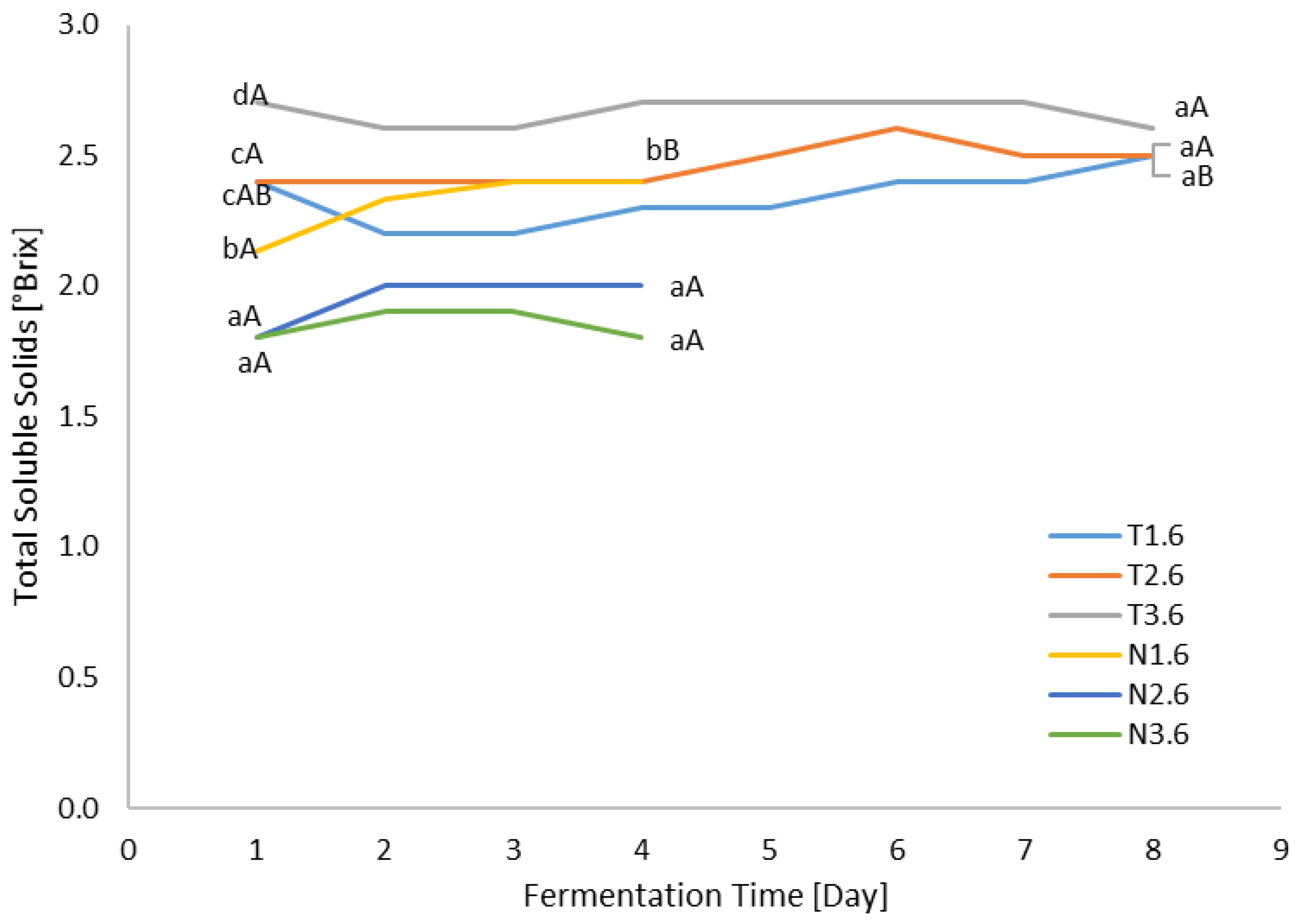
| Sample Abbreviation | Sample Abbreviation Explanation |
|---|---|
| T1.4 | Traditional method of fermentation/5% sugar/4% coffee |
| T2.4 | Traditional method of fermentation/6% sugar/4% coffee |
| T3.4 | Traditional method of fermentation/7% sugar/4% coffee |
| T1.6 | Traditional method of fermentation/5% sugar/6% coffee |
| T2.6 | Traditional method of fermentation/6% sugar/6% coffee |
| T3.6 | Traditional method of fermentation/7% sugar/6% coffee |
| N1.4 | Fermentation in the bioreactor/5% sugar/4% coffee |
| N2.4 | Fermentation in the bioreactor/6% sugar/4% coffee |
| N3.4 | Fermentation in the bioreactor/7% sugar/4% coffee |
| N1.6 | Fermentation in the bioreactor/5% sugar/6% coffee |
| N2.6 | Fermentation in the bioreactor/6% sugar/6% coffee |
| N3.6 | Fermentation in the bioreactor/7% sugar/6% coffee |
| Time of Measurement | SCOBY Mass Change [g] | |||||
|---|---|---|---|---|---|---|
| T1.4 | T2.4 | T3.4 | N1.4 | N2.4 | N3.4 | |
| Before fermentation | 25.5 bA ±0.1 | 25.4 abcA ±0.3 | 25.3 aA ±0.1 | 25.2 aA ±0.1 | 25.3 aA ±0.1 | 25.7 cA ±0.1 |
| After fermentation | 33.9 aB ±0.3 | 36.8 bB ±0.3 | 42.7 eB ±1.2 | 36.7 bB ±0.6 | 39.7 cB ±0.1 | 38.6 dB ±0.6 |
| T1.6 | T2.6 | T3.6 | N1.6 | N2.6 | N3.6 | |
| Before fermentation | 26.3 cA ±1.0 | 25.4 abcA ±0.2 | 24.8 abA ±0.7 | 24.3 aA ±1.0 | 25.6 bcA ±0.3 | 25.4 abcA ±0.3 |
| After fermentation | 28.1 aA ±2.6 | 29.1 aA ±3.6 | 28.4 aB ±0.9 | 29.0 aB ±2.2 | 30.1 aA ±4.4 | 26.9 aA ±2.1 |
| Coffee Concentration | SCOBY Mass Increase (Δm) [g] | |||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | N1 | N2 | N3 | |
| 4% | 8.36 aB ±0.46 | 11.32 bB ±0.03 | 17.39 eB ±1.28 | 11.45 bB ±0.44 | 14.42 dB ±0.12 | 12.82 cB ±0.70 |
| 6% | 3.15 aA ±0.03 | 6.38 cA ±0.46 | 4.91 bA ±0.13 | 6.98 cA ±0.69 | 7.03 cA ±1.66 | 3.10 aA ±0.21 |
| Day of Measurement | Total Polyphenol Concentration [mg GAE/100 mL] | ||||||
|---|---|---|---|---|---|---|---|
| Control 4% * | T1.4 | T2.4 | T3.4 | N1.4 | N2.4 | N3.4 | |
| 4 | 124.87 a ±1.58 | 170.54 bcA ±9.81 | 183.03 bcdA ±18.73 | 179.16 cdA ±8.68 | 186.11 cd ±12.41 | 187.22 d ±7.20 | 162.93 b ±1.32 |
| 8 | NA | 193.14 aB ±8.02 | 209.99 aA ±23.93 | 189.01 aA ±7.96 | NA | NA | NA |
| Control 6% * | T1.6 | T2.6 | T3.6 | N1.6 | N2.6 | N3.6 | |
| 4 | 257.26 b ±4.21 | 227.44 aA ±8.00 | 225.98 aA ±2.36 | 234.14 abA ±20.83 | 234.62 a ±12.53 | 231.08 a ±7.10 | 212.48 a ±23.68 |
| 8 | NA | 233.28 aA ±12.66 | 265.74 bB ±0.01 | 277.80 bB ±31.80 | NA | NA | NA |
| Day of Measurement | DPPH * Scavenging Capacity [%] | ||||||
|---|---|---|---|---|---|---|---|
| Control 4% * | T1.4 | T2.4 | T3.4 | N1.4 | N2.4 | N3.4 | |
| 4 | 15.03 a ±0.69 | 32.89 cA ±3.76 | 34.69 cA ±0.97 | 33.84 cA ±3.19 | 37.48 c ±5.19 | 32.12 b ±2.35 | 31.97 b ±0.18 |
| 8 | NA | 43.55 aB ±0.09 | 59.44 bB ±0.19 | 65.72 bB ±0.13 | NA | NA | NA |
| Control 6% * | T1.6 | T2.6 | T3.6 | N1.6 | N2.6 | N3.6 | |
| 4 | 44.36 c ±0.43 | 58.10 dB ±0.48 | 41.06 bB ±0.47 | 39.32 aA ±0.33 | 52.94 cd ±10.56 | 72.44 f ±0.53 | 71.64 e ±0.20 |
| 8 | NA | 51.10 cA ±0.24 | 36.77 aA ±0.19 | 46.71 bB ±4.14 | NA | NA | NA |
| Day of Measurement | Caffeine Concentration [ppm] | ||||||
|---|---|---|---|---|---|---|---|
| Control 4%* | T1.4 | T2.4 | T3.4 | N1.4 | N2.4 | N3.4 | |
| 4 | 3.77 a ±0.01 | 4.55 aA ±1.38 | 4.78 aA ±1.62 | 4.50 aA ±1.73 | 4.58 a ±1.89 | 4.75 a ±1.81 | 4.77 a ±2.23 |
| 8 | NA | 3.10 aA ±0.54 | 2.92 aA ±0.68 | 2.45 aA ±0.18 | NA | NA | NA |
| Control 6%* | T1.6 | T2.6 | T3.6 | N1.6 | N2.6 | N3.6 | |
| 4 | 3.34 abc ±0.01 | 3.62 abcA ±0.24 | 3.00 aA ±0.57 | 3.62 abcA ±0.63 | 5.19 bc ±1.58 | 4.90 bc ±1.40 | 3.79 abc ±0.76 |
| 8 | NA | 3.27 aA ±0.73 | 2.75 aA ±0.64 | 3.36 aA ±0.83 | NA | NA | NA |
| Colour Coordinate | Coffee Concentration | Control | T1 | T2 | T3 | N1 | N2 | N3 |
|---|---|---|---|---|---|---|---|---|
| L* | 4% | 29.15 aA ±0.01 | 30.61 gA ±0.01 | 30.35 dA ±0.01 | 30.46 eA ±0.01 | 30.26 cB ±0.01 | 29.96 bA ±0.01 | 30.50 fB ±0.01 |
| 6% | 29.14 aA ±0.01 | 30.56 deA ±0.16 | 30.55 eB ±0.01 | 30.53 dB ±0.01 | 30.12 cA ±0.01 | 29.96 bA ±0.01 | 30.12 cA ±0.01 | |
| a* | 4% | 1.01 aB ±0.03 | 1.31 bA ±0.03 | 1.49 dB ±0.03 | 1.39 cB ±0.01 | 1.99 eB ±0.01 | 2.48 fB ±0.01 | 2.01 eB ±0.02 |
| 6% | 0.74 aA ±0.01 | 1.34 cA ±0.02 | 1.31 bA ±0.02 | 1.30 bA ±0.02 | 1.69 eA ±0.03 | 1.42 dA ±0.01 | 1.63 eA ±0.04 | |
| b* | 4% | −0.98 aB ±0.02 | 0.32 dA ±0.01 | 0.17 bA ±0.01 | 0.20 cA ±0.01 | 0.61 fB ±0.01 | 0.40 eB ±0.02 | 0.81 gB ±0.01 |
| 6% | −0.92 aA ±0.01 | 0.50 dB ±0.01 | 0.30 cB ±0.02 | 0.30 cB ±0.02 | 0.29 cA ±0.01 | 0.06 bA ±0.01 | 0.29 cA ±0.01 |
| Control 4% | Control 6% | T1.4 | T1.6 | T2.4 | T2.6 | T3.4 | T3.6 | N1.4 | N2.6 | N2.4 | N2.6 | N3.4 | N3.6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control 4% | 0 | |||||||||||||
| Control 6% | 0.4 | 0 | ||||||||||||
| T1.4 | 2.0 | 1.9 | 0 | |||||||||||
| T1.6 | 2.1 | 2.0 | 0.2 | 0 | ||||||||||
| T2.4 | 1.7 | 1.7 | 0.3 | 0.4 | 0 | |||||||||
| T2.6 | 1.9 | 1.8 | 0.1 | 0.2 | 0.3 | 0 | ||||||||
| T3.4 | 1.8 | 1.7 | 0.2 | 0.3 | 0.2 | 0.2 | 0 | |||||||
| T3.6 | 1.9 | 1.8 | 0.1 | 0.2 | 0.3 | 0.0 | 0.2 | 0 | ||||||
| N1.4 | 2.2 | 2.1 | 0.8 | 0.7 | 0.7 | 0.8 | 0.8 | 0.8 | 0 | |||||
| N1.6 | 1.7 | 1.7 | 0.6 | 0.6 | 0.3 | 0.6 | 0.5 | 0.6 | 0.5 | 0 | ||||
| N2.4 | 2.2 | 2.2 | 1.3 | 1.3 | 1.1 | 1.3 | 1.2 | 1.3 | 0.6 | 0.8 | 0 | |||
| N2.6 | 1.4 | 1.3 | 0.7 | 0.7 | 0.4 | 0.6 | 0.5 | 0.6 | 0.8 | 0.4 | 1.1 | 0 | ||
| N3.4 | 2.5 | 2.4 | 0.9 | 0.7 | 0.8 | 0.9 | 0.9 | 0.9 | 0.3 | 0.7 | 0.8 | 1.1 | 0 | |
| N3.6 | 1.7 | 1.7 | 0.6 | 0.6 | 0.3 | 0.5 | 0.4 | 0.5 | 0.5 | 0.1 | 0.9 | 0.4 | 0.7 | 0 |
| Colour |
| Coffee Concentration | Total Number of Microorganisms [CFU/mL] | ||||||
|---|---|---|---|---|---|---|---|
| Control | T1 | T2 | T3 | N1 | N2 | N3 | |
| 4% | ND | 1.3 × 106 | 1.2 × 107 | 8.9 × 108 | 2.0 × 104 | 8.9 × 105 | 1.8 × 105 |
| 6% | ND | 8.1 × 108 | 1.3 × 109 | 5.4 × 109 | 1.0 × 103 | 6.1 × 103 | 8.9 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błaszak, B.; Dorawa, P.; Sudoł, P.; Fabiszak, K.; Świadek, M.; Witucka, K.; Zimnicka, J.; Brudnicki, M.; Maciejewski, B.; Bovkun, D.; et al. Kombucha Fermentation in Coffee: Application of Constant Air Flow Reactor. Processes 2024, 12, 2159. https://doi.org/10.3390/pr12102159
Błaszak B, Dorawa P, Sudoł P, Fabiszak K, Świadek M, Witucka K, Zimnicka J, Brudnicki M, Maciejewski B, Bovkun D, et al. Kombucha Fermentation in Coffee: Application of Constant Air Flow Reactor. Processes. 2024; 12(10):2159. https://doi.org/10.3390/pr12102159
Chicago/Turabian StyleBłaszak, Błażej, Piotr Dorawa, Paweł Sudoł, Karolina Fabiszak, Martyna Świadek, Klaudia Witucka, Julia Zimnicka, Mateusz Brudnicki, Bartosz Maciejewski, Daniil Bovkun, and et al. 2024. "Kombucha Fermentation in Coffee: Application of Constant Air Flow Reactor" Processes 12, no. 10: 2159. https://doi.org/10.3390/pr12102159
APA StyleBłaszak, B., Dorawa, P., Sudoł, P., Fabiszak, K., Świadek, M., Witucka, K., Zimnicka, J., Brudnicki, M., Maciejewski, B., Bovkun, D., Cierach, M., Gozdecka, G., & Szulc, J. (2024). Kombucha Fermentation in Coffee: Application of Constant Air Flow Reactor. Processes, 12(10), 2159. https://doi.org/10.3390/pr12102159






