Gas Chromatography Tandem Mass Spectrometry (GC-MS/MS) for High-Throughput Screening of Pesticides in Rice Samples Collected in Bangladesh and Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Origin
2.2. Sample Preparation
2.3. Gas Chromatography Mass Spectrometry: Experimental Conditions
2.4. Method Development
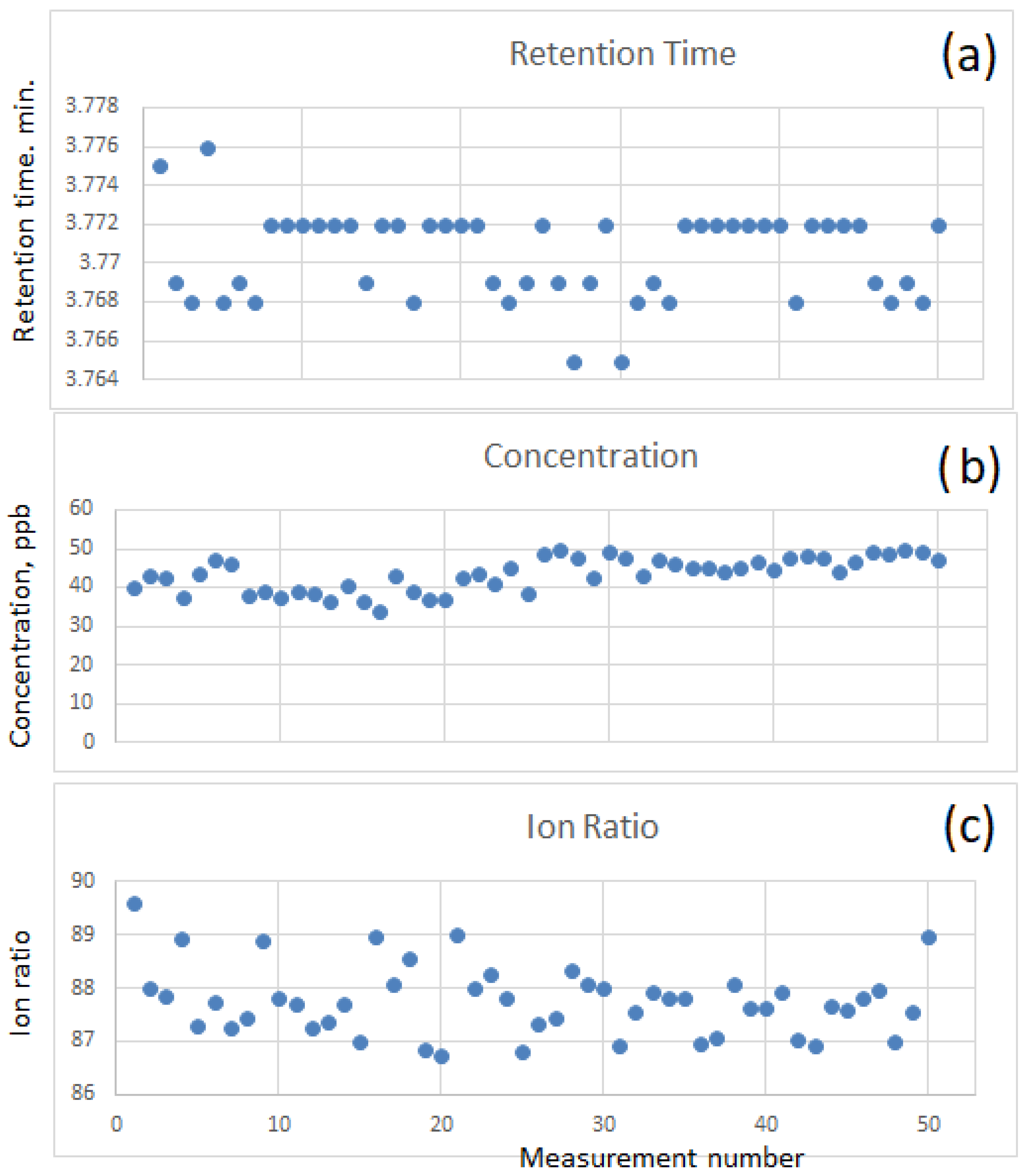
2.5. Calibration Procedure and Long Stability Tests
3. Results
3.1. Estimation of Limits of Detection (LOD) and Quantification (LOQ)
3.2. Recoveries of Pesticides after Sample Preparation
3.3. Analysis of the Rice Samples Collected in Bangladesh and Saudi Arabia
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Quantifier Transition | Qualifier Transition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound Name | RT, min | Dwell Time, ms | Ion1, a.m.u. | Ion 2, a.m.u. | CE1, eV | Ion1, a.m.u. | Ion2, a.m.u. | CE2, eV | Ion Ratio | |
| 1 | 2,4′ DDD | 4.7 | 18.0 | 235 | 199 | 15 | 235 | 165 | 20 | 31 |
| 2 | 2,4′ DDE | 4.4 | 12.0 | 246 | 211 | 20 | 246 | 176 | 30 | 10 |
| 3 | 2,4′ DDT | 5.0 | 14.6 | 235 | 199 | 20 | 235 | 165 | 20 | 26 |
| 4 | 2,4′ Methoxychlor | 5.3 | 44.2 | 227 | 169 | 25 | 227 | 115 | 40 | 95 |
| 5 | 4,4′ DDD | 4.9 | 15.2 | 235 | 199 | 15 | 235 | 165 | 20 | 25 |
| 6 | 4,4′ DDE | 4.6 | 16.6 | 246 | 211 | 20 | 246 | 176 | 30 | 9 |
| 7 | 4,4′ DDT | 5.2 | 26.1 | 235 | 199 | 20 | 235 | 165 | 20 | 7 |
| 8 | 4,4′ Dichlorobenzophenone | 4.0 | 23.9 | 250 | 139 | 8 | 139 | 111 | 8 | 15 |
| 9 | 4,4′ Methoxychlor olefin | 5.1 | 16.9 | 227 | 169 | 25 | 227 | 115 | 40 | 42 |
| 10 | Acrinathrin | 6.2 | 40.7 | 289 | 93 | 5 | 208 | 181 | 5 | 40 |
| 11 | Aldrin | 4.0 | 25.1 | 293 | 257 | 8 | 293 | 186 | 40 | 22 |
| 12 | Allethrin | 4.2 | 40.8 | 123 | 107 | 5 | 123 | 79 | 10 | 0.8 |
| 13 | alpha BHC | 3.2 | 57.6 | 219 | 183 | 5 | 219 | 145 | 25 | 29 |
| 14 | Anthraquinone | 4.0 | 65.9 | 208 | 180 | 10 | 208 | 152 | 22 | 90 |
| 15 | Azinphos ethyl | 6.5 | 40.7 | 160 | 132 | 5 | 160 | 105 | 12 | 50 |
| 16 | Azinphos methyl | 6.1 | 74.1 | 160 | 132 | 5 | 160 | 105 | 12 | 50 |
| 17 | beta BHC | 3.3 | 32.6 | 219 | 183 | 5 | 219 | 145 | 25 | 40 |
| 18 | Bifenthrin | 5.6 | 49.2 | 181 | 166 | 10 | 181 | 115 | 50 | 19 |
| 19 | Bromfenvinfos | 4.4 | 15.2 | 323 | 170 | 10 | 267 | 170 | 10 | 50 |
| 20 | Bromfenvinfos methyl | 4.2 | 21.1 | 295 | 170 | 10 | 295 | 109 | 10 | 2 |
| 21 | Bromphos ethyl | 4.4 | 17.4 | 331 | 316 | 14 | 329 | 314 | 14 | 99 |
| 22 | Bromphos methyl | 4.1 | 32.5 | 331 | 316 | 14 | 329 | 314 | 14 | 73 |
| 23 | Bupirimate | 4.7 | 21.5 | 273 | 193 | 5 | 273 | 108 | 5 | 54 |
| 24 | Carbophenothion | 5.1 | 29.7 | 342 | 157 | 10 | 199 | 143 | 10 | 78 |
| 25 | Chlorbenside | 4.3 | 15.2 | 125 | 99 | 18 | 125 | 89 | 16 | 36 |
| 26 | Chlorfenapyr | 4.7 | 38.2 | 247 | 227 | 15 | 247 | 200 | 25 | 84 |
| 27 | Chlorfenson (Ovex) | 4.5 | 13.0 | 175 | 111 | 10 | 111 | 75 | 14 | 100 |
| 28 | Chlorfenvinphos | 4.2 | 22.3 | 295 | 267 | 5 | 267 | 81 | 40 | 66 |
| 29 | Chlorpyrifos methyl | 3.6 | 60.2 | 286 | 271 | 26 | 288 | 93 | 26 | 50 |
| 30 | Chlorpyrifos | 4.0 | 65.9 | 314 | 286 | 5 | 314 | 258 | 5 | 31 |
| 31 | Chlorthiophos | 4.9 | 29.8 | 325 | 269 | 12 | 269 | 205 | 14 | 72 |
| 32 | cis Chlordane | 4.4 | 11.7 | 373 | 301 | 10 | 373 | 266 | 20 | 34 |
| 33 | cis Nonachlor | 5.0 | 15.3 | 409 | 300 | 18 | 263 | 193 | 28 | 82 |
| 34 | cis Permethrin | 6.8 | 74.1 | 183 | 153 | 15 | 163 | 128 | 5 | 6 |
| 35 | Coumaphos | 7.1 | 98.9 | 362 | 109 | 15 | 210 | 182 | 15 | 49 |
| 36 | Cyfluthrin | 5.9 | 36.6 | 263 | 127 | 10 | 226 | 206 | 10 | 50 |
| 37 | Cypermethrin | 6.2 | 35.2 | 209 | 141 | 20 | 163 | 127 | 20 | 5 |
| 38 | Cyprodinil | 4.1 | 18.2 | 224 | 208 | 15 | 224 | 197 | 20 | 29 |
| 39 | delta BHC | 3.4 | 32.6 | 219 | 183 | 10 | 219 | 145 | 20 | 40 |
| 40 | Deltamethrin | 10.1 | 99.2 | 253 | 172 | 5 | 253 | 93 | 5 | 67 |
| 41 | Diazinon | 3.3 | 73.9 | 304 | 179 | 15 | 137 | 84 | 15 | 68 |
| 42 | Dieldrin | 4.5 | 14.5 | 345 | 263 | 8 | 279 | 243 | 8 | 50 |
| 43 | Edifenphos | 5.2 | 44.8 | 310 | 201 | 8 | 310 | 173 | 14 | 28 |
| 44 | Endosulfan ether | 3.5 | 38.8 | 241 | 206 | 14 | 239 | 204 | 12 | 165 |
| 45 | Endosulfan I | 4.4 | 12.2 | 239 | 204 | 15 | 195 | 160 | 5 | 165 |
| 46 | Endosulfan II | 4.8 | 17.3 | 207 | 172 | 15 | 195 | 159 | 10 | 24 |
| 47 | Endosulfan sulfate | 5.2 | 20.1 | 387 | 289 | 5 | 272 | 237 | 15 | 22 |
| 48 | Endrin | 4.9 | 15.8 | 263 | 193 | 35 | 245 | 173 | 30 | 42 |
| 49 | Endrin aldehyde | 5.0 | 15.2 | 250 | 215 | 24 | 173 | 138 | 16 | 95 |
| 50 | Endrin ketone | 5.7 | 74.0 | 250 | 215 | 24 | 173 | 138 | 16 | 96 |
| 51 | EPN | 5.7 | 40.8 | 157 | 110 | 15 | 157 | 77 | 25 | 50 |
| 52 | Ethion | 4.9 | 44.8 | 231 | 175 | 5 | 231 | 129 | 25 | 84 |
| 53 | Ethylan (Perthane) | 4.7 | 18.5 | 223 | 179 | 20 | 223 | 167 | 12 | 87 |
| 54 | Etofenprox | 8.0 | 99.3 | 163 | 135 | 5 | 163 | 107 | 15 | 81 |
| 55 | Fenamiphos | 4.4 | 16.5 | 303 | 195 | 8 | 303 | 154 | 18 | 92 |
| 56 | Fenarimol | 6.4 | 99.0 | 219 | 107 | 10 | 139 | 111 | 15 | 58 |
| 57 | Fenchlorphos (Ronnel) | 3.7 | 34.1 | 285 | 270 | 15 | 285 | 240 | 30 | 80 |
| 58 | Fenitrothion | 3.7 | 43.5 | 277 | 260 | 5 | 277 | 109 | 20 | 55 |
| 59 | Fenson | 4.1 | 23.9 | 141 | 77 | 8 | 77 | 51 | 14 | 19 |
| 60 | Fenthion | 3.9 | 35.2 | 278 | 169 | 20 | 278 | 109 | 20 | 43 |
| 61 | Fenvalerate | 8.9 | 99.1 | 167 | 125 | 12 | 125 | 89 | 20 | 69 |
| 62 | Fipronil | 4.3 | 13.3 | 213 | 178 | 10 | 213 | 143 | 20 | 6 |
| 63 | Flucythrinate | 8.1 | 99.1 | 199 | 157 | 5 | 157 | 107 | 15 | 87 |
| 64 | Fludioxonil | 4.5 | 19.3 | 248 | 154 | 25 | 248 | 127 | 30 | 46 |
| 65 | Fluridone (Sonar) | 8.5 | 99.1 | 328 | 154 | 10 | 310 | 154 | 10 | 50 |
| 66 | Flusilazole | 4.6 | 21.1 | 233 | 165 | 20 | 233 | 152 | 20 | 55 |
| 67 | Flutriafol | 4.5 | 18.5 | 219 | 123 | 12 | 219 | 95 | 20 | 54 |
| 68 | Folpet | 4.3 | 13.8 | 260 | 130 | 14 | 260 | 95 | 20 | 14 |
| 69 | gamma BHC (Lindane) | 3.4 | 28.4 | 219 | 183 | 5 | 219 | 145 | 5 | 16 |
| 70 | Heptachlor | 3.7 | 50.8 | 272 | 237 | 10 | 272 | 143 | 40 | 96 |
| Heptachlor epoxide (isomer B) | 4.2 | 18.7 | 217 | 182 | 22 | 183 | 119 | 25 | 78 | |
| 71 | Hexazinone | 5.3 | 43.6 | 171 | 128 | 5 | 171 | 83 | 10 | 10 |
| 72 | Iodofenphos | 4.5 | 16.6 | 377 | 125 | 10 | 377 | 109 | 15 | 50 |
| 73 | Iprodione | 5.9 | 74.0 | 314 | 245 | 10 | 314 | 56 | 20 | 87 |
| 74 | Isazophos | 3.4 | 74.0 | 257 | 162 | 5 | 161 | 119 | 5 | 12 |
| 75 | Isodrin | 4.2 | 20.8 | 193 | 157 | 20 | 193 | 123 | 28 | 78 |
| 76 | lambda Cyhalothrin | 6.2 | 28.3 | 197 | 161 | 8 | 197 | 141 | 12 | 29 |
| 77 | Lenacil | 5.2 | 60.3 | 153 | 136 | 5 | 153 | 110 | 5 | 54 |
| 78 | Leptophos | 6.1 | 99.2 | 171 | 124 | 10 | 171 | 77 | 18 | 23 |
| 79 | Malathion | 3.8 | 34.1 | 173 | 99 | 15 | 158 | 125 | 15 | 18 |
| 80 | Methacrifos | 3.3 | 60.3 | 208 | 180 | 8 | 208 | 110 | 19 | 12 |
| 81 | MGK-264 | 4.1 | 24.5 | 164 | 98 | 10 | 164 | 93 | 10 | 65 |
| 82 | Mirex | 6.4 | 99.1 | 274 | 239 | 14 | 272 | 237 | 14 | 62 |
| 83 | Myclobutanil | 4.6 | 18.8 | 179 | 152 | 5 | 179 | 125 | 10 | 14 |
| 84 | Paclobutrazol | 4.4 | 17.3 | 236 | 167 | 20 | 236 | 125 | 10 | 6 |
| 85 | Penconazole | 4.2 | 15.0 | 248 | 192 | 15 | 248 | 157 | 25 | 76 |
| 86 | Pentachlorothioanisole | 3.9 | 30.2 | 296 | 263 | 5 | 296 | 246 | 5 | 7 |
| 87 | Phenothrin | 5.9 | 36.6 | 183 | 153 | 15 | 123 | 81 | 8 | 22 |
| 88 | Phosalone | 6.1 | 73.9 | 367 | 182 | 5 | 182 | 138 | 8 | 50 |
| 89 | Phosmet | 5.7 | 60.3 | 160 | 133 | 15 | 160 | 77 | 30 | 50 |
| 90 | Pirimiphos ethyl | 4.1 | 74.1 | 333 | 180 | 5 | 318 | 180 | 5 | 0.2 |
| 91 | Pirimiphos methyl | 3.8 | 43.6 | 305 | 180 | 5 | 290 | 151 | 15 | 87 |
| 92 | Procymidone | 4.3 | 13.8 | 283 | 255 | 8 | 283 | 96 | 8 | 47 |
| 93 | Profenofos | 4.6 | 36.8 | 337 | 309 | 5 | 337 | 267 | 15 | 16 |
| 94 | Prothiofos | 4.5 | 22.5 | 309 | 239 | 15 | 309 | 221 | 25 | 79 |
| 95 | Pyraclofos | 6.5 | 40.7 | 360 | 194 | 5 | 360 | 138 | 5 | 50 |
| 96 | Pyrazophos | 6.4 | 65.8 | 221 | 193 | 10 | 221 | 149 | 15 | 31 |
| 97 | Pyridaphenthion | 5.6 | 65.9 | 340 | 199 | 5 | 340 | 188 | 5 | 17 |
| 98 | Pyrimethanil | 3.3 | 43.6 | 198 | 156 | 25 | 198 | 118 | 25 | 58 |
| 99 | Pyriproxyfen | 6.1 | 74.1 | 136 | 96 | 10 | 136 | 78 | 20 | 63 |
| 100 | Quinalphos | 4.3 | 99.1 | 157 | 129 | 15 | 146 | 91 | 30 | 44 |
| 101 | Resmethrin | 4.2 | 40.8 | 171 | 143 | 6 | 171 | 128 | 12 | 18 |
| 102 | Sulfotepp | 3.4 | 40.8 | 238 | 146 | 10 | 202 | 146 | 10 | 21 |
| 103 | Sulprofos | 5.0 | 26.4 | 322 | 156 | 10 | 156 | 141 | 14 | 35 |
| 104 | tau Fluvalinate | 9.2 | 99.1 | 250 | 200 | 20 | 250 | 55 | 15 | 47 |
| 105 | Tebuconazole | 5.3 | 60.3 | 250 | 153 | 12 | 250 | 125 | 20 | 18 |
| 106 | Tefluthrin | 3.3 | 74.2 | 177 | 137 | 15 | 177 | 127 | 15 | 32 |
| 107 | Terbacil | 3.3 | 57.5 | 161 | 117 | 5 | 160 | 118 | 5 | 24 |
| 108 | Terbufos | 3.5 | 36.6 | 231 | 175 | 10 | 231 | 129 | 25 | 50 |
| 109 | Terbuthylazine | 3.2 | 60.2 | 214 | 173 | 5 | 173 | 132 | 5 | 14 |
| 110 | Tetrachlorvinphos | 4.4 | 16.0 | 329 | 109 | 25 | 329 | 79 | 35 | 28 |
| 111 | Tetradifon | 6.0 | 74.1 | 356 | 229 | 10 | 356 | 159 | 10 | 90 |
| 112 | Tetramethrin | 5.6 | 49.2 | 164 | 123 | 5 | 164 | 81 | 10 | 0.2 |
| 113 | Tolclofos methyl | 3.7 | 34.1 | 265 | 250 | 15 | 265 | 220 | 25 | 52 |
| 114 | trans Chlordane | 4.4 | 13.6 | 373 | 301 | 10 | 373 | 266 | 20 | 51 |
| 115 | trans nonachlor | 4.5 | 12.0 | 409 | 300 | 18 | 237 | 143 | 24 | 53 |
| 116 | trans Permethrin | 6.9 | 74.1 | 183 | 153 | 15 | 163 | 128 | 5 | 7 |
| 117 | Transfluthrin | 3.6 | 49.2 | 163 | 143 | 14 | 163 | 91 | 12 | 97 |
| 118 | Triadimefon | 4.0 | 44.2 | 208 | 181 | 5 | 208 | 127 | 15 | 46 |
| 119 | Triadimenol | 4.2 | 13.3 | 168 | 112 | 4 | 168 | 70 | 10 | 88 |
| 120 | Triflumizole | 4.3 | 12.9 | 278 | 73 | 6 | 278 | 55 | 12 | 50 |
| 121 | Vinclozolin | 3.6 | 74.2 | 212 | 172 | 15 | 212 | 109 | 40 | 78 |
Appendix B
| Origin | Rice Type | Crop Year | |
|---|---|---|---|
| SR1 | India | Yellow long grain | 2019 |
| SR2 | India | Yellow long grain | 2021 |
| SR3 | India | Yellow long grain | 2020 |
| SR4 | India | White long grain | 2021 |
| SR5 | India | White long grain | 2020 |
| SR6 | India | White long grain | 2020 |
| SR7 | India | White long grain | 2019 |
| SR8 | Al Hasa (Saudi Arabia) | Red medium grain | 2020 |
| SR9 | Australia | White short grain | 2021 |
| SR10 | Al Hasa (Saudi Arabia) | Red medium grain | 2020 |
| SR11 | Pakistan | White long grain | 2020 |
| SR12 | Thailand | White medium grain | 2021 |
| SR13 | India | Yellow long grain | 2021 |
| SR14 | India | Yellow long grain | 2020 |
| SR15 | India | Yellow long grain | 2021 |
| SR16 | India | White long grain | 2020 |
| SR17 | Al Hasa (Saudi Arabia) | Red medium grain | 2020 |
| SR18 | USA | Whit medium grain | 2021 |
| SR19 | India | brown long grain | 2019 |
| SR20 | Al Hasa (Saudi Arabia) | Red medium grain | 2021 |
| SR21 | Al Hasa (Saudi Arabia) | Red medium grain | 2021 |
| SR22 | Al Hasa (Saudi Arabia) | Red medium grain | 2019 |
| SR23 | Al Hasa (Saudi Arabia) | Red medium grain | 2019 |
| SR24 | India | White long grain | 2021 |
| SR25 | India | White long grain | 2021 |
| SR26 | Al Hasa (Saudi Arabia) | Red medium grain | 2022 |
| SR27 | Al Hasa (Saudi Arabia) | Red medium grain | 2022 |
| SR28 | USA | Yellow medium grain | 2020 |
| SR29 | Al Hasa (Saudi Arabia) | Red medium grain | 2019 |
| SR30 | India | White long grain | 2021 |
Appendix C
| ID | Origin | Rice Type | Crop Year |
|---|---|---|---|
| BR1 | Rice Mill/Market | White medium grain | 2022 |
| BR2 | Farmers | White medium long grain | 2022 |
| BR3 | Rice Mill/Market | Off-white small grain | 2022 |
| BR4 | Farmers | Off-white small grain | 2022 |
| BR5 | Farmers | Light brown medium grain | 2022 |
| BR6 | Farmers | Off-white medium grain | 2022 |
| BR7 | Farmers | Off-white medium grain | 2022 |
| BR8 | Farmers | Light brown small grain | 2022 |
| BR9 | Rice Mill/Market | White small grain | 2022 |
| BR10 | Rice Mill/Market | Off-white medium grain | 2022 |
| BR11 | Farmers | Yellowish white long grain | 2022 |
| BR12 | Farmers | Off-white medium grain | 2022 |
| BR13 | Farmers | Off-white small grain | 2022 |
| BR14 | Rice Mill/Market | White medium grain | 2022 |
| BR15 | Rice Mill/Market | White medium grain | 2022 |
| BR16 | Farmers | Off-white medium grain | 2022 |
| BR17 | Farmers | Light brown small grain | 2022 |
| BR18 | Farmers | Off-white medium grain | 2022 |
| BR19 | Farmers | Off-white medium grain | 2022 |
| BR20 | Farmers | Light brown small grain | 2022 |
| BR21 | Rice Mill/Market | Light brown small grain | 2022 |
| BR22 | Rice Mill/Market | White small grain | 2022 |
| BR23 | Farmers | Yellowish white medium grain | 2022 |
| BR24 | Farmers | Off-white medium grain | 2022 |
| BR25 | Farmers | Off-white small grain | 2022 |
References
- Lee, S.J.; Park, H.J.; Kim, W.; Jin, J.S.; Abd El-Aty, A.M.; Shim, J.-H.; Shin, S.C. Multiresidue analysis of 47 pesticides in cooked wheat flour and polished rice by liquid chromatography with tandem mass spectrometry. Biomed. Chromatogr. 2009, 23, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Esturk, O.; Yakar, Y.; Ayhan, Z. Pesticide residue analysis in parsley, lettuce and spinach by LC-MS/MS. J. Food Sci. Technol. 2014, 51, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Maul, J.; Berg, T.; Eberhardt, K.; Hoog, I.; Ferrer, G. A laser desorption/resonance enhanced photoionisation TOF-system for the spatially resolved trace analysis of elements. Nucl. Instrum. Methods Phys. Res. 2004, 226, 644–650. [Google Scholar] [CrossRef]
- Guan, W.; Li, C.; Liu, X.; Zhou, S.; Ma, Y. Graphene as dispersive solid phase extraction materials for pesticides LC-MS/MS multi-residue analysis in leek, onion and garlic. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 250–261. [Google Scholar] [CrossRef]
- Shin, J.m.; Choi, S.J.; Park, Y.h.; Kwak, B.; Moon, S.H.; Yoon, Y.T.; Jo, S.A.; Yi, H.; Kim, S.J.; Park, S.K.; et al. Comparison of QuEChERS and Liquid–Liquid extraction methods for the simultaneous analysis of pesticide residues using LC-MS/MS. Food Control 2022, 141, 109202. [Google Scholar] [CrossRef]
- Bhuiya, A.; Yasmin, S.; Shaikh, M.A.A.; Mustafa, M.G.; Kabir, M.H. Method development of multi pesticide residue analysis in country beans collected from Dhaka, Bangladesh, and their dietary risk assessment. Food Chem. 2024, 445, 138741. [Google Scholar] [CrossRef]
- Jahan, T.; Yasmin, S.; Ali Shaikh, M.A.; Ibn Yousuf, M.J.; Islam, M.S.; Islam Choudhury, M.T.; Kabir, M.H. Development and validation of a modified QuEChERS method coupled with LC-MS/MS for simultaneous determination of difenoconazole, dimethoate, pymetrozine, and chlorantraniliprole in brinjal collected from fields and markets places to assess human health risk. Heliyon 2023, 9, e14972. [Google Scholar] [CrossRef]
- Tauseef, M.; Rafique, N.; Ahmad, I.; Ishtiaq, M.; Samad, A.; Saba, S.; Ahad, K.; Mehboob, F. Analysis of multiple pesticide residues in rice by LC–MS/MS. Chem. Pap. 2021, 75, 2871–2879. [Google Scholar] [CrossRef]
- Hou, X.; Han, M.; Dai, X.; Yang, X.; Yi, S. A multi-residue method for the determination of 124 pesticides in rice by modified QuEChERS extraction and gas chromatography–tandem mass spectrometry. Food Chem. 2013, 138, 1198–1205. [Google Scholar] [CrossRef]
- Strashnov, I.; Karunarathna, N.B.; Fernando, B.R.; Dissanayake, C.; Binduhewa, K.M. An isotope dilution liquid chromatography—Mass spectrometry method for detection of melamine in milk powder. Food Addit. Contam. Part A 2021, 38, 1805–1816. [Google Scholar] [CrossRef]
- Lee, J.; Kim, L.; Shin, Y.; Lee, J.; Lee, J.; Kim, E.; Moon, J.-K.; Kim, J.-H. Rapid and Simultaneous Analysis of 360 Pesticides in Brown Rice, Spinach, Orange, and Potato Using Microbore GC-MS/MS. J. Agric. Food Chem. 2017, 65, 3387–3395. [Google Scholar] [CrossRef]
- Lin, X.-Y.; Mou, R.-X.; Cao, Z.-Y.; Cao, Z.-Z.; Chen, M.-X. Analysis of pyrethroid pesticides in Chinese vegetables and fruits by GC–MS/MS. Chem. Pap. 2018, 72, 1953–1962. [Google Scholar] [CrossRef]
- Ahammed Shabeer, T.P.; Girame, R.; Utture, S.; Oulkar, D.; Banerjee, K.; Ajay, D.; Arimboor, R.; Menon, K.R.K. Optimization of multi-residue method for targeted screening and quantitation of 243 pesticide residues in cardamom (Elettaria cardamomum) by gas chromatography tandem mass spectrometry (GC-MS/MS) analysis. Chemosphere 2018, 193, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Hoque, M.S.; Bhowmik, S.; Ferdousi, S.; Kabiraz, M.P.; van Brakel, M.L. Monitoring of pesticide residues from fish feed, fish and vegetables in Bangladesh by GC-MS using the QuEChERS method. Heliyon 2021, 7, e06390. [Google Scholar] [CrossRef]
- Hasan, G.M.M.A.; Das, A.K.; Satter, M.A. Human Health Risk Assessment through the Detection of Organochlorine Pesticides in Vegetables and fruits from Dhaka, Bangladesh by Gas Chromatography Tandem Mass Spectrometry (GC-MS/MS). Curr. Res. Nutr. Food Sci. 2022, 10, 720–732. [Google Scholar] [CrossRef]
- Abo-Gaida, A.-A.H.; Shendy, A.H.; Taha, S.M.; Mahmoud, H.A.; Attallah, E.R.; Fernandez-Alba, A.R. Fennel-seeds extract as an analyte protectant for the GC-MS/MS residue analysis of 182 pesticide in strawberries: Comparing the manual mixing and sandwich injection. J. Chromatogr. Open 2022, 2, 100056. [Google Scholar] [CrossRef]
- BS EN 15662:2018; Foods of Plant Origin. Multimethod for the Determination of Pesticide Residues Using GC- and LC-Based Analysis Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE. Modular QuEChERS-Method. British Standards Institute: London, UK, 2018.
- Alamri, Y.; Reed, M.; Saghaian, S. Competition in the Saudi Arabian rice market. Bulg. J. Agric. Sci. 2020, 26, 275–281. Available online: https://www.agrojournal.org/26/02-03.pdf (accessed on 13 August 2024).
- Almutairi, M.; Alsaleem, T.; Jeperel, H.; Alsamti, M.; Alowaifeer, A.M. Determination of inorganic arsenic, heavy metals, pesticides and mycotoxins in Indian rice (Oryza sativa) and a probabilistic dietary risk assessment for the population of Saudi Arabia. Regul. Toxicol. Pharmacol. 2021, 125, 104986. [Google Scholar] [CrossRef]
- Shraim, A.M. Arsenic and other metals in rice. In Understanding the Geological and Medical Interface of Arsenic, Proceedings of the 4th International Congress on Arsenic in the Environment, Cairns, Australia, 22–27 July 2012; Ng, J.C., Noller, B.N., Naidu, R., Bundschuh, J., Bhattacharya, P., Eds.; CRC Press: London, UK, 2012; Volume 1, pp. 373–375. [Google Scholar]
- Shraim, A.M. Rice is a potential dietary source of not only arsenic but also other toxic elements like lead and chromium. Arab. J. Chem. 2017, 10, S3434–S3443. [Google Scholar] [CrossRef]
- Bangladesh Bureau of Statistics (BBS). Yearbook of Agricultural Statistics-2023. Statistics and Informatics Division (SID), Ministry of Planning, Government of the People’s Republic of Bangladesh. 2023. Available online: https://bbs.gov.bd/site/page/3e838eb6-30a2-4709-be85-40484b0c16c6/Yearbook-of-Agricultural-Statistics (accessed on 29 July 2024).
- Sayeed, K.A.; Yunus, M.M. Rice Prices and Growth, and Poverty Reduction in Bangladesh. In Background Paper to the UNCTAD-FAO, Commodities and Development Report 2017, Commodity Markets, Economic Growth and Development; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2018; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/613ea845-eab1-4e7f-bab8-a53b3874eb90/content (accessed on 29 July 2024).
- Bangladesh Bureau of Statistics (BBS). District Report: Tangail. Agricultural Census 2019, Statistics and Informatics Division, Ministry of Planning, Government of the People’s Republic of Bangladesh. 2022. Available online: http://203.112.218.65:8008/WebTestApplication/userfiles/Image/agri_2023/Tangail.pdf (accessed on 29 July 2024).
- AOAC International. AOAC Official Method 2007.01: Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate. Gas Chromatography/Mass Spectrometry and Liquid Chromatography/Tandem Mass Spectrometry 2007. Available online: https://nucleus.iaea.org/sites/fcris/Shared%20Documents/SOP/AOAC_2007_01.pdf (accessed on 29 July 2024).
- Lehotay, S.J. Determination of Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate: Collaborative Study. J. AOAC Int. 2007, 90, 485–520. [Google Scholar] [CrossRef]
- Hakme, E.; Lozano, A.; Uclés, S.; Fernández-Alba, A.R. Further improvements in pesticide residue analysis in food by applying gas chromatography triple quadrupole mass spectrometry (GC-QqQ-MS/MS) technologies. Anal. Bioanal. Chem. 2018, 410, 5491–5506. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Kim, M.; Kim, E.J.; Choe, W.-J. Determination of 66 pesticide residues in livestock products using QuEChERS and GC–MS/MS. Food Sci. Biotechnol. 2020, 29, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Jang, S.H.; Hur, S.H.; Bang, H.Y.; Bae, I.-K.; Kim, H.J. LC-MS/MS and GC-MS/MS cross-checking analysis method for 247 pesticide residues in sweet pepper (Capsicum annuum). Int. J. Food Prop. 2021, 24, 1758–1776. [Google Scholar] [CrossRef]
- Budakoti, S.K.; Sarvendra, P.S.; Oulkar, D. Large scale screening and quantitation of pesticide residues in rice using GC-(EI)-MS/MS. Application Note: 72952, Thermo Fisher Scientific, India. 2019. Available online: https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/an-72952-gc-ms-pesticides-rice-an72952-en.pdf (accessed on 29 July 2024).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Validation of Analytical Procedures: Text and Methodology Q2(R1). ICH Harmonised Tripartite Guideline. 2005. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 14 August 2024).
- FAO. Understanding International Harmonization of Pesticide Maximum Residue Limits with Codex Standards: A Case Study on Rice; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2020. [Google Scholar] [CrossRef]
- European Commission. EU Pesticides Database. 2024. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls (accessed on 14 August 2024).
- Environmental Protection Agency. Tolerances and Exemptions for Pesticide Chemical Residues in Food. 40 CFR Part 180: Subchapter E—Pesticide Programs; Code of Federal Regulations. 2024. Available online: https://www.ecfr.gov/current/title-40 (accessed on 14 August 2024).
- Saudi Food and Drug Authority. Maximum limits of Pesticide Residues in Agricultural and Food Products. SFDA.FD 382/2018. 2018. Available online: https://apeda.gov.in/apedawebsite/HACCP/SFDA_FD_382_eng.pdf (accessed on 14 August 2024).
- Bangladesh Food Safety Authority. Food Safety (Contaminants, Toxins and Harmful Residues) Regulations, 2017. Ministry of Food, Government of the People’s Republic of Bangladesh. 2017. Available online: https://bfsa.portal.gov.bd/site/view/law/Food-Safety-Related-Law-Rules-Regulations (accessed on 19 August 2024).
- Ahmed, S.; Siddique, M.A.; Rahman, M.; Bari, M.L.; Ferdousi, S. A study on the prevalence of heavy metals, pesticides, and microbial contaminants and antibiotics resistance pathogens in raw salad vegetables sold in Dhaka, Bangladesh. Heliyon 2019, 5, e01205. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Chowdhury, M.A.Z.; Hossain, M.S.; Mijanur Rahman, M.; Rahman, M.A.; Gan, S.H.; Khalil, M.I. Detection of Residual Levels and Associated Health Risk of Seven Pesticides in Fresh Eggplant and Tomato Samples from Narayanganj District, Bangladesh. J. Chem. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahman, M.R.; Prodhan, M.D.H.; Sarker, D.; Rahman, M.M.; Uddin, M.K. Human health risk assessment of pesticide residues in pointed gourd collected from retail markets of Dhaka City, Bangladesh. Accredit. Qual. Assur. 2021, 26, 201–210. [Google Scholar] [CrossRef]
- Nisha, U.S.; Khan, M.S.I.; Prodhan, M.D.H.; Meftaul, I.M.; Begum, N.; Parven, A.; Shahriar, S.; Juraimi, A.S.; Hakim, M.A. Quantification of Pesticide Residues in Fresh Vegetables Available in Local Markets for Human Consumption and the Associated Health Risks. Agronomy 2021, 11, 1804. [Google Scholar] [CrossRef]
- Prodhan, M.D.H.; Afroze, M.; Begum, A.; Sarker, D. Determination of organophosphorus and synthetic pyrethroid pesticide residues and their variability in large size fruit crops. J. Sci. Food Agric. 2021, 101, 4847–4854. [Google Scholar] [CrossRef]
- Habibullah-Al-Mamun, M.; Tanima, S.A.; Paul, B.; Al Zahid, M.; Kabir, M.H.; Ahmed, S.; Mandal, S.C.; Hossain, A. Occurrence of Organochlorine Pesticides (OCPs) Residues in Farmed and Wild Fish in Bangladesh and Implications for Human Health. Expo. Health 2023, 15, 425–437. [Google Scholar] [CrossRef]
- Hasan, G.M.M.A.; Das, A.K.; Satter, M.A. Multi residue analysis of organochlorine pesticides in fish, milk, egg and their feed by GC-MS/MS and their impact assessment on consumers health in Bangladesh. NFS J. 2022, 27, 28–35. [Google Scholar] [CrossRef]
- Hoque, M.S.; Tamanna, F.; Hasan, M.M.; Al Banna, M.H.; Mondal, P.; Prodhan, M.D.H.; Rahman, M.Z.; van Brakel, M.L. Probabilistic public health risks associated with pesticides and heavy metal exposure through consumption of common dried fish in coastal regions of Bangladesh. Environ. Sci. Pollut. Res. Int. 2022, 29, 20112–20127. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, M.; Afsana, M.; Mamun, M.I.R.; Nilufar, N. Organochlorine pesticide residues in poultry meats of Bangladesh. Croat. J. Food Sci. Technol. 2016, 8, 30–33. [Google Scholar] [CrossRef]
- Huda, M.N.; Harun-Ur-Rashid, M.; Hosen, A.; Akter, M.; Islam, M.M.; Emon, S.Z.; Rahman, A.; Jashim, Z.B.; Shahrukh, S.; Ismail, M. A potential toxicological risk assessment of heavy metals and pesticides in irrigated rice cultivars near industrial areas of Dhaka, Bangladesh. Environ. Monit. Assess. 2024, 196, 794. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Hasan, G.M.M.A. Analysis of Pesticide Residual Levels In Maize (Zea mays L.) Grain, Flour and Processed Items in Selected Areas of Dhaka, Bangladesh. Orient. J. Chem./Orient. J. Chem. 2022, 38, 681–687. [Google Scholar] [CrossRef]
- Chowdhury, F.R.; Dewan, G.; Verma, V.R.; Knipe, D.W.; Isha, I.T.; Faiz, M.A.; Gunnell, D.J.; Eddleston, M. Bans of WHO Class I Pesticides in Bangladesh—Suicide prevention without hampering agricultural output. Int. J. Epidemiol. 2018, 47, 175–184. [Google Scholar] [CrossRef]
- Robinson, E.J.Z.; Das, S.R.; Chancellor, T.B.C. Motivations behind farmers’ pesticide use in Bangladesh rice farming. Agric. Hum. Values 2007, 24, 323–332. [Google Scholar] [CrossRef]
- Ali, M.P.; Kabir, M.M.M.; Haque, S.S.; Qin, X.; Nasrin, S.; Landis, D.; Holmquist, B.; Ahmed, N. Farmer’s behavior in pesticide use: Insights study from smallholder and intensive agricultural farms in Bangladesh. Sci. Total Environ. 2020, 747, 141160. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Islam, K.S.; Jahan, M. Rice Farmers’ knowledge of the risks of pesticide use in Bangladesh. J. Health Pollut. 2018, 8, 181203–181209. [Google Scholar] [CrossRef]
- Fan, L.; Niu, H.; Yang, X.; Qin, W.; Bento, C.P.M.; Ritsema, C.J.; Geissen, V. Factors affecting farmers’ behaviour in pesticide use: Insights from a field study in northern China. Sci. Total Environ. 2015, 537, 360–368. [Google Scholar] [CrossRef]
- Houbraken, M.; Bauweraerts, I.; Fevery, D.; Van Labeke, M.-C.; Spanoghe, P. Pesticide knowledge and practice among horticultural workers in the Lâm Đồng region, Vietnam: A case study of chrysanthemum and strawberries. Sci. Total Environ. 2016, 550, 1001–1009. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; AlShahrani, A.M.; Muzammil, K.; Saati, A.A.; Wahab, S.; Elbendary, E.Y.; Kambal, N.; et al. Pesticides impacts on human health and the environment with their mechanisms of action and possible countermeasures. Heliyon 2024, 10, e29128. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mariano, J.Á.; Baltazar-Reyes, M.C.; Salazar-Martínez, E.; Cupul-Uicab, L.A. Exposure to the pesticide DDT and risk of diabetes and hypertension: Systematic review and meta-analysis of prospective studies. Int. J. Hyg. Environ. Health 2022, 239, 113865. [Google Scholar] [CrossRef] [PubMed]
- Jaacks, L.M.; Staimez, L.R. Association of persistent organic pollutants and non-persistent pesticides with diabetes and diabetes-related health outcomes in Asia: A systematic review. Environ. Int. 2015, 76, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Perrin, L.; Spinosi, J.; Chaperon, L.; Kab, S.; Moisan, F.; Ebaz, A. Pesticides expenditures by farming type and incidence of Parkinson disease in farmers: A French nationwide study. Environ. Res. 2021, 197, 111161. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]


| Pesticides | RT, min | LOD, µg/kg | LOQ, µg/kg | Maximum Residue Level (MRL), µg/kg | |||||
|---|---|---|---|---|---|---|---|---|---|
| FAO/WHO Codex | EU | Saudi Arabia | USA | Bangladesh | |||||
| 1 | Azinphos ethyl | 6.5 | 32.3 | 97.8 | 50 | ||||
| 2 | Chlorpyrifos | 3.9 | 3.2 | 9.8 | 500 | 500 | 500 | 100 | 500 |
| 3 | Chlorpyrifos methyl | 3.6 | 7.4 | 22.6 | 3000 | 100 | 6000 | ||
| 4 | Diazinon | 3.2 | 4 | 12.1 | 10 | ||||
| 5 | EPN | 5.6 | 28.8 | 87.3 | |||||
| 6 | Fenitrothion | 3.7 | 10.6 | 32.4 | 6000 | 50 | 20 | ||
| 7 | Isazophos | 3.3 | 14.6 | 44.4 | |||||
| 8 | Phosalone | 6 | 36.6 | 111.0 | 10 | ||||
| 9 | Phosmet | 5.6 | 50.5 | 153 | 10 | ||||
| 10 | Pirimiphos ethyl | 4 | 8.7 | 26.6 | |||||
| 11 | Pirimiphos methyl | 3.7 | 4.3 | 13.1 | 7000 | 500 | 7000 | 500 | |
| 12 | Pyraclofos | 6.5 | 49.8 | 151.1 | |||||
| 13 | Pyrazophos | 6.3 | 33.7 | 102.3 | 10 | ||||
| 14 | Pyridaphenthion | 5.5 | 32.2 | 97.6 | |||||
| 15 | Quinalphos | 4.2 | 55.4 | 167.9 | 10 | 10 | |||
| 16 | Acrinathrin | 6.2 | 12.4 | 37.6 | |||||
| 17 | Allethrin | 4.1 | 51.6 | 156.5 | |||||
| 18 | Anthraquinone | 3.9 | 2.5 | 7.7 | |||||
| 19 | Bifenthrin | 5.5 | 1.1 | 3.3 | 10 | 50 | |||
| 20 | cis- Permethrin | 6.7 | 2.3 | 7.2 | 2000 | 50 | 50 | ||
| 21 | Cypermethrin | 6.1 | 9.9 | 30.1 | 2000 | 2000 | 2000 | 2000 | |
| 22 | Deltamethrin | 9.9 | 22.3 | 67.7 | 2000 | 1000 | 2000 | 1500 | |
| 23 | Fenvalerate | 8.8 | 15.9 | 48.3 | 20 | 2000 | 2500 | ||
| 24 | Flucythrinate | 8 | 13.2 | 40 | |||||
| 25 | lambda Cyhalothrin | 6.1 | 9 | 27.4 | 1000 | 200 | 1000 | 1000 | |
| 26 | Phenothrin | 5.8 | 11 | 33.5 | 50 | 10 | |||
| 27 | Resmethrin | 4.2 | 158.3 | 479.8 | 20 | 3000 | |||
| 28 | tau- Fluvalinate | 9.1 | 21.6 | 65.7 | 10 | ||||
| 29 | Tefluthrin | 3.2 | 18.5 | 56 | 10 | ||||
| 30 | Tetramethrin | 5.5 | 14.2 | 43.2 | |||||
| 31 | trans- Permethrin | 6.8 | 4 | 12.3 | 2000 | 50 | 2000 | ||
| 32 | Transfluthrin | 3.5 | 27.8 | 84.3 | |||||
| 33 | 2,4′ DDD | 4.7 | 2.4 | 7.3 | 50 | ||||
| 34 | 2,4′ DDE | 4.3 | 2.8 | 8.7 | |||||
| 35 | 2,4′ DDT | 4.9 | 4.4 | 13.5 | 100 | 100 | 500 | ||
| 36 | 2,4′ Methoxychlor | 5.2 | 21.1 | 64.2 | |||||
| 37 | 4,4’ DDD | 4.8 | 59.8 | 181.3 | 50 | ||||
| 38 | 4,4’ DDE | 4.5 | 2.3 | 7.2 | |||||
| 39 | 4,4’ DDT | 5.2 | 9.4 | 28.6 | 100 | 100 | 500 | ||
| 40 | 4,4’ Dichlorobenzophenone | 3.9 | 12.2 | 37.1 | |||||
| 41 | Aldrin | 3.9 | 23.6 | 71.6 | 20 | 10 | 20 | 20 | |
| 42 | alpha- BHC | 3.1 | 6.7 | 20.5 | 10 | ||||
| 43 | beta- BHC | 3.2 | 71.5 | 216.7 | 10 | ||||
| 44 | Chlorbenside | 4.3 | 2.8 | 8.7 | 10 | ||||
| 45 | Chlorfenson (Ovex) | 4.5 | 4.2 | 12.7 | 10 | ||||
| 46 | cis- Chlordane | 4.4 | 5.7 | 17.3 | 20 | 20 | |||
| 47 | cis- Nonachlor | 4.9 | 6.2 | 18.8 | |||||
| 48 | delta- BHC | 3.2 | 6 | 18.2 | 10 | ||||
| 49 | Dieldrin | 4.4 | 5.7 | 17.5 | 20 | 10 | 20 | 20 | |
| 50 | Endosulfan ether | 3.5 | 3.8 | 11.6 | |||||
| 51 | Endosulfan I | 4.3 | 16.5 | 50.1 | 50 | ||||
| 52 | Endosulfan II | 4.8 | 13.6 | 41.3 | |||||
| 53 | Endosulfan sulfate | 5.2 | 12.7 | 38.7 | |||||
| 54 | Endrin | 4.8 | 1.4 | 4.3 | 10 | ||||
| 55 | Endrin aldehyde | 5 | 3.4 | 10.5 | |||||
| 56 | Endrin ketone | 5.7 | 3.1 | 9.6 | |||||
| 57 | Ethylan (Perthane) | 4.7 | 2.5 | 7.7 | |||||
| 58 | Fenson | 4.1 | 2.1 | 6.4 | |||||
| 59 | Gamma BHC (Lindane) | 3.4 | 8.6 | 26 | 10 | ||||
| 60 | Heptachlor | 3.7 | 36.8 | 111.7 | 20 | 10 | 20 | 30 | |
| 61 | Heptachlor epoxide (isomer B) | 4.2 | 32.3 | 98 | |||||
| 62 | Isodrin | 4.1 | 6.1 | 18.7 | |||||
| 63 | Mirex | 6.3 | 2.2 | 6.7 | |||||
| 64 | Pentachlorothioanisole | 3.9 | 3.8 | 11.6 | |||||
| 65 | Tetradifon | 5.9 | 1.8 | 5.7 | 10 | ||||
| 66 | trans- Chlordane | 4.3 | 2.2 | 6.6 | 20 | 20 | |||
| 67 | trans- Nonachlor | 4.4 | 8 | 24.4 | |||||
| 68 | Bupirimate | 4.6 | 3.8 | 11.4 | 10 | ||||
| 69 | Chlorfenapyr | 4.7 | 6.1 | 18.5 | 20 | ||||
| 70 | Cyprodinil | 4.1 | 4.1 | 12.5 | 10 | ||||
| 71 | Etofenprox | 7.9 | 6.7 | 20.4 | 10 | 10 | 10 | 10 | 10 |
| 72 | Fenarimol | 6.4 | 2.3 | 6.9 | 20 | ||||
| 73 | Fipronil | 4.1 | 4.3 | 13.1 | 10 | 5 | 10 | 40 | 10 |
| 74 | Fludioxonil | 4.5 | 3.7 | 11.2 | 50 | 10 | 50 | 20 | |
| 75 | Fluridone (Sonar) | 8.5 | 28.3 | 86 | 100 | ||||
| 76 | Flusilazole | 4.6 | 1.5 | 4.5 | 10 | 10 | |||
| 77 | Flutriafol | 4.4 | 4.6 | 14 | 1000 | 500 | |||
| 78 | Folpet | 4.2 | 19.1 | 58 | |||||
| 79 | Hexazinone | 5.2 | 11.6 | 35.2 | |||||
| 80 | Lenacil | 5.1 | 2.4 | 7.4 | 100 | ||||
| 81 | MGK-264 | 4 | 5.2 | 15.9 | |||||
| 82 | Myclobutanil | 4.6 | 4.8 | 14.7 | 10 | 30 | |||
| 83 | Paclobutrazol | 4.3 | 5.1 | 15.4 | 10 | ||||
| 84 | Penconazole | 4.1 | 6.4 | 19.5 | 10 | ||||
| 85 | Procymidone | 4.2 | 6.3 | 19.1 | |||||
| 86 | Pyrimethanil | 3.2 | 2.5 | 7.8 | 50 | ||||
| 87 | Pyriproxyfen | 6 | 3.6 | 10.9 | 50 | 1100 | |||
| 88 | Tebuconazole | 5.3 | 5.3 | 16.1 | 1500 | 1500 | 1500 | 1500 | |
| 89 | Terbacil | 3.3 | 46.2 | 140.2 | |||||
| 90 | Terbuthylazine | 3.1 | 1.8 | 5.6 | 10 | ||||
| 91 | Triadimefon | 3.9 | 4.7 | 14.4 | 10 | ||||
| 92 | Triadimenol | 4.2 | 4.4 | 13.6 | 10 | ||||
| 93 | Triflumizole | 4.2 | 14.4 | 43.6 | 20 | ||||
| 94 | Vinclozolin | 3.5 | 1.5 | 4.6 | 10 | ||||
| 95 | Bromfenvinfos methyl | 4.2 | 10.6 | 32.2 | |||||
| 96 | Bromphos ethyl | 4.3 | 4.3 | 13.2 | 10 | ||||
| 97 | Bromphos methyl | 4 | 18 | 54.7 | |||||
| 98 | Carbophenothion | 5 | 5.4 | 16.4 | |||||
| 99 | Chlorfenvinphos | 4.1 | 5.1 | 15.4 | 10 | ||||
| 100 | Chlorthiophos | 4.9 | 3.1 | 9.3 | |||||
| 101 | Coumaphos | 7 | 16.3 | 49.4 | |||||
| 102 | Edifenphos | 5.1 | 14.5 | 44.1 | 20 | ||||
| 103 | Ethion | 4.8 | 5.3 | 16 | 10 | 30 | |||
| 104 | Fenamiphos | 4.4 | 8.4 | 25.4 | |||||
| 105 | Fenchlorphos (Ronnel) | 3.7 | 8.3 | 25.2 | |||||
| 106 | Fenthion | 3.9 | 10 | 30.5 | 50 | 10 | 50 | 50 | |
| 107 | Iodofenphos | 4.5 | 13.8 | 42 | |||||
| 108 | Leptophos | 6 | 6.4 | 19.4 | |||||
| 109 | Malathion | 3.8 | 7.8 | 23.6 | 8000 | 8000 | |||
| 110 | Profenofos | 4.5 | 6.2 | 18.9 | 10 | ||||
| 111 | Prothiofos | 4.5 | 2.1 | 6.3 | |||||
| 112 | Sulfotepp | 3.2 | 223 | 676 | |||||
| 113 | Sulprofos | 4.9 | 4.4 | 13.4 | |||||
| 114 | Tetrachlorvinphos | 4.3 | 10.6 | 32.3 | |||||
| 115 | Tolclofos methyl | 3.6 | 7.9 | 14.2 | 10 | ||||
| Name | Recovery, % | St. Deviation, n = 3 |
|---|---|---|
| 2,4′ DDD | 85 | 2 |
| 2,4′ DDE | 84 | 4 |
| 2,4′ DDT | 89 | 4 |
| 2,4′ Methoxychlor | 85 | 4 |
| 4,4′ DDD | 81 | 4 |
| 4,4′ DDE | 75 | 3 |
| 4,4′ DDT | 88 | 5 |
| 4,4′ Dichlorobenzophenone | 79 | 3 |
| Acrinathrin | 112 | 13 |
| Aldrin | 76 | 2 |
| Allethrin | 53 | 13 |
| alpha BHC | 96 | 15 |
| Anthraquinone | 82 | 7 |
| Azinphos ethyl | 107 | 21 |
| beta BHC | 63 | 32 |
| Bifenthrin | 89 | 2 |
| Bromfenvinfos methyl | 93 | 23 |
| Bromphos ethyl | 102 | 28 |
| Bromphos methyl | 87 | 8 |
| Bupirimate | 105 | 6 |
| Carbophenothion | 91 | 6 |
| Chlorbenside | 76 | 11 |
| Chlorfenapyr | 104 | 5 |
| Chlorfenson (Ovex) | 95 | 5 |
| Chlorfenvinphos | 95 | 13 |
| Chlorpyrifos methyl | 107 | 23 |
| Chlorpyrifos | 109 | 1 |
| Chlorpyrifos methyl | 88 | 7 |
| Chlorthiophos | 89 | 4 |
| cis Nonachlor | 67 | 4 |
| cis Permethrin | 86 | 4 |
| Coumaphos | 111 | 15 |
| Cypermethrin | 122 | 12 |
| Cyprodinil | 102 | 5 |
| delta BHC | 75 | 3 |
| Deltamethrin | 116 | 13 |
| Diazinon | 85 | 13 |
| Dieldrin | 87 | 8 |
| Edifenphos | 96 | 24 |
| Endosulfan ether | 98 | 2 |
| Endosulfan I | 109 | 16 |
| Endosulfan II | 76 | 7 |
| Endosulfan sulfate | 100 | 9 |
| Endrin | 88 | 4 |
| Endrin aldehyde | 45 | 5 |
| Endrin ketone | 91 | 5 |
| EPN | 93 | 5 |
| Ethion | 97 | 5 |
| Ethylan (Perthane) | 88 | 2 |
| Etofenprox | 104 | 5 |
| Fenarimol | 114 | 9 |
| Fenchlorphos (Ronnel) | 80 | 4 |
| Fenitrothion | 78 | 10 |
| Fenson | 69 | 5 |
| Fenthion | 81 | 6 |
| Fenvalerate | 112 | 9 |
| Fipronil | 109 | 4 |
| Flucythrinate | 115 | 13 |
| Fludioxonil | 110 | 11 |
| Flusilazole | 116 | 13 |
| Flutriafol | 112 | 6 |
| Folpet | 12 | 2 |
| gamma BHC (Lindane) | 144 | 85 |
| Heptachlor | 75 | 17 |
| Heptachlor epoxide (isomer B) | 38 | 10 |
| Hexazinone | 127 | 22 |
| Iodofenphos | 99 | 6 |
| Iprodione | 102 | 5 |
| Isazophos | 97 | 18 |
| Isodrin | 77 | 20 |
| lambda Cyhalothrin | 118 | 9 |
| Lenacil | 128 | 29 |
| Leptophos | 90 | 7 |
| Malathion | 92 | 12 |
| MGK-264 | 73 | 4 |
| Mirex | 53 | 5 |
| Myclobutanil | 115 | 10 |
| Paclobutrazol | 116 | 4 |
| Penconazole | 107 | 13 |
| Pentachlorothioanisole | 33 | 18 |
| Phenothrin | 82 | 4 |
| Phosalon | 132 | 28 |
| Phosalone | 130 | 26 |
| Phosmate | 159 | 36 |
| Phosmet | 162 | 34 |
| Pirimiphos ethyl | 76 | 3 |
| Pirimiphos methyl | 83 | 18 |
| Procymidone | 110 | 5 |
| Profenofos | 90 | 16 |
| Prothiofos | 82 | 3 |
| Pyrazophos | 107 | 14 |
| Pyridaphenthion | 107 | 14 |
| Pyrimethanil | 96 | 14 |
| Pyriproxyfen | 114 | 6 |
| Quinalphos | 77 | 9 |
| Sulfotepp | 96 | 19 |
| Sulprofos | 79 | 4 |
| tau Fluvalinate | 126 | 26 |
| Tebuconazole | 134 | 13 |
| Tefluthrin | 92 | 4 |
| Terbacil | 163 | 99 |
| Terbuthylazine | 96 | 49 |
| Tetrachlorvinphos | 90 | 25 |
| Tetradifon | 94 | 3 |
| Tetramethrin | 163 | 25 |
| Tolclofos methyl | 74 | 5 |
| trans Chlordane | 84 | 6 |
| trans nonachlor | 90 | 11 |
| trans Permethrin | 88 | 5 |
| Transfluthrin | 88 | 18 |
| Triadimefon | 102 | 8 |
| Triadimenol | 113 | 10 |
| Triflumizole | 110 | 18 |
| Vinclozolin | 106 | 4 |
| Sample Number | Exp. Run 1, µg/kg | Exp. Run 2, µg/kg | Exp. Run 3, µg/kg | Mean, µg/kg | St. Dev |
|---|---|---|---|---|---|
| Chlorpyrifos | |||||
| 3 | 28.6 | 18.8 | 16.4 | 21.3 | 6.5 |
| 15 | 29.8 | 26.8 | 24.6 | 27.1 | 2.6 |
| 20 | 75.4 | 70.6 | 69.8 | 71.9 | 3.0 |
| 56 | 23.4 | 23.2 | 21.2 | 22.6 | 1.2 |
| Tebuconazole | |||||
| 2 | 66.2 | 63.8 | 68.0 | 66.0 | 2.1 |
| 7 | 48.4 | 49.2 | 48.8 | 48.8 | 0.4 |
| 14 | 69.4 | 67.6 | 70.0 | 69.0 | 1.2 |
| 17 | 39 | 39.4 | 37.4 | 38.6 | 1.1 |
| 25 | 38.2 | 40 | 40.2 | 39.5 | 1.1 |
| 32 | 43.4 | 42.6 | 44.3 | 43.4 | 0.9 |
| 37 | 35 | 32.8 | 36.4 | 34.7 | 1.8 |
| 41 | 43.8 | 42.6 | 38.6 | 41.7 | 2.7 |
| 45 | 38.6 | 37.4 | 37 | 37.7 | 0.8 |
| 53 | 50.2 | 49.2 | 45.4 | 48.3 | 2.5 |
| Pirimiphos methyl | |||||
| 17 | 12.6 | 10.6 | 8.8 | 10.7 | 1.9 |
| 36 | 14.4 | 11.6 | 10.4 | 12.1 | 2.1 |
| 46 | 22.4 | 20.8 | 19 | 20.7 | 1.7 |
| Pesticide | MRL FAO/WHO Codex, µg/kg | MRL EU, µg/kg | MRL Saudi Arabia, µg/kg | MRL USA, µg/kg | MRL Bangladesh, µg/kg |
|---|---|---|---|---|---|
| Chlorpyrifos | 500 | 500 | 500 | 100 | 500 |
| Tebuconazole | 1500 | 1500 | 1500 | 1500 | |
| Pirimiphos methyl | 7000 | 500 | 7000 | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strashnov, I.; Ahmed, F.T.; Alrashdi, M.M.; Nesmiyan, I.; Polya, D.A. Gas Chromatography Tandem Mass Spectrometry (GC-MS/MS) for High-Throughput Screening of Pesticides in Rice Samples Collected in Bangladesh and Saudi Arabia. Processes 2024, 12, 2170. https://doi.org/10.3390/pr12102170
Strashnov I, Ahmed FT, Alrashdi MM, Nesmiyan I, Polya DA. Gas Chromatography Tandem Mass Spectrometry (GC-MS/MS) for High-Throughput Screening of Pesticides in Rice Samples Collected in Bangladesh and Saudi Arabia. Processes. 2024; 12(10):2170. https://doi.org/10.3390/pr12102170
Chicago/Turabian StyleStrashnov, Ilya, Farah T. Ahmed, May M. Alrashdi, Inna Nesmiyan, and David A. Polya. 2024. "Gas Chromatography Tandem Mass Spectrometry (GC-MS/MS) for High-Throughput Screening of Pesticides in Rice Samples Collected in Bangladesh and Saudi Arabia" Processes 12, no. 10: 2170. https://doi.org/10.3390/pr12102170
APA StyleStrashnov, I., Ahmed, F. T., Alrashdi, M. M., Nesmiyan, I., & Polya, D. A. (2024). Gas Chromatography Tandem Mass Spectrometry (GC-MS/MS) for High-Throughput Screening of Pesticides in Rice Samples Collected in Bangladesh and Saudi Arabia. Processes, 12(10), 2170. https://doi.org/10.3390/pr12102170








