Cost-Effective Optimization of the Transfructosylation Activity of an Invertase Produced from Aspergillus carbonarius PC-4 Using Pineapple Crown and Determination of Its Biochemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Microorganism and Maintenance
2.2.2. Submerged Culture Conditions
2.2.3. The Transfructosylation Activity of the Invertase from A. carbonarius PC-4
2.2.4. Optimization of the Production of Transfructosylation Activity
Plackett–Burman Design
Central Composite Rotatable Design
2.2.5. The Biochemical Properties of the Invertase
Effect of pH and Temperature on Invertase Activity
The Effects of pH and Temperature on the Stability of the Transfructosylation Activity
2.2.6. Statistical Analysis
3. Results and Discussion
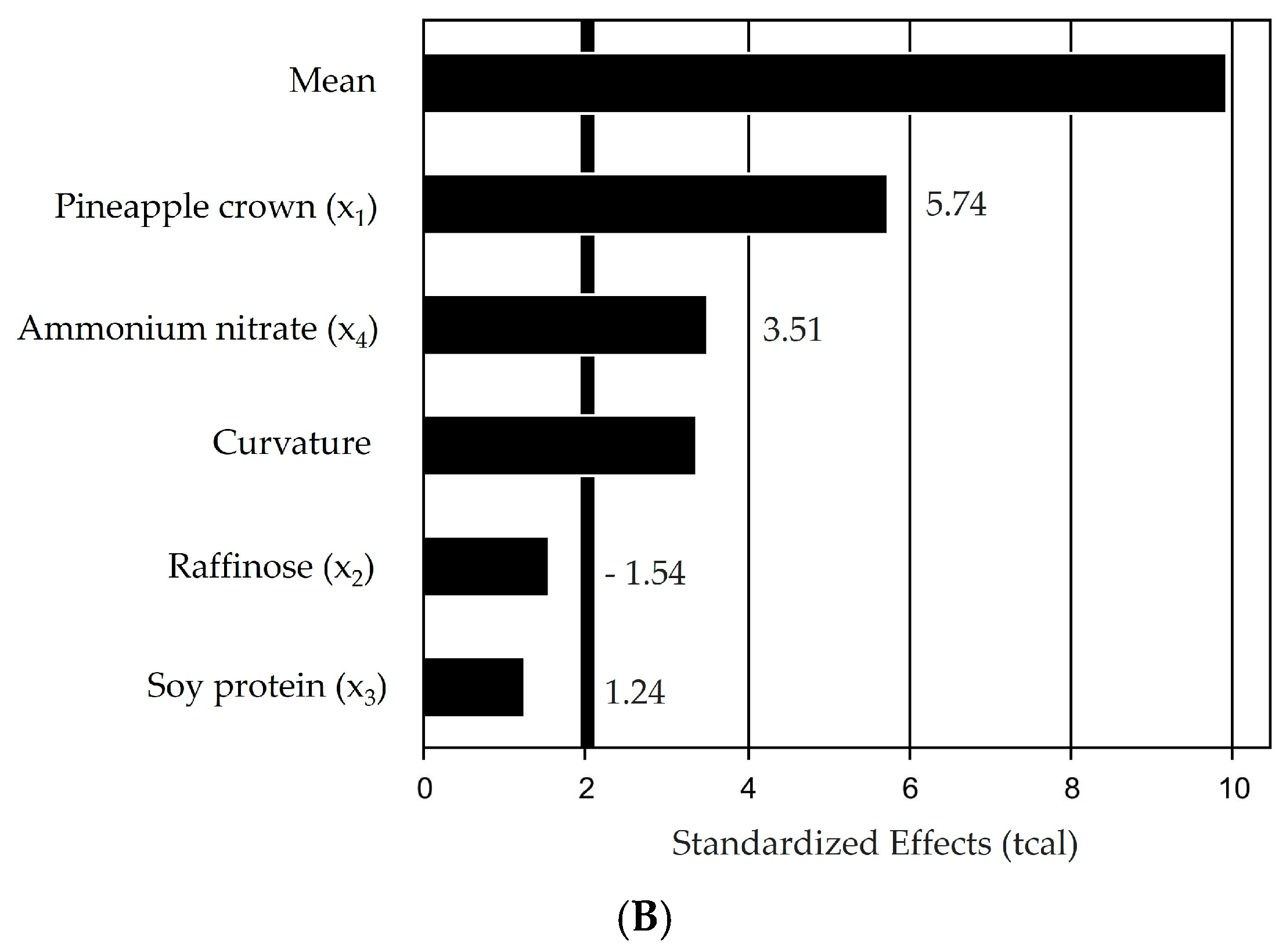
3.1. Screening of Carbon and Nitrogen Sources by the Plackett–Burman Design
3.2. Medium Composition Optimization by Central Composite Rotational Design (CCRD)
3.3. Validation
3.4. The Physical and Chemical Properties of the Transfructosylation Activity
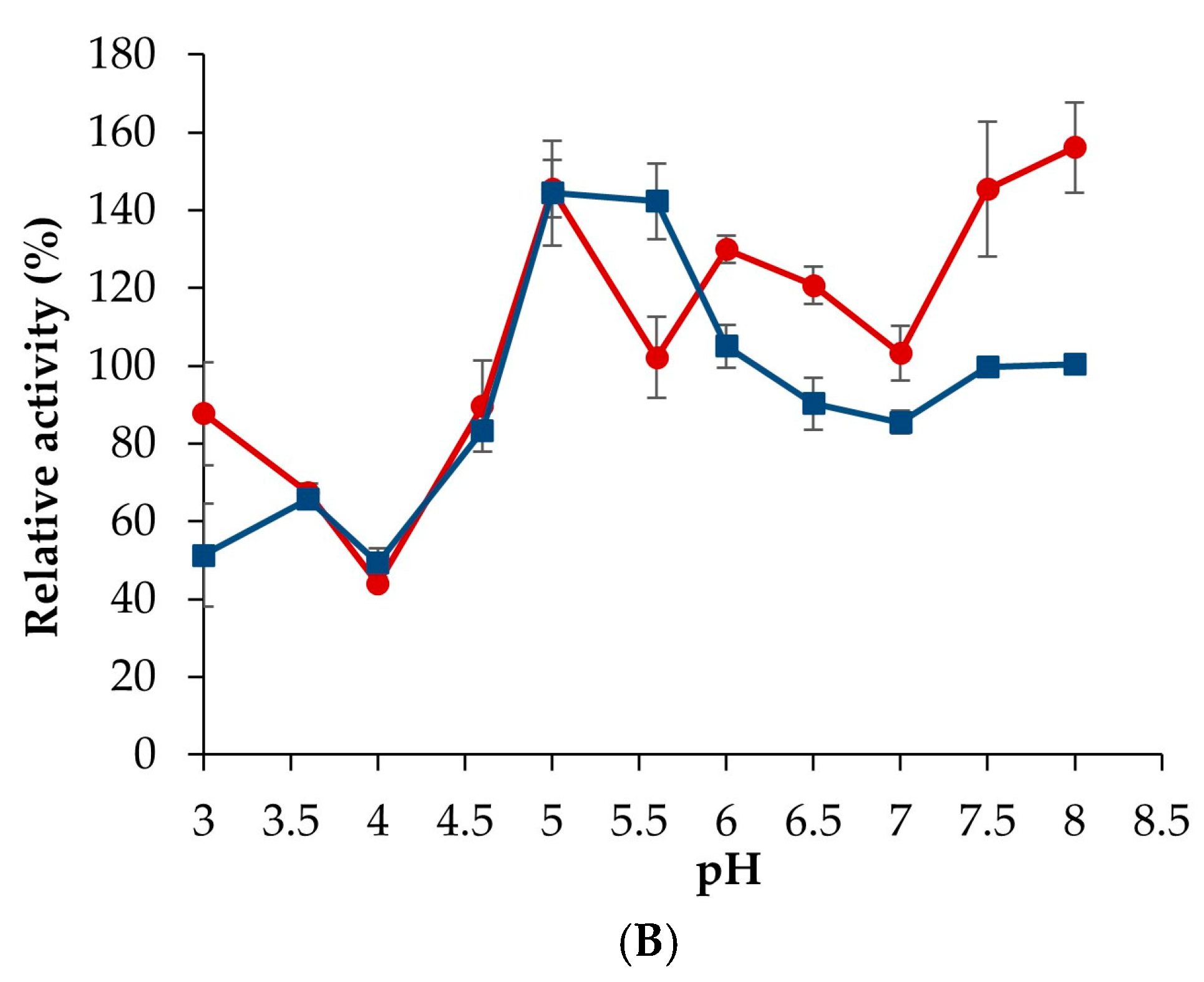
3.4.1. Influence of Temperature and pH on Enzyme Activity
3.4.2. Validation
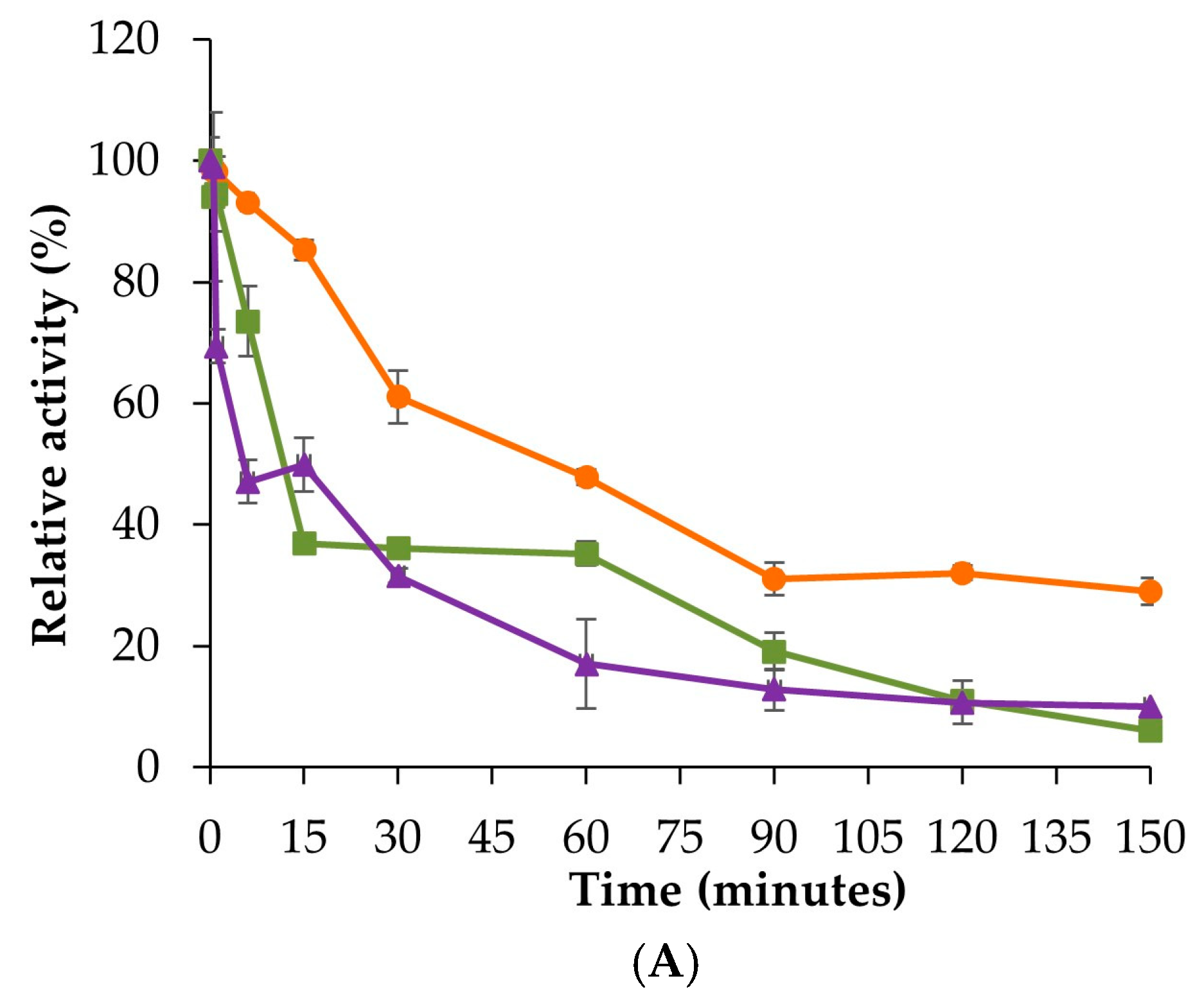
3.5. Thermal Stability and pH Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manoochehri, H.; Hosseini, N.F.; Saidijam, M.; Taheri, M.; Rezaee, H.; Nouri, F. A review on invertase: Its potentials and applications. Biocatal. Agric. Biotechnol. 2020, 25, 101599–101610. [Google Scholar] [CrossRef]
- Panetta, J.C.; Barbuto, O.J.M.; Riccetti, R.V.; Moreno, A.G. Ocorrência de microrganismos responsáveis pela deterioração de um produto cárneo de baixa acidez. Rev. Fac. Med. Vet. Zootec. Univ. São Paulo 1976, 13, 241–247. [Google Scholar] [CrossRef][Green Version]
- Karkeszová, K.; Polakovic, M. Production of Fructooligosaccharides Using a Commercial Heterologously Expressed Aspergillus sp. Fructosyltransferase. Catalysts 2023, 13, 843. [Google Scholar] [CrossRef]
- Han, S.; Ye, T.; Leng, S.; Pan, L.; Zeng, W.; Chen, G.; Liang, Z. Purification and biochemical characteristics of a novel fructosyltransferase with a high FOS transfructosylation activity from Aspergillus oryzae S719. Protein Expr. Purif. 2020, 167, 105549–105558. [Google Scholar] [CrossRef]
- Nascimento, G.C.; Batista, R.D.; Santos, C.C.A.D.A.; Silva, E.M.; Paula, F.C.; Mendes, D.B.; Oliveira, D.P.; Almeida, A.F. β-Fructofuranosidase and β-D-Fructosyltransferase from New Aspergillus carbonarius PC-4 Strain Isolated from Canned Peach Syrup: Effect of Carbon and Nitrogen Sources on Enzyme Production. Sci. World J. 2019, 1, 6956202. [Google Scholar] [CrossRef]
- Osiebe, O.; Adewale, I.O.; Omafuvbe, B.O. Production and characterization of intracellular invertase from Saccharomyces cerevisiae (OL629078.1), using cassava-soybean as a cost-effective substrate. Sci. Rep. 2023, 13, 16295–16304. [Google Scholar] [CrossRef]
- Michel, M.R.; Flores-Gallegos, A.C.; Villarreal-Morales, S.L.; Aguilar-Zárate, P.; Aguilar, C.N.; Riutort, M.; Rodríguez-Herrera, R. Fructosyltransferase production by Aspergillus oryzae BM-DIA using solid-state fermentation and the properties of its nucleotide and protein sequences. Folia Microbiol. 2021, 66, 469–481. [Google Scholar] [CrossRef]
- Belmonte-Izquierdo, Y.; Salomé-Abarca, L.F.; González-Hernández, J.C.; Francisco, L.; López, M.G. Fructooligosaccharides (FOS) production by microorganisms with fructosyltransferase activity. Fermentation 2023, 9, 968. [Google Scholar] [CrossRef]
- Correa, A.C.; Lopes, M.S.; Perna, R.F.; Silva, E.K. Fructan-type prebiotic dietary fibers: Clinical studies reporting health impacts and recent advances in their technological application in bakery, dairy, meat products and beverages. Carbohydr. Polym. 2024, 323, 121396. [Google Scholar] [CrossRef]
- Ho, J.; Nicolucci, A.C.; Virtanen, H.; Schik, A.; Meddings, J.; Reimer, R.A.R.; Huang, C. Effect of prebiotic on microbiota, intestinal permeability, and glycemic control in children with type 1 diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 4427–4440. [Google Scholar] [CrossRef]
- Sheu, D.-C.; Chang, J.-Y.; Chen, Y.-J.; Lee, C.-W. Production of high-purity neofructooligosaccharides by culture of Xanthophyllomyces dendrorhous. Bioresour. Technol. 2013, 132, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, A.L.; Rodrigues, L.R.; Lima, N.M.; Teixeira, J.A. An Overview of the recent developments on fructooligosaccharide production and applications. Food Bioprocess Technol. 2014, 7, 324–337. [Google Scholar] [CrossRef]
- Choukade, R.; Kango, N. Production, properties, and applications of fructosyltransferase: A current appraisal. Crit. Rev. Biotechnol. 2021, 41, 1178–1193. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, R.; Avonts, L.; De Vuyst, L. Short fractions of oligofructose are preferentially metabolized by Bifidobacterium animalis DN-173 010. Appl. Environ. Microbiol. 2004, 70, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- De La Rosa, O.; Flores-Gallegos, A.C.; Muñiz-Márquez, D.B.; Ochoa-Zarzosa, A.; Rodríguez-Herrera, R.; Aguilar, C.N. Fructooligosaccharides production from agro-wastes as alternative low-cost source. Trends Food Sci. Technol. 2019, 91, 139–146. [Google Scholar] [CrossRef]
- Ganaie, M.A.; Soni, H.; Naikoo, G.A.; Oliveira, L.T.S.; Rawat, H.K.; Mehta, P.K.; Narain, N. Screening of low-cost agricultural wastes to maximize the fructosyltransferase production and its applicability in generation of fructooligosaccharides by solid state fermentation. Int. Biodeterior. Biodegrad. 2017, 118, 19–26. [Google Scholar] [CrossRef]
- Ojwach, J.; Kumar, A.; Mutanda, T.; Mukaratirwa, S. Fructosyltransferase and inulinase production by indigenous coprophilous fungi for the biocatalytic conversion of sucrose and inulin into oligosaccharides. Biocatal. Agric. Biotechnol. 2020, 30, 101867–101875. [Google Scholar] [CrossRef]
- Uday, U.S.P.; Choudhury, P.; Bandyopadhyay, T.K.; Bhunia, B. Classification, mode of action and production strategy of xylanase and its application for biofuel production from water hyacinth. Int. J. Biol. Macromol. 2016, 82, 1041–1054. [Google Scholar] [CrossRef]
- Maiorano, A.E.; Piccoli, R.M.; Silva, E.S.; Rodrigues, M.F.A. Microbial production of fructosyltransferases for synthesis of pre-biotics. Biotechnol. Lett. 2008, 30, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Kavuthodi, B.; Sebastian, D. Biotechnological valorization of pineapple stem for pectinase production by Bacillus subtilis BKDS1: Media formulation and statistical optimization for submerged fermentation. Biocatal. Agric. Biotechnol. 2018, 16, 715–722. [Google Scholar] [CrossRef]
- IBGE—Instituto Brasileiro de Geografia e Estatística. Produção Agrícola. Lavoura Temporária. 2023. Available online: https://cidades.ibge.gov.br/brasil/pesquisa/14/0 (accessed on 28 August 2024).
- Almeida, J.M.; Lima, V.A.; Lima, P.C.G.; KNOB, A. Effective and low-cost saccharification of pineapple peel by Trichoderma viride crude extract with enhanced beta-glucosidase activity. BioEnergy Res. 2016, 9, 701–710. [Google Scholar] [CrossRef]
- Ademakinwa, A.N.; Ayinla, Z.A.; Agboola, F.K. Strain improvement and statistical optimization as a combined strategy for improving fructosyltransferase production by Aureobasidium pullulans NAC8. J. Genet. Eng. Biotechnol. 2017, 15, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Castellani, A. Maintenance and cultivation of the common pathogenic fungi of man in sterile distilled water. J. Trop. Med. Hyg. 1967, 70, 181–184. [Google Scholar]
- Vogel, H.J. A convenient growth medium for Neurospora crassa (medium N). Microbiol. Genet. Bull. 1956, 13, 42–43. [Google Scholar]
- Rawat, H.K.; Ganaie, M.A.; Kango, N. Production of inulinase, fructosyltransferase and sucrase from fungi on low-value inulin-rich substrates and their use in generation of fructose and fructo-oligosaccharides. Antonie Van Leeuwenhoek 2015, 107, 799–811. [Google Scholar] [CrossRef]
- Plackett, R.L.; Burman, J.P. The design of optimum multifactorial experiments. Biometrika 1946, 33, 305–325. [Google Scholar] [CrossRef]
- Rodrigues, M.I.; Iemma, A.F. Experimental Design and Process Optimization, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–336. [Google Scholar] [CrossRef]
- Tan, T.; Zhang, M.; Xu, J.; Zhang, J. Optimization of culture conditions and properties of lipase from Penicillium camembertii Thom PG-3. Process Biochem. 2004, 39, 1495–1502. [Google Scholar] [CrossRef]
- Garai, D.; Kumar, V. A Box-Behnken design approach for the production of xylanase by Aspergillus candidus under solid state fermentation and its application in saccharification of agro residues and Parthenium hysterophorus L. Ind. Crops Prod. 2013, 44, 352–363. [Google Scholar] [CrossRef]
- Suganthi, R.; Benazir, J.F.; Santhi, R.; Ramesh Kumar, V.; Hari, A.; Meenakshi, N.; Nidhyia, K.A.; Kavitha, G.; Lakshmi, R. Amylase production by Aspergillus niger under solid state fermentation using agroindustrial wastes. Int. J. Eng. Sci. Technol. 2011, 3, 1756–1760. [Google Scholar]
- Almeida, A.F.; Tauk-Tornisielo, S.M.; Carmona, E.C. Influence of carbon and nitrogen sources on lipase production by a newly Candida viswanathii strain. Ann. Microbiol. 2013, 63, 1225–1234. [Google Scholar] [CrossRef]
- Siti Roha, A.M.; Zainal, S.; Noriham, A.; Nadzirah, K.Z. Determination of sugar content in pineapple waste variety N36. Int. Food Res. J. 2013, 20, 1941–1943. [Google Scholar]
- Oyedeji, O.; Bakare, M.K.; Adewale, I.O.; Olutiola, P.O.; Omoboye, O.O. Optimized production and characterization of thermostable invertase from Aspergillus niger IBK1, using pineapple peel as alternate substrate. Biocatal. Agric. Biotechnol. 2017, 9, 218–223. [Google Scholar] [CrossRef]
- Knob, A.; Fortkamp, D.; Prolo, T.; Izidoro, S.C.; Almeida, J.M. Agro-residues as alternative for xylanase production by filamentous fungi. Bioresour. Technol. 2014, 9, 5738–5773. [Google Scholar]
- Saravanan, P.; Muthuvelayudham, R.; Viruthagiri, T. Enhanced production of cellulase from pineapple waste by response surface methodology. J. Eng. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Nascimento, A.K.C.; Nobre, C.; Cavalcanti, M.T.H.; Teixeira, J.A.; Porto, A.L.F. Screening of fungi from the genus Penicillium for production of β-fructofuranosidase and enzymatic synthesis of fructooligosaccharides. J. Mol. Catal. B Enzym. 2016, 134, 70–78. [Google Scholar] [CrossRef][Green Version]
- Amin, F.; Bhatti, H.N.; Rehman, S. Optimization of growth parameters for lipase production by Ganoderma lucidum using response surface methodology. Afr. J. Biotechnol. 2011, 10, 5514–5523. [Google Scholar]
- Park, J.P.; Bae, J.T.; Yun, J.W. Critical effect of ammonium ions on the enzymatic reaction of a novel transfructosylating enzyme for fructooligosaccharide production from sucrose. Biotechnol. Lett. 1999, 21, 987–990. [Google Scholar] [CrossRef]
- Ghazi, I.; Fernandez-Arrojo, L.; Garcia-Arellano, H.; Ferrer, M.; Ballesteros, A.; Plou, F.J. Purification and kinetic characterization of a fructosyltransferase from Aspergillus aculeatus. J. Biotechnol. 2007, 128, 204–211. [Google Scholar] [CrossRef]
- Nemukula, A.; Mutanda, T.; Wilhelmi, B.S.; Whiteley, C.G. Response surface methodology: Synthesis of short chain fructooligosaccharides with a fructosyltransferase from Aspergillus aculeatus. Bioresour. Technol. 2009, 100, 2040–2045. [Google Scholar] [CrossRef]
- Aguiar-Oliveira, E.; Maugeri, F. Characterization of the Immobilized Fructosyltransferase from Rhodotorula sp. Int. J. Food Eng. 2010, 6, 1–21. [Google Scholar] [CrossRef]
- Kashyap, R.; Palai, T.; Bhattacharya, P.K. Kinetics and model development for enzymatic synthesis of fructo-oligosaccharides using fructosyltransferase. Bioprocess Biosyst. Eng. 2015, 38, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- L’Hocine, L.; Wang, Z.; Jiang, B.; Xu, S. Purification and partial characterization of fructosyltransferase and invertase from Aspergillus niger AS0023. J. Biotechnol. 2000, 81, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-A.; Zhou, K.; Liu, D.-L.; Brennan, C.S.; Brennan, M.; Zhou, J.-S.; Yu, S.-J. Preparation of fructooligosaccharides using Aspergillus niger 6640 whole-cell as catalyst for bio-transformation. Food Sci. Technol. 2016, 65, 1072–1079. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Zhang, L.; Shen, W. Heterologous expression and enzymatic characterization of fructosyltransferase from Aspergillus niger in Pichia pastoris. New Biotechnol. 2016, 33, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.M. Princípios de Bioquímica de Lehninger, 5th ed.; Artmed: Porto Alegre, Brasil, 2011; pp. 189–193. [Google Scholar]
- Keramat, A.; Kargari, A.; Sohrabi, M.; Mirshekar, H.; Sanaeepur, H. Kinetic model for invertase-induced sucrose hydrolysis: Initial time lag. Chem. Eng. Technol. 2017, 40, 529–536. [Google Scholar] [CrossRef]



| Factors | Code | −1 | 0 | 1 |
|---|---|---|---|---|
| Pineapple crown | X1 | 0 | 5 | 10 |
| Raffinose | X2 | 0 | 5 | 10 |
| Soy protein | X3 | 0 | 1 | 2 |
| Ammonium nitrate | X4 | 0 | 1 | 2 |
| Factors | Code | −1.41 | −1 | 0 | 1 | 1.41 |
|---|---|---|---|---|---|---|
| Pineapple crown | X1 | 6 | 10 | 20 | 30 | 34 |
| Ammonium nitrate | X2 | 0.5 | 3 | 9 | 15 | 17.5 |
| Factors | Code | −1.41 | −1 | 0 | 1 | 1.41 |
|---|---|---|---|---|---|---|
| pH | X1 | 2.17 | 3 | 5 | 7 | 7.83 |
| Temperature (°C) | X2 | 14.64 | 25 | 50 | 75 | 85.36 |
| Run | Pineapple Crown (X1, g/L) | Ammonium Nitrate (X2, g/L) | TFA (U/mL) | Yp/s (U/g) | Pp (U/h) |
|---|---|---|---|---|---|
| 1 | 10 | 3 | 35.18 ± 1.50 | 2526.17 | 7.02 |
| 2 | 30 | 3 | 16.73 ± 0.35 | 409.49 | 3.41 |
| 3 | 10 | 15 | 29.88 ± 5.42 | 1711.80 | 4.75 |
| 4 | 30 | 15 | 40.83 ± 7.61 | 941.26 | 7.84 |
| 5 | 6 | 9 | 24.92 ± 3.23 | 2891.45 | 4.82 |
| 6 | 34 | 9 | 16.73 ± 3.58 | 309.41 | 2.92 |
| 7 | 20 | 0.5 | 32.76 ± 0.69 | 1443.03 | 8.02 |
| 8 | 20 | 17.5 | 65.86 ± 0.12 | 2958.73 | 16.44 |
| 9 | 20 | 9 | 68.98 ± 3.92 | 2916.05 | 16.20 |
| 10 | 20 | 9 | 69.44 ± 3.00 | 2657.67 | 14.76 |
| 11 | 20 | 9 | 52.48 ± 3.35 | 2233.18 | 12.41 |
| Factors | Coefficient | Error | t | p-Value |
|---|---|---|---|---|
| Média | 63.63 | 4.66 | 13.65 | 0.000 |
| X1 | −2.38 | 2.85 | −0.84 | 0.441 |
| X12 | −22.51 | 3.40 | −6.63 | 0.001 |
| X2 | 8.20 | 2.85 | 2.87 | 0.035 |
| X22 | −8.26 | 3.40 | −2.43 | 0.059 |
| X1.X2 | 7.35 | 4.04 | 1.82 | 0.128 |
| Variation Source | Sum of Squares | Degree of Freedom | Mean Square | Fcal | p-Value |
|---|---|---|---|---|---|
| Regression | 3415.5 | 3 | 1138.5 | 13.6 | 0.00265 |
| Residue | 587.4 | 7 | 83.9 | ||
| Lack of fit | 400.7 | 5 | 80.1 | 0.9 | 0.61566 |
| Pure error | 186.7 | 2 | 93.4 |
| Variable | Experimental Conditions | Predicted Value | Experimental Value |
|---|---|---|---|
| Pineapple crown | 20 g/L | 65.33 ± 4.62 U/mL | 66.46 ± 2.67 U/mL |
| Ammonium nitrate | 10.5 g/L |
| Run | pH X1 | Temperature (°C) X2 | TFA (U/mL) | Relative Activity (%) |
|---|---|---|---|---|
| 1 | 3 | 25 | 0.00 | 0.00 |
| 2 | 7 | 25 | 2.01 | 3.49 |
| 3 | 3 | 75 | 0.00 | 0.00 |
| 4 | 7 | 75 | 0.86 | 1.47 |
| 5 | 2.17 | 50 | 0.01 | 0.01 |
| 6 | 7.83 | 50 | 1.36 | 2.35 |
| 7 | 5 | 14.64 | 2.09 | 3.62 |
| 8 | 5 | 85.36 | 0.09 | 0.15 |
| 9 | 5 | 50 | 54.55 | 89.48 |
| 10 | 5 | 50 | 57.65 | 94.62 |
| 11 | 5 | 50 | 51.59 | 100 |
| Factors | Coefficient | Error | t | p-Value |
|---|---|---|---|---|
| Mean | 54.60 | 1.12 | 48.64 | 0.000 |
| X1 | 0.60 | 0.69 | 0.87 | 0.4243 |
| X12 | −27.00 | 0.82 | −33.00 | 0.0000 |
| X2 | −0.50 | 0.69 | −0.73 | 0.4997 |
| X22 | −26.80 | 0.82 | −32.75 | 0.0000 |
| X1.X2 | −0.29 | 0.97 | −0.30 | 0.7778 |
| Variation Source | Sum of Squares | DF | Mean Square | Fcal | p-Value |
|---|---|---|---|---|---|
| Regression | 6314.5 | 2 | 3157.3 | 1048.5 | 0.00000 |
| Residue | 24.1 | 8 | 3.0 | ||
| Lack of fit | 5.7 | 6 | 1.0 | 0.0 | 0.99683 |
| Pure error | 18.4 | 2 | 9.2 | ||
| Total | 6338.6 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, R.D.; Carvalho do Nascimento, G.; Carvalho, N.B.; Leite, P.C.; Basso, R.C.; Morales, S.A.V.; Xavier, M.d.C.A.; Perna, R.F.; Almeida, A.F.d. Cost-Effective Optimization of the Transfructosylation Activity of an Invertase Produced from Aspergillus carbonarius PC-4 Using Pineapple Crown and Determination of Its Biochemical Properties. Processes 2024, 12, 2255. https://doi.org/10.3390/pr12102255
Batista RD, Carvalho do Nascimento G, Carvalho NB, Leite PC, Basso RC, Morales SAV, Xavier MdCA, Perna RF, Almeida AFd. Cost-Effective Optimization of the Transfructosylation Activity of an Invertase Produced from Aspergillus carbonarius PC-4 Using Pineapple Crown and Determination of Its Biochemical Properties. Processes. 2024; 12(10):2255. https://doi.org/10.3390/pr12102255
Chicago/Turabian StyleBatista, Ryhára Dias, Gustavo Carvalho do Nascimento, Nayara Bezerra Carvalho, Paula Candido Leite, Rodrigo Correa Basso, Sergio Andres Villalba Morales, Michelle da Cunha Abreu Xavier, Rafael Firmani Perna, and Alex Fernando de Almeida. 2024. "Cost-Effective Optimization of the Transfructosylation Activity of an Invertase Produced from Aspergillus carbonarius PC-4 Using Pineapple Crown and Determination of Its Biochemical Properties" Processes 12, no. 10: 2255. https://doi.org/10.3390/pr12102255
APA StyleBatista, R. D., Carvalho do Nascimento, G., Carvalho, N. B., Leite, P. C., Basso, R. C., Morales, S. A. V., Xavier, M. d. C. A., Perna, R. F., & Almeida, A. F. d. (2024). Cost-Effective Optimization of the Transfructosylation Activity of an Invertase Produced from Aspergillus carbonarius PC-4 Using Pineapple Crown and Determination of Its Biochemical Properties. Processes, 12(10), 2255. https://doi.org/10.3390/pr12102255






